Abstract
The effects of the immunosuppressant drugs cyclosporin A and tacrolimus (FK506) on nitric oxide synthesis were examined in a murine macrophage cell line (J774) and rat vascular smooth muscle cells (VSMC) in culture for 24 and 48 h, respectively.
Cyclosporin A (0.01–10 μM) inhibited by up to 90% accumulation of nitrite induced by lipopolysaccharide (LPS) in both cell lines, but FK506 (0.01–10 μM) had a weaker effect on nitrite accumulation in these cells. Cyclosporin A and FK506 (at 1 μM) also significantly inhibited nitrite production induced by recombinant murine interferon-γ (rIFNγ) and recombinant murine interleukin-1β (rIL-1β) in J774 and VSMC, respectively.
In J774 cells, cyclosporin A (but not FK506) at 1 μM was inhibitory when co-incubated with the inducing agents but not when the cells were treated with the immunosuppressant before or after the inducer. In VSMC, nitrite production was inhibited by co-incubation of cyclosporin A or FK506 with the inducer, or when the immunosuppressants were pre-incubated with cells. In contrast, N-monomethyl L-arginine (NMMA) abolished nitrite production when incubated with either cell type during or after addition of inducing agent, but not if cells were preincubated with NMMA.
RNA extracted from treated J774 and VSMC was subjected to reverse transcription–polymerase chain reaction (RT–PCR). Cyclosporin A, but not FK506, suppressed expression of mRNA for NOS2 in a concentration-dependent manner when co-incubated with LPS.
The fact that the potency difference between cyclosporin A and FK506 for NO suppression is the opposite to that for inhibition of interleukin-2 generation suggests that the immunosuppressants act in J774 macrophages and VSMC through intracellular mechanisms that differ from those elucidated in T-cells. Cyclosporin A suppresses NOS2 gene transcription, but FK506 acts post-transcriptionally to suppress NO generation in VSMC.
Taken together the present data suggest that therapeutic concentrations of cyclosporin A, but not FK506, might well suppress NO production, but FK506 would not have this effect. Suppression of NO might contribute to the side effects of hypertension and nephrotoxicity associated with long-term use of cyclosporin A to prevent transplant rejection.
Keywords: Cyclosporin A, FK506, immunosuppressants, J774 macrophages, nitric oxide synthase, tacrolimus, vascular smooth muscle
Introduction
The use of powerful immunosuppressants such as cyclosporin A and tacrolimus (FK506) has permitted organ transplantation to become widely practiced (Anonymous, 1994; Oellerich, 1998). It has been speculated that nitric oxide (NO), in particular that generated via the induction of the NOS2 enzyme isoform, is an important cytotoxic contributor to the process of allograft rejection (Langrehr et al., 1992; Winlaw et al., 1994). For example, in the transplanted heart it has been demonstrated that the NOS2 isoform of NO synthase is induced following increased cytokine activation, both in myocytes and in immunocompetent cells infiltrating the graft (Yang et al., 1994), whilst serum nitrite and nitrate levels rise in the acute phase of rejection of vascularized allografts (Langrehr et al., 1992; Winlaw et al., 1994). Moreover, FK506 and cyclosporin A inhibit graft rejection and the latter has been shown to prevent the elevation of serum nitrite and nitrate, and this has been attributed to inhibition by this immunosuppressant of cytokine production by T-cells (Winlaw et al., 1994). Thus the therapeutic application of cyclosporin appears to suppress NO production in allografts, but the mechanisms involved have not been well defined. Lastly, well known side effects associated with chronic treatment with cyclosporin include nephrotoxicity and hypertension, and any effects of such immunosuppressants on NO production in vivo could conceivably contribute to these adverse consequences (Mason, 1989). In this paper we confirm our previous finding that cyclosporin A inhibits NO production and NO synthase transcription in a murine macrophage cell line (J774) as well as in rat cultured VSMC (Akita et al., 1994), but we also show that FK 506 is less potent and does not suppress NOS2 transcription.
