Abstract
The entorhinal cortex (EC), main input structure to the hippocampus, gets innervated by serotonergic terminals from the raphe nuclei and expresses 5-HT-receptors at high density. Using extra- and intracellular recording techniques we here investigated the effects of serotonin on population and cellular responses within the EC.
Stimulation in the lateral entorhinal cortex resulted in complex field potential responses in the superficial EC. The potentials are composed of an early antidromic and a late orthodromic component reflecting the efferent and afferent circuitry.
Serotonin (5-HT) reduced synaptic potentials of the stimulus evoked extracellular field potential at all concentrations tested (0.1–100 μM; 59%-depression by 10 μM serotonin), while the antidromic response was not significantly changed by up to 50 μM 5-HT. Depression of field potential responses by serotonin was associated with a significant increase in paired-pulse facilitation from 1.15 to 1.88.
The effects of serotonin on field potential responses were mimicked by 5-HT1A-receptor agonists (8-OH-DPAT, 5-CT) and partially prevented by the 5-HT1A-receptor antagonist (S-UH-301). Moreover, the 5-HT1A-receptor antagonist WAY100635 reduced the effect of 5-CT.
Fenfluramine, a serotonin releaser, mimics the effects of serotonin on stimulus-evoked field potential responses, indicating that synaptically released serotonin can produce the changes in reactivity to afferent stimulation.
Depression of isolated AMPA-receptor mediated EPSCs by serotonin as well as fenfluramine was associated with an increase in paired pulse facilitation, indicating a presynaptic locus of action.
We conclude that physiological concentrations of serotonin potently suppresses excitatory synaptic transmission in the superficial entorhinal cortex by a presynaptic mechanism.
Keywords: Serotonin, entorhinal cortex, synaptic transmission, fenfluramine, paired pulse
Introduction
The entorhinal cortex (EC) is the major gateway for sensory information both to and from the hippocampus (Alonso & Köhler, 1984). Far from simply being a passive funnel for these in- and outputs, it is becoming clearer that the EC has its own active properties that contribute to signal processing (Insausti et al., 1993; Jones, 1993). The perforant path, which originates in the superficial layers of the EC provides an input from layer II cells to the dentate gyrus and CA3 regions and from layer III cells to CA1 and the subiculum (Steward & Scoville, 1976).
In addition to the large amount of information from the cortical mantle the EC receives numerous afferents from brain stem regions. The serotonergic system attracts attention in two terms. First, the cells of the superficial layers of the EC receive a strong serotonergic projection from the raphe nuclei (Bobillier et al., 1975) and also express a high density of 5-HT receptors (Pazos et al., 1985; Pazos & Palacios, 1985). Second, the EC shows characteristic lesions in certain forms of schizophrenia, Alzheimer's and Korsakow's disease which are associated with changes in serotonergic transmission and serotonin turnover (Langlais et al., 1987; Tejani-Butt et al., 1995).
Therefore, we here investigated the effects of serotonin on neuronal activity in the superficial layers of the entorhinal cortex.
Methods
Slice preparation
Horizontal slices containing the hippocampus, entorhinal, perirhinal and temporal cortices were prepared from adult Wistar rats (>6 weeks; 180–250 g). In brief, the animals were deeply anaesthetized with ether, decapitated and the brain removed. Tissue blocks containing the temporal cortex and hippocampus were mounted on a Vibratome (Campden Instruments, Loughborough, U.K.) in a chamber filled with cold (∼4°C) artificial cerebrospinal fluid, ACSF, containing (in mM): NaCl, 129; NaHCO3, 21; KCl, 3; NaH2PO4, 1.25; CaCl2, 1.6; MgSO4, 1.8; glucose, 10, saturated with 95% O2-5% CO2, pH 7.4. Horizontal slices were cut at 400 μm thickness and transferred to an interface chamber where they were maintained at 35°C and perfused with ACSF at a rate of 1.5–1.8 ml min−1. The slices were allowed to rest for at least 1 h after the preparation before recording.
