Abstract
Receptors mediating CGRP-induced vasorelaxation were investigated in rat thoracic aorta and porcine left anterior descending (LAD) coronary artery and anterior interventricular artery (AIA), using CGRP agonists, homologues and the antagonist hα CGRP8–37.
In the endothelium-intact rat aorta, hα CGRP, hβ CGRP, rat β CGRP and human adrenomedullin caused relaxation with similar potencies. Compared with hα CGRP, rat amylin was about 25 fold less potent, while [Cys(ACM2,7)] hα CGRP and salmon calcitonin were at least 1000 fold weaker.
Hα CGRP8–37 (up to 10−5 M) did not antagonize responses to hα CGRP, hβ CGRP or rat β CGRP (apparent pKB <5). Peptidase inhibitors did not increase either the effect of hα CGRP or [Cys(ACM,2,7)] hα CGRP, while hα CGRP8–37 remained inactive. Endothelium-dependent relaxation produced by hα CGRP was accompanied by increases in cyclic AMP and cyclic GMP, that were not inhibited by hα CGRP8–37 (10−5 M).
In porcine LAD and AIA, hα CGRP produced relaxation in an endothelium-independent manner. Hα CGRP8-37 competitively antagonized hα CGRP responses (pA2 6.3 and 6.7 (Schild slope 0.9±0.1, each), in LAD and AIA, respectively). In LAD artery, hα CGRP-induced relaxation was accompanied by increases in cyclic AMP that were inhibited by hα CGRP8–37 (10−7–10−5 M).
In conclusion, the antagonist affinity for hα CGRP8–37 in porcine coronary artery is consistent with a CGRP1 receptor, while the lack of hα CGRP8–37 antagonism in rat aorta could suggest either a CGRP receptor different from CGRP1 and CGRP2 type, or a non-CGRP receptor.
Keywords: Human CGRP; rat CGRP; human adrenomedullin; rat amylin; [Cys(ACM2,7)] hα CGRP; hα CGRP8–37; peptidase inhibitors; CGRP receptor subtypes; rat aorta; porcine coronary artery
Introduction
CGRP is a 37 residue neuropeptide with numerous actions, including cardiostimulation (Sigrist et al., 1986) and vasodilation (Brain et al., 1985; Marshall et al., 1986a,1986b). CGRP receptors have been sub-divided into CGRP1 and CGRP2 (Dennis et al., 1989; 1990; Mimeault et al., 1991; Quirion et al., 1992), largely based on studies using the C-terminal fragment CGRP8–37 which acts as an antagonist with higher affinity value at the guinea-pig atrium CGRP1 receptor (pA2 7.2–7.7) than at the rat vas deferens CGRP2 receptor (pA2 6.2–6.6). However, antagonist affinities for CGRP8–37 from various species and tissue preparations range from less than 6 (e.g. Giuliani et al., 1992; Tomlinson & Poyner, 1996) up to 9.3 (Longmore et al., 1994), and do not fit into discrete groups. PA2/pKB values for hα CGRP8–37 have shown heterogeneity within the same organ, such as in porcine large and small coronary arteries covering values from 5.7 to 7.0 (Foulkes et al., 1991). The presence of peptidases metabolizing CGRP and analogues may contribute to the variability in the antagonist affinity value (Longmore et al., 1994).
In the vasculature, CGRP induces both endothelium-dependent and independent relaxation, depending on species, size and location of vessels (Marshall, 1992). For instance, in porcine coronary artery CGRP does not require the endothelium to produce relaxation (Franco-Cereceda et al., 1987), whereas the sensitivity of the rat pulmonary artery to CGRP depends entirely on the presence of an intact endothelium (Wisskirchen et al., 1998), consistent with that in the rat aorta (Gray & Marshall, 1992a,1992b). In the rat aorta, our previous studies indicated that hα CGRP8–37 might behave as a non-competitive antagonist (Gray et al., 1991), but a detailed pharmacological characterization of CGRP receptors has not been done. Therefore, the purpose of this study was to characterize the receptor mediating CGRP relaxation and its transduction mechanism of the rat thoracic aorta, compared with that of an endothelium-independent mechanism by CGRP in porcine coronary arteries.
Methods
Rat isolated thoracic aorta
Male Sprague Dawley rats (300–450 g) were stunned and killed by cervical dislocation. The thoracic aorta was isolated, cleared of fat and connective tissue and cut into rings (2–3 mm in length). The endothelium was removed in some experiments by gently abrading the intimal surface of the aortic rings with fine wires. The rings were suspended on tungsten wires (0.125 mm diameter) under 0.5 g resting tension and allowed to equilibrate for 60 min in Krebs solution containing (mM): Na+ 143, K+ 5.9, Ca2+ 2.5, Mg2+ 1.2, Cl− 128, HCO3− 25, HPO42− 1.2, SO42− 1.2 and glucose 11, at 37°C and oxygenated with 95% O2 and 5% CO2. Tension was recorded with Grass FT.03 isometric transducers connected to a Grass 7D polygraph.
Aortic rings were contracted with noradrenaline (10−7 M) which produced approximately 75% of the maximal response. Contractile responses were assessed for stability over a period of 10 min, followed by addition of acetylcholine (10 −6 M). In rings which had been rubbed, the failure of acetylcholine (10−6 M) to produce relaxation (<5%) of noradrenaline (10−7 M)-induced tone was taken as an indication of endothelium removal. In endothelium-denuded tissues, the effect of hα CGRP was tested on noradrenaline-induced tone, 30 min later.
Tissues with intact endothelium were contracted with noradrenaline (30 min after the test of endothelium–integrity), and a cumulative concentration response curve to one agonist (hα CGRP, hβ CGRP, rat β CGRP, [Cys(ACM2,7)] hα CGRP, human adrenomedullin, rat amylin, or salmon calcitonin) was constructed. Agonist concentrations were added in half log molar increments, allowing time for the effect to plateau (Figure 1). Concentration response curves to either hα CGRP or hβ CGRP or rat β CGRP or [Cys(ACM2,7)] hα CGRP were repeated 30 min later. In separate experiments, the effect of hα CGRP8–37, equilibrated for 20 min, was studied either on second curves to hα CGRP or on first agonist curves in separate tissues. The effect of equilibrating hα CGRP8–37 (10−5 M) for either 2 or 60 min was assessed against hα CGRP and compared with single agonist curves from separate tissues. The effect of hα CGRP8–37 (10−5 M; 20 min pretreatment) was studied on hβ CGRP and rat β CGRP by constructing single curves which were compared with agonist curves alone from separate tissues. Hα CGRP8–37 (10−7–10−5 M) was tested on the basal (i.e. unstimulated preparation) and on the noradrenaline-induced tone.
Figure 1.
