Abstract
We have used isolated arterial preparations from the rabbit and dog to investigate whether non-ionic iodinated radiographic contrast media (IRCM) modulate nitric oxide (NO) release. The tri-iodinated monomers iopromide and iohexol were compared with the hexa-iodinated dimer iodixanol.
The vasodilator effects of iohexol (300 mg ml−1) and iodixanol (320 mg ml−1) were assessed in cascade bioassay. Increasing concentrations of iohexol or iodixanol caused concentration-dependent relaxations of the detector tissue which were insensitive to 100 μM NG-nitro L-arginine methyl ester (L-NAME) and 10 μM indomethacin, whereas viscosity-associated relaxations induced by the ‘inert' agent dextran (MW 80,000; 1–4%) were attenuated by inhibition of NO synthesis.
Relaxations of endothelium-intact rings to acetylcholine (ACh) were attenuated by preincubation with iohexol or iodixanol, whereas relaxations to sodium nitroprusside (SNP) in endothelium-denuded rings were unaffected. Inhibitory activity did not correlate with either molarity or iodine concentration. Mannitol caused inhibition of both ACh- and SNP-induced responses.
In isolated perfused arteries the depressor responses to iodixanol (320 mg ml−1) and iopromide (300 mg ml−1) administered as close arterial bolus attained a plateau with maximal dilatations of ∼25% and ∼60%, respectively. Addition of 100 μM NG-nitro L-arginine (L-NOARG) and/or 10 μM indomethacin to the perfusate had no effect on the responses to either agent.
We conclude that IRCM exert direct effects on the endothelium that inhibit NO production rather than its action on vascular smooth muscle. Shear stress-induced stimulation of NO production by IRCM is unlikely to contribute to their vasodilator activity in vivo when administered during angiography despite high intrinsic viscosity.
Keywords: Iodixanol, iohexol, iopromide, nitric oxide, endothelium, vascular smooth muscle
Introduction
All types of iodinated radiographic contrast media (IRCM) cause vasodilatation, regardless of molecular structure, although it has become apparent both clinically and in animal models that this phenomenon is less pronounced with nonionic hexa-iodinated dimers such as iotrolan and iodixanol than nonionic tri-iodinated compounds at iodine concentrations that provide equivalent angiographic contrast (for review see Pugh & Karlsson, 1997). Previous generations of ionic IRCM often caused severe pain during intra-arterial injection, and the main factor responsible for vasodilatation was thought to be the high osmolality of such compounds which can be 7–8 times that of plasma (Almén, 1995). However, vasodilatation is still observed following intravascular injection of isoosmotic formulations of nonionic dimeric IRCM, so that such agents cannot be considered as haemodynamically inert (Pugh & Karlsson, 1997). Such observations suggest that vasodilatation may result, at least partly, from intrinsic pharmacological properties of the IRCM molecule itself. Indeed, as early as 1970, Lindgren questioned whether the vasodilator properties of IRCM were attributable exclusively to osmotic effects (Lindgren, 1970), concluding that vasodilatation was largely due to pharmacological actions of IRCM on the basis of pronounced differences in the in vivo vasodilator activities of diatrizoate and acetrizoate, two agents which have almost identical osmolalities.
Organ bath experiments have demonstrated that a direct endothelium-independent action on vascular smooth muscle plays an important role in the mediation of IRCM-induced vasorelaxation in rabbit aortic rings (Pugh et al., 1995; Pitman et al., 1996). However, the development from tri-iodinated ionic monomers to nonionic monomers and subsequently to hexa-iodinated nonionic dimers has resulted in increased viscosity (Almén, 1995), which may be as high as 15 mPa s−1 for clinically-used formulations of some modern IRCM. During angiography large volumes are administered as a single bolus at rapid injection rates. In theory, such high viscosity solutions could therefore stimulate release of NO from the vascular endothelium which is regulated not only by activation of specific pharmacological receptors (Furchgott & Zawadzki, 1980; Ignarro et al., 1987; Palmer et al., 1987; Moncada et al., 1991) but also by the longitudinal intimal shear stress that results from fluid flow (Pohl et al., 1986; Tesfamariam & Cohen, 1988; Hutcheson & Griffith, 1991; 1994). Cascade bioassay experiments with rabbit aorta, employing dextran to increase perfusate viscosity, have thus demonstrated incremental increases in shear stress-induced NO release from the donor segments until a plateau is attained at viscosities ca. 2 mPa s−1, which correspond to a mean endothelial shear stress of ∼10 dyne cm−2 (Hutcheson & Griffith, 1994). To determine whether this phenomenon contributes to the vasodilatation induced by IRCM, in the present study we have employed cascade bioassay experiments with perfused rabbit aorta to compare the effects of tri- and hexa-iodinated nonionic IRCM on NO release with those of dextran at equivalent viscosities. In parallel experiments, isolated endothelium-intact rabbit aortic rings were used to assess the effects of IRCM and mannitol, an agent widely used to investigate the effects of increased osmolality, on receptor-mediated NO release evoked by acetylcholine. Finally, isolated dog ear arteries were used to study the effects of bolus administration of IRCM on arterial tone under conditions that mimic those employed during clinical arteriography.
