Abstract
This study examined the impact of allopurinol on the renal functional responses to a 30 min period of ischaemia in anaesthetized rats.
Immediately on reperfusion, blood pressure rose transiently, while renal blood flow remained stable throughout at control values. Glomerular filtration rate was decreased by some 90% over the first and 80% over the sixth hour (P<0.001).
Allopurinol, 50 or 100 mg kg−1, had no effect on the blood pressure or renal blood flow responses over the 6 h reperfusion period but glomerular filtration decreased by 60% initially, and to less than 30% of basal at 6 h.
Urine flow and absolute sodium excretion increased 2–3 fold in the first 2 h but decreased thereafter. Fractional sodium excretion was 30 times higher for the first 2 h but decreased reaching some 10 fold higher at 6 h. In the presence of allopurinol, urine flow and absolute sodium excretion increased by 5–6 fold in the first 2 h, and fell by half by 6 h which was greater than in the vehicle group (P<0.01). Fractional sodium excretion increased 20 fold in the allopurinol animals in the first 2 h period, but fell at a faster rate (P<0.01) than in untreated rats.
Potassium excretion decreased (P<0.05) by one half for the 6 h reperfusion period but in the allopurinol animals it was minimally altered.
Allopurinol largely ameliorated the decrease in kidney haemodynamic and excretory function following an ischeamic period for the initial few hours of reperfusion.
Keywords: Renal blood flow, glomerular filtration rate, sodium excretion, renal ischaemia, xanthine oxidase
Introduction
Hypoxic damage to the kidneys occurs during cardiovascular surgery, shock or following trauma and may lead to acute renal failure. This situation may develop in up to 5% of hospitalized patients and has a substantial morbidity and high mortality rate and can be related to a single event of renal hypoperfusion. Moreover, maintenance of the integrity of kidney function prior to and during transplantation is critically important to the success of the grafting process. It is the period of hypoxia or ischaemia followed by reperfusion which initiates the damage and subsequent failure in function and because the kidney is a complex tissue comprising vascular and tubular structures, both are susceptible to injury. It is recognized that the consequences of the ischaemia-reperfusion insult is a renal vasoconstriction, but more importantly at the tubular level, the epithelial cells swell, may slough off into the tubular lumen causing obstruction and thereby preventing filtration at the glomerulus. This is further exacerbated by an increase back leakage of filtered fluid from the tubules into the interstitium and peritubular capillaries (Donohoe et al., 1978).
The mechanisms underlying the ischaemic damage are complex and poorly understood. Nonetheless, it is not only the period of anoxia or hypoxia which initiates the injury, but it is also compounded when oxygen supply is restored in the immediate reperfusion period. One of the major contributors to cell damage is the production of oxygen free radicals which causes peroxidation of many of the cell components (Weinberg, 1991). There is evidence that during the period of anoxia, ATP is degraded to hypoxanthine which is the substrate for xanthine oxidase. Once an oxygen supply is re-established, xanthine oxidase has the ability to generate the superoxide anion, O2−, and hydrogen peroxide, H2O2. O2− and H2O2 react in the presence of iron and other metals (Halliwell & Gutteridge, 1984) to form hydroxyl radicals (OH) which are one of the most potent radicals known. It has been suggested that, amongst the various potential sources of free radicals, xanthine oxidase mediated degradation of purine bases is one of the most important and is therefore a potential target for therapeutic intervention.
Allopurinol (4-hydroxypyrazolo(3,4-d)pyrimidine) is a potent inhibitor of xanthine oxidase (Kelley & Beardmore, 1970) and has been utilized in a range of experimental models of ischaemia-reperfusion injury (Leff & Repine, 1990). Studies have shown that it is effective in preventing damage arising from reperfusion in the liver and heart. However, its effectiveness in preventing damage to the kidney following a period of ischaemia has been only poorly studied and in a relatively superficial way (Leff & Repine 1990), that is, kidneys were subjected to an ischaemic insult but their haemodynamic function was measured 24 h later. What has not been investigated to date has been the renal haemodynamic and tubular changes which develop immediately on release of the clamp. The question which arises is whether the deficit in haemodynamic function becomes evident immediately on release of the clamp or whether it takes several hours to develop and whether an agent such as allopurinol could be of immediate benefit.
The aim of this study was to utilize an acute rat model of renal ischaemia-reperfusion injury to categorize the renal vascular and tubular responses immediately on removal of the clamp and up to 6 h subsequently, and to determine whether blockade of xanthine oxidase with allopurinol in any way ameliorated the deterioration in kidney function following ischaemia. This was done by using the anaesthetized rat in which one renal artery was occluded for a 30 min period and haemodynamic and excretory function followed for 2–6 h in groups of rats which either received saline or allopurinol to block xanthine oxidase activity.