Electrophysiological recordings
For extracellular field potential recordings, electrodes were pulled from borosilicate glass (o.d.: 1.5 mm or 2.0 mm) and filled with 154 mM NaCl. Intracellular electrodes were pulled from borosilicate glass (o.d.: 1.2 mm) and filled with 2 M K-acetate and 50 μM of the lidocaine derivate QX-314 (blocker of Na+- and K+-channels). Electrode resistances were especially low (20–50 MΩ) to enhance the diffusion of QX-314. Extra- and intracellular recordings were performed in an interface chamber using a home-made ion-sensitive amplifier and a SEC10L-amplifier (NPI Instruments, Tamm, Germany), respectively. The presence of QX-314 led to a blockade of action potentials in the cells 2–10 min after impalement. In voltage clamp experiments the cells were at first clamped close to resting membrane potential, the gain, capacity compensation and switching frequency (20–38 kHz) were then optimised. The head stage voltage was continuously monitored and always fully decayed before the next current injection during a ¼ duty cycle. Clamp efficiency estimated from the difference between the clamped and unclamped EPSP ranged from 85–93% (89.2±1.4%). Compound polysynaptic potentials were evoked by electrical stimulation (0.05 ms duration, 1–30 V) at 0.1–0.5 Hz via a bipolar insulated stimulation electrode placed in the lateral entorhinal cortex. We used a stimulation intensity of 70–80% of that required to evoke maximal amplitude response. The signals were filtered at 3 kHz, digitised at 8–10 kHz by a CED 1401 (Cambridge Electronic Design, Cambridge, U.K.) or an ITC-16 (Instrutech Corp. NY, U.S.A.) interface and subsequently stored on an IBM-compatible computer.
Drugs and solutions
Drugs were bath-applied at the concentrations indicated. Bicuculline methiodide (5 μM), fenfluramine (FFA, 200 μM) and 5-hydroxytryptamine creatine sulphate complex (5-HT, 0.1–100 μM) were purchased from SIGMA (Deisenhofen, Germany). (±)-2-amino-5-phosphonopentanoic acid (APV, 60 μM), (±)8-hydroxy-2-(di-n-propylamino)tetralin HBr (8-OH-DPAT, 10, 100 μM), CGS-12066A maleate (10 μM), 2-methyl-5-hydroxytryptamine maleate (50 μM), αmethyl-5-hydroxytryptamine maleate (50 μM), 5-carboxamido-tryptamine maleate (5-CT, 1, 10 μM), S-5-fluoro-8-hydroxy-DPAT hydrochloride (S-UH-301, 20 μM), ritanserin (10, 100 μM), clozapine (10, 100 μM), methysergide maleate (10 μM) and WAY100635 (40 μM) were all from Research Biochemicals (Natick, MA, U.S.A.). 1,2,3,4-tetrahydro-6-Nitro-2,3-dioxo-benzol[f]quinoxaline-7-sulphonamide (NBQX, 10 μM) was a kind gift from Novo Nordish (Denmark) and CGP55845A (2 μM) was a kind gift from Ciba-Geigy (Basel, Switzerland).
Data analysis and statistics
Data were analysed off line using Sigavg (CED, U.K.) or Wintida (HEKA, Germany). Amplitudes of the complex field potentials were measured at different times after the stimulation artefact (see Results). Percentage changes were analysed for the anti- and orthodromic potentials. The orthodromic response was analysed as the sum of the second negative response and the late positive component (see Results and Figure 2). Data are expressed as mean±standard error of the mean. Drug effects were analysed with Student's t-test (Sigmaplot, Jandel, Corte Madera, U.S.A.) for paired data and an error probability of P<0.05 was regarded as significant.
Figure 2.
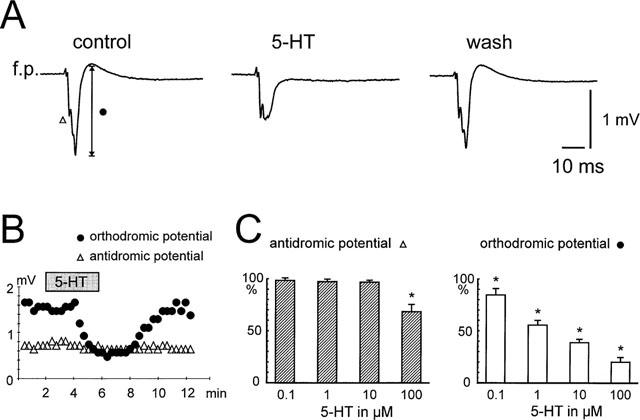
Effects of serotonin on stimulus-induced field potential responses. (A) Serotonin (5-HT, 10 μM) reversibly reduced the synaptic component of the field potential response (circle), while the antidromic response (triangle) were not affected. (B) Time course of the effect of serotonin on the synaptic component and antidromic response of the field potential. (C) Histograms summarizing the effects on both types of responses in different serotonin concentrations (0.1–100 μM). *P<0.05.