Methods
NO bioassay
A previously described cascade bioassay system was used to monitor shear stress-induced release of NO (Hutcheson & Griffith, 1991). Briefly, male New Zealand White rabbits (2.0–2.5 kg) were sacrificed by an overdose of sodium pentobarbital (120 mg kg−1 i.v.) and the thoracic aorta rapidly removed and placed in Holman's buffer of the following composition (mM): NaCl 120; KCl 5.0; CaCl2 2.5; NaH2PO4 1.3; NaHCO3 25; glucose 11; and sucrose 10. An 8 cm length of donor aorta possessing an intact endothelium was perfused at a constant 9 ml min−1 with Holman's buffer (37°C) containing increasing concentrations of iohexol 300 mg ml−1 (7.8–473 mM) or iodixanol 320 mg ml−1 (4.2–252 mM) or dextran 80 (1–4% w v−1) in the absence and presence of 100 μM NG-nitro L-arginine methyl ester (L-NAME) to inhibit NO synthesis by the endothelium. An endothelium-denuded recipient ring of the rabbit aorta, constricted by superfusion with 300 nM phenylephrine (PhE), served to monitor NO release from the perfused donor aorta. Changes in tension were measured by a force transducer (FT 102, CB Sciences Inc.) connected to a Maclab/4e (ADInstruments, NSW, Australia). All experiments were performed in the presence of 10 μM indomethacin to inhibit the synthesis of prostaglandins. Viscosity of IRCM and dextran solutions was measured using a clinical viscometer (Luckham, U.K.).
Isolated rabbit aortic rings
Two-millimetre-wide isolated rings of rabbit thoracic aorta were preconstricted with 300 nM PhE and cumulative concentration-relaxation curves to acetylcholine (ACh; 1 nM–3 μM) and sodium nitroprusside (SNP; 0.1 nM–3 μM) then constructed. In the case of SNP, all rings were denuded of endothelium by gentle abrasion with a wooden probe, denudation being confirmed by lack of relaxation to ACh. Following washout of all drugs and re-constriction, the effects of IRCM on both ACh- and SNP-induced relaxations were assessed with iohexol (10 or 30%) and iodixanol (30%) present in the organ bath. As both agents depress constriction of the rabbit aorta directly (Pitman et al., 1996), tone was returned to its original level by increasing the concentration of PhE administered to 3 μM. The effects of 10, 30 and 50% of a 300 mM solution of mannitol on ACh- and SNP-evoked relaxations were also investigated. In these studies further addition of PhE to the organ bath was not required to attain the initial level of tone.
Perfused dog ear arteries
Dog ears were obtained from mongrel dogs which had been sacrificed with pentobarbital/KCl. Specimens were immersed in a Krebs' buffer solution, of the following composition (mM): NaCl 122, KCl 4.7; CaCl2 2.5; MgCl2 1.2; KH2PO4 1.2; NaHCO3 15.4; and glucose 5.6. The Krebs buffer was equilibrated with 5% CO2 and 95% O2 at 37°C and 2–3 cm of the central ear arteries were dissected free from surrounding tissue and cannulated at both ends with thin polypropylene tubing. The arteries were then mounted in a Radnoti 158730 blood vessel perfusion system (Radnoti, Monrovia, CA, U.S.A.) containing Krebs' buffer solution. The perfusion pressure was measured using a Radnoti 159050 blood pressure transducer and a Gould Windograph (Gould Inc., Valley View, Ohio, U.S.A.). Following an equilibration period of 1 h, PhE (0.3–3 μM) was added to the perfusate and dose-response curves constructed by adding increasing volumes of iodixanol 320 mg ml−1 and iopromide 300 mg ml−1 to the perfusate as boluses. In a separate series of experiments the effects of bolus injections of 300 μl iopromide and 300 μl iodixanol were studied, in the presence and absence of 100 μM NG-nitro L-arginine (L-NOARG)±10 μM indomethacin, to inhibit synthesis of NO and prostanoids, respectively. Addition of 500 μl ACh (0.1 μM) was used to test endothelium-dependent vasodilatation.
Drugs
The following agents were employed: iodixanol (Visipaque 320 mg ml−1, viscosity at 37°C=ca. 11 mPa s−1, Nycomed Imaging, Oslo, Norway); iohexol (Omnipaque 300 mg ml−1, viscosity at 37°C=ca. 6 mPa s−1, Nycomed Imaging); iopromide (Ultravist 300 mg ml−1, viscosity at 37°C=ca. 5 mPa s−1, Schering AG, Berlin, Germany); acetylcholine, indomethacin, sodium nitroprusside (SNP), mannitol, dextran (80,000 MW), NG-nitro L-arginine (L-NOARG) and NG-nitro L-arginine methyl ester (L-NAME) (Sigma Chemical Co., St. Louis, MO, U.S.A.). Where discussed, increases in molarity due to IRCM and mannitol are given as additional to the molarity of Holman's buffer.