Methods
All procedures were permitted under the U.K. Government Home Office Project Licence No. PPL 40/1367 and Personal Licence No. PIL. 40/00371 issued to E.J. Johns and Personal Licence No. PIL 40/5302 issued to D. Hestin.
Animal preparation
Male Wistar rats (250–350 g), were initially anaesthetized with halothane in O2-N2O. The right femoral vein was immediately cannulated and chloralose-urethane was given i.v. at a dose of 12 and 180 mg kg−1, respectively, over 30 min, followed by supplementary doses every 30 min. During this period the gaseous anaesthetic was gradually removed such that the animal respired air after 15–30 mins. An infusion of inulin (saline containing 15 mg ml−1 inulin) was begun at 3 ml h−1 and maintained at this level for the duration of the study. The right femoral artery was cannulated for measurement of mean blood pressure. The left kidney was exposed through a flank incision and its artery was cleared of connective tissue so that an electromagnetic flowmeter probe (Carolina Medical Electronic, King, NC, U.S.A.) could be placed around it for the measurement of renal blood flow. The ureter of the left kidney was cannulated to enable collection of urine samples. Body temperature was checked periodically with a rectal probe and maintained by means of gentle heat from operating lamps placed over the animal.
Experimental protocol
The animals were allowed to stabilize for 60–90 min before experimental procedures were begun. Arterial blood pressure and renal blood flow were monitored (recorded on a Macintosh computer running custom software written in Labview) from the stabilization period, up to the end of the experiment. After stabilization, two 15-min clearance periods were taken to establish baseline levels. The left renal artery was occluded for a period of 30 min with a non-traumatic clamp, or left untouched in sham experiments. On removal of the clamp, renal haemodynamic and excretory functions were followed for 2 or 6 h. Additional clearances were taken as follows: two 15 min plus two 30 min clearances in the 2 h study: two 30 min clearances beginning 1 and 5 h after reperfusion in the 6 h experiments. Thereafter the rats were killed and the kidneys removed for histological examination. The right kidney was left untouched in all experiments. Different groups of animals were given either 50 or 100 mg kg−1 allopurinol or pH- adjusted saline in equal volume.
Drug preparation, dosage and administration
Allopurinol (Sigma, Poole, Dorset, U.K.) was dissolved in 200 μl of a 1 M NaOH sodium hydroxide solution, and brought up to a final volume of 2 ml with saline. Untreated animals were given pH-adjusted saline in equal volume. A preliminary study was carried out to determine an appropriate regimen for administration of allopurinol. The compound was given i.v. as a bolus over 1 min, 50% of the drug being given either 5 min (n=4), 10 min (n=4) or 15 min (n=4) before clamping the renal artery. The remaining 50% was administered as a bolus i.v. 5 min before removal of the clamp and reperfusion. This preliminary study (data not presented) showed that exposure to drug in the 15 min period before the ischaemic challenge was necessary to exert a significant effect on renal function. Therefore, in the experiments reported herein, the first injection of allopurinol was given 15 min before inducing renal ischaemia.
The following groups were studied
Group A: eight rats given saline were subjected to the 30 min period of renal ischaemia and function measured over the subsequent 2 h; group B: eight rats underwent the same procedure as A and function measured for 6 h after ischaemia; groups C and D: six rats in each group were subjected to renal ischaemia and given allopurinol 50 mg kg−1, and renal function measured over 2 h (group C) and 6 h (group D); groups E and F were similar to groups C and D but given allopurinol 100 mg kg−1. Two additional sham-operated groups of animals were evaluated: in group G, five rats given pH adjusted saline and underwent the experimental procedure described above, but with no occlusion of renal artery and kidney function followed for 6 h after reperfusion. Group H was similar to group G, but the animals were given allopurinol 100 mg kg−1.
Renal haemodynamic and excretory function
Plasma samples were taken at the beginning and end of each pair of clearance periods. Arterial blood samples (0.3 ml) were withdrawn from the femoral cannula, centrifuged for 1 min (16,000 r.p.m.), and plasma removed. The remaining packed erythrocytes were resuspended in an equal volume of saline and reinfused into the animal within 1 min. The urine produced during each clearance period was measured gravimetrically. Plasma and urine samples were assayed for inulin, and electrolytes (Huang & Johns, 1998). Inulin clearance was used to estimate glomerular filtration rate and to calculate fractional excretion of sodium.