Results
Effects of serotonin on stimulus-induced field potentials in the superficial EC
Stimulation in the lateral entorhinal cortex resulted in complex field potential responses in the superficial layers II and III of the medial entorhinal cortex. These potentials are composed of anti- and orthodromic components reflecting the efferent and afferent circuitry of the superficial EC (Figure 1). An early negative component peaked at 2.9±0.2 ms from the stimulus artefact while a second negative response was measured at 5.8±0.3 ms and a late slow positive component at 12.3±0.5 ms. Application of the glutamate-receptor antagonists NBQX and APV blocked both the second negative and the late positive components while the early negative component was unchanged (n=4). Experiments with the NMDA-receptor antagonist APV alone (n=6) resulted in a selective reduction of the late positive response (from 0.34±0.1 mV to 0.1±0.04 mV; P<0.05, Student's paired t-test), whereas the early (−1.2±0.2 mV to −1.3±0.2 mV, P=0.3) and the second (−1.5±0.2 mV to −1.6±0.3 mV, P=0.5) negative responses were not significantly changed. The selective AMPA-antagonist NBQX markedly reduced both the second negative component (−1.7±0.2 mV to −0.1±0.03 mV, n=5, P<0.05) as well as the late positive response (0.34±0.1 mV to −0.04±0.04 mV, P<0.05) whereas the early negative component was not significantly changed (−1.2±0.2 mV to −1.1±0.4 mV, P=0.5, Figure 1).
Figure 1.
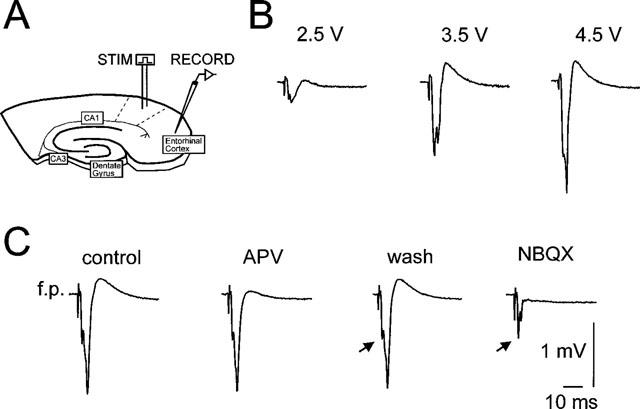
(A) Schematical drawing of the combined hippocampal-entorhinal cortex slice preparation. Extra- and intracellular recordings were made in the superficial medial entorhinal cortex, while synaptic fibres were stimulated in the lateral entorhinal cortex. (B) Field potential responses in dependency of three different stimulus intensities. (C) Effects of APV and NBQX on the shape of the stimulus-evoked field potential responses. Note that APV only affected the positive component of the potential, while NBQX left the early negative response (arrows) unchanged and reduced the second negative response to a great extent.
We tested the effect of serotonin on the complex field potentials in a concentration-range of 0.1–100 μM in 40 slices. Serotonin dose-dependently reduced the second negative response and the later positive component, while the early negative response was not significantly changed by up to 50 μM 5-HT. This component was only significantly affected at a concentration of 100 μM 5-HT. The differential effects of serotonin on the antidromic and orthodromic responses are illustrated in detail in Figure 2.
Pharmacology of the serotonin effect
In order to identify the subtype of the 5-HT-receptor mediating the depression of field potential responses we applied various specific agonists: 8-OH-DPAT and 5-CT (both agonists of the 5-HT1A/7-receptor), CGS-12066 (5-HT1B), α-methyl-5-HT (5-HT2/1C) and 2-methyl-5-HT (5-HT3). However, only 8-OH-DPAT (10, 100 μM) and 5-CT (1, 10 μM) mimicked the effect of 5-HT (Figure 3A and Ba). In contrast, all other specific agonistic drugs (CGS-12066, α-methyl-5-HT, 2-methyl-5-HT) were unable to mimic the serotonin effect (n⩾4 for each substance, P⩾0.1, see histograms in Figure 3B). This result suggests that 5-HT1A- and/or 5-HT7-receptors are involved in the effect of serotonin. The 5-HT7-receptor is a recently identified 5-HT-receptor subtype (Lovenberg et al., 1993; Ruat et al., 1993), which has an agonist profile closely related to the 5-HT1A-receptor (Lovenberg et al., 1993; Ruat et al., 1993). Binding profiles of the 5-HT7-receptor revealed high binding affinities for several antagonists, for example clozapine, ritanserin and methysergide (Lovenberg et al., 1993; Ruat et al., 1993). Therefore, we tested the possible antagonism of these drugs. Methysergide was applied at 10 μM, while clozapine and ritanserin were applied at 10 and 100 μM. However, none of the drugs could antagonize the effect of serotonin (n⩾3 for each substance and each concentration; P⩾0.1, Figure 3Bb). On the other hand, co-application of S-UH-301 (20 μM), a highly potent and selective 5-HT1A-receptor antagonist, with 5-HT (10 μM) reduced the effects of serotonin from 52 to 28%-reduction (n=5, P<0.05, Figure 3Bb). Moreover, the 5-HT1A-receptor antagonist WAY100635 reduced the effect of 5-CT (10 μM) significantly from 69 to 36%-reduction (n=5, P<0.05, Figure 3Bc).
Figure 3.