Statistical analysis
Results were expressed as mean±s.e.mean, where n denotes the number of animals studied for each data point. The significance of differences between groups in cascade bioassay and organ bath studies was tested by repeated measures of ANOVA followed by Bonferroni multiple comparisons test and for perfused canine ear artery and organ bath studies the Student's t-test for paired data was also used. P<0.05 was considered as significant.
Results
Cascade bioassay
Increments in the concentration of iohexol and iodixanol present in the perfusate passing through the donor rabbit aorta resulted in concentration-dependent relaxations of the recipient ring that were unaffected by L-NAME (100 μM) (Figure 1). The relaxations obtained with both agents were similar in magnitude, being of the order of 35–40% at the highest concentration employed (60% v v−1). In experiments with dextran, administered in concentrations (1–4% w v−1) that resulted in increases in viscosity that were comparable with those obtained with iohexol and iodixanol, relaxation of the recipient ring was significantly attenuated by L-NAME (100 μM, P<0.05), although a small L-NAME-insensitive component remained evident (Figure 1), as previously reported (Hutcheson & Griffith, 1994). In the absence of L-NAME, relaxations to dextran were comparable with those obtained with the iohexol and iodixanol only at concentrations resulting in a viscosity >2 mPa s−1.
Figure 1.
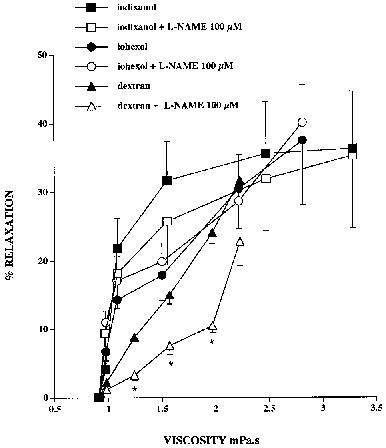
Relaxations of the detector ring induced by iohexol, iodixanol and dextran 80 in cascade bioassay, plotted as a function of the viscosity of the buffer perfusing the donor rabbit aorta. Increasing concentrations of each agent evoked progressive relaxation of the recipient aortic ring. Inclusion of L-NAME (100 μM) in the buffer had no effect on relaxations evoked by iohexol or iodixanol, but significantly attenuated those induced by dextran. *Denotes P<0.05 for dextran in the presence and absence of L-NAME.
Effects of IRCM on ACh- and SNP-induced relaxations
Preliminary experiments showed that addition of 10 and 30% iohexol and 30% iodixanol to the organ bath caused sustained relaxations of 46+4, 67±4 and 59±5% respectively in preparations constricted by 300 nM PhE (n=4 in each case). To determine the effects of these IRCM on responses to ACh, the concentration of PhE used to generate tone in their presence was increased in order to achieve levels of tone similar to the control situation and thus exclude effects attributable to functional antagonism. Following an increase in the concentration of PhE used to preconstrict the rings from 300 nM to 3 μM, there was no significant difference in the initial constrictor tone used to construct the concentration-relaxation curves to ACh in the absence or presence of 10 and 30% iohexol (2.8±0.1 g cf. 2.7±0.2 g, n=4; 3.2±0.2 g cf. 3.1±0.3 g, n=4, respectively) or 30% iodixanol (3.2±0.1 g cf. 3.4±0.3 g, n=4). Concentration-relaxation curves to ACh in endothelium-intact rings were markedly depressed by iohexol and iodixanol with both IRCM causing a shift to the right from an EC50 of 81±7 to 210±30 nM for 10% iohexol (P<0.01, n=4) and from 93±13 to 175±26 nM for 30% iodixanol (P<0.05, n=4) (Figure 2a). Maximum relaxations to ACh were equivalently depressed ∼30% by concentrations of 10% iohexol (from 77±11 to 54±8%, P<0.05, n=4) and 30% iodixanol (from 75±7 to 51±4%, P<0.05, n=4). The inhibition of ACh-induced relaxations did not correlate with iodine concentration or molarity as 10% iohexol and 30% iodixanol in Holman's buffer contain 30 and 96 mg ml−1 with final molarities of 78.8 and 126 mM, respectively. This is further emphasised by the fact that 30% iohexol in Holman's buffer, which has an iodine concentration of 90 mg ml−1 and molarity of 236 mM, depressed maximum relaxation by ∼80% to 17±3% (n=4) in association with a further shift in the EC50 value to 320±70 nM (n=4) (Figure 2a).
Figure 2.
