Abstract
Exposure to epinephrine has been shown to have a range of effects on cells and tissues. A recent study suggested that the proliferative ability of CF epithelial cells, exposed to high concentrations of epinephrine (200–300 μM), was reduced when compared to that of normal cells. This approach could potentially provide a means to effectively separate cells with functional cyclic AMP-dependent Cl-ion transport from those defective in this pathway.
The sensitivity to killing by epinephrine is reported here for four different CF cell lines, three normal cell lines, and two CF epithelial cell lines complemented with wild-type (wt) CF transmembrane conductance regulator (CFTR) cDNA.
While each cell line exhibited varying sensitivity to 200 μM epinephrine, no predictable pattern was observed between the expression of wt-CFTR and cell survival following epinephrine exposure. Overall, normal cell lines did exhibit a greater resistance to epinephrine-induced cell death although, the most resistant cell line was derived from CF tracheal epithelium (ΣCFTE29o-).
The expression of exogenous wt-CFTR increased the survival of one cell line (CFDEo-) when compared to the parent line, but in another complemented line, survival was reduced.
These findings suggest that while epinephrine induces cell killing, it is not consistently effective for preferential selection of normal over CF cells. Although CFTR may play a role in the mechanism(s) of epinephrine killing, other factors such as cell density, proliferative ability, cell type origin and phenotype are involved.
Keywords: Cell selection, gene therapy, adrenergic receptor, adrenaline, cell proliferation
Introduction
Gene therapy for cystic fibrosis (CF) has progressed rapidly since the isolation of the cystic fibrosis transmembrane conductance regulator (CFTR) gene (Alton et al., 1992; Boucher, 1996; Wagner & Gardner, 1997; Wilson, 1995). Restoration of the cyclic AMP-dependent Cl-ion transport defect associated with CF has been achieved through complementation with expression vectors or recombinant viral constructs containing wild-type (wt) CFTR cDNA (Caplen et al., 1995; Crystal et al., 1994; Zabner et al., 1993) and gene targeting (Goncz & Gruenert, 1999; Goncz et al., 1998; Kunzelmann et al., 1996). Currently, the success of gene complementation and the detection of corrected cells both in vitro (Egan et al., 1992; Ferkol et al., 1993; Johnson et al., 1992; Lei et al., 1996; Rich et al., 1990) and in vivo (Rosenfeld et al., 1992; Yei et al., 1994) is primarily assessed using ion transport techniques, such as patch clamp or SPQ fluorescence. However, such techniques have been ineffective for use in the separation and/or enrichment of cells with functional cyclic AMP-dependent Cl-ion transport (CFTR+) from a population that contains both CFTR+ and cells defective in cyclic AMP-dependent Cl-transport (CFTR−). Enrichment would facilitate isolation of corrected (CFTR+) cells for further characterization in the assessment of gene therapy analysis.
The presence of selectable marker genes as part of the expression vector or recombinant viral construct used in gene therapy can facilitate enrichment. However, in the absence of such genes, as is the case with CF gene targeting, enriching for and isolating corrected cells has been difficult (Gruenert, 1998). Until recently, the expression of wtCFTR alone did not appear to be sufficient to give CFTR+ cells a proliferative or survival advantage over CFTR− cells. A novel study suggested that exposure to high concentrations (200 μM) of epinephrine can selectively kill cells that are CFTR−, while CFTR+ cells are more resistant to epinephrine induced-killing (Vega et al., 1994). Differential survival of CFTR− and CFTR+ cells was observed when the exposure time and concentration of epinephrine was varied. This study was the first indication that preferential killing of CFTR− cells could be achieved when grown concomitantly with CFTR+ cells.
The studies reported here test the hypothesis that epinephrine selection can be used to enrich for CFTR+ cells as indicated previously (Vega et al., 1994). In addition to the 56FHTE8o- cell line used in the previous study, a number of other normal as well as CF epithelial cell lines were used. The cytotoxic effects of epinephrine treatment were determined in normal: CFTR+ [(56FHTE8o-, 9HTEo-, 16HBE14o-)], CF:CFTR− [(ΣCFTE29o-, CFBE41o-, CFDEo-, CFSMEo-)] and CF expressing wild-type (wt) CFTR:CFTR+ [(CFDEo-/pREP-CFTR, ΣCFTR29o-/pREP-CFTR,)] human airway epithelial cells and did not necessarily correlate with the presence of wtCFTR.
Methods
Tissue culture
Transformed human epithelial cell lines derived from the airways of non-CF (CFTR+) and CF (CFTR−) individuals were used. The 56FHTE8o-, 16HBE14o-, and 9HTEo- are normal cell lines and show intact cyclic AMP-dependent Cl-transport (Cozens et al., 1992, 1994). The genotype of the CF cell lines are as follows: ΣCFTE 29o- (Gruenert et al., 1988) and CFBE41o- (unpublished data) are homozygous for the ΔF508 allele (ΔF508/ΔF508), CFSMEo- (Kunzelmann et al., 1993) is compound heterozygote with one unknown CFTR mutation (ΔF508/?), CFDEo- (Chao et al., 1994; Lei et al., 1996) is a non-ΔF508 CF cell line with unknown CFTR mutations (?/?). All the CF cell lines are defective in cyclic AMP-dependent Cl-transport. Two of the CFTR− cell lines (ΣCFTE29o- and CFDEo-) were complemented with wt-CFTR cDNA cloned into an Epstein-Barr virus based episomal expression vector (pREP4β, Invitrogen) under the regulation of the Rous Sarcoma Virus (RSV) promoter. Both corrected cell lines display cyclic AMP-dependent Cl-transport as determined by 36Cl− efflux (Lei et al., 1996) and/or SPQ (unreported observations).