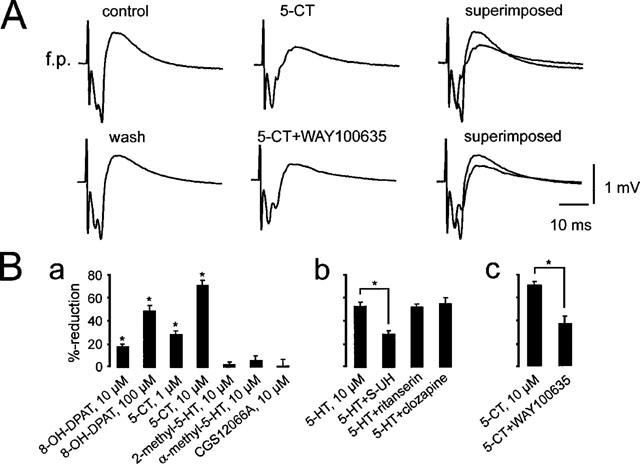
Effects of 5-HT receptor agonists and antagonists on stimulus-evoked field potential responses. (A) 5-CT (10 μM) strongly reduced the stimulus-evoked synaptic field potential response in the superficial layers of the medial entorhinal cortex. Note that the antidromic response was not affected. After washout of 5-CT and following the application of the 5-HT1A-receptor antagonist WAY100635 a second application still reduced the response, but to a smaller extent (see also the summarizing histogram in Bc). (Ba) Histograms summarizing the lack of effect (CGS12066A, α-methyl-5-HT, 2-methyl-5-HT) and effects (5-CT, 8-OH-DPAT) of different 5-HT receptor agonists. (Bb) The 5-HT7-receptor antagonists ritanserin and clozapine (both applied at 100 μM) had no antagonistic effect, while the 5-HT1A-receptor antagonist, S-UH-301, reduced the serotonin-effect significantly. (Bc) The 5-HT1A-receptor antagonist WAY100635 reduced significantly the effect of 10 μM 5-CT. *Mean ratios significantly different from control (P<0.05).
Effects of synaptically released serotonin
One way to verify if the effect of 5-HT could be ascribed to a physiological release of serotonin from synaptic terminals we used a selective 5-HT releaser–fenfluramine (FFA). 200 μM FFA mimicked the effect of serotonin on stimulus-evoked field potential responses in the entorhinal cortex in vitro. The field potential responses were reduced from 1.35±0.13 mV to 0.67±0.06 (=50.4%-reduction, P<0.01) and recovered partially after washout to 1.2±0.09 mV (Figure 4). These effects were observed in 6 out of 8 slices. In the presence of the 5-HT1A-receptor antagonist WAY100635 the effect of fenfluramine was reduced to 26±2.8%-reduction. A striking feature in these kind of experiments was, that the effects of FFA were far slower to develop than the effects of 5-HT (see also Figure 6).
Figure 4.
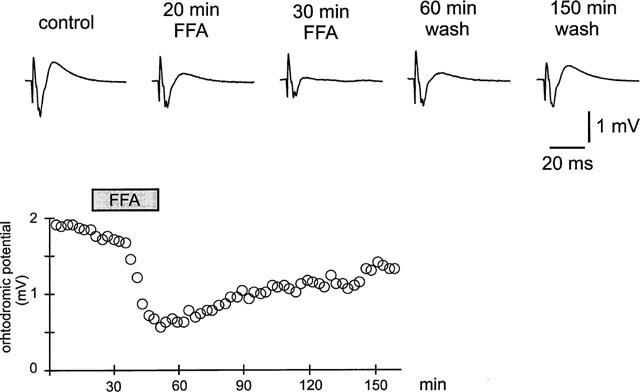
Effects of fenfluramine (200 μM) on stimulus-evoked field potential responses. Note the time course of the effect is much slower compared to the effect of 5-HT (see Figures 2, 4, 6 for comparison).
Figure 6.
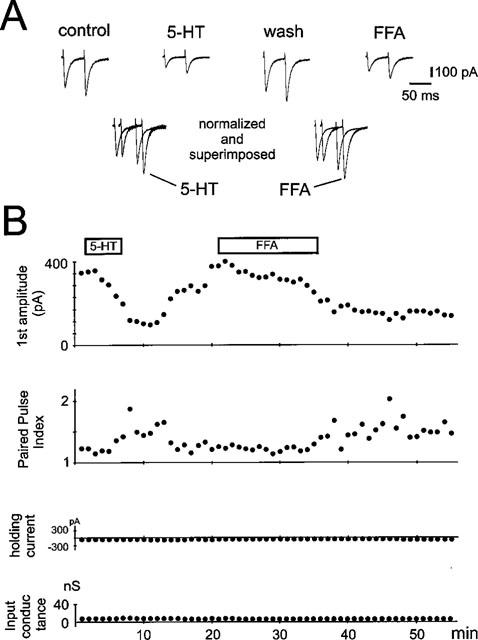
Effects of serotonin and fenfluramine (FFA) on AMPA-receptor mediated EPSCs and paired pulse facilitation. (A) AMPA-receptor mediated EPSCs were evoked by stimulating synaptic fibres in the presence of APV, bicuculline and CGP55845A. 5-HT and fenfluramine depressed both AMPA-receptor mediated EPSCs. In the lower panel the first EPSCs under 5-HT and FFA have been scaled to match the size of the first EPSCs under control and wash, respectively. Note that the second EPSC is enhanced. (B) Plots of the time course of the first EPSC, the paired pulse ratio, holding current and input conductance. Note that following the application of serotonin EPSC-amplitude and paired pulse index were significantly changed, while holding current and input conductance were not affected.