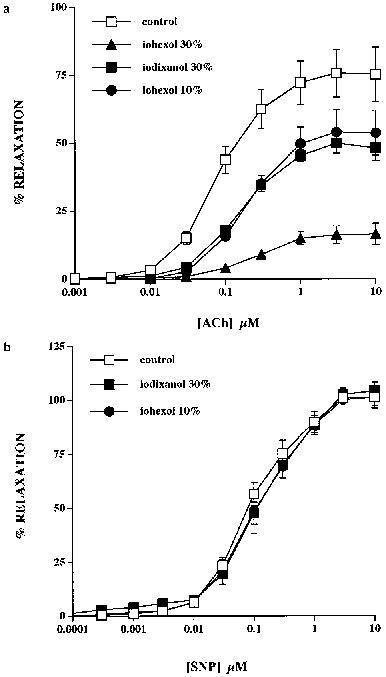
Effects of iohexol and iodixanol on ACh- and SNP-induced relaxations of isolated rings of rabbit aorta. (a) Pre-incubation with 10% iohexol and 30% iodixanol attenuated control ACh-induced relaxations to an equivalent degree. Thirty per cent iohexol further reduced the ACh-evoked response. (b) Control SNP-induced relaxations were unaffected by pre-incubation with either 10% iohexol or 30% iodixanol.
Concentration-relaxation curves to SNP in endothelium-denuded rings were unaffected by 10% iohexol with EC50 values (control: 80±6 nM, iohexol: 120±45 nM, n=4) and maximum relaxations (control: 106±4%, iohexol: 102±4%, n=4) being unchanged (Figure 2b). Similarly, 30% iodixanol was without effect on SNP-induced responses either in terms of the EC50 value (control: 79±7 nM, iodixanol: 101±25 nM, n=4) or maximal relaxations (control: 102±4%, iodixanol: 105±1%, n=4) (Figure 2b). There was no significant difference in the initial tone used to construct the concentration-relaxation curves in the absence or presence of iohexol (3.3±0.5 g cf. 3.2±0.4 g, n=4) or iodixanol (3±0.3 g cf. 3.2±0.4 g, n=4) as the concentration of PhE used to preconstrict the rings was again increased from 300 nM to 3 μM.
Effects of mannitol on ACh- and SNP-induced relaxations
Relaxations to ACh in endothelium-intact rings were depressed by mannitol in a concentration-dependent fashion in association with a progressive shift in the response curve to the right (Figure 3a). Maximum relaxation in the control situation was 60±6%, and was depressed ∼15% to 51±4% (n=3) by 10% mannitol, ∼80% to 14±3% (P<0.01, n=3) by 30% mannitol and almost abolished by 50% mannitol (molarities 30, 90 and 150 mM, respectively). EC50 values under these conditions were correspondingly increased from 60±6 to 98±16 nM by 10% mannitol and 120±0.03 nM by 30% mannitol. Incubation with 10, 30 and 50% mannitol evoked relaxations of PhE-induced tone of the order of 17±3, 32±5 and 39±5% respectively (n=6 for all concentrations). These relaxations were transient, however, with tone returning to pre-mannitol values within ∼10 mins, so there was no significant difference in tension prior to and following incubation with 10% (3.7±0.3 g cf. 3.6±0.2 g), 30% (3.6±0.1 g cf. 3.5±0.1 g) and 50% (3.5±0.3 g cf. 3.6±0.2 g) mannitol. It was therefore unnecessary to increase the concentration of PhE used to determine initial tone.
Figure 3.
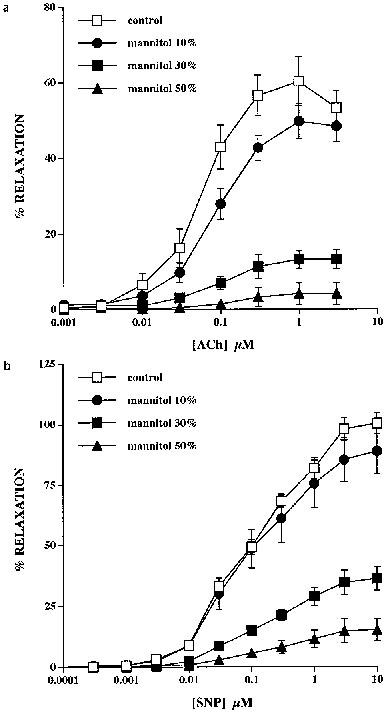
Effects of mannitol on ACh- and SNP-induced relaxations of isolated rings of rabbit thoracic aorta. (a) Incubation of the rings with mannitol at concentrations of 10, 30 and 50% caused progressive inhibition of control relaxations to ACh. (b) SNP-induced relaxations were similarly susceptible to concentration-dependent inhibition by mannitol.
Relaxations to SNP in endothelium-denuded rings were similarly depressed by mannitol (Figure 3b). Maximum relaxation in the control situation was 98±5%, and was depressed ∼15% to 83±7% by 10% mannitol, ∼65% to 35±5% (P<0.001, n=3) by 30% mannitol and ∼85% to 15±5% (P<0.001, n=3) by 50% mannitol, respectively, with EC50 values being increased from 78±7 to 100±17, 160±60 and 210±15 nM, respectively. In this series of experiments mannitol was again without effect on the steady-state tension response to 300 nM PhE, this being 3.8±0.4 g cf. 3.7±0.4 g with 10% mannitol, 4±0.3 g cf. 4.2±0.2 g with 30% mannitol and 4.1±0.4 g cf. 4.1±0.2 g with 50% mannitol.