All cells were grown in Eagle's Minimal essential medium (MEM) supplemented with 10% foetal calf serum, L-glutamine and penicillin/streptomycin at 37°C under 5% CO2. The complemented cell lines (CFDEo-/pREP-N and ΣCFTE29o-/pREP-N) were grown in 300 μM Hygromycin B. Cultures were routinely grown on tissue culture plastic coated with an extracellular matrix comprised of fibronectin/vitrogen/bovine serum albumin (Gruenert et al., 1990; Lechner & LaVeck, 1985). Growth medium was replaced every other day.
Epinephrine treatment
Experiments were performed at least three times and for each experiment, cells were plated in 60 mm Petri dishes at various densities (5×105 to 3×106) in triplicate (n=3). Complemented cell lines were fed with regular growth medium, without Hygromycin B, 3 days prior to plating. After an initial 24 h incubation that allowed for cells attachment and the start of exponential growth, the growth medium was replaced with serum free medium containing either 100, 200 or 300 μM epinephrine (Biofluids, Rockville, MD, U.S.A.). Control cultures were identical to treated cultures except that the fresh, serum-free growth medium did not contain epinephrine. After a 48 h exposure, treatment was terminated and cells were harvested by trypsinization. The cells were then resuspended in growth medium as single-cell suspensions and counted with a particle counter (Model ZF, Coulter Electronics, Hialeah, FL, U.S.A.).
Cell survival analysis
Cell survival was calculated by dividing the number of cells in treated dishes (S) by the average number of cells in duplicate control dishes (So) at the end of the experiment. The proliferative ability of each cell line was determined by comparing the average number of cells initially plated (Po) with the average number present in control dishes (So) at the end of the experiment. The ratio of these two numbers (So/Po) is expressed as the cell proliferation (CP) index. Values greater than 1 define highly proliferative cells whereas values less than 1 define less proliferative cells.
Mixed cell population
CFTR+ cells (16HBE14o- or 9HTEo-) were seeded at low density in 100 mm dishes and grown until colonies appeared. The position of the colonies was marked on the bottom of the dish. CFTR− cells (ΣCFTE29o- or CFBE41o-) were then plated evenly over the CFTR+ colonies at 70–80% confluence. The cells were then treated with epinephrine (200 μM for 48 h). Control dishes received no epinephrine.
Statistics
Results are expressed as mean±s.e.mean. Unpaired Student's t-tests were performed on the data and the null hypothesis was rejected at P<0.05.
Results
Epinephrine dose response
Initial cell survival analysis for three different concentrations of epinephrine (100, 200 and 300 μM) with two cell lines, 9HTEo- (N/N) and ΣCFTE29o- (ΔF508/ΔF508), defined the sensitivity of both cell lines to epinephrine exposure. After a 48 h exposure, both cell lines showed an increase in sensitivity to killing with increasing epinephrine concentration (Figure 1). Survival for 9HTEo- cells dropped from 64% at 100 μM to 40% at 300 μM, whereas cell survival for ΣCFTE29o- cells showed even greater sensitivity, falling from 78 to 19% over the same range of epinephrine concentrations. However, unlike previous observations (Vega et al., 1994) the CF cell line (ΣCFTE29o-) was more resistant than the normal cell line (9HTEo-) at the 100 and 200 μM concentrations.
Figure 1.
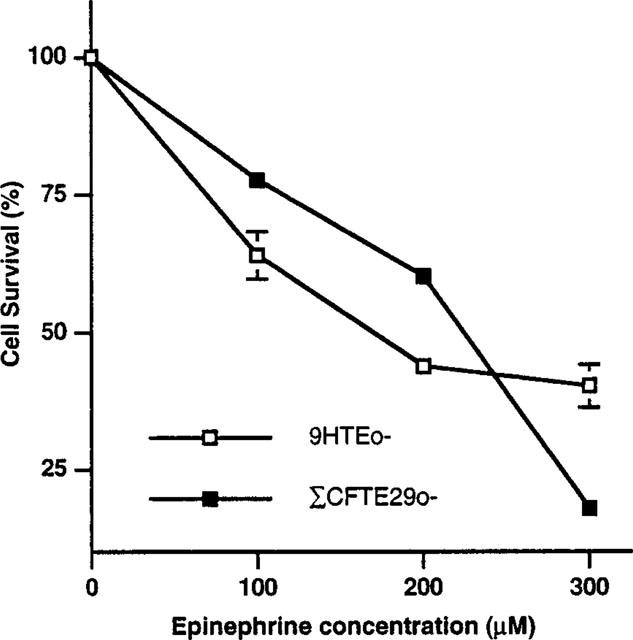
Cell survival of 9HTEo- and ΣCFTE29o- cells exposed to three different concentrations of epinephrine. Cells were initially plated at a density of 200,000 cells per dish and then exposed to epinephrine for 48 h.
Cell survival
Sensitivity to 200 μM epinephrine was determined for all seven cell lines at different cell densities (Figure 2). Overall, cell survival decreased by an average of 70% when compared to untreated controls. From the previous study (Vega et al., 1994), it was expected that normal cells would be significantly more resistant than the CF cells. The average survival for cells with normal genotype, at all of the plating densities, was higher than the average calculated for all the CF cells (37.1±4.7% versus 22.2±4.8%, respectively). However, there is substantial variation in cell survival when each cell line is considered separately.
Figure 2.