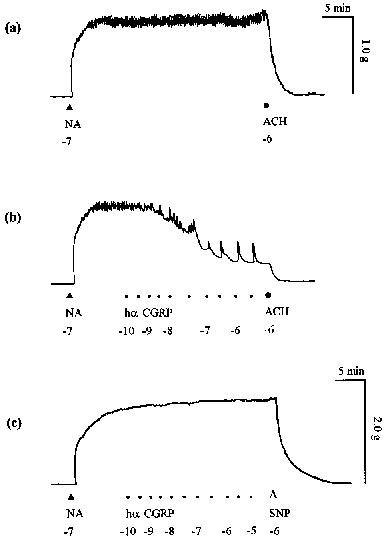
Endothelium-dependent vasorelaxation to hα CGRP in rat isolated thoracic aorta. Traces showing (a) contraction to noradrenaline (NA; 10−7 M), relaxation to acetylcholine (ACH; 10−6 M) and (b) concentration-dependent relaxation to hα CGRP in endothelium-intact rings. (c) Traces showing no effect to hα CGRP in an endothelium-denuded ring but relaxation to sodium nitroprusside (SNP; 10−6 M) on noradrenaline (10−7 M)-induced tone. All drugs were added at the points labelled; hα CGRP was added in half-log molar increments; numbers represent log molar concentrations.
A mixture of the peptidase inhibitors amastatin, bestatin, captopril, phosphoramidon and thiorphan (10−6 M each; 30 min pretreatment) was studied on responses to either hα CGRP, [Cys(ACM2,7)] hα CGRP or to hα CGRP in the presence of hα CGRP8–37. For hα CGRP, responses in the absence and presence of peptidase inhibitors were examined either within a single tissue by construction of second curves or in separate tissues by comparing single curves. For [Cys(ACM2,7)] hα CGRP and those studies where the antagonist hα CGRP8–37 (10−5 M) was assayed against hα CGRP, single curves were constructed, where peptidase inhibitors were present throughout the experiment and compared with results obtained in their absence. The effect of the peptidase inhibitors was tested on basal tone and on the spasmogen-induced tone.
Porcine isolated coronary arteries
Porcine hearts from mixed strain and sex pigs (6–12 months of age) were obtained fresh from the abattoir. The left anterior descending (LAD) coronary artery was removed from just below the branch of the left circumflex artery close to the base of the heart. The LAD coronary artery preparation (internal diameter 4 mm) was taken between the top of the artery and the first branch, the conus artery. A second, smaller preparation, termed the anterior interventricular artery (AIA; internal diameter 1 mm) was taken as far down the arterial trunk as possible. Both preparations were cut into rings (3–4 mm) and denuded of endothelium by gently abrading the intimal surface with fine wire. Rings were mounted on steel wires (0.40 mm diameter) in organ baths in oxygenated Krebs solution under 1.0 g resting tension as described above. After 75 min equilibration, LAD and AIA rings were contracted with the thromboxane analogue U46,619 (10−8 M) and acetylcholine (2×10−7 M), respectively (concentrations evoking around 80% of the maximum response to these spasmogens). Tissues relaxing to bradykinin (10−6 M) were discarded as having functional endothelium remaining. A cumulative concentration response curve to hα CGRP was measured on the spasmogen-induced tone. In LAD rings, single agonist curves were constructed in either the absence or presence of hα CGRP8–37 (10−6–10−5 M) after exposure to the antagonist for 15 min. In the AIA, following a 30 min interval, a second agonist curve was measured in the presence of hα CGRP8–37 (10−7–10−5 M; 15 min pretreatment).
Cyclic nucleotide determination
Isolated rings of rat thoracic aorta and porcine LAD coronary artery were prepared as described above. After exposure of the vascular rings to hα CGRP in absence and presence of hα CGRP8–37 at the concentrations and times indicated in the results, rings were rapidly removed from the baths, immediately frozen in liquid nitrogen, and cyclic nucleotides were determined as described previously (Gray & Marshall, 1992a). Briefly, each tissue was ground in 95% ethanol (pH 3.0) in a mortar and pestle and left overnight for extraction of the cyclic nucleotides. The samples were centrifuged to pellet the residual tissue fragments. The supernatant was decanted, evaporated to dryness under nitrogen, and each sample was then resuspended in sodium acetate (50 mM at pH 5.0). Porcine LAD coronary artery samples were used for measurements of cyclic AMP levels and rat aortic samples were split into two aliquots for simultaneous measurements of both cyclic AMP and cyclic GMP levels by scintillation proximity assay (Amersham) using the acetylation protocol. The tissue residue was dissolved in sodium hydroxide solution (0.5 M) and the protein content determined by the method of Lowry et al. (1951) with bovine serum albumin as the standard.
Chemicals
Hα CGRP, hβ CGRP, rat β CGRP and hα CGRP8–37 were donated by GlaxoWellcome Research Laboratories (Beckenham, Kent), having been synthesized and purified as previously described (Wisskirchen et al., 1998). All other chemicals were obtained from Sigma, U.K. Peptides were diluted in distilled water to form a 10−2 M stock solution and stored in aliquots at −20°C. The peptidase inhibitors (amastatin, bestatin, captopril, phosphoramidon and thiorphan) were dissolved and diluted in DMSO, to form a stock solution of 10−4 M and kept stored at −20 °C. Acetylcholine chloride, noradrenaline bitartrate (with added ascorbic acid to prevent oxidation), bradykinin were prepared daily in distilled water (10−3 M), and U46,619 (9,11-dideoxy-11α,9α epoxymethanprostaglandin F2α) was prepared in ethanol (100%).
Data analysis
All values are given as mean±s.e.mean. Responses to vasodilator drugs in rat thoracic aorta and porcine coronary arteries are expressed as a percentage relaxation of the noradrenaline- and U46,619- or acetylcholine-induced tone, respectively. Levels of cyclic nucleotides are expressed in fmol/mg proteins. Differences between log concentration response curves or cyclic nucleotide levels were tested for significance using two-way ANOVA and Student's t-test (paired and unpaired groups, as appropriate), respectively. For all tests the significance level was set at P<0.05.
The pEC50 (−log of EC50; concentration of the agonist that produced 50% of the maximal response) was determined by non-linear regression curve fitting, using Graphpad Prism 2.0 (Graphpad software, U.S.A.). The Hill slope of each non-linear regression curve was determined (Graphpad Prism 2.0), to check whether curves in the absence and presence of antagonist were parallel. With a single concentration of antagonist, an apparent pKB value was calculated using the Gaddum equation:
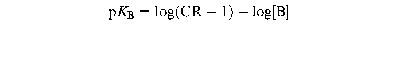 |
where CR is the concentration ratio of the EC50 values in the presence and absence of the antagonist and [B] is the molar concentration of the antagonist.
Where multiple concentrations of antagonist were used, a Schild plot of log (CR-1) against log [B] was plotted, and the pA2 and Schild slope determined by linear regression. (Arunlakshana & Schild, 1959), using Graphpad Prism 2.0. The pA2 values were calculated from the individual control concentration response curves and the respective curves obtained in the presence of hα CGRP8–37, using one antagonist concentration per tissue.
Results
Rat aorta
In rat isolated thoracic aorta, noradrenaline (10−7 M) evoked a stable concentration of 1.02±0.07 g (n=12) and 1.62±0.15 g (n=5) in endothelium-intact (Figure 1a) and denuded rings (Figure 1c), respectively, for at least 30 min.