Statistics
Data are reported as means±s.e.mean and tested using analysis of variance (ANOVA) for repeated measures, to test three hypotheses, (1) treatment effect, (2) time effect and (3) interaction between treatments and time. As unequal group sizes were used, adjustment to correct multisample aspericity (Greenhouse-Geisser ε) was applied to guarantee control of a type I error (Ludbrook, 1994). Vertical comparisons were made at certain time points using F or t-tests for these comparisons, depending on whether three values (F test) or two values (t-test) were compared at each time point. Bonferroni adjustment was used to correct the resultant P value. Five per cent was taken as the level of statistical significance.
Results
The levels of blood pressure and renal haemodynamic and excretory variables at basal and at the end of the 2 and 6 h experiments are given in Table 1. The values for all variables were very similar for all groups of animals during the baseline period.
Table 1.
Renal haemodynamic and excretory function in rats given saline or 50 or 100 mg kg−1 allopurinol. Baseline values and values 2 h (groups A, C and E) and 6 h (groups B, D and F) after reperfusion

Renal haemodynamic function
Once the clamp was applied to the renal artery urine excretion and renal blood flow fell to zero where they remained for the duration of the occlusion. Over the first 5 min after removing the clamp blood pressure increased by some 20% of the basal levels (P<0.001) and thereafter gradually returned towards the basal levels. This blood pressure response to renal artery occlusion did not differ between the groups of rats receiving either the allopurinol or saline (Figure 1). Once reperfusion had commenced, blood pressure gradually decreased and by 2 h it had returned to values similar to basal and remained stable at this level until 6 h after reperfusion (Table 1 and Figure 1). The pattern and magnitude of blood pressure alteration over the study period did not differ between groups of rats given saline or allopurinol.
Figure 1.
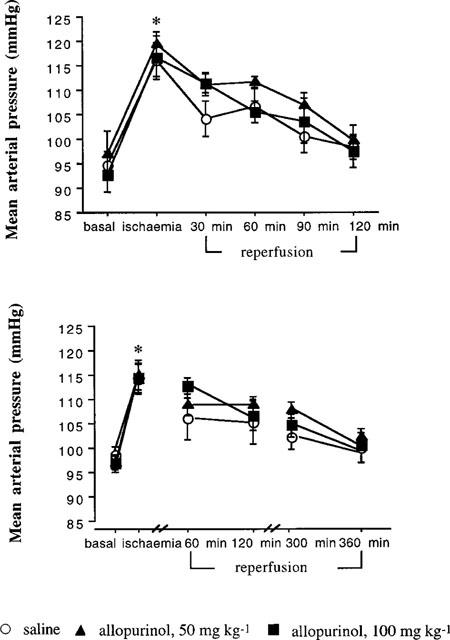
This presents the blood pressure response to renal ischaemia-reperfusion in rats given saline or allopurinol. The upper graph shows the changes over the 2 h study and the lower graph the 6 h study. There was a significant rise in blood pressure after the ischaemic challenge (ANOVA, P<0.001) which persisted over the study period in both experiments. *P<0.01 compared with basal.
On removal of the renal artery clamp, renal blood flow rapidly increased, to values slightly higher than basal, but thereafter it fell back to values similar to basal (Figure 2). Although the rise in renal blood flow seemed to be higher in rats receiving 100 mg kg−1 allopurinol this did not reach a significant level.
Figure 2.
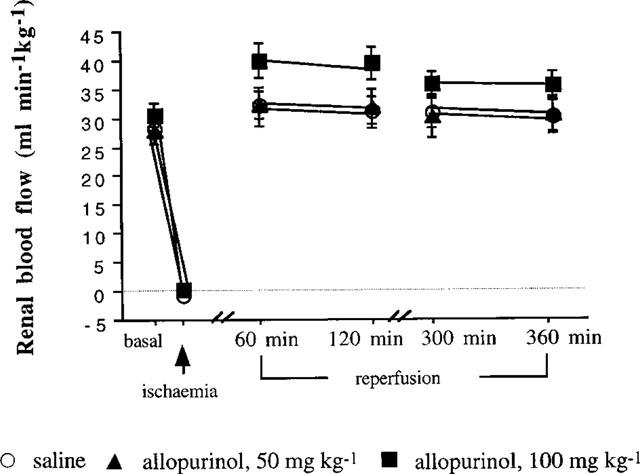
This illustrates the renal blood flow (RBF) changes over the 6 h study in rats given saline or 50 or 100 mg kg−1 allopurinol.