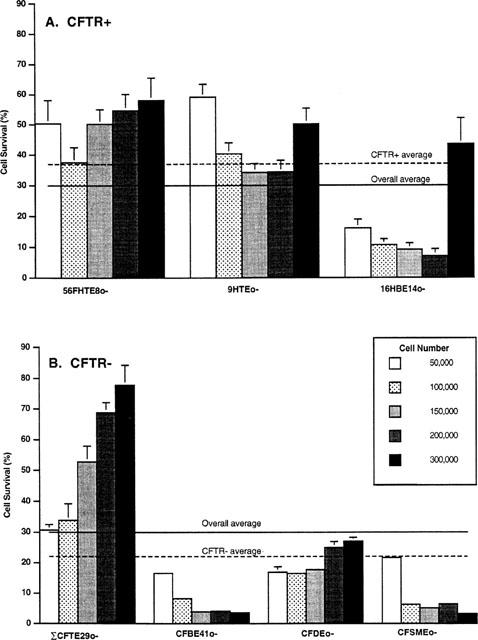
Cell survival of three CFTR+ (56FHTE8o-, 9HTEo-, 16HBE14o-) and four CFTR− (ΣCFTE29o-, CFDEo-, CFBE41o-, CFSMEo-) cell lines exposed to 200 μM epinephrine for 48 h. Cells were plated at five different plating densities. Cell survival for three CFTR+ cell lines (A) and four CFTR− cell lines (B) after a 48 h exposure to 200 μM epinephrine are shown. The average cell survival for all of the cell lines at the different plating densities was approximately 30%. Cells with wtCFTR generally showed a higher cell survival rate than cells with mutant CFTR however, there is considerable variation with regards to both plating density and cell type. Results are from three separate experiments except for the data from ΣCFTE29o- cells, which is combined from six separate experiments.
The most resistant cell line is a CF cell line (ΣCFTE29o-). The average cell survival for all cell densities is approximately 50%. Cell lines ranked from most resistant to least resistant, are as follows: ΣCFTE29o- (52.2±9.1%)>56FHTE8o- (50.2±3.5%.)>9HTEo- (43.7±4.8%)>CFDEo- (20.6± 2.2%.)>16HBE14o- (17.5±6.6%.)>CFSMEo- (8.6± 3.3%.) Σ4CFBE41o- (7.2±2.5%).
Differences in cell survival were observed for the same cell line as a function of the initial plating density (Figure 2). For example, the survival of 16HBE14o- cells plated at a density of 50,000 cells per dish is only 16.1±5.6%. However, when these cells are plated at a 6 fold higher density (300,000 cells per dish), survival almost triples (43.5±16.7%). Evidence for plating density-dependent epinephrine sensitivity can be seen in each cell line to varying degrees. Cell lines that show a significant increase in survival between the lowest and the highest plating densities include the ΣCFTE29o- and CFDEo- cell lines. A significant decrease in survival with increasing density was observed for the CFBE41o- and CFSMEo- cell lines. The 16HBE14o- and 9HTEo- cell lines also exhibited a significant decrease in survival with increasing plating density except at the highest plating density when cell survival was statistically similar (9HTEo-) or greater than (16HBE14o-) the lowest plating density. Only one cell line (56FHTE8o-) was relatively unaffected by cell density and cell survival remained the same (P<0.05) for all but one plating density (100,000 cells/well). These results are unlike the previous study (Vega et al., 1994) in which both CFTR+ and CFTR− cells were most sensitive to killing at low cell densities whereas at a higher plating density, cell survival for both types of cells was observed to be 100%.
The density-dependent survival of individual cell lines to epinephrine changes the cell sensitivity ranking according to cell density. While 9HTEo- cells are more resistant to epinephrine killing than ΣCFTE29o- cells at a plating density of 50,000 cells per dish, ΣCFTE29o- cells show a greater resistance to epinephrine-mediated killing than their normal counterparts (9HTEo-) when both cell types are plated at 300,000 cells per dish.
Correction of the CFTR− phenotype
The presence of functional CFTR influences the sensitivity of CFTR− cells to epinephrine. The survival of two CF cell lines (CFDEo- and ΣCFTE29o-) stably transfected with wtCFTR cDNA (CFDEo-/pREP-N and ΣCFTE29o-/pREP-N, respectively), was found to be different from that of the parental lines. Correction of the Cl-transport defect by this complementation was sufficient to render the CFDEo-/pREP-N cells statistically less sensitive to epinephrine at all plating densities (Figure 3A). The observed increase in cell survival was greatest at cell plating densities below 150,000 cells per dish. Under these conditions, cell survival was approximately three times that of uncorrected parental cells. At the higher plating densities, the differences in survival between corrected and uncorrected cells was reduced to a factor of two. This variation in survival can be attributed to the fact that CFDEo- cells are more resistant to epinephrine at higher plating densities whereas plating density does not appear to play a role in the survival of the corrected CFDEo-/pREP-N cells.
Figure 3.