Perfused dog ear arteries
Perfusion of the arteries at a constant rate of 5±1 ml min−1 resulted in a pressure of 69±3 mmHg which increased to 236±32 mmHg following addition of PhE (3 μM) to the perfusate. Increasing volumes of iopromide (300 mg ml−1) and iodixanol (320 mg ml−1) caused dose-dependent decreases in PhE-induced pressure (Figure 4), with iopromide resulting in a significantly larger depressor response of 69±9 compared to 36±6% with iodixanol, when both IRCM were injected as a 1500 μl bolus (P<0.05, n=6).
Figure 4.
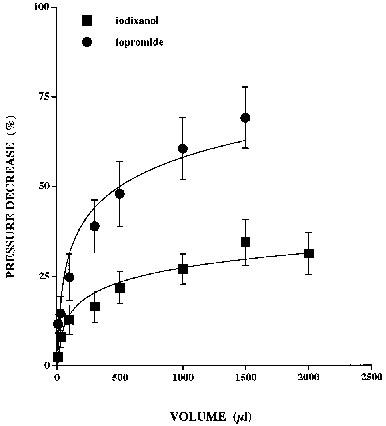
Depressor responses induced by bolus injections of iopromide (300 mg ml−1) and iodixanol (320 mg ml−1) in perfused (5±1 ml min−1) isolated dog ear arteries constricted by PhE. Increasing volumes of the IRCM caused dose-dependent reductions in perfusion pressure, with the responses to both agents approaching a plateau.
In a separate series of experiments the phenylephrine concentration was titrated over the range 300 nM–3 μM to increase pressure to the lower level of 142±10 mmHg. Bolus injections of 300 μl iopromide (300 mg ml−1) caused a 26±2% (n=5) decrease in PhE-induced pressure which was unaffected by addition of either 100 μM L-NOARG (38.6±3.4%, n=5) or a combination of L-NOARG and 10 μM indomethacin (32±5%, n=5) (Figure 5). Bolus injections of 300 μl iodixanol (320 mg ml−1) caused a 4±0.6% (n=3) pressure decrease in the absence of 100 μM L-NOARG, a 6.3±4.1% (n=3) decrease in its presence, and a 3.3±0.3% (n=3) decrease in the presence of both L-NOARG and indomethacin. Figure 5 shows that neither inhibition of NO synthase nor cyclo-oxygenase affected the vasodilator activity of iopromide or iodixanol, whereas L-NOARG alone and L-NOARG and indomethacin significantly attenuated the vasodilator effect of acetylcholine (from 45±4 to 31±8 and 25±11% respectively, P<0.05 and n=5 for both).
Figure 5.
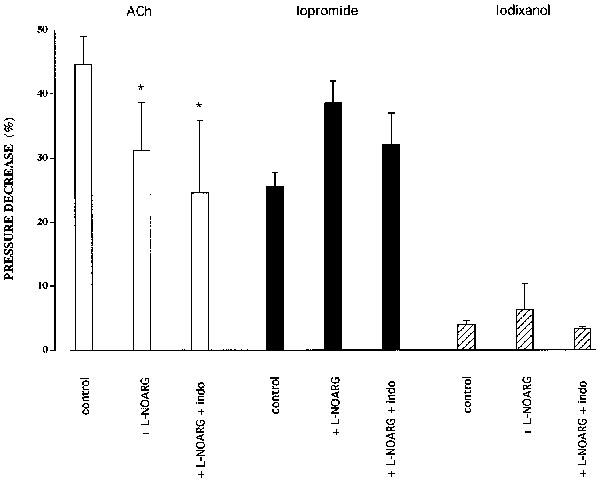
The effects of bolus injections of 500 μl ACh (0.1 μM) and of 300 μl iopromide or iodixanol (both 100% v v−1) on perfusion pressure in isolated dog ear arteries constricted by PhE, in the absence and presence of either 100 μM L-NOARG alone or L-NOARG and 10 μM indomethacin (Indo). Bars marked with asterisks differ significantly from their respective controls (P<0.05).
Discussion
The major finding of the present study is that iodinated radiographic contrast media (IRCM) exert direct pharmacological effects on the endothelium that depress production of NO in response to the mechanical stimulus of shear stress and the endothelium-dependent agonist acetylcholine (ACh). In cascade bioassay NO release was enhanced following increases in the viscosity of the donor perfusate by graded additions of dextran, whereas a similar phenomenon was absent with iohexol or iodixanol. In isolated arterial rings both IRCM inhibited endothelium-dependent relaxations to ACh without affecting smooth muscle relaxation to sodium nitroprusside, an exogenous donor of NO. Consistent results were obtained in experiments with isolated ear arteries in which depressor responses evoked by close arterial injection of iopromide and iodixanol were unaffected by inhibition of NO synthesis.