In the first 30 min of reperfusion following the ischaemic period, glomerular filtration rate was decreased by some 90% in the rats given saline and was still reduced 2 and 6 h later, by 83 and 77% respectively (Figure 3). In the groups of rats receiving 50 and 100 mg kg−1 allopurinol, glomerular filtration rate was decreased by 59 and 47%, respectively over the first 30 min (both P<0.001), had partially recovered at 2 h, being reduced by 47 and 39% and further improved at 6 h with reductions of 32 and 27% respectively (Figure 3). The magnitudes of the decreases in glomerular filtration rate were significantly less and the rate of recovery faster (P<0.001) for both the allopurinol treated groups compared with the saline infused groups. Moreover, the recovery in glomerular filtration rate was somewhat greater (P<0.05) in the 100 mg kg−1 group compared with the 50 mg kg−1 group in the 2 h study (Figure 3).
Figure 3.
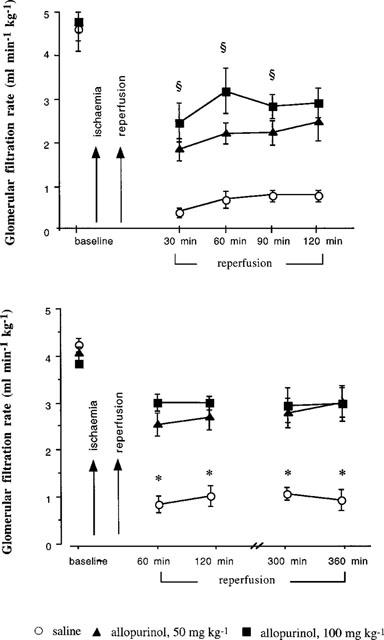
This demonstrates the alteration in glomerular filtration rate in rats receiving saline or allopurinol (AL) after ischaemia for up to 2 h (top graph) or 6 h (bottom graph) following reperfusion. The ischaemic challenge to the kidney caused a marked fall in GFR. ≈rcub;P<0.05 comparing 100 mg kg−1 Al to 50 mg kg−1 and saline groups. *P<0.001 between the saline group and the 50 and 100 mg kg−1 allopurinol treated group.
Excretory function
The basal levels of urine flow, absolute and fractional sodium excretions were very comparable in all groups of animals studied (Table 1). Figure 4 shows that in the saline infused group subjected to the ischaemic period and followed for 2 h, over the first 30 min, urine flow approximately doubled (P<0.001) and then reached a peak of 4–5 fold higher (P<0.0001) which was sustained until the end of the study. A similar situation was observed in the 6 h experiment where urine flow was some 4–5 fold higher than basal for the first 2 h, but fell slightly, to about 3–4 fold higher than basal for the 6 h post-ischaemia time period. By contrast, the increase in urine flow in the 50 and 100 mg kg−1 allopurinol treated groups, reached some 8–10 fold higher than basal over the 2 h study which was significantly (both P<0.001) greater compared with the saline infused group (Figure 4). A similar situation was observed in the 6 h study in which there were large increases in urine flow over the first 2 h period which partially recovered by 6 h post-ischaemia but the magnitudes of the changes were larger and the rate of return towards basal level faster (both P<0.001) in the groups receiving allopurinol compared with the saline infused group (Figure 4).
Figure 4.
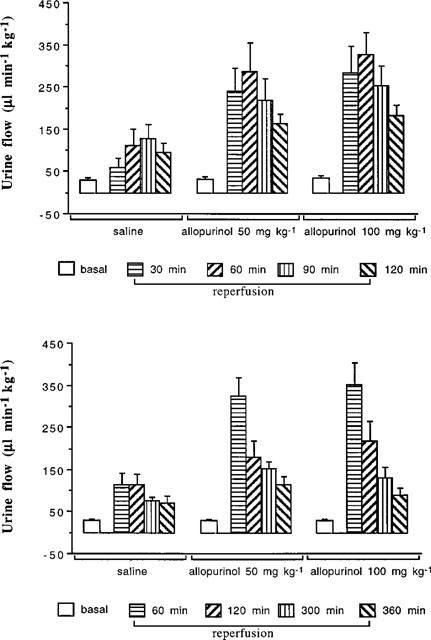
This presents the changes in urine flow rate after the ischaemic challenge to the kidney, up to 2 h (top figure) and 6 h (bottom) of reperfusion. In rats receiving allopurinol (AL), urine flow rate was significantly higher than in rats given saline (ANOVA, P<0.001 between the saline group and both drug treatments over time).
Basal levels of absolute and fractional sodium excretions were comparable across all groups of rats (Table 1). In the 2 and 6 h studies in which the animals received saline following the ischaemic insult, absolute sodium excretion increased by some 5–6 fold over the first 2 h but in the subsequent 4 h gradually decreased but was still raised some 2–3 fold over basal levels at the 5–6 h time period (Figure 5). In the groups of rats receiving either 50 or 100 mg kg−1 allopurinol, following the ischaemic insult absolute sodium excretion increased 10–12 fold over the first 2 h, which was approximately double (both P<0.01) that observed in the saline infused group (Figure 5). In the 6 h study, in the 2–6 h post-ischaemic period, absolute excretion gradually fell towards basal level in the groups of rats receiving 50 and 100 mg kg−1 allopurinol, but was still some 5–6 fold higher than basal values at 6 h and were both significantly (P<0.01) higher than the group which only received saline.