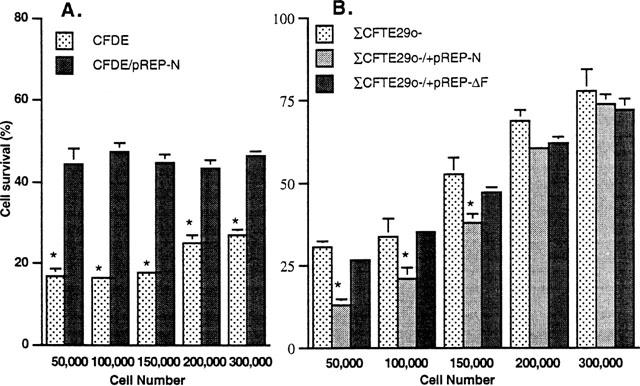
Cell survival analysis of CF cell lines complemented with wtCFTR as compared to parental cell lines after exposure to 200 μM epinephrine for 48 h. Results for (A) CFDEo- cells and (B) ΣSCFTE29o- cells plated at five different cell densities are shown. The presence of functional CFTR in the CFDEo- cell line statistically improved cell survival at all of the plating densities. This was not the case with the ΔCFTE29o- cell line. In fact, complementation at a plating density of 150,000 cells per dish and lower resulted in a statistically lower survival. Survival of ΣCFTE29o- cells stably transfected with cDNA encoding for Δ508 (pREP-ΔF) was not statistically different from the parental ΣCFTE29o- cell lines. Results are from three separate experiments, except for the data from ΣCFTE29o- cells, which is combined from six separate experiments. Asterisks indicate a statistically significant difference (P<0.05) in survival between CFTR− and CFTR+ cells at the respective population densities.
Expression of wtCFTR had a different effect in the complemented ΣCFTE29o- /pREP-N cells (Figure 3B). At the higher plating densities, there was no statistical difference in cell survival between the corrected and uncorrected parental line. However, at densities of 150,000 cells per dish and lower, corrected cells were statistically more sensitive to epinephrine-mediated killing than the parental ΣCFTE29o- cell line. In addition, both corrected, ΣCFTE29o- /pREP-N, and parental ΣCFTE29o- cells, unlike the CFDEo- cells, show an increase in resistance to epinephrine with increasing cell density.
Preferential selection of CFTR+ over CFTR−
Under specific conditions, it was possible to select CFTR+ clones from a mixed population of CFTR+ and CFTR− cells. Figure 4 shows the results of two cell mixing experiments using 9HTEo-/CFBE41o- and 16HBE14o-/ΣCFTE29o- after a 48 h incubation in the presence of epinephrine. Results from the 9HTEo-/CFBE41o- treated dish (Figure 4B) were as expected, in that at least half of the 9HTEo- colonies survived exposure to 200 μM epinephrine while CFBE41o- cells died. The same killing pattern was observed when CFBE41o- cells were first grown as colonies and 9HTEo- cells plated on top (data not shown). In this case, after epinephrine exposure, the CFBE41o- colonies disappeared and the 9HTEo- cells proliferated.
Figure 4.
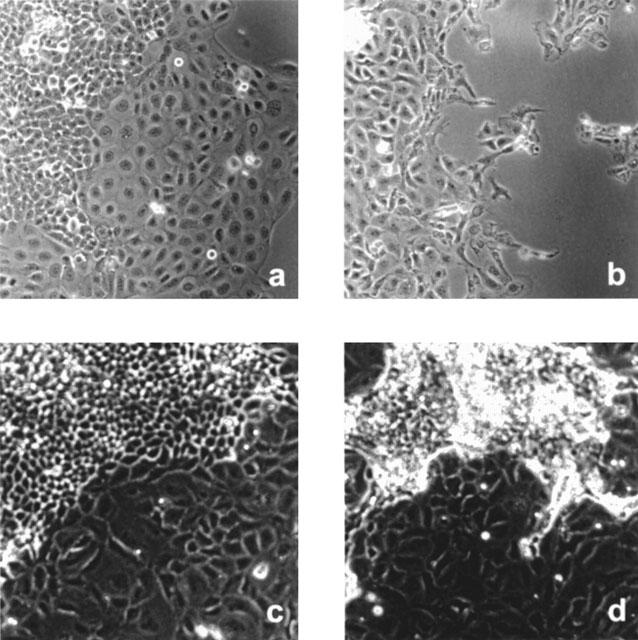
Morphologic analysis of three mixed cultures of CFTR+ and CFTR− cells with and without exposure to 200 μM epinephrine for 48 h. 9HTEo- colonies with CFBE41o- plated over top (A) control and (B) post-epinephrine exposure. 16HBE14o- colonies with ΣCFTE29o- cells seeded over top (C) control and (D) post-epinephrine exposure. As predicted, epinephrine selectively kills the CFTR− cell line (CFBE41o-) whereas the CFTR+ cell line (9HTEo-) remains relatively intact (A). The cells have appeared to swell slightly over controls after exposure. However, similar exposure is also sufficient to selectively kill a CFTR+ (16HBE14o-) cell line when co-plated with another CFTR− cell line (ΣCFTE29o-). The distinct morphological differences between the cell lines is useful for identifying which population of cells is sensitive to epinephrine-induced cell killing. (Original magnification: ×200).
However, using different cell lines, it is also possible to preferentially select CFTR− cells. In the 16HBE14o-/ΣCFTE29o- dish, the majority (88%) of CFTR+ colonies died after exposure to epinephrine whereas there was no obvious decrease in the number or in the proliferative rate of ΣCFTE29o- cells (Figure 4D). This result is consistent with the data presented above (Figure 2). At low density, 16HBE14o- cells are quite sensitive to epinephrine whereas the ΣCFTE29o- cells, when plated at a high density, are insensitive to epinephrine-mediated cell killing.
Cell proliferation
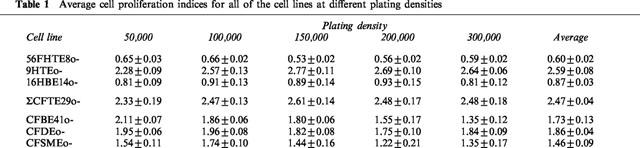
Although all cell lines were initially plated at identical densities, the effective number of cells treated with epinephrine after 24 h varied according to the CP index of a given cell line (see Methods). A CP index of 1.0 indicates that after 72 h (at the end of the 48 h epinephrine exposure) the number of cells in the experimental dishes were the same as that initially plated. Only two cell lines showed an average CP index of lower than 1.0; the 56FHTE8o- (0.60±0.02) and the 16HBE14o- (0.87±0.03.). The CP index was calculated for each cell line at each plating density (Table 1). As can be seen, the values remain relatively constant and vary little between experiments.