Agonist activity of hα CGRP and related peptides in rat aorta
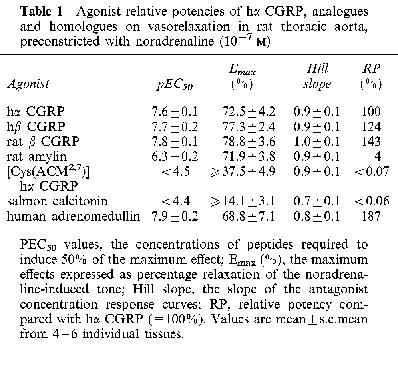
Cumulative addition of hα CGRP to the noradrenaline-induced tone relaxed the endothelium-intact aorta (Figure 1b); but not endothelium-denuded rings (Figure 1c). Endothelium-dependent relaxation to hα CGRP gave a pEC50 of 7.6±0.1 and 73% maximum response (Figure 2a; Table 1). The effect of a given concentration began within 10–15 s of administration and reached its maximum after about 170 s. Agonist responses to hβ CGRP and rat β CGRP were similar in onset, equilibration, potency and maximum response to those of hα CGRP (Figure 2a; Table 1). Human adrenomedullin produced relaxation with a similar potency and maximum response as compared with hα CGRP (Figure 2a; Table 1), while the maximum effects of the two peptides were not additive with each other (data not shown). Relaxant responses to rat amylin were around 25 fold weaker in potency than those to hα CGRP (Figure 2a; Table 1). The linear analogue [Cys(ACM2,7)] hα CGRP was at least 1000 fold less potent than hα CGRP, while salmon calcitonin was even weaker (Figure 2a; Table 1).
Figure 2.

Relaxation evoked by CGRP analogues and homologues in rat thoracic aorta. (a) Concentrations response curves to hα CGRP, hβ CGRP, rat β CGRP, human adrenomedullin, rat amylin, [Cys(ACM2,7)] hα CGRP and salmon calcitonin on noradrenaline-induced tone. (b) Concentration response curve to hα CGRP on the spasmogen-evoked tone, and a second curve 30 min later (in the same tissue). Results are expressed as percentage relaxation of the spasmogen-induced tone. Points represent the mean±s.e.mean of 4–6 separate experiments.
Table 1.
Agonist relative potencies of hα CGRP, analogues and homologues on vasorelaxation in rat thoracic aorta, preconstricted with noradrenaline (10−7 M)
Concentration response curves to hα CGRP repeated after 30 min (in the same tissue) were not reproducible (P<0.0001; pEC50 7.5±0.1 and 7.1±0.1 in first and second curves, respectively), and showed approximately 14% reduction in maximum response (Figure 2b). A change in potency and maximum of hβ CGRP and rat β CGRP was observed with concentration effect curves in the same tissues (data not shown), suggesting tachyphylaxis. Preliminary experiments with hα CGRP using either shorter (20 min) or longer intervals (60 min) between construction of successive agonist curves did not prevent the fall in maximum response (data not shown).
Effect of CGRP8–37 in rat aorta
Addition of hα CGRP8–37 (10−7–10−5 M) alone had no effect on basal or spasmogen-induced tone. Treatment with hα CGRP8–37 (10−5 M) for either 2, 20 or 60 min, before addition of hα CGRP did not significantly effect the agonist curves (P>0.05; n=4 each, using separate tissues). Subsequent results were obtained by incubating hα CGRP8–37 for 20 min prior to the addition of agonists.
In the presence of hα CGRP8–37 (10−5 M), there was a non-parallel rightward shift and a reduction in the maximum response to hα CGRP when controls and antagonist curves were carried out in the same tissue (Figure 3a), similar to that seen when hα CGRP curves were repeated (Figure 2b). The pEC50 values for hα CGRP in the absence and presence of hα CGRP8–37 were 7.5±0.1 and 7.1±0.2, respectively. However, when first concentration response curves to hα CGRP in the presence of hα CGRP8–37 (10−5 M) were constructed and compared with single agonist curves from separate tissues, these were not significantly different from each other (P>0.05; Figure 3b). The pEC50 values for hα CGRP were 7.6±0.1 and 7.3±0.1 in the absence and presence of hα CGRP8–37, respectively (apparent pKB <5 for hα CGRP8–37). Subsequent results were obtained by constructing single agonist curves in either the absence or presence of antagonist. Hα CGRP8–37 (10−5 M) did not significantly modify responses to either hβ CGRP or rat β CGRP (Figure 4a,b). The pEC50 values of hβ CGRP were 7.8±0.1 and 7.6±0.1, and of rat β CGRP were 7.6±0.1 and 7.4±0.1 in the absence and presence of hα CGRP8–37, respectively, and maximum responses to agonists were unaltered.
Figure 3.
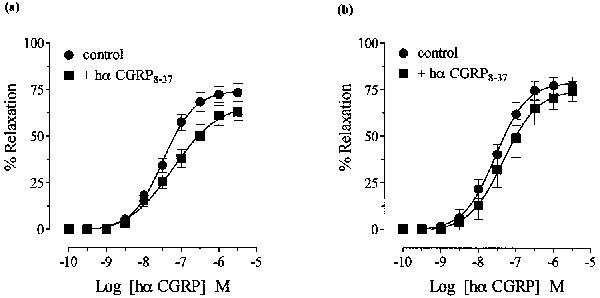
Effect of hα CGRP8–37 (10−5 M) on hα CGRP-induced relaxation in noradrenaline-contracted rat thoracic aorta in (a) the same or (b) separate groups of tissues (see text). Results are expressed as percentage relaxation of the spasmogen-induced tone. Points represent the mean±s.e.mean of four separate experiments.
Figure 4.
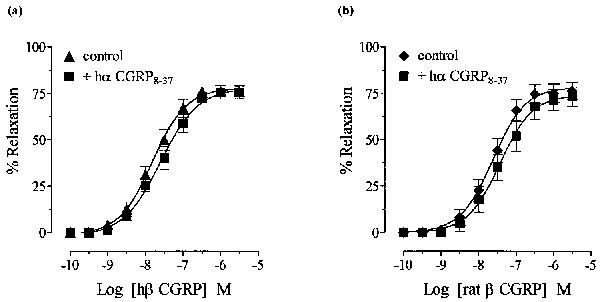
Effect of hα CGRP8–37 (10−5 M) on (a) hβ CGRP and (b) rat β CGRP relaxation of noradrenaline-induced tone in rat thoracic aorta, obtained from separate tissues. Results are expressed as percentage relaxation of the spasmogen-induced tone. Points represent the mean±s.e.mean of four separate experiments.
Effect of peptidase inhibitors of rat aorta
A mixture of peptidase inhibitors (amastatin, bestatin, captopril, phosphoramidon, thiorphan; 10−6 M each) had no effect on either basal or spasmogen-induced tone. Pretreatment (30 min) with the peptidase inhibitors did not potentiate relaxation to either hα CGRP or [Cys(ACM2,7)] hα CGRP, when tested in the same (Figure 5a) or separate (Figure 5b) tissues. The inactivity of hα CGRP8–37 (10−5 M) against hα CGRP was not affected by peptidase inhibitors (pEC50 values of hα CGRP in the presence of hα CGRP8–37 were 7.4±0.1 and 7.3±0.2 before and after treatment with peptidase inhibitors, respectively; Figure 6).
Figure 5.