Rocha et al. (1995) have previously suggested that high molarity solutions inhibit NO production on the basis that the enhancement of phenylephrine-induced contraction normally evoked by L-NAME was attenuated by 100 mM mannitol or sucrose. However, in the present study there appeared to be no direct correlation between inhibition of NO production and the ionic concentration of the solutions employed. Buffer containing 10% v v−1 iohexol or 30% v v−1 iodixanol, which differ in molarity by ∼50 mM, inhibited the response to ACh to an identical extent, whereas 30% v v−1 iohexol exerted markedly greater inhibition than 30% v v−1 iodixanol despite having a similar molarity. This suggests that IRCM exert specific effects on the endothelium that are not primarily related to high osmolality. Previous studies have shown that concentration-relaxation curves for iohexol, iopromide and iodixanol in endothelium-intact and -denuded rabbit aorta can be superimposed if plotted on a molar basis, with EC50 values for all three agents being in the range 50–60 mM and maximum relaxations being >50% of developed tone (Pugh et al., 1995; Pitman et al., 1996). However, a direct relationship between osmolality and the effects of IRCM was not evident as mannitol-induced relaxations of the rabbit aorta were maximally of the order of 10–20%, even at concentrations of 100 mM (Pitman et al., 1996). The transient nature of the relaxations observed with mannitol in the present study are consistent with this conclusion. The effects of IRCM on intrinsic smooth muscle contractility are not attributable to the presence of the iodine atom in the IRCM molecule (Pugh et al., 1995; Pitman et al., 1996). The present results similarly suggest that iodine concentration is not correlated with the attenuation of NO production by the endothelium. Although containing similar iodine concentrations, 30% iohexol exerted significantly greater inhibition of ACh-induced relaxations than 30% iodixanol, whereas 10% iohexol and 30% iodixanol, which differ in composition by 70 mg ml−1, were equieffective.
Differences between mannitol and IRCM were also evident in their effects on relaxations evoked by ACh and SNP, with mannitol markedly attenuating relaxations to SNP in endothelium-denuded rings as well as responses to ACh in endothelium-intact preparations. These observations are consistent with a previous study with canine coronary artery that demonstrated impaired vasodilatation to the nitrovasodilator glyceryl trinitrate in the presence of mannitol (Krishnamurty et al., 1978). It is possible that specific chemical properties of mannitol, such as an ability to modulate free radical reactions, influence the response to NO as the degree of inhibition of ACh and SNP relaxations was similar at equivalent concentrations. Mannitol scavenges the hydroxyl (•OH) radical, and in common with other compounds that possess an alcohol functional group (e.g. sorbitol and glucose) reacts with peroxynitrite which is formed by the reaction of NO and the superoxide (•O2−) radical (Dowell & Martin, 1997). Although iohexol and iodixanol are ‘sugar-like' in the sense that they both contain -OH groups, the present findings indicate that these moieties do not contribute to the pharmacological properties of IRCM by interacting with NO as there was no impairment of relaxations to SNP. Analogously, Rocha et al. (1995) found that relaxations to the NO donor 3-morpholinosydnonimine (SIN-1) were unaffected by 100 mM sucrose.
In theory, in the experiments with ear arteries, which mimic administration of IRCM during clinical arteriography, iodixanol (320 mg ml−1) might be expected to enhance NO release to a greater extent than iopromide (300 mg ml−1). Differences in the viscosity of the parent solutions (ca. 11 mPa s−1 cf. 5 mPa s−1) imply that there will be a ∼2 fold greater peak shear stress with iodixanol following the administration of equivalent volumes. However, the depressor responses actually observed were significantly greater with iopromide. This parallels the greater relaxant effects of iopromide than iodixanol (up to 3 fold at any given iodine concentration) in isolated rings of rabbit aorta (Pugh et al., 1995; Pitman et al., 1996), and suggests that the responses observed in ear arteries were attributable to direct effects of IRCM on vascular smooth muscle. This would be expected if such agents inhibit NO production. These experiments also showed that the initial level of constriction influences the magnitude of relaxations to IRCM. Although the differential potency of iopromide and iodixanol was preserved over a 2 fold range in perfusion pressures under variation in phenylephrine concentration, on a relative basis depressor responses were greater at high levels of constriction. This potential source of variability was eliminated in experiments with aortic rings by employing different concentrations of phenylephrine to standardize initial tone.