Table 1.
Average cell proliferation indices for all of the cell lines at different plating densities
The dependence of cell survival on proliferation is indicated in Figure 5. There is a direct correlation between these two parameters for six out of seven cell lines. Cells with low CP indices exhibit lower cell survival, while cells with high CP indices have increased cell survival after exposure to epinephrine. The same correlation was not observed for 56FHTE8o- cells. This cell line has the lowest CP index, yet cell survival is average after epinephrine exposure.
Figure 5.
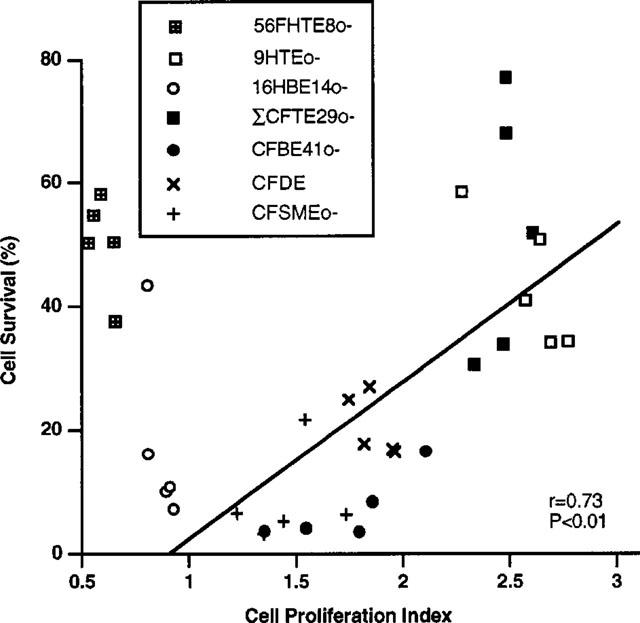
Cell survival after exposure to epinephrine as a function of the cell proliferation (CP) index of each cell line for all of the plating densities. Standard regression analysis indicates a correlation between cell survival and CP for six out of the seven cell lines (with the exception of a data point corresponding to 16HBE14o- cells plated at a density of 300,000). The 56FHTE8o- cell line differs from the other cell lines in that it is derived from foetal tissue.
Discussion
The development of human airway epithelial cell lines has been important for enhancing the understanding of cell biology, CF and airway disease processes (Gruenert et al., 1990; Hopfer et al., 1996). Such cell lines have been critical in characterizing the efficacy of CF gene therapy strategies and the mechanisms whereby exogenous DNA is delivered to the nucleus. However, since there appears to be no obvious difference between the proliferative potential of CF and normal epithelial cells, it has not been possible to selectively enrich for either type of cell in gene therapy experiments.
Research in development of the SFHR gene targeting strategy for correction of CFTR mutations (Goncz & Gruenert, 1999; Goncz et al., 1998; Kunzelmann et al., 1996) would be greatly facilitated by a method that selectively enriches for normal cells over CF cells. The initial report that exposure to epinephrine would cause selective killing of CF cells (Vega et al., 1994) was of particular interest. To ascertain whether this approach was generally applicable, several normal and CF epithelial cell lines developed within the laboratory and, currently utilized by a number of researchers, were evaluated. The results presented here cannot confirm a direct correlation between the expression of wtCFTR and resistance to epinephrine-induced cell killing. Treatment with epinephrine (200 μM), previously shown to be preferentially cytotoxic for CF cells (CFTR−), was not consistently detrimental to cells that did not express wtCFTR. Therefore, the presence or absence of wtCFTR alone cannot be used as a criterion for epinephrine sensitivity.
Reduced or defective Cl-transport due to defective CFTR cannot be completely ruled out as contributing to epinephrine-mediated cell death. Results from experiments on corrected CFDEo-/pREP-N cells suggest that CFTR-mediated Cl-transport may influence cell survival as corrected cells had better survival after exposure to epinephrine as compared to their parental counterparts. However, the observation that corrected ΣCFTE29o- cells displayed more sensitivity to epinephrine, indicates that the presence of wtCFTR does not always improve cell survival.
It is possible that the activity of other ion channels stimulated by epinephrine could account for the observed results. Epinephrine induces a number of salt currents in a variety of cells and tissues (Galietta et al., 1991; Greger et al., 1996; Halm et al., 1993; Hawk et al., 1993; Hyun et al., 1994). This stimulatory process occurs via both β- and α-adrenergic receptors present in surface and submucosal gland epithelial cells from both normal and cystic fibrosis subjects (Merten et al., 1993; Welsh, 1987; Widdicombe, 1986). As such, cell survival is dependent on the coordination of a number of ion fluxes (Haas et al., 1995; McCann & Welsh, 1990). Adrenergic stimulation of other permeability pathways that result in asynchronous activation of a salt influx and/or efflux could cause an imbalance in cell osmolytes and ultimately change cell volume culminating in cell death (Halm & Halm, 1994). Accumulation of calcium and sodium after epinephrine exposure in dog cardiac cells (Lee et al., 1980) and in goldfish melanocytoma cells (Shih & Lo, 1993) has been shown to be detrimental after exposure to epinephrine. Thus, it is possible that the differential sensitivity to epinephrine observed in this study is due to asynchronous stimulation of different ion channels in individual cell lines. Differences in ion channel composition have been observed in cells from different origins. Resistance to epinephrine could also be attributed to the presence of ion channels that compensate for defective cyclic AMP-stimulated Cl-efflux and could explain why cell lines derived from tracheal origin (ΣCFTE29o-, 56FHTE8o- and 9HTEo-) were more resistant to epinephrine than bronchial derived cell lines (16HBE14o- and CFBE41o-). The specific phenotypic attributes of individual clones, such as cell polarity, may also play a role in defining ion channel composition, distribution and the resultant epinephrine sensitivity.