Effect of peptidase inhibitors (amastatin, bestatin, captopril, phosphoramidon, thiorphan; 10−6 M each) on hα CGRP and [Cys(ACM2,7)] hα CGRP responses in the rat thoracic aorta. Concentration response curves to hα CGRP and [Cys(ACM2,7) hα CGRP alone on noradrenaline-induced tone, and the effect of peptidase inhibitors (hollow symbols) on (a) hα CGRP responses in the same tissue or (b) responses to hα CGRP and [Cys(ACM2,7)] hα CGRP, obtained from separate tissues. Results are expressed as percentage relaxation of the spasmogen-induced tone. Points represent the mean±s.e.mean of four or five individual experiments.
Figure 6.
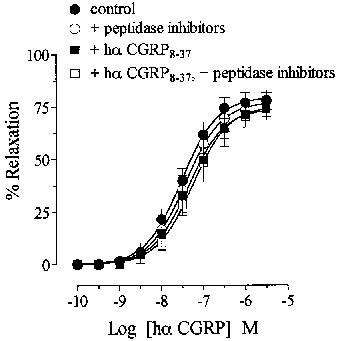
Effects of peptidase inhibitors (amastatin, bestatin, captopril, phosphoramidon, thiorphan; 10−6 M each) on hα CGRP responses in the absence and presence of hα CGRP8–37 in rat thoracic aorta. Single concentration response curve to hα CGRP on noradrenaline-induced tone, and in the presence of hα CGRP8–37 (10−5 M; from separate tissues) before and after addition of peptidase inhibitors (10−6 M; from separate tissues). Results are expressed as percentage relaxation of the spasmogen-induced tone. Points represent the mean±s.e.mean of four or five individual experiments.
Effect of CGRP and CGRP8–37 in porcine coronary artery
In porcine left anterior descending (LAD) artery, U46,619 (10−8 M) induced a stable contractile tone of 2.7±0.2 g and 3.7±0.3 g (n=6 each), in endothelium-intact and -denuded tissues, respectively. In rings without endothelium hα CGRP produced a concentration-dependent relaxation (pEC50 8.3±0.1; 101% maximum relaxation; Figure 7a). The effect of a given concentration began within 10–15 s of administration and reached its maximum after about 300 s. Construction of second and third concentration response curves to hα CGRP (after 30 min) were not reproducible (P<0.05), and showed a 3–10 fold reduction in pEC50 values and maximum response, suggesting tachyphylaxis. Subsequent results were obtained by comparing single agonist curves to hα CGRP in either the absence or the presence hα CGRP8–37. Pretreatment (15 min) with hα CGRP8–37 (10−6–10−5 M) produced a concentration-dependent parallel rightward shift of the hα CGRP curve, without reducing the maximum response (pA2 6.3, slope 0.9±0.1; Figure 7a). In porcine anterior interventricular artery (AIA) without endothelium, acetylcholine (2×10−7 M) induced a stable contractile response of 3.4±0.5 g (n=6). Cumulative addition of hα CGRP induced an endothelium-independent relaxation (pEC50 8.3±0.1, maximum 106% relaxation; Figure 7b), as observed in the LAD artery. In contrast to LAD rings, second concentration response curves to hα CGRP in the AIA were reproducible, and showed no tachyphylaxis (data not shown). Hα CGRP8–37 (10−7–10−5 M; 15 min pretreatment) antagonized hα CGRP responses, concentration-dependently, with no decrease in maximum response (pA2 6.7, slope 0.9±0.1; Figure 7b).
Figure 7.
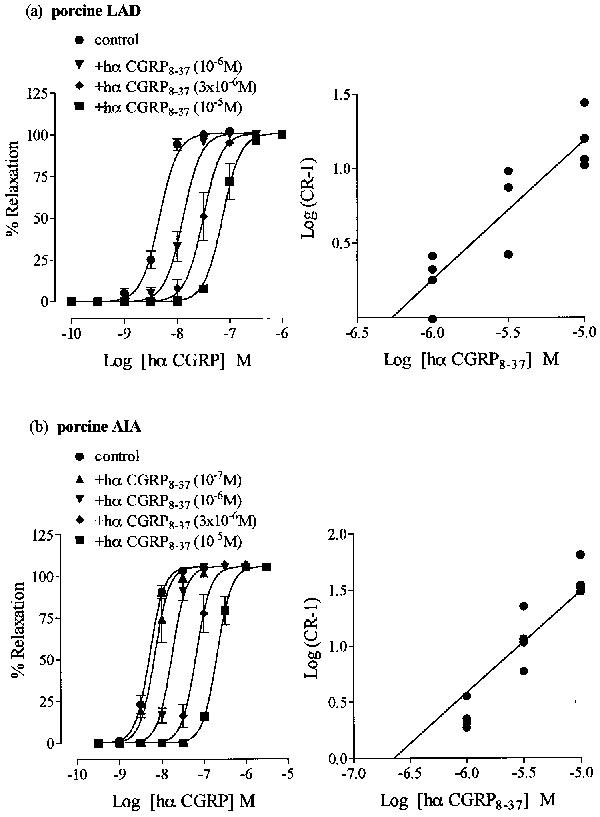
Antagonist effect of hα CGRP8–37 against hα CGRP-induced relaxation of U46,619-preconstructed porcine left anterior descending coronary artery (LAD) and of acetylcholine-precontracted porcine anterior interventricular artery (AIA). Concentration response curves to hα CGRP (left panel) on (a) U46,619-induced tone in LAD and (b) acetylcholine-induced tone in AIA, and in the presence of hα CGRP8–37 at (a) 10−6–10−5 M and (b) 10−7–10−5 M. Results are expressed as percentage relaxation of the spasmogen-induced tone. Points represent the mean±s.e.mean of four individual experiments. The Schild plots for hα CGRP8–37 (right panel) in porcine (a) LAD and (b) AIA, where points represent individual values from four experiments.
Effect of hα CGRP8–37 on cyclic nucleotide accumulation induced by hα CGRP in rat aorta and porcine coronary artery
Cyclic AMP and cyclic GMP control levels in rat aortic rings with intact endothelium and constricted with noradrenaline (10−7 M) were 645±38 fmol mg−1 protein (n=4) and 175±35 fmol mg−1 protein (n=4), respectively, at 30 s. Hα CGRP (3×10−7 M) relaxed the spasmogen-induced tone by 66±3%, and caused a 4 fold increase in cyclic AMP and a 10 fold increase in cyclic GMP, at 30 s. Preincubation with hα CGRP8–37 (10−5 M; 20 min) did not affect hα CGRP-induced accumulation of either cyclic AMP or cyclic GMP levels (P>0.05; Figure 8a).
Figure 8.