In the isolated perfused kidney Morcos et al. (1997) found that the monomeric ionic IRCM, diatrizoate, did not attenuate the vasodilatory response to ACh at an iodine concentration of 20 mg ml−1, concluding that the overall decrease in renal blood flow caused by this agent was not due to inhibition of NO synthesis. However, this concentration is lower than used in the present study, and Schwartz et al. (1994) demonstrated a marked fall in urinary NO2− and NO3− excretion, which reflects both systemic and renal NO production, following administration of diatrizoate to anaesthetized rats. Indeed, excretion of these metabolites fell to levels equivalent to those observed on administration of NG-nitro L-arginine, consistent with direct inhibitory effects of IRCM on eNOS in vivo. Paradoxically, other workers have hypothesized that IRCM stimulate NO production on the basis that the vasodilator response to iohexol and iothalamate injected into the bronchial circulation of anaesthetized sheep is attenuated by blockade of eNOS (Baile et al., 1997). Whether these differences involve release of endogenous mediators such as acetylcholine, bradykinin, serotonin and histamine, which have been suggested to contribute to the in vivo vasodilator activity of IRCM, remains to be established (see Pugh & Karlsson, 1997 for review). However, a major role for such secondary mediators, which generally cause vasodilatation by stimulating NO synthesis, seems unlikely given that the present findings indicate that IRCM attenuate NO synthesis. There is also evidence that IRCM can promote vasodilatation through NO-independent mechanisms that involve activation of adenosine A2 receptors (Arakawa et al., 1996), while coronary microvascular dilatation to hyperosmolar solutions of glucose or sucrose (300–345 mosM) is an endothelium-dependent phenomenon associated with smooth muscle hyperpolarization mediated by activation of ATP-sensitive K+ channels, rather than NO (Ishizaka & Kuo, 1997). The incomplete inhibition of ACh-induced vasodilatation observed in ear arteries in the presence of L-NOARG and indomethacin is attributable to an endothelium-derived hyperpolarizing factor (EDHF) that may diffuse from the endothelium to subjacent smooth muscle cells via gap junctions (Garland et al., 1995; Chaytor et al., 1998; Hutcheson et al., 1999). However, the close similarity of IRCM-induced relaxations in endothelium-intact and endothelium-denuded rabbit aortic rings (Pugh et al., 1995; Pitman et al., 1996), provide no evidence that this factor contributes to the vasodilatation induced by IRCM.
In conclusion, non-ionic IRCM may inhibit shear stress- and agonist-induced release of NO and their vasodilator properties thus appear to be dominated by an action on vascular smooth muscle rather than the endothelium. The cellular mechanisms that underlie the ability of IRCM to inhibit NO production do not correlate directly with osmolality or iodine concentration. It remains to be determined whether the effects of IRCM on ion exchange mechanisms and transport systems, which are known to contribute to relaxation of vascular smooth muscle (Pitman et al., 1996), also modulate endothelial cell function.
Abbreviations
- ACh
acetylcholine
- IRCM
iodinated radiographic contrast media
- L-NAME
NG-nitro L-arginine methyl ester
- L-NOARG
NG-nitro L-arginine
- NO
nitric oxide
- PhE
phenylephrine
- SNP
sodium nitroprusside
References
- ARAKAWA K., SUZUKI H., NAITOH M., MATSUMOTO A., HAYASHI K., MATSUDA H., ICHIHARA A., KUBATO E., SARUTA T. Role of adenosine in the renal responses to contrast medium. Kidney Int. 1996;49:1199–1206. doi: 10.1038/ki.1996.173. [DOI] [PubMed] [Google Scholar]
- ALMÉN T. Visipaque – a step forward. A historical review. Acta Radiol. 1995;36:2–18. [PubMed] [Google Scholar]
- BAILE E.M., WANG L., VERBURGT L., PARE P.D. Bronchial vasodilatory response to ionic and nonionic contrast media. J. Appl. Physiol. 1997;82:841–845. doi: 10.1152/jappl.1997.82.3.841. [DOI] [PubMed] [Google Scholar]
- CHAYTOR A.T., EVANS W.H., GRIFFITH T.M. Central role of heterocellular gap junctional communication in endothelium-dependent relaxations of rabbit arteries. J. Physiol. (London) 1998;508:561–573. doi: 10.1111/j.1469-7793.1998.561bq.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DOWELL F.J., MARTIN W. The effects of peroxynitrite on rat aorta: interaction with glucose and related substances. Eur. J. Pharmacol. 1997;338:43–53. doi: 10.1016/s0014-2999(97)01320-4. [DOI] [PubMed] [Google Scholar]