Effects of serotonin on paired pulse facilitation
To characterize the effect of serotonin on paired-pulse facilitation of stimulus-evoked field potential responses we applied two stimuli of identical strength with an interval of 50 ms. Under control conditions, the ratio (f.p.2/f.p.1) of the paired stimulus-evoked field potentials varied from slice to slice between 0.96 and 1.6 with a mean of 1.15±0.07. Following application of serotonin the paired pulse ratio significantly increased to 1.88±0.15 (P<0.001, n=9, Figure 5). After washout the ratio recovered to a value of 1.09±0.09. An altered paired pulse ratio of complex field potential responses might not be exclusively indicative for a presynaptic mechanism, since the different components of a complex shaped field potential show different paired pulse characteristics (Clark et al., 1994). Therefore, we characterized the effects of serotonin on paired pulse facilitation on isolated α-amino-3-hydroxy-5-methyl-4-isoxazole-propionic acid receptor (AMPAR)-mediated excitatory postsynaptic currents, in which changes in the paired pulse ratio are believed to be solely presynaptic (Clark et al., 1994). Due to the fact that the effects of serotonin are critically dependent on the intracellular milieu, we made intracellular recordings from neurones of layer II and III of the medial entorhinal cortex in switched electrode voltage clamp mode and in the presence of APV, bicuculline and CGP55845A. The cells were held at a potential of −80 mV with a mean holding current of −196±44 pA and had an input resistance of 119±30 MΩ (n=8, Figure 6). Following application of serotonin the holding current and the input resistance did not change significantly to −206±42 pA and 127±30 MΩ. When two stimuli of identical strength were applied in close succession (interstimulus-interval: 50 ms) the paired pulse ratio (EPSC2/EPSC1) varied from cell to cell between 1.09 and 1.55. Following application of serotonin (10 μM) the reduction of the isolated AMPAR-mediated currents (from −495±119 pA to −209±65 pA; P<0.001, n=8) were accompanied by a significant increase in the paired pulse ratio from 1.29±0.05 to 1.68±0.09 (P<0.001; n=8). A similar result could be observed in three out of four cells with fenfluramine, where the AMPA-EPSC reductions (55±13%) were accompanied with an increase in the paired pulse ratio from 1.2±0.08 to 1.6±0.1.
Figure 5.
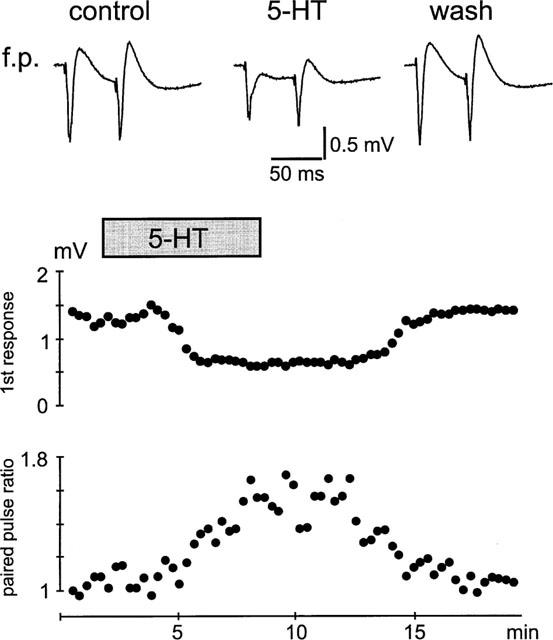
Influence of 5-HT on paired pulse facilitation of stimulus-evoked field potential responses. Note that the first response is stronger affected than the second response, yielding in an increase in the paired pulse ratio following the application of 5-HT.