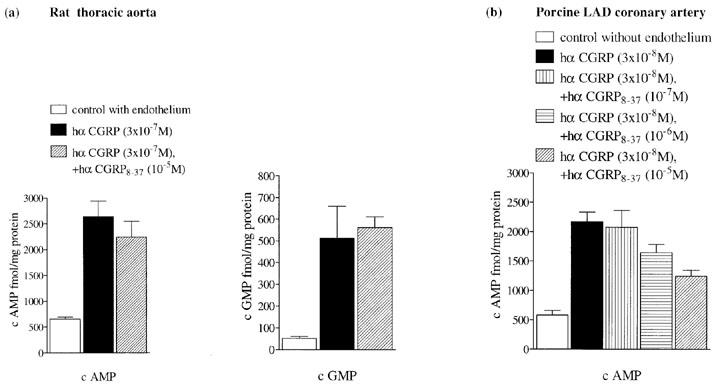
Effect of (a) hα CGRP8–37 (10−5 M) on cyclic AMP (left panel) and cyclic GMP (right panel) levels induce by hα CGRP (3×10−7 M) in rat thoracic aortic rings with intact endothelium preconstricted with noradrenaline (10−7 M), at 30 s and effect of (b) hα CGRP8–37 (10−7–10−5 M) on cyclic AMP levels induced by hα CGRP (3×10−8 M) in porcine left anterior descending (LAD) coronary arterial rings with denuded endothelium preconstricted with U46,619 (10−8 M), at 180 s. Levels of cyclic AMP and cyclic GMP are expressed as fmol mg−1 protein. Bars represent the mean±s.e.mean of between five and ten separate experiments. *P<0.05, ***P<0.001.
In porcine left anterior descending coronary artery without endothelium, control levels of cyclic AMP constricted with U46,619 (10−8 M) were 580±73 fmol mg−1 protein (n=6). Hα CGRP (3×10−8 M) gave a relaxation of 46±4%, and caused a 4 fold increase in cyclic AMP, without affecting levels of cyclic GMP, at 180 s. After pretreatment (15 min) with hα CGRP8–37 at 10−7 M, 10−6 M and 10−5 M, hα CGRP-induced elevation of cyclic AMP was either unaffected (P>0.05), or significantly reduced by 24% (P<0.05) and 43% (P<0.001), respectively.
Discussion
The classification of CGRP receptors into CGRP1 and CGRP2 subtypes has been proposed on the basis of a 10- to 50 fold difference in antagonist affinity for hα CGRP8–37 between the guinea-pig atrium and the rat vas deferens (Dennis et al., 1990). The present study from the rat aorta, however, shows an affinity for CGRP8–37 lower than reported for either of the two CGRP receptor subtypes, while in the porcine coronary artery the affinity value could match a CGRP1 receptor type.
In the rat thoracic aorta, the antagonist hα CGRP8–37 demonstrates an apparent pKB of less than five against endothelium-dependent relaxation of three CGRP isoforms, indicating that the lack of effect is not agonist-dependent. The reactivity of the aorta to CGRP showed a fall in maximum response when hα CGRP8–37 was present in second against curves, but not when the antagonist was measured against first curves to CGRP from separate tissues (but was seen in second control curves with no antagonist present). Thus, the decrease in CGRP response reflects factors independent of hα CGRP8–37. The mechanism involved in the reduction of tissue sensitivity to CGRP is not fully known, but one possibility may be loss of endothelium. It is interesting that the maximum relaxation to CGRP in the rat aorta (75% in present results) ranges from less than 20% up to nearly 80% in the literature (e.g. Brain et al., 1985; Grace et al., 1987; Fiscus et al., 1991; Gray & Marshall, 1992b; Zygmunt et al., 1995; Yoshimoto et al., 1998).
There are some contrasting results for hα CGRP8–37, in the rat aorta. For instance, recently Yoshimoto et al. (1998) reported an apparent pKB value of seven, although it is unclear whether results were obtained from single or separate tissues. Our previous studies indicated that hα CGRP8–37 behaves like a non-competitive antagonist against second hα CGRP curves (Gray et al., 1991). The present study, however, indicates that the fall in tissue sensitivity to CGRP is not due to hα CGRP8–37, having compared first and second agonist curves. Therefore, this illustrates how an apparent shift of hα CGRP8–37 in the rat aorta could be mistaken as receptor antagonism.
Failure to reach equilibrium at its receptor is unlikely to explain the relative inactivity of hα CGRP8–37 in the present study of the rat aorta, since preincubation from 2 min up to 60 min did not alter the inactivity of hα CGRP8–37, and previous studies have shown that equilibration had largely occurred within 2–3 min, in the rat pulmonary artery and vas deferens (Wisskirchen et al., 1998).
In rat aortic smooth muscle cells, it has been shown that endopeptidase 24.11 and aminopeptidase N are involved in CGRP metabolism (Mentlein & Roos, 1996). Thiorphan increased the affinity value of hα CGRP8–37 by more than 10% (Longmore et al., 1994) and phosphoramidon potentiated the effect of CGRP (Chatelain et al., 1995). However, the peptidase inhibitors did not modify either the potency of hα CGRP or the apparent affinity value of hα CGRP8–37, in rat aorta, consistent with rat pulmonary artery and vas deferens (Wisskirchen et al., 1998). Thus, it appears that metabolism by peptidases does not account for the low affinity value of hα CGRP8–37 in the aorta.
The relatively inactivity for hα CGRP8–37 is not consistent with the current receptor classification, with an at least 10 fold lower apparent pKB value than at the CGRP2 receptor (pA2 6.2–6.6, Dennis et al., 1990; Mimeault et al., 1991). CGRP2 receptors have been suggested in tissues where no affinity value for hα CGRP8–37 was demonstrated, but most studies used concentrations only down to 3×10−6 M (Quirion et al., 1992; Giuliani et al., 1992; Tomlinson & Poyner, 1996). In the present experiments, hα CGRP8–37 was inactive at the even higher concentration of 10−5 M, which could suggest that receptors in the rat aorta differ from both CGRP1 and CGRP2 receptors.
In the rat, antagonist pKB/pA2 values for hα CGRP8–37 range from less than five to 8.5 (Table 2). Values around six (e.g. vas deferens) and seven (e.g. pulmonary artery) might match a CGRP2 and a CGRP1 receptor, respectively, but there are several values at least an order of magnitude higher than that at the pulmonary artery CGRP1 receptor (Chin et al., 1994; Entzeroth et al, 1995; Bell & McDermott, 1994; Aiyar et al., 1992; Poyner et al., 1992). This range of hα CGRP8–37 affinities has been obtained from rat in vitro and perfused tissues, and isolated cells.
Table 2.
Antagonist affinities for hα CGRP8–37 against CGRP in the rat from isolated tissues, perfused tissues and cell preparations
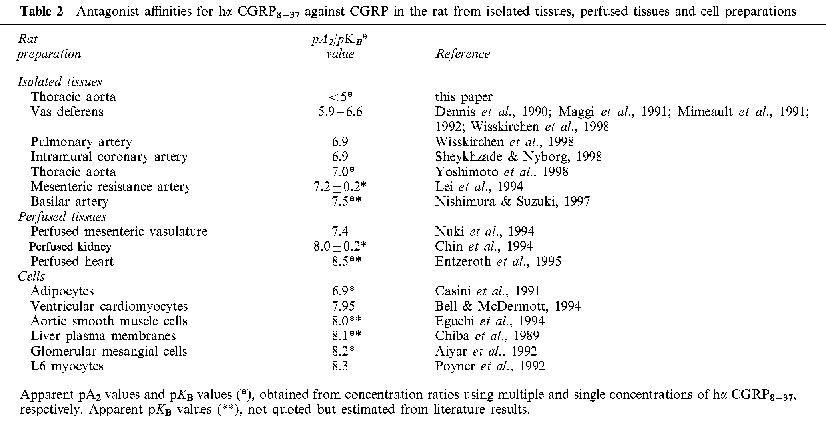
In rings of rat aorta, elevation in both cyclic AMP and cyclic GMP occurs but only when the endothelium is present (Gray & Marshall, 1992a). The same result was found in the present experiments. However, in addition the antagonist hα CGRP8–37 did not attenuate the CGRP-evoked rises in either cyclic nucleotides. In recent experiments a rise in aortic endothelial [Ca2+]i was associated with CGRP but the effect of the antagonist was not reported (Yoshimoto et al., 1998). There are contrasting results between cultured cells and isolated tissues. For example, CGRP binding sites were not detected in sections of rat aorta but were present in cultured rat aortic smooth muscle cells (Connat et al., 1992), suggesting the latter may not be a realistic model of the former. Furthermore, in rat cultured aortic smooth muscle cells, CGRP elicits a selective increase in cyclic AMP (Kubota et al., 1985; Hirata et al., 1988), which is antagonized by hα CGRP8–37 with an affinity value of around eight (Eguchi et al., 1994). The present study, however, demonstrates a pKB value for hα CGRP8–37 at least three orders of magnitude lower against endothelium-dependent relaxation of CGRP. These conflicting results between cultured systems and more complex tissues highlight the difficulties of extrapolating from cultured systems to tissues. However, in this study of rat aortic tissue the inactivity of hα CGRP8–37 against CGRP-relaxation is consistent with the finding that CGRP-induced accumulation of cAMP and cGMP was not antagonized by hα CGRP8–37.