Figure 5.
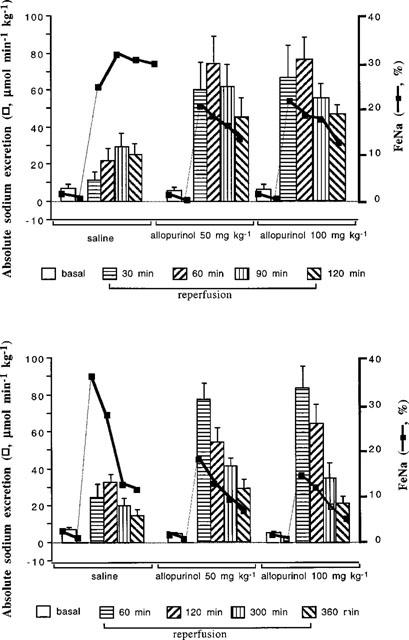
This illustrates the changes in absolute urinary sodium excretion and in fractional sodium excretion (FeNa) after ischaemia-reperfusion of the kidney. The top section reports the 2 h reperfusion study and bottom section the 6 h reperfusion study. Rats given 50 or 100 mg kg−1 allopurinol (AL) excreted more sodium than rats given saline (ANOVA, P<0.01 between the saline and both allopurinol groups over time).
Fractional sodium excretion, representing the fraction of filtered sodium load excreted, rose some 25–30 fold in the 2 h following the ischaemic insult in the saline infused rat (Figure 5) whereas in the groups receiving 50 and 100 mg kg−1 allopurinol, fractional sodium excretion rose less (both P<0.05), by only 15–20 fold, compared with the saline infused group, and gradually declined towards basal levels. An essentially similar pattern was seen in the 6 h study (Figure 5), in which there was a progressive decrease in fractional sodium excretion in the saline infused group over the 6 h post ischaemia period ending some 10 fold above basal levels. In the groups receiving 50 and 100 mg kg−1 allopurinol, the increases in fractional sodium excretion in the post-ischaemic period were significantly less and the rate of return to basal levels was faster (P<0.05) and reached values closer to basal (some 2–3 fold higher) than that attained by the saline infused group (Figure 5).
Table 1 shows that basal potassium excretion was similar in all groups studied. Potassium excretion in the saline infused group (Figure 6) was significantly (P<0.05) reduced over the whole 6 h period following the ischaemic period. By contrast, in the groups or rats receiving both the low and high doses of allopurinol, potassium excretion was higher (P<0.05) over the 6 h period following ischaemia compared to that of the group receiving saline (Figure 6).
Figure 6.
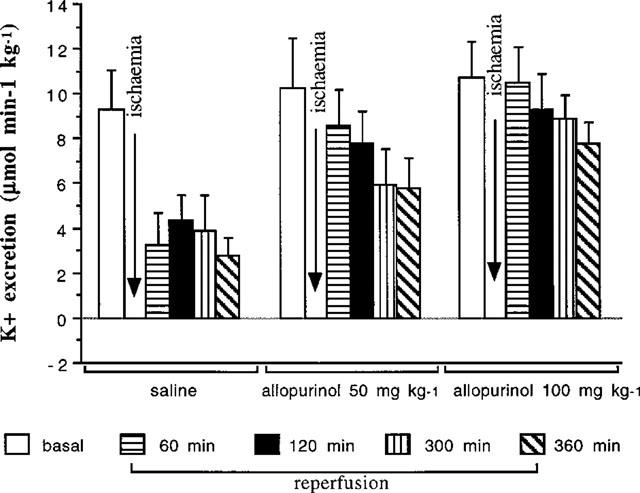
This gives the absolute potassium (K+) excretion in rats given saline, 50 or 100 mg kg−1 allopurinol. Over the 6 h reperfusion period potassium excretion was significantly higher in rats receiving allopurinol compared to rats given saline (ANOVA, P<0.05).
Two groups of rats were subjected to the sham clamping of the renal artery in which the kidney was not subjected to the ischaemia, one was given saline and the other 100 mg kg−1 allopurinol. In both groups of rats renal haemodynamic and excretory function remained stable over the 6 h study period (data not shown).