The most direct result from this study is the correlation between cell proliferation and sensitivity to epinephrine. Cells exhibiting a lower proliferative potential, as determined by CP index, are more sensitive to epinephrine-induced killing than those with a higher CP index. The notion that cell viability plays a role in cell survival after epinephrine exposure has been found in other experiments (Brenner et al., 1987). The only exception to this pattern was the 56FHTE8o- cell line. This cell line is different from the other six in that it is derived from foetal tissue. Interestingly, this was the only naturally occurring CFTR+ cell line used in the previous study (Vega et al., 1994). The differences between the results presented here and the previous study could be attributed to differences in experimental methodology and/or the limited scope of the previous study.
In conclusion, differences in epinephrine sensitivity appear to be the result of cell origin and/or the phenotypic characteristics of an individual clone and, that the proliferative potential of a given epithelial cell line is a better indicator for sensitivity to epinephrine than the presence of wtCFTR. While it was possible to isolate a normal cell line (9HTEo-) from a mixed population of normal and CF (CFBE41o-) cells, selection was only possible because the cells grow in morphologically distinct colonies and because the differences in sensitivity to epinephrine were large. Such differences would not be found in vivo or in cell culture because the corrected and parental cell lines share the same morphological features. Furthermore, epinephrine can be used to select for CF cells (ΣCFTE29o-) by preferentially killing normal (16HBE14o-) cells. While the differential sensitivity of cells to epinephrine is inherently interesting for airway epithelial cell biology, especially in treatment of asthma and emphysema (Green et al., 1994), there is as yet, no significant evidence to conclude that exposure to high doses of epinephrine will be useful to select cells based on cyclic AMP-dependent Cl-transport. Further study will be necessary to elucidate the mechanisms underlying the observed differences in epinephrine sensitivity.
Acknowledgments
Drs Karim Dabbagh and Alessia Colosimo are thanked for helpful comments and discussions regarding the manuscript. This work was supported by grants from the National Institutes of Health DK46002, American Cystic Fibrosis Foundation, Cystic Fibrosis Research Inc. DK47766 (D.C. Gruenert), and DK09392 (K.K. Goncz).
Abbreviations
- cyclic AMP
3′,5′ cyclic adenosine monophosphate
- CF
cystic fibrosis
- CFTR
cystic fibrosis transmembrane conductance regulator
- SPQ
6-methoxy-N-(3-sulphopropyl)quinolinium
References
- ALTON E., CAPLEN N., GEDDES D., WILLIAMSON R. New treatments for cystic fibrosis. Nature. 1992;360:785–804. doi: 10.1093/oxfordjournals.bmb.a072578. [DOI] [PubMed] [Google Scholar]
- BOUCHER R.C. Current status of CF gene therapy. Trends Genet. 1996;12:81–84. doi: 10.1016/0168-9525(96)81410-7. [DOI] [PubMed] [Google Scholar]
- BRENNER D.J., ZAIDER M., GEARD C.R., GEORGSSON M.A. Cell survival and plating efficiency. Rad. Res. 1987;111:572–576. [PubMed] [Google Scholar]
- CAPLEN N.J., ALTON E.W., MIDDLETON P.G., DORIN J.R., STEVENSON B.J., GAO X., DURHAM S.R., JEFFERY P.K., HODSON M.E., COUTELLE C., HUANG L., PORTEOUS D.J., WILLIAMSON R., GEDDES D.M. Liposome-mediated CFTR gene transfer to the nasal epithelium of patients with cystic fibrosis. Nature Med. 1995;1:39–46. doi: 10.1038/nm0195-39. [DOI] [PubMed] [Google Scholar]
- CHAO A.C., ZIFFERBLATT J.B., WAGNER J.A., DONG Y.-J., GRUENERT D.C., GARDNER P. Stimulation of chloride secretion by P1 purinoceptor agonist in cystic fibrosis phenotype airway epithelial cell line CFPEo- Br. J. Pharmacol. 1994;112:169–175. doi: 10.1111/j.1476-5381.1994.tb13047.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- COZENS A.L., YEZZI M.J., CHIN L., FINKBEINER W.E., WAGNER J.A., GRUENERT D.C. Characterization of immortal cystic fibrosis tracheobronchial gland epithelial cells. Proc. Natl. Acad. Sci. U.S.A. 1992;89:5171–5175. doi: 10.1073/pnas.89.11.5171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- COZENS A.L., YEZZI M.J., KUNZELMANN K., OHRUI T., CHIN L., ENG K., FINKBEINER W.E., WIDDICOMBE J.H., GRUENERT D.C. CFTR expression and chloride secretion in polarized immortal human bronchial epithelial cells. Am. J. Respir. Cell. Mol. Biol. 1994;10:38–47. doi: 10.1165/ajrcmb.10.1.7507342. [DOI] [PubMed] [Google Scholar]