The lack of effect of hα CGRP8–37 in the aorta might reflect the absence of CGRP receptors. The present results, suggesting that CGRP is a full agonist, are consistent with literature data (Gray & Marshall, 1992b), but this is not direct evidence for a CGRP receptor. Therefore, it is possible that CGRP interacts with non-CGRP receptors, including those for CGRP homologues. For instance, CGRP and adrenomedullin demonstrated equal potencies, which might suggest cross-reaction with adrenomedullin receptors, but further work with adrenomedullin antagonists would be needed to evaluate this possibility.
The agonist potency of [Cys(ACM2,7)] hα CGRP relative to hα CGRP has been suggested as a criterion to characterize CGRP receptors (Dennis et al., 1989), although this was not confirmed at either rat CGRP1 or CGRP2 receptors (Wisskirchen et al., 1998). Similarly, in the present study, [Cys(ACM2,7)] hα CGRP was over 1000 fold weaker than hα CGRP, and was not increased by peptidase inhibitors. Therefore, the weak activity of [Cys(ACM2,7)] hα CGRP in the aorta cannot be used to either support or refute the involvement of a CGRP receptor.
Porcine coronary arteries were different from the rat aorta, as CGRP induced an endothelium-independent vasorelaxation in both left anterior descending coronary and anterior interventricular arteries. However, results from porcine left anterior descending artery and rat aorta were similar in regard to CGRP showing tachyphylaxis in both vessels.
The pA2 values for hα CGRP8–37 in both porcine coronary artery preparations (pA2 6.3 and 6.7) are consistent with previous reports (apparent pKB 6.7; Saha et al., 1998), and agree with values from, for instance, the rat intramural coronary artery (pA2 6.9; Sheykhzade & Nyborg, 1998), and the guinea-pig left atrium (pA2 6.9; Dennis et al., 1990), indicating that the type of CGRP receptor in porcine and rat coronary circulation might match that of a CGRP1 receptor.
The relaxation to CGRP was associated with an accumulation of cyclic AMP in porcine left anterior descending coronary artery. Like the relaxation, the rise in cyclic AMP was antagonized by hα CGRP8–37 suggesting that the CGRP receptor mediated both effects. This contrasts with the rat aorta where neither relaxation nor rises in cyclic nucleotides were inhibited by the antagonist.
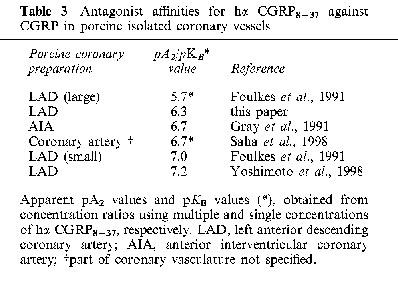
In vascular preparations, reported affinities for hα CGRP8–37 have shown heterogeneity of pKB/pA2 values, ranging from below five (e.g. rat aorta) up to around nine (e.g. canine lingual artery; Kobayashi et al., 1995), covering a range of species and tissue and making CGRP receptor classification more difficult. Part of this range may reflect differences in CRLR (calcitonin receptor like receptor) and the accessory protein RAMP, the combination of these two being required for a functional CGRP receptor (McLatchie et al., 1998). For CGRP receptors in rat vascular tissues, the 100 fold plus difference in hα CGRP8–37 affinity between the aorta and other arteries such as the pulmonary artery or mesenteric resistance artery (Table 2), could suggest the presence of heterogenous CGRP receptors. However, because an affinity value for hα CGRP8–37 was not determined in the present study of the rat aorta, it remains unclear whether the inactivity of hα CGRP8–37 reflects a CGRP receptor other than CGRP1 or CGRP2 or a non-CGRP receptor. At present, there is no porcine data to compare with the coronary vessel (Table 3), making it difficult to decide whether this is a CGRP1 receptor, or perhaps a CGRP2 receptor, that has a higher affinity value in this species compared with the rat.
Table 3.
Antagonist affinities for hα CGRP8–37 against CGRP in porcine isolated coronary vessels
In conclusion, the high potency of CGRP and the relative inactivity of hα CGRP8–37 in the rat aorta suggest that this may be a CGRP receptor distinct from both the CGRP1 and CGRP2 subtypes which has implications for CGRP receptor classification. However, it cannot be excluded that CGRP interacts with a non-CGRP receptor in the rat aorta. In the porcine coronary artery, the antagonist affinity value for hα CGRP8–37 is consistent with a CGRP1 type, with the assumption that species differences do not exist between rat and pig.
Acknowledgments
We thank Dr Paul Doyle, Dr John Harris and Sharon Gough (GlaxoWellcome) for synthesis and supply of peptides.