Discussion
Hypoxic injury to the kidney is a particularly serious event as not only is there a short-term risk of acute renal failure, but in the longer term the initial damage can act as a focus for fibrotic and sclerotic changes which may develop and progress into chronic renal failure. The issue considered in this study was whether the xanthine oxidase inhibitor, allopurinol, could ameliorate or prevent the haemodynamic and tubular derangements which occurred during the first 2–6 h of reperfusion after a 30 min period of ischaemia. When a tissue becomes ischaemic, oxidation of cytochrome C fails (Weinberg, 1991) with the result that ATP levels become depleted (Jennings & Steenbergen, 1985). The consequence is that there is a build up of hypoxanthine and xanthine which are oxidizable purine substances. During hypoxia, calcium also accumulates within cells which converts xanthine dehydrogenase to xanthine oxidase which is oxygen-dependent (Della Corte & Stirpe, 1972). Thus, when perfusion is restored, xanthine oxidase activity utilizes molecular oxygen together with hypoxanthine and xanthine substrates with the resultant generation of superoxide radicals. It is these reactive oxygen species which are capable of irreversibly damaging most classes of macromolecules within the cells (Del Maestro, 1980).
Importantly, allopurinol has been recognized as having a number of actions: it blocks xanthine oxidase, thus limiting superoxide formation: it is able to act as a scavenger of the highly reactive hydroxy radicals (Moorhouse et al., 1989; Karwinski & Soreide, 1997) and facilitates ATP resynthesis (Karwinski et al., 1991). Enhancement of hypoxanthine reutilization may save a substantial quantity of energy which may be important in limiting damage following ischaemia and reperfusion (Puig et al., 1989a). Thus, Puig et al. (1989b) have demonstrated that allopurinol prevents adenine nucleotide depletion in the dog liver and rat kidney. (Cunningham et al., 1974) during ischaemic injury. It was for these reasons that allopurinol was utilized to investigate `its impact on the immediate functional responses in the reperfusion period after ischaemia. Importantly, our preliminary studies identified the fact that in order for allopurinol to be effective in this study it had to be present within the kidney before the start of the ischaemic insult, achieved by giving the first dose, and present at high levels when reperfusion commenced, hence the second dose was given just before the clamp was removed from the renal artery. This would suggest that for the compound to be effective it needed to be present in the kidney before the start of the ischaemic period and at a high concentration once reperfusion began. Indeed, this approach has also been found to be effective in the protection of the liver from ischaemic damage by allopurinol (Karwinski et al., 1991).
The experimental design used in the present study was to occlude the renal artery for a 30 min period which was comparable to that utilized in a longer time-frame study to generate renal damage leading to acute renal failure in the rat (Gellai et al., 1994). It was evident that immediately upon reperfusion, renal blood flow was restored back to levels close to baseline, or slightly higher, which was similar to that reported in the rabbit (Hansson et al., 1982) and dog (Williams et al., 1987) kidneys subjected to 60 min ischaemia. Moreover, the studies herein showed that the renal blood flow was maintained at a relatively normal level for at least 6 h after the ischaemia. There is good evidence to show that the renal vasculature is damaged by the ischaemia (Conger et al., 1991) insofar as renal blood flow tends to follow blood pressure passively, autoregulatory ability is depressed and the responses to adrenergic agonists is raised. It was of interest to note that neither the low nor high dose of allopurinol altered the pattern of renal blood flow response over the 6 h period after the ischaemic insult.
A major finding was that in the saline perfused rats, once the kidneys were subjected to reperfusion, glomerular filtration rate was decreased markedly, by some 90%, during the first 30 min period of measurement and recovered only slowly over the subsequent 6 h, still being depressed by approximately 80% at the end of the study. Importantly, this occurred at a time when blood flow was at a relatively normal level. There are probably multiple reasons for this, all relating to epithelial cell function and tubular integrity. One consequence of oxygen deprivation at the epithelial cells is that as ATP becomes depleted, the Na+/K+ ATPase activity will cease and sodium and water will accumulate in the cells causing them to swell (Weinberg, 1991). This cellular swelling not only causes a narrowing of the tubular lumen, but if the insult is severe, may also result in cells sloughing off and totally blocking the tubule. Both of these events will raise tubular hydrostatic pressure thereby preventing filtration. It is also likely that these events raise tubular permeability and back-leak of filtrate into the renal interstitium further exacerbating the failure of filtration. It would seem that it is the S3 segment of the proximal tubule which is the most susceptible to this ischaemia-mediated damage (Weinberg, 1991).