Discussion
In this study we have shown that serotonin reliably reduced stimulus-evoked field potential responses in the medial entorhinal cortex. The synaptic potentials of the response were affected by serotonin concentrations as low as 0.1 μM, while the antidromic early response were only affected with a concentration >50 μM. The effect was mimicked by the 5-HT1A-receptor agonists 8-OH-DPAT and 5-CT and was partially reduced by the 5-HT1A-antagonist S-UH-301. We suggest that the mechanism is presynaptic since 5-HT significantly enhanced paired pulse facilitation of both field potential responses as well as isolated AMPA-receptor mediated EPSCs. In addition we provide evidence that synaptically released 5-HT can mimic the effects of exogeneously applied serotonin.
Composition of stimulus-evoked field potential responses within the superficial entorhinal cortex
Mimicking the activation of one of the pathways on superficial entorhinal cortex neurones by stimulation in the lateral entorhinal cortex resulted in multiphasic complex field potential responses. Two early negative components, a late and slow positive response and in some occasions a third negative response can be differentiated. The obvious experiment for interpretation of pharmacological effects on field potential responses is to record both intra- and extracellular potentials simultaneously from the same site. However, since there are many different cell types within the superficial entorhinal cortex (Lorente de Nó, 1933; Alonso & Klink, 1993; Jones, 1994; Gloveli et al., 1997a) with different synaptic responses upon electrical stimulation (Jones, 1994; Gloveli et al., 1997a,1997b), we will here only state that the application of the glutamate-receptor antagonists NBQX and APV blocked both the second negative and the late positive components while the early negative component was unchanged. Therefore, the first negative response most likely reflects an antidromic activation upon electrical stimulation.
Serotonin potently depresses the synaptic components of stimulus-evoked field potential responses in the superficial entorhinal cortex
Serotonin dose-dependently reduced both synaptic components of the stimulus-evoked field potential responses in the superficial EC. Even concentrations tested down to 0.1 μM were effective (see Figure 2). In contrast, the early negative response of the field potential, which most likely reflects an antidromic activation, was only affected by much higher concentrations of serotonin (100 μM) and to a much smaller extent. Therefore, one can conclude that the main effect of serotonin in the EC is linked to a depression of excitatory synaptic transmission and is not due to alterations of intrinsic properties.
In order to identify the subtype of the 5-HT-receptor mediating the depression of field potential responses we applied various specific agonists and antagonists for different subtypes of 5-HT-receptors with the highest densities for the entorhinal cortex. However, only the 5-HT1A/7-receptor agonists 8-OH-DPAT and 5-CT could mimic the effect of serotonin, while the specific agonists for 5-HT1B-, 5-HT2C- or 5-HT3-receptors were ineffective. Since 8-OH-DPAT as well as 5-CT bind with high affinity to 5-HT1A- and 5-HT7-receptors (Lovenberg et al., 1993; Ruat et al., 1993) and the limbic structures express significant levels of both 5-HT-receptor subtypes (Pazos & Palacios, 1985; Lovenberg et al., 1993; Ruat et al., 1993), we tested both 5-HT1A- and 5-HT7-receptor antagonists. However, the effects of serotonin or 5-CT were only antagonized by 5-HT1A-receptor antagonists (S-UH-301, WAY100635) and was not affected by the three different 5-HT7-receptor antagonists (methysergide, clozapine, ritanserin) tested. These results suggest that activation of the 5-HT1A-receptor is most likely responsible for the depression of field potential responses. However, since the effects of serotonin and 5-CT were only partially antagonized by the 5-HT1A-receptor antagonists and the 5-HT1A-receptor agonists 8-OH-DPAT and 5-CT were only effective in relative high concentrations, it is feasible that the effect is mediated by an additional 5-HT1A-receptor subtype (Liu & Albert, 1991), which is only partially sensitive to the commonly used agonists and antagonists.
Locus of action
Several mechanisms might account for the effect of 5-HT. One important issue is the question what is a primary and what are secondary effects of serotonin. The 5-HT-induced decrease in input resistance associated with a K+-conductance increase could shunt the membrane and prevent EPSP-propagation from remote dendrites to the soma. However, we have recently shown that this is not an issue for the depression of excitatory synaptic currents within the EC (Schmitz et al., 1998). In addition, we here demonstrate that the synaptic potentials were particularly sensitive to serotonin, while the antidromic component was only affected by much higher concentrations and then only to a small extent. Moreover, our intracellular recordings with low resistance electrodes and high concentrations of QX-314 (blockade of 5-HT-induced outward currents) demonstrate that the serotonin effect was not accompanied by changes in holding current and intrinsic membrane conductance increases. Therefore, our data can largely exclude any postsynaptic mechanism underlying the serotonin effect on synaptic field potentials and EPSCs. Moreover, our experiments on paired pulse facilitation on field potential responses and even stronger on isolated AMPA-receptor mediated EPSCs revealed that serotonin significantly enhanced the paired pulse ratios, a result consistent with a presynaptic locus of action for serotonin (Zucker, 1989; Clark et al., 1994). This and the reduction of the frequency of miniature EPSCs (Schmitz et al., 1998) makes it very likely that serotonin suppresses the release of glutamate from synaptic terminals.