Abbreviations
- AIA
anterior interventricular artery
- LAD
left anterior descending
- U46,619
9,11-dideoxy-11α,9α epoxymethanprostaglandin F2α
References
- AIYAR N., GRIFFIN E., ALBRIGHTSON-WINSLOW C., FEUERSTEIN G., NAMBI P. Homologous desensitization of calcitonin gene-related peptidase response in rat glomerular mesangial cells in culture. Mol. Cell. Biochem. 1992;113:17–23. doi: 10.1007/BF00230881. [DOI] [PubMed] [Google Scholar]
- ARUNLAKSHANA O., SCHILD H.O. Some quantitative uses of drug antagonists. Br. J. Pharmacol. Chemother. 1959;14:48–57. doi: 10.1111/j.1476-5381.1959.tb00928.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BELL D., MCDERMOTT B.J. Calcitonin gene-related peptide stimulates a positive contractile response in rat ventricular cardiomyocytes. J. Cardiovasc. Pharmacol. 1994;23:1011–1021. doi: 10.1097/00005344-199406000-00021. [DOI] [PubMed] [Google Scholar]
- BRAIN D., WILLIAMS T.J., TIPPINS J.R., MORRIS H.R., MACINTYRE I. Calcitonin gene-related peptide is a potent vasodilator. Nature. 1985;313:54–56. doi: 10.1038/313054a0. [DOI] [PubMed] [Google Scholar]
- CASINI A., GALLI G., SALZANO R., SANTICIOLI P., MAGGI C.A., ROTELLA C.M., SURRENTI C. Calcitonin gene-related peptide increases the production of glycosaminoglycans but not of collagen type I and III in cultures of rat fat-storing cells. Life Sci. 1991;49:PL163–PL168. doi: 10.1016/0024-3205(91)90331-5. [DOI] [PubMed] [Google Scholar]
- CHATELAIN C., POCHON N., LACROIX J.S. Functional effects of phosphoramidon and captopril on exogenous neuropeptides in human nasal mucosa. Eur. Arch. Otorhinolaryngol. 1995;252:83–85. doi: 10.1007/BF00168025. [DOI] [PubMed] [Google Scholar]
- CHIBA T., YAMAGUCHI A., YAMATANI T., NAKAMURA A., MORISHITA T., INUI T., FUKASE M., NODA T., FUJITA T. Calcitonin gene-related peptide receptor antagonist human CGRP-(8–37) Am. J. Physiol. 1989;256:E331–E335. doi: 10.1152/ajpendo.1989.256.2.E331. [DOI] [PubMed] [Google Scholar]
- CHIN S.Y., HALL J.M., BRAIN S.D., MORTON I.K.M. Vasodilator responses to calcitonin gene-related peptide (CGRP) and amylin in the rat isolated perfused kidney are mediated via CGRP 1 receptors. J. Pharmacol. Exp. Ther. 1994;269:989–992. [PubMed] [Google Scholar]
- CONNAT J.L., THIEVENT A., CARRIER N., HUGGEL H. Occurrence of CGRP receptors on dedifferentiated smooth muscle cells from media of rat thoracic aorta. Ann. N.Y. Acad. Sci. 1992;657:444–448. doi: 10.1111/j.1749-6632.1992.tb22795.x. [DOI] [PubMed] [Google Scholar]
- DENNIS T., FOURNIER A., CADIEUX A., POMERLEAU F., JOLICEUR F.B., ST-PIERRE S., QUIRION R. hCGRP8-37, a calcitonin gene-related peptide antagonist revealing calcitonin gene-related peptide receptor heterogeneity in brain and periphery. J. Pharmacol. Exp. Ther. 1990;254:123–128. [PubMed] [Google Scholar]
- DENNIS T., FOURNIER A., ST-PIERRE S., QUIRION R. Structure-activity profile of calcitonin gene-related peptide in peripheral and brain tissues. Evidence for receptor multiplicity. J. Pharmacol. Exp. Ther. 1989;251:718–725. [PubMed] [Google Scholar]
- EGUCHI S., HIRATA Y., KANO H., SATO K., WATANABE T.X., NAKAJIMA K., SAKAKIBARA S., MARUMO F. Specific receptors for adrenomedullin in cultured rat vascular smooth muscle cells. FEBS Lett. 1994;340:226–230. doi: 10.1016/0014-5793(94)80143-6. [DOI] [PubMed] [Google Scholar]
- ENTZEROTH M., DOODS H.N., WIELAND H.A., WIENEN W. Adrenomedullin mediates vasodilatation via CGRP1 receptors. Life Sci. 1995;56:19–25. doi: 10.1016/0024-3205(94)00412-l. [DOI] [PubMed] [Google Scholar]
- FISCUS R.R., ZHOU H.L., WANG X., HAN C., ALI S., JOYCE C.D., MURAD F. Calcitonin gene-related peptide (CGRP)-induced cyclic AMP, cyclic GMP and vasorelaxant responses in rat thoracic aorta are antagonized by blockers of endothelium-derived relaxant factor (EDRF) Neuropeptides. 1991;20:133–143. doi: 10.1016/0143-4179(91)90063-o. [DOI] [PubMed] [Google Scholar]
- FOULKES R., SHAW N., BOSE C., HUGHES B. Differential vasodilator profile of calcitonin gene-related peptide in porcine large and small diameter coronary artery rings. Eur. J. Pharmacol. 1991;201:143–149. doi: 10.1016/0014-2999(91)90337-p. [DOI] [PubMed] [Google Scholar]
- FRANCO-CERECEDA A., RUDENHILL A., LUNDBERG J.M. Calcitonin gene-related peptide but not substance P mimics capsaicin-induced coronary vasodilation in the pig. Eur. J. Pharmacol. 1987;142:235–243. doi: 10.1016/0014-2999(87)90112-9. [DOI] [PubMed] [Google Scholar]
- GIULIANI S., WIMALAWANSA S.J., MAGGI C.A. Involvement of multiple receptors in the biological effects of calcitonin gene-related peptide and amylin in rat and guinea-pig preparations. Br. J. Pharmacol. 1992;107:510–514. doi: 10.1111/j.1476-5381.1992.tb12775.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GRACE G.C., DUSTING G.J., KEMP B.E., MARTIN T.J. Endothelium and the vasodilator action of rat calcitonin gene-related peptide (CGRP) Br. J. Pharmacol. 1987;91:729–733. doi: 10.1111/j.1476-5381.1987.tb11270.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GRAY D.W., MARSHALL I. Human α-calcitonin gene-related peptide stimulates adenylate cyclase and relaxes rat thoracic aorta by releasing nitric oxide. Br. J. Pharmacol. 1992a;107:691–696. doi: 10.1111/j.1476-5381.1992.tb14508.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GRAY D.W., MARSHALL I. Nitric oxide synthesis inhibitors attenuate calcitonin gene-related peptide endothelium-dependent vasorelaxation in rat aorta. Eur. J. Pharmacol. 1992b;212:37–42. doi: 10.1016/0014-2999(92)90069-g. [DOI] [PubMed] [Google Scholar]
- GRAY D.W., MARSHALL I., BOSE C., FOULKES R., HUGHES B. Subtypes of the calcitonin-gene related peptide (CGRP) receptor in vascular tissues. Br. J. Pharmacol. 1991;102:189P. [Google Scholar]
- HIRATA Y., TAKAGI Y., SHOICHIRO T., FUKUDA Y., YOSHIMI H., FUJITA T. Calcitonin gene-related peptide receptor in cultured vascular smooth muscle and endothelial cells. Biochem. Biophys. Res. Commun. 1988;151:113–1121. doi: 10.1016/s0006-291x(88)80481-9. [DOI] [PubMed] [Google Scholar]
- KOBAYASHI D., TODOKI K., OZONO S., OKABE E. Calcitonin gene-related peptide mediating neurogenic vasorelaxation in the isolated canine lingual artery. Jpn. J. Pharmacol. 1995;67:329–339. doi: 10.1254/jjp.67.329. [DOI] [PubMed] [Google Scholar]