It was evident that in the rats given allopurinol, the reductions in glomerular filtration rate over the whole period of reperfusion, from the first 30 min to the 6 h time point, was relatively well preserved, only falling by some 50–60% at the start of reperfusion and then showing a recovery, being only 30% below basal values at 6 h. This marked preservation of glomerular filtration following the ischaemic period is compatible with there being less damage to the epithelial cells such that the cells do not detach from the basement membrane or swell to a sufficient degree to cause a serious block of the tubules. Indeed, preliminary histological evaluation found a good preservation of tubular structure in the kidneys derived from the allopurinol treated compared with the saline infused animals. These findings would indicate that the ability of allopurinol to block xanthine oxidase and scavenge reactive oxygen species is a very effective means to preventing damage to the haemodynamic function both during the ischaemic period as well as in the crucial reperfusion period. These observations are supported by previous reports which showed that 24 h after a period of renal artery clamping in rats, plasma creatinine was elevated less in allopurinol treated animals (Alatas et al., 1996). It was apparent that both doses of allopurinol gave similar responses even though the higher dose gave a slightly better recovery over the earlier period of reperfusion (2–4 h), which would suggest that the compound could be used at slightly lower doses and still be protective.
Removal of the renal artery clamp was associated with a prompt and sustained increase in water and sodium excretion, of some 2–4 fold which lasted for 2–3 h and then gradually fell back to basal levels. Thus, even though filtered load of fluid was decreased by some 90%, of that fluid filtered a relatively large proportion could not be reabsorbed. In fact, the fractional sodium excretion reached a value of around 30% which means that only 70% of the filtrate was reabsorbed as against 99% under normal conditions. In the presence of allopurinol, both water and absolute sodium excretion increased by almost twice as much as compared to the untreated rats, but when considered as fractional sodium excretion, the proportion of the filtered load reabsorbed was greater, being about 85%, and in the first 2 h showed a sharp increase which continued until the end of the 6 h observation period. These observations were compatible with a good recovery of the reabsorptive function of the tubules in the presence of allopurinol. Although there appeared to be recovery of reabsorptive capability in the untreated rat, it was much less complete than that occurring in the allopurinol treated animals. Again, the data suggest that both the 50 and 100 mg kg−1 doses of allopurinol were equally effective and may indicate that the maximum dose of the compound had been used. There remains the possibility that even lower doses could be useful.
The handling of potassium by the kidney in the post-occlusion reperfusion period was only minimally affected. The reason for this is unclear, but may relate to the fact that potassium handling occurs at the distal regions of the nephron which are much less susceptible to the ischaemic insult. One possibility is that not only is potassium actively secreted by the distal nephron, it can leak down its concentration gradient into the tubular lumen.
In summary, studies were performed on anaesthetized rats in which the renal artery was occluded for 30 min and renal haemodynamic and excretory function followed for up to 6 h. It was evident that in the immediate reperfusion period and up to 6 h later, whereas renal blood flow was relatively unaffected there were marked reductions in glomerular filtration rate associated with large increases in urine flow, absolute and fractional sodium excretion. If allopurinol was given prior to and during the ischaemic period, the depression of renal function in the reperfusion state was attenuated and although urine flow and absolute sodium excretion were higher, fractional sodium excretion was lower and recovered more rapidly than compared to untreated rats. These data indicate that allopurinol has great potential as a useful compound for minimizing ischaemia-reperfusion injury in the kidney, for example, as may occur in cardiovascular shock or during the transplantation process. However, allopurinol only provides partial protection from the ischaemic damage and clearly there are other factors activated which also participate in the injury processes. These other contributory processes will also need to be explored.