In summary, our data describe strong depressant effects of serotonin on the superficial layers of the entorhinal cortex. Therefore, serotonin might modulate not only excitability of the mEC but also influence synaptic plasticity and prevent neurotoxic effects of glutamate in this area. This is of specific interest because the entorhinal cortex shows a high degree of synaptic plasticity (Alonso et al., 1990; Insausti et al., 1993; Jones, 1993), which may relate not only to learning and memory but also contribute to various forms of disease (Langlais et al., 1987; Tejani-Butt et al., 1995; Schmitz et al., 1997). We are aware that studies in vitro may not accurately reflect the situation in vivo where the release of 5-HT will depend upon the activity within the raphe nuclei. In addition, the concentrations of 5-HT used may be very different from those encountered in vivo. However, previous studies have shown that full equilibration of applied drugs within slices takes at least 1 h (Müller et al., 1988). We recorded after only 5–8 min of wash-in and together with 5-HT uptake and oxidation mechanisms the nominal concentrations of 5-HT were considerably more than actually ‘seen' by the neurones. Interestingly, the 5-HT releasing drug fenfluramine, which so far has been mostly used in in vivo experiments, could mimic in our experiments the effects of serotonin on field potential responses as well as on AMPA-EPSCs. These experiments firstly indicate that serotonergic fibres functionally survive in our in vitro slice preparation and secondly that 5-HT released from its stores is capable of mimicking the effects of exogenous application of 5-HT.
Acknowledgments
This work was supported by a Royal Society Exchange Program Fellowship to Ruth M. Empson and DFG grant INK21/A1-1.
Abbreviations
- APV
(±)-2-amino-5-phosphonopentanoic acid
- 5-CT
5-carboxamido-tryptamine maleate
- EC
entorhinal cortex
- FFA
fenfluramine
- 5-HT
5-hydroxytryptamine
- NBQX
1,2,3,4-tetrahydro-6-nitro-2,3-dioxo-benzol[f]quinoxaline-7-sulphonamide
- 8-OH-DPAT
(±)8-hydroxy-2-(di-n-propylamino)tetralin
- S-UH-301
S-5-fluoro-8-hydroxy-DPAT hydrochloride
References
- ALONSO A., DE CURTIS M., LLINAS R.R. Postsynaptic Hebbian and non-Hebbian long-term potentiation of synaptic efficacy in the entorhinal cortex in slices and in the isolated adult guinea pig brain. Proc. Natl. Acad. Sci. U.S.A. 1990;87:9280–9284. doi: 10.1073/pnas.87.23.9280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ALONSO A., KLINK R. Differential electroresponsiveness of stellate and pyramidal-like cells of medial entorhinal cortex layer II. J. Neurophysiol. 1993;70:128–143. doi: 10.1152/jn.1993.70.1.128. [DOI] [PubMed] [Google Scholar]
- ALONSO A., KÖHLER C. A study of the reciprocal connections between the septum and the entorhinal area using anterograde and retrograde axonal transport methods in the rat brain. J. Compar. Neurol. 1984;225:327–343. doi: 10.1002/cne.902250303. [DOI] [PubMed] [Google Scholar]
- BOBILLIER P., PETITJEAN F., SALVERT D., LIGIER M., SEGUIN S. Differential projections of the nucleus Raphe dorsalis and nucleus Raphe centralis as revealed by autoradiography. Brain Res. 1975;85:205–210. doi: 10.1016/0006-8993(75)90071-2. [DOI] [PubMed] [Google Scholar]
- CLARK K.A., RANDALL A.D., COLLINGRIDGE G.L. A comparison of paired-pulse facilitation of AMPA and NMDA receptor-mediated excitatory postsynaptic currents in the hippocampus. Exp. Brain. Res. 1994;101:272–278. doi: 10.1007/BF00228747. [DOI] [PubMed] [Google Scholar]
- GLOVELI T., SCHMITZ D., EMPSON R.M., DUGLADZE T., HEINEMANN U. Morphological and electrophysiological characterisation of layer III cells of the medial entorhinal cortex of the rat. Neuroscience. 1997a;77:629–648. doi: 10.1016/s0306-4522(96)00494-0. [DOI] [PubMed] [Google Scholar]
- GLOVELI T., SCHMITZ D., HEINEMANN U. Prolonged inhibitory potentials in layer III projection cells of the rat medial entorhinal cortex induced by synaptic stimulation in vitro. Neuroscience. 1997b;80:119–131. doi: 10.1016/s0306-4522(97)00104-8. [DOI] [PubMed] [Google Scholar]