- FURCHGOTT R.F., ZAWADZKI J.V. The obligatory role of endothelial cells in the relaxation of arterial smooth muscle by acetylcholine. Nature. 1980;228:373–376. doi: 10.1038/288373a0. [DOI] [PubMed] [Google Scholar]
- GARLAND C.J., PLANE F., KEMP B.K., COCKS T.M. Endothelium-dependent hyperpolarization: a role in the control of vascular tone. TiPS. 1995;16:23–30. doi: 10.1016/s0165-6147(00)88969-5. [DOI] [PubMed] [Google Scholar]
- HUTCHESON I.R., CHAYTOR A.T., EVANS W.H., GRIFFITH T.M. Nitric-oxide-independent relaxations to acetylcholine and A23187 involve different routes of heterocellular communication: Role of gap junctions and phospholipase A2. Circ. Res. 1999;84:53–63. doi: 10.1161/01.res.84.1.53. [DOI] [PubMed] [Google Scholar]
- HUTCHESON I.R., GRIFFITH T.M. Release of endothelium-derived relaxing factor is modulated by both frequency and amplitude of pulsatile flow. Am. J. Physiol. 1991;261:H257–H262. doi: 10.1152/ajpheart.1991.261.1.H257. [DOI] [PubMed] [Google Scholar]
- HUTCHESON I.R., GRIFFITH T.M. Heterogeneous populations of K+ channels mediate EDRF release to flow but not antagonists in rabbit aorta. Am. J. Physiol. 1994;266:H590–H596. doi: 10.1152/ajpheart.1994.266.2.H590. [DOI] [PubMed] [Google Scholar]
- IGNARRO L.J., BUGA G.M., WOOD K.S., BYRNS R.E., CHAUDHURI G. Endothelium-derived relaxing factor (EDRF) produced and released from artery and vein is nitric oxide. Proc. Natl. Acad. Sci. U.S.A. 1987;84:9265–9269. doi: 10.1073/pnas.84.24.9265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ISHIZAKA H., KUO L. Endothelial ATP-sensitive potassium channels mediate coronary microvascular dilation to hyperosmolarity. Am. J. Physiol. 1997;273:H104–H112. doi: 10.1152/ajpheart.1997.273.1.H104. [DOI] [PubMed] [Google Scholar]
- KRISHNAMURTY V.S., ADAMS H.R., TEMPLETON G.H., WILLERSON J.T. Inhibitory effect of hypertonic mannitol on vasoconstrictor and vasodilator responses of isolated coronary arteries. Am. J. Physiol. 1978;235:H728–H735. doi: 10.1152/ajpheart.1978.235.6.H728. [DOI] [PubMed] [Google Scholar]
- LINDGREN P. Hemodynamic responses of contrast media. Invest. Radiol. 1970;5:424–435. doi: 10.1097/00004424-197011000-00007. [DOI] [PubMed] [Google Scholar]
- MONCADA S., PALMER R.M.J., HIGGS E.A. Nitric oxide: Physiology, pathophysiology and pharmacology. Pharmacol. Rev. 1991;43:109–142. [PubMed] [Google Scholar]
- MORCOS S.K., OLDROYD S., HAYLOR J. Effect of radiographic contrast media on endothelium derived nitric oxide-dependent renal vasodilatation. Br. J. Radiol. 1997;70:154–159. doi: 10.1259/bjr.70.830.9135441. [DOI] [PubMed] [Google Scholar]
- PALMER R.M.J., FERRIGE A.G., MONCADA S. Nitric oxide release accounts for the biological activity of endothelium-derived relaxing factor. Nature. 1987;327:5254–5260. doi: 10.1038/327524a0. [DOI] [PubMed] [Google Scholar]
- PITMAN M.R., KARLSSON J.O.G., GRIFFITH T.M. Ionic mechanisms contributing to the vasorelaxant properties of iodinated contrast media: a comparison of iohexol and iodixanol in the rabbit isolated aorta. Br. J. Pharmacol. 1996;119:685–690. doi: 10.1111/j.1476-5381.1996.tb15727.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- POHL U., BUSSE R., KUON E., BASSENGE E. Pulsatile perfusion stimulates the release endothelial autocoids. J. Appl. Cardiol. 1986;1:215–235. [Google Scholar]
- PUGH N.D., HUTCHESON I.R., EDWARDS D.H., NOSSEN J.Ø., KARLSSON J.O.G., GRIFFITH T.M. Angiographic contrast media relax isolated rabbit aorta through an endothelium-independent mechanism that may not depend on the presence of the iodine atom. Br. J. Radiol. 1995;6:23–26. doi: 10.1259/0007-1285-68-805-23. [DOI] [PubMed] [Google Scholar]
- PUGH N.D., KARLSSON J.O.G. Vasodilator and hemorheological effects of iodinated contrast media. Drugs Today. 1997;33:191–203. [Google Scholar]
- ROCHA G., BUCHER B., TSCHOPL M., STOCLET J.C. Hyperosmolarity enhances smooth muscle contractile responses to phenylephrine and partially impairs nitric oxide production in the rat tail artery. J. Vasc. Res. 1995;32:58–65. doi: 10.1159/000159078. [DOI] [PubMed] [Google Scholar]
- SCHWARTZ D., BLUM M., PEER G., WOLLMAN Y., MAREE A., SERBAN I., GROSSKOPF I., CABILI S., LEVO Y., IAINA A. Role of nitric oxide (EDRF) in radiocontrast acute renal failure in rats. Am. J. Physiol. 1994;267:F374–F379. doi: 10.1152/ajprenal.1994.267.3.F374. [DOI] [PubMed] [Google Scholar]
- TESFAMARIAM B., COHEN R.A. Inhibition of adrenergic vasoconstriction by endothelial shear stress. Circ. Res. 1988;63:720–725. doi: 10.1161/01.res.63.4.720. [DOI] [PubMed] [Google Scholar]