Abbreviations
- ATP
adenosine triphosphate
References
- ALATAS O., SAHIN A., COLAK O., INAL M., KOEN T., YASAR B., KARAHUSEYINOGLU E. Beneficial effects of allopurinol on glutathione levels and glutathione–peroxidase activity in rat ischemic acute renal failure. J. Int. Med. Res. 1996;24:33–39. doi: 10.1177/030006059602400105. [DOI] [PubMed] [Google Scholar]
- CONGER J.D., ROBINETTE J.B., HAMMOND W.S. Differences in vascular reactivity in models of ischaemic acute renal failure. Kidney Int. 1991;39:1089–1097. doi: 10.1038/ki.1991.138. [DOI] [PubMed] [Google Scholar]
- CUNNINGHAM S.K., KEAVENY T.V., FITZGERALD P. Effect of allopurinol on tissue ATP, ADP and AMP concentrations in renal ischaemia. Br. J. Surg. 1974;61:562–565. doi: 10.1002/bjs.1800610716. [DOI] [PubMed] [Google Scholar]
- DELLA CORTE E., STIRPE F. The regulation of rat liver xanthine oxidase: involvement of thiol groups in the conversion of the enzyme activity from dehydrogenase (type D) into oxidase (type O) and purification of the enzyme. Biochem. J. 1972;126:739–745. doi: 10.1042/bj1260739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DEL MAESTRO RF. An approach to free radicals in medicine and biology. Acta Physiol. Scand. 1980;492:153–168. [PubMed] [Google Scholar]
- DONOHOE J.F, , VENKATACHALAM M.A, , BERNARD D.B, LEVINSKY N.G. Tubular leakage and obstruction after renal ischaemia: Structural-functional correlations. Kidney Int. 1978;13:208–222. doi: 10.1038/ki.1978.31. [DOI] [PubMed] [Google Scholar]
- GELLAI M., JUGUS M., FLETCHER T., DEWOLF R, NAMBI P. Reversal of postischemic acute renal failure with a selective endothelin A receptor antagonist in the rat. J. Clin. Invest. 1994;93:900–906. doi: 10.1172/JCI117046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HALLIWELL B., GUTTERIDGE J.M.C. Oxygen toxicity, oxygen radicals. Transition metals and disease. Biochem. J. 1984;219:1–14. doi: 10.1042/bj2190001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HANSSON R., GUSTAFSSON B., JONSSON O., LUNDSTROM S., PETTERSON S., SCHERSTEN T., WALDENSTROM J. Transpl. Proc. 1982. pp. 51–58.
- HUANG C., JOHNS E.J. Role of angiotensin II in mediating somatosensory-induced renal nerve-dependent antinatriuresis in the rat. Am. J. Physiol. 1998;275:R194–R202. doi: 10.1152/ajpregu.1998.275.1.R194. [DOI] [PubMed] [Google Scholar]
- JENNINGS R.B., STEENBERGEN C. Nucleotide metabolism and cellular damage in myocardial ischemia. Annu. Rev. Physiol. 1985;4:727–749. doi: 10.1146/annurev.ph.47.030185.003455. [DOI] [PubMed] [Google Scholar]
- KARWINSKI W., DRANGE A., FARSTAD M., ULVIK R., SØREIDE O. Sixty-minute normothermic liver ischaemia in rats. Transplantation. 1991;52:231–234. [PubMed] [Google Scholar]
- KARWINSKI W., SOREIDE O. Allopurinol improves scavenging ability of the liver after ischaemia/reperfusion injury. Liver. 1997;17:139–143. doi: 10.1111/j.1600-0676.1997.tb00796.x. [DOI] [PubMed] [Google Scholar]
- KELLEY W.N., BEARDMORE T.D. Allopurinol: alteration in pyrimidine metabolism in man. Science. 1970;169:388–390. doi: 10.1126/science.169.3943.388. [DOI] [PubMed] [Google Scholar]
- LEFF J.A., REPINE E. Blood cells and ischaemia - reperfusion injury. Blood cells. 1990;16:183–192. [PubMed] [Google Scholar]
- LUDBROOK J. Repeated measurements and multiple comparisons in cardiovascular research. Cardiovasc. Res. 1994;28:303–311. doi: 10.1093/cvr/28.3.303. [DOI] [PubMed] [Google Scholar]
- MOORHOUSE P.C., GROOTVELD M., HALLIWELL B., QUINLAN J.G., GUTTERIDGE J.M.C. Allopurinol and oxypurinol are hydroxyl radical scavengers. FEBS Lett. 1989;213:23–28. doi: 10.1016/0014-5793(87)81458-8. [DOI] [PubMed] [Google Scholar]
- PUIG J.G., JIMÉNEZ M.L., MATEOS F.A., FOX I.H. Adenine nucleotide turnover in hypoxanthine-guanine phosphoribosyl-transferase (HGPRT) deficiency: evidence for an increased contribution of purine biosynthesis de novo. Metabolism. 1989a;38:410–418. doi: 10.1016/0026-0495(89)90189-3. [DOI] [PubMed] [Google Scholar]
- PUIG J.G., MATEOS F.A., DIAZ V.D. Inhibition of xanthine oxidase by allopurinol: a therapeutic option for ischaemia induced pathological processes. Ann. Rheum. Dis. 1989b;48:883–888. doi: 10.1136/ard.48.11.883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WEINBERG J.M. The cell biology of ischemic renal injury. Kidney Int. 1991;39:476–500. doi: 10.1038/ki.1991.58. [DOI] [PubMed] [Google Scholar]
- WILLIAMS R.H., THOMAS C.E., NAVAR L.G., EVAN A.P. Hemodynamic and single nephron function during the maintenance phase of ischemic acute renal failure. Kidney Int. 1987;19:503–515. doi: 10.1038/ki.1981.48. [DOI] [PubMed] [Google Scholar]