- CRYSTAL R.G., MCELVANEY N.G., ROSENFELD M.A., CHU C.-S., MASTRANGELI A., HAY J.G., BRODY S.L., JAFFE H.A., EISSA N.T., DANEL C. Administration of an adenovirus containing the human CFTR cDNA to the respiratory tract of individuals with cystic fibrosis. Nature Genet. 1994;8:42–51. doi: 10.1038/ng0994-42. [DOI] [PubMed] [Google Scholar]
- EGAN M., FLOTTE T., AFIONE S., SOLOW R., ZEITLIN P.L., CARTER B.J., GUGGINO W.B. Correction of defective PKA regulation of outwardly rectifying chloride channels by insertion of cystic fibrosis transmembrane regulator into CF airway epithelial cells. Nature. 1992;358:581–584. doi: 10.1038/358581a0. [DOI] [PubMed] [Google Scholar]
- FERKOL T., KAETZEL C.S., DAVIS P.B. Gene transfer into respiratory epithelial cells by targeting the polymorphic immunoglobulin receptor. J. Clin. Invest. 1993;92:2394–2400. doi: 10.1172/JCI116845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GALIETTA L.J., BARONE V., GRUENERT D.C., ROMEO G. A chloride conductance evoked by hypotonic shock in epithelial cells. Adv. Exp. Med. Biol. 1991;290:307–316. doi: 10.1007/978-1-4684-5934-0_29. [DOI] [PubMed] [Google Scholar]
- GONCZ K.K., GRUENERT D.C.Site-directed alteration of DNA by small fragment homologous replacement (SFHR) Gene Targeting Vector Protocols 1999Humana Press: Totowa, N.J; 85–99.In: Gruenert, D.C. & Kmiec, E.B. (eds) [DOI] [PubMed] [Google Scholar]
- GONCZ K.K., KUNZELMANN K., XU Z., GRUENERT D.C. Targeted replacement of normal and mutant CFTR sequences in human airway epithelial cells using DNA fragments. Hum. Mol. Genet. 1998;7:1913–1919. doi: 10.1093/hmg/7.12.1913. [DOI] [PubMed] [Google Scholar]
- GREEN S.A., TURKI J., INNES M., LIGGETT S.B. Amino terminal polymorphisms of the human β2-adrenergic receptor impart distinct agonist-promoted regulatory properties. Biochemistry. 1994;33:9414–9419. doi: 10.1021/bi00198a006. [DOI] [PubMed] [Google Scholar]
- GREGER R., MALL M., BLEICN M., ECKE D., WARTH R., RIEDEMANN N., KUNZELMANN K. Regulation of epithelial ion channels by the cystic fibrosis transmembrane conductance regulator. J. Mol. Med. 1996;74:527–534. doi: 10.1007/BF00204979. [DOI] [PubMed] [Google Scholar]
- GRUENERT D.C. Gene correction with small DNA fragments. Curr. Res. Mol. Ther. 1998;1:607–613. [Google Scholar]
- GRUENERT D.C., BASBAUM C.B., WELSH M.J., LI M., FINKBEINER W.E., NADEL J.A. Characterization of human tracheal epithelial cells transformed by an origin-defective simian virus 40. Proc. Natl. Acad. Sci. U.S.A. 1988;85:5951–5955. doi: 10.1073/pnas.85.16.5951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GRUENERT D.C., BASBAUM C.B., WIDDICOMBE J.H. Long-term culture of normal and cystic fibrosis epithelial cells grown under serum-free conditions. In Vitro Cell. Dev. Biol. 1990;26:411–418. doi: 10.1007/BF02623833. [DOI] [PubMed] [Google Scholar]
- HAAS M., MCBRAYER D., LYTLE C. [Cl−]i-dependent phosphorylation of the Na-K-Cl cotransport protein of dog tracheal epithelial cells. J. Biol. Chem. 1995;270:28955–28961. doi: 10.1074/jbc.270.48.28955. [DOI] [PubMed] [Google Scholar]
- HALM D.R., HALM S.T. Aldosterone stimulates K+ secretion prior to onset of Na absorption in guinea pig distal colon. Am. J. Physiol. 1994;266:C552–C558. doi: 10.1152/ajpcell.1994.266.2.C552. [DOI] [PubMed] [Google Scholar]
- HALM D.R., KIRK K.L., SATHIAKUMAR K.C. Stimulation of Cl permeability in colonic crypts of Lieberkuhn measured with a fluorescent indicator. Am. J. Physiol. 1993;265:G423–G431. doi: 10.1152/ajpgi.1993.265.3.G423. [DOI] [PubMed] [Google Scholar]
- HAWK C.T., KUDO L.H., ROUCH A.J., SCHAFER J.A. Inhibition by epinephrine of AVP- and cAMP-stimulated Na+ and water transport in Dahl rat CCD. Am. J. Physiol. 1993;265:F448–F460. doi: 10.1152/ajprenal.1993.265.3.F449. [DOI] [PubMed] [Google Scholar]
- HOPFER U., JACOBBERGER J.W., GRUENERT D.C., ECKERT R.L., JAT P.S., WHITSETT J.A. Immortalization of epithelial cells. Am. J. Physiol. 1996;270:C1–C11. doi: 10.1152/ajpcell.1996.270.1.C1. [DOI] [PubMed] [Google Scholar]
- HYUN C.S., JIYONG A., MINHAS B.S., CRAGOE J., FIELD M. Ion transport in rabbit proximal colon: effects of sodium, amiloride, cAMP, and epinephrine. Am. J. Physiol. 1994;29:G1071–G1082. doi: 10.1152/ajpgi.1994.266.6.G1071. [DOI] [PubMed] [Google Scholar]