- KUBOTA M., MOSELEY J.M., BOTERA L., DUSTING G.M., MACDONALD P.S., MARTIN T.S. Calcitonin gene-related peptide stimulates cAMP in rat aortic smooth muscle cells. Biochem. Biophys. Res. Commun. 1985;132:88–94. doi: 10.1016/0006-291x(85)90992-1. [DOI] [PubMed] [Google Scholar]
- LEI S., MULVANEY M.J., NYBORG N.C. Characterisation of the CGRP receptor and mechanisms of action in rat mesenteric small arteries. Pharmacol. Toxicol. 1994;74:130–135. doi: 10.1111/j.1600-0773.1994.tb01087.x. [DOI] [PubMed] [Google Scholar]
- LONGMORE J., HOGG J.E., HUSTON P.H., HILL R.G. Effects of two truncated forms of human calcitonin gene-related peptide: implications for receptor classification. Eur. J. Pharmacol. 1994;265:53–59. doi: 10.1016/0014-2999(94)90222-4. [DOI] [PubMed] [Google Scholar]
- LOWRY O.A., ROSEBROUGH N.J., FARR A.L., RANDALL A.J. Protein measurement with the Folin phenol reagent. J. Biol. Chem. 1951;193:265–275. [PubMed] [Google Scholar]
- MAGGI C.A., CHIBA T., GIULIANI S. Human α-calcitonin gene-related peptide (8–37) as an antagonist of exogenous and endogenous calcitonin gene-related peptide. Eur. J. Pharmacol. 1991;192:85–88. doi: 10.1016/0014-2999(91)90072-x. [DOI] [PubMed] [Google Scholar]
- MARSHALL I. Mechanism of vascular relaxation by the calcitonin gene-related peptide. Ann. N.Y. Acad. Sci. 1992;657:194–203. doi: 10.1111/j.1749-6632.1992.tb22769.x. [DOI] [PubMed] [Google Scholar]
- MARSHALL I., AL-KAZWINI S.J., HOLMAN J.J., CRAIG R.K. Human and rat α-CGRP but not calcitonin cause mesenteric vasodilatation in rats. Eur. J. Pharmacol. 1986a;123:217–222. doi: 10.1016/0014-2999(86)90662-x. [DOI] [PubMed] [Google Scholar]
- MARSHALL I., AL-KAZWINI S.J., ROBERTS P.M., SHEPPERSON N.B., ADAMS A., CRAIG R.K. Cardiovascular effects of human and rat CGRP compared in the rat and other species. Eur. J. Pharmacol. 1986b;123:207–216. doi: 10.1016/0014-2999(86)90661-8. [DOI] [PubMed] [Google Scholar]
- MCLATCHIE L.M., FRASER N.J., MAIN M.J., WISE A., BROWN J., THOMPSON N., SOLARI R., LEE M.G., FOORD S.M. RAMPs regulate the transport and ligand specificity of the calcitonin-receptor-like receptor. Nature. 1998;393:333–339. doi: 10.1038/30666. [DOI] [PubMed] [Google Scholar]
- MENTLEIN R., ROOS T. Proteases involved in the metabolism of angiotensin II, bradykinin, calcitonin gene-related peptide (CGRP), and neuropeptide Y by vascular smooth muscle cells. Peptides. 1996;17:709–720. doi: 10.1016/0196-9781(96)00066-6. [DOI] [PubMed] [Google Scholar]
- MIMEAULT M., FOURNIER A., DUMONT Y., ST-PIERRE S., QUIRION R. Comparative affinities and antagonistic potencies of various human calcitonin gene-related peptide fragments on calcitonin gene-related peptide receptors in brain and periphery. J. Pharm. Exp. Ther. 1991;258:1084–1090. [PubMed] [Google Scholar]
- MIMEAULT M., QUIRION R., DUMONT Y., ST-PIERRE S., FOURNIER A. Structure-activity study of hCGRP8-37, a calcitonin gene-related peptide receptor antagonist. J. Med. Chem. 1992;35:2165–2168. doi: 10.1021/jm00090a003. [DOI] [PubMed] [Google Scholar]
- NISHIMURA Y., SUZUKI A. Relaxant effects of vasodilator peptides on isolated basilar arteries from Stroke-Prone spontaneously hypertensive rats. Clin. Exp. Pharmacol. Physiol. 1997;24:157–161. doi: 10.1111/j.1440-1681.1997.tb01800.x. [DOI] [PubMed] [Google Scholar]
- NUKI C., KAWASAKI H., TAKASAKI K., WADA A. Structure-activity study of chicken calcitonin gene-related peptide on vasorelaxation in rat mesenteric resistance vessels. Jpn. J. Pharmacol. 1994;65:99–105. doi: 10.1254/jjp.65.99. [DOI] [PubMed] [Google Scholar]
- POYNER D.R., ANDREW D.P., BROWN D., BOSE C., HANLEY M.R. Pharmacological characterisation of a receptor for calcitonin gene related peptide on rat, L6 myocytes. Br. J. Pharmacol. 1992;105:441–447. doi: 10.1111/j.1476-5381.1992.tb14272.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- QUIRION R., VAN ROSSUM D., DUMONT Y., ST-PIERRE S., FOURNIER A. Characterization of CGRP1 and CGRP2 receptor subtypes. Ann. N.Y. Acad. Sci. 1992;657:88–105. doi: 10.1111/j.1749-6632.1992.tb22759.x. [DOI] [PubMed] [Google Scholar]
- SAHA S., WAUGH D.J.J., ZHAO P., ABEL P.W., SMITH D.D. Role of conformational constrains of position 7 of the disulphide bridge of h-α-CGRP derivatives in their agonist versus antagonist properties. J. Peptides Res. 1998;52:112–120. doi: 10.1111/j.1399-3011.1998.tb01365.x. [DOI] [PubMed] [Google Scholar]
- SHEYKHZADE M., NYBORG N.C.B. Characterization of calcitonin gene-related peptide (CGRP) receptors in intramural coronary arteries from male and female Sprague Dawley rats. Br. J. Pharmacol. 1998;123:1464–1470. doi: 10.1038/sj.bjp.0701760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SIGRIST S., FRANCO-CERECEDA A., MUFF R., HENKE H., LUNDBERG J.M., FISCHER J.A. Specific receptor and cardiovascular effects of calcitonin gene-related peptides. Endocrinology. 1986;119:381–389. doi: 10.1210/endo-119-1-381. [DOI] [PubMed] [Google Scholar]
- TOMLINSON A.E., POYNER D.R. Multiple receptors for calcitonin gene-related peptide and amylin on guinea-pig ileum and vas deferens. Br. J. Pharmacol. 1996;117:1362–1368. doi: 10.1111/j.1476-5381.1996.tb16737.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WISSKIRCHEN F.M., BURT R.P., MARSHALL I. Pharmacological characterization of CGRP receptors mediating relaxation of the rat pulmonary artery and inhibition of twitch responses of the rat vas deferens. Br. J. Pharmacol. 1998;123:1673–1683. doi: 10.1038/sj.bjp.0701783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- YOSHIMOTO R., MITSUI-SAITO M., OZAKI H., KARAKI H. Effect of adrenomedullin and calcitonin gene-related peptide on contractions of the rat aorta and porcine coronary artery. Br. J. Pharmacol. 1998;123:1645–1654. doi: 10.1038/sj.bjp.0701805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ZYGMUNT P.M., RYMAN T., HOGESTATT E.D. Regional differences in endothelium-dependent relaxation in the rat: contribution of nitric oxide and nitric oxide-independent mechanisms. Acta Physiol. Scand. 1995;155:257–266. doi: 10.1111/j.1748-1716.1995.tb09972.x. [DOI] [PubMed] [Google Scholar]