- INSAUSTI R., BELICHENKO P.V., FROTSCHER M., MATUS A., MONYER H., PALM G., STEINHÄUSER C. Plasticity in the entorhinal-hippocampal system. Hippocampus. 1993;3 Suppl.:289–292. [PubMed] [Google Scholar]
- JONES R.S.G. Entorhinal-hippocampal connections: A speculative view of their function. Trends Neurosci. 1993;16:58–64. doi: 10.1016/0166-2236(93)90018-h. [DOI] [PubMed] [Google Scholar]
- JONES R.S.G. Synaptic and intrinsic properties of neurons of origin of the perforant path in layer II of the rat entorhinal cortex in vitro. Hippocampus. 1994;4:335–353. doi: 10.1002/hipo.450040317. [DOI] [PubMed] [Google Scholar]
- LANGLAIS P.J., MAIR R.G., ANDERSON C.D., MCENTEE W.J. Monoamines and metabolites in cortex and subcortical structures: normal regional distribution and the effects of thiamine deficiency in the rat. Brain Res. 1987;421:140–149. doi: 10.1016/0006-8993(87)91284-4. [DOI] [PubMed] [Google Scholar]
- LIU Y.F., ALBERT P.R. Cell-specific signaling of the 5-HT1A receptor. J. Biol. Chem. 1991;266:23689–23697. [PubMed] [Google Scholar]
- LORENTE DE NO R. Studies on the structure of the cerebral cortex. J. Psychol. Neurol. 1933;45:381–438. [Google Scholar]
- LOVENBERG T.W., BARON B.M., DE LECEA L., MILLER J.D., PROSSER R.A., REA M.A., FOYE P.E., RACKE M., SLONE A.L., SIEGEL B.W., DANILESON P.E., SUTCLIFFE J.G., ERLANDER M.G. A novel adenylyl cyclase-activating serotonin receptor (5-HT7) implicated in the regulation of mammalian circadian rhythms. Neuron. 1993;11:449–458. doi: 10.1016/0896-6273(93)90149-l. [DOI] [PubMed] [Google Scholar]
- MÜLLER W., MISGELD U., HEINEMANN U. Carbachol effects on hippocampal neurons in vitro: dependence on the rate of rise of carbachol tissue concentration. Exp. Brain Res. 1988;72:287–298. doi: 10.1007/BF00250251. [DOI] [PubMed] [Google Scholar]
- PAZOS A., CORTÉS R., PALACIOS J.M. Quantitative autoradiographic mapping of serotonin receptors in the rat brain. II. Serotonin-2-receptors. Brain Res. 1985;346:231–249. doi: 10.1016/0006-8993(85)90857-1. [DOI] [PubMed] [Google Scholar]
- PAZOS A., PALACIOS J.M. Quantitative autoradiographic mapping of serotonin receptors in the rat brain. I. Serotonin-1 receptors. Brain Res. 1985;346:205–230. doi: 10.1016/0006-8993(85)90856-x. [DOI] [PubMed] [Google Scholar]
- RUAT M., TRAIFFORT E., LEURS R., TARDIVEL-LACOMBE J., DIAZ J., ARRANG J.-M., SCHWARTZ J.-C. Molecular cloning, characterization, and localization of a high-affinity serotonin receptor (5-HT7) activating cAMP formation. Proc. Natl. Acad. Sci. U.S.A. 1993;90:8547–8551. doi: 10.1073/pnas.90.18.8547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SCHMITZ D., EMPSON R.M., GLOVELI T., HEINEMANN U. Serotonin blocks different pattern of low Mg2+-induced epileptiform activity in rat entorhinal cortex, but not hippocampus. Neuroscience. 1997;76:449–458. doi: 10.1016/s0306-4522(96)00302-8. [DOI] [PubMed] [Google Scholar]
- SCHMITZ D., GLOVELI T., EMPSON R.M., DRAGUHN A., HEINEMANN U. Serotonin reduces synaptic excitation in the superficial medial entorhinal cortex of the rat via a presynaptic mechanism. J. Physiol. (London) 1998;508:119–129. doi: 10.1111/j.1469-7793.1998.119br.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- STEWARD O., SCOVILLE S.A. Cells of origin of entorhinal cortical afferents to the hippocampus and fascia dentata of the rat. J. Compar. Neurol. 1976;169:347–370. doi: 10.1002/cne.901690306. [DOI] [PubMed] [Google Scholar]
- TEJANI-BUTT S.M., YANG J., PAWLYK A.C. Altered serotonin transporter sites in Alzheimer's disease raphe and hippocampus. NeuroReport. 1995;6:1207–1210. doi: 10.1097/00001756-199505300-00033. [DOI] [PubMed] [Google Scholar]
- ZUCKER R.S. Short-term synaptic plasticity. Annu. Rev. Neurosci. 1989;12:13–31. doi: 10.1146/annurev.ne.12.030189.000305. [DOI] [PubMed] [Google Scholar]