- JOHNSON L.G., OLSEN J.C., SARKADI B., MOORE K.L., SWANSTROM R., BOUCHER R.C. Efficiency of gene transfer for restoration of normal airway epithelial function in cystic fibrosis. Nature Genet. 1992;2:21–25. doi: 10.1038/ng0992-21. [DOI] [PubMed] [Google Scholar]
- KUNZELMANN K., LEGENDRE J.-Y., KNOELL D.L., ESCOBAR L.C., XU Z., GRUENERT D.C. Gene targeting of CFTR DNA in CF epithelial cells. Gene Therapy. 1996;3:859–867. [PubMed] [Google Scholar]
- KUNZELMANN K., SCHWIEBERT E., KUO W.-L., STANTON B.A., GRUENERT D.C. An immortalized cystic fibrosis tracheal epithelial cell line homozygous for the ΔF508 CFTR mutation. Am. J. Respir. Cell. Mol. Biol. 1993;8:522–529. doi: 10.1165/ajrcmb/8.5.522. [DOI] [PubMed] [Google Scholar]
- LECHNER J.F., LAVECK M.A. A serum-free method for culturing normal human bronchial epithelial cells at clonal density. J. Tiss. Cult. Meth. 1985;9:43–48. [Google Scholar]
- LEE W.-K., HAIDER B., AHMED S.S., OLDEWURTEL H.A., LYONS M.M., REGAN T.J. Cell sodium and the induction of myocardial injury after adrenaline. Cardiovasc. Res. 1980;14:661–670. doi: 10.1093/cvr/14.11.661. [DOI] [PubMed] [Google Scholar]
- LEI D.C., KUNZELMANN K., KOSLOWSKY T., YEZZI M.J., ESCOBAR L.C., XU Z.D., ROMMENS J.M., TSUI L.-C., TYKOCINSKI M., GRUENERT D.C. Episomal expression of wild-type CFTR corrects cAMP-dependent chloride transport in respiratory epithelial cells. Gene Therapy. 1996;3:427–436. [PubMed] [Google Scholar]
- MCCANN J.D., WELSH M.J. Regulation of Cl− and K+ channels in airway epithelium. Ann. Rev. Physiol. 1990;52:115–135. doi: 10.1146/annurev.ph.52.030190.000555. [DOI] [PubMed] [Google Scholar]
- MERTEN M. D., TOURNIER J.-M., MECKLER Y., FIGARELLA C. Epinephrine promotes growth and differentiation of human tracheal gland cells in culture. Am. J. Resp. Cell Mol. Biol. 1993;9:172–178. doi: 10.1165/ajrcmb/9.2.172. [DOI] [PubMed] [Google Scholar]
- RICH D.P., ANDERSON M.P., GREGORY R.J., CHENG S.H., PAUL S., JEFFERSON D.M., MCCANN J.D., KLINGER K.W., SMITH A.E., WELSH M.J. Expression of cystic fibrosis transmembrane conductance regulator corrects defective chloride channel regulation in cystic fibrosis airway epithelial cells [see comments] Nature. 1990;347:358–363. doi: 10.1038/347358a0. [DOI] [PubMed] [Google Scholar]
- ROSENFELD M.A., YOSHIMURA K., TRAPNELL B.C., YONEYAMA K., ROSENTHAL E.R., DALEMANS W., FUKAYAMA M., BARGON J., STIER L.E., STRATFORD P.L. In vivo transfer of the human cystic fibrosis transmembrane conductance regulator gene to the airway epithelium. Cell. 1992;68:143–155. doi: 10.1016/0092-8674(92)90213-v. [DOI] [PubMed] [Google Scholar]
- SHIH Y.L., LO S.J. Induction of cell expansion of goldfish melanocytoma cells (GMM-1) by epinephrine and dexamethasone requires external calcium. Cell Biol. Int. 1993;17:533–542. doi: 10.1006/cbir.1993.1095. [DOI] [PubMed] [Google Scholar]
- VEGA M.A., GOOSENS M., BESMOND C. A powerful method for in vitro selection of normal versus cystic fibrosis airway epithelial cells. Gene Therapy. 1994;1:59–63. [PubMed] [Google Scholar]
- WAGNER J.A., GARDNER P. Toward cystic fibrosis gene therapy. Ann. Rev. Med. 1997;48:203–216. doi: 10.1146/annurev.med.48.1.203. [DOI] [PubMed] [Google Scholar]
- WELSH J.M. Electrolyte transport by airway epithelium. Physiol. Rev. 1987;67:1143–1184. doi: 10.1152/physrev.1987.67.4.1143. [DOI] [PubMed] [Google Scholar]
- WIDDICOMBE J. Cystic fibrosis and ß-adrenergic response of airway epithelial cell cultures. Am. J. Physiol. 1986;251:R818–R822. doi: 10.1152/ajpregu.1986.251.4.R818. [DOI] [PubMed] [Google Scholar]
- WILSON J.M. Gene therapy for cystic fibrosis: challenge and future directions. J. Clin. Invest. 1995;96:2547–2554. doi: 10.1172/JCI118318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- YEI S., MITTEREDER N., TANG K., O'SULLIVAN C., TRAPNELL B.C. Adenovirus-mediated gene transfer for cystic fibrosis: quantitative evaluation of repeated in vivo vector administration to the lung. Gene Therapy. 1994;1:192–200. [PubMed] [Google Scholar]
- ZABNER J., COULTURE L.A., GREGORY R.J., GRAHAM S.M., SMITH A.E., WELSH M.J. Adenovirus-mediated gene transfer transiently corrects the chloride transport defect in nasal epithelia of patients with cystic fibrosis. Cell. 1993;75:207–216. doi: 10.1016/0092-8674(93)80063-k. [DOI] [PubMed] [Google Scholar]