Methods
Drugs, cytokines and materials
Dulbecco's Modified Eagle's Medium (DMEM) and Fetal Bovine Serum (FBS) were from ICN Biomedicals Inc. (Costa Mesa, CA, U.S.A.). 94 mm petri dishes, 96-well flat-bottom plates and tissue culture flasks were from Greiner Labortechnik (Frickenhausen, Germany). Recombinant murine IFNγ was purchased from Boehringer (Mannheim, Germany). Recombinant human IL-1β was kindly provided by Dr Ian Campbell (Royal Melbourne Hospital, Melbourne). NG-monomethyl-L-Arginine (NMMA) was from the Institute of Drug Technology (Boronia, Victoria, Australia). Lipopolysaccharide (E. coli. serotype 0111 : B4) and L-glutamine were from Sigma Chemicals (St. Louis, MO, U.S.A.). Cyclosporin A (Sandoz Australia Pty Ltd) was a kind gift from Dr Tom Mandel (Walter and Eliza Hall Institute, Melbourne). FK506 was generously provided by Fujisawa Pharmaceutical (Osaka, Japan). Both cyclosporin A and FK506 were made as stock solutions with absolute ethanol at 10 mM. Stock solutions were diluted with media at appropriate concentrations each day before use.
Cell culture
Both cell types were incubated in DMEM supplemented with 10% FBS, 100 mg ml−1 streptomycin, 100 μ ml−1 penicillin and 2 mM L-glutamine at 37°C in a humidified incubator containing 5% CO2. Endotoxin levels were measured by a Spectrozyme Limulus amebocyte lysate assay (American Diagnostica) and media were found to be free of endotoxin (less than 100 pg ml−1).
Rat vascular smooth muscle cells (VSMC)
VSMC were isolated from the thoracic aortas of six male Sprague-Dawley rats by enzymic digestion. They were identified as smooth muscle cells by their typical growth pattern of ‘hills and valleys' and by immunohistochemistry with monoclonal antibody to α-smooth muscle actin (from Sigma, St. Louis, MO, U.S.A.) and alkaline phosphatase conjugated secondary antibody. This was further confirmed by fluoresceine immunolocalization. Cultures at passages 3–10 were used for the experiments.
Cells were incubated in DMEM supplemented with 10% FBS, 100 mg ml−1 streptomycin, 100 μ ml−1 penicillin and 2 mM L-glutamine into 25 or 75 cm2 culture flasks. After confluence, VSMC were harvested by trypsinization, and plated into 96-well flat bottom culture plates at a density of 1×104 cells per well with 200 ml of media. After culturing for 4–6 days, VSMC reached confluence. For experiments, VSMC were washed with fresh media once and replaced with 100 μl of fresh media with appropriate concentrations of inducers (LPS or IL-1β) with or without cyclosporin A or FK506 (see results section). After 24 or 48 h incubation, the supernatants were collected for nitrite assay. When adding different compounds at different times, the first compounds were washed out with fresh media at least three times. More than 95% of the cells were viable as determined by trypan blue exclusion test after incubation with all reagents, including concentrations of cyclosporin A and FK506 up to 1.0 μM. Higher concentrations of cyclosporin A and FK506 compromised cell viability in some cultures, and these were excluded from further analysis in the study. Respiratory activity of all cells at the end of each study was measured by incubation with the tetrazolium salt XTT. This XTT assay gives a measure of cell viability for XTT is cleaved by dehydrogenase enzymes of metabolically active cells, yielding a highly coloured formazan product which is water soluble and assayed spectrophotometrically (Roehm et al., 1991; Jost et al., 1992). As measured by this assay, proliferation and respiratory activity of all cells included in the study after incubation with these reagents were the same as controls.
Murine macrophage cell line-J774
J774 murine macrophage cell line (American Tissue Culture Catalogue T1B) were cultured in 94 mm petri dishes. After reaching confluence, J774 were resuspended by pipetting and passaged at 1/10∼1/20 twice a week. J774 were collected by pipetting and plated into 96-well flat bottom culture plates at density of 1×105 cells per well with 100 μl of media. After allowing 2–3 h to attach to the bottom, media was replaced with 200 μl of fresh media and appropriate concentrations of various compounds were added. The supernatants were collected for assay 24 or 48 h later. When adding different compounds at different times, the first compounds were washed out with fresh media at least three times. More than 90% of the cells were viable as determined by the trypan blue exclusion test after incubation with all reagents, including concentrations of cyclosporin A and FK506 up to 10 μM. Proliferation and respiratory activity of cells after incubation with all reagents were the same as control as determined by XTT assay (Roehm et al., 1991; Jost et al., 1992).
Nitrite assay
The induction of NO synthesis was evaluated by quantifying the accumulation of nitrite in the culture media (Ding et al., 1990; Gross & Levi, 1992). Nitrite concentrations were determined by a microplate assay. Cell-free supernatant aliquots (50- or 100-μl) were mixed with an equal volume of Griess reagent (1% sulphanilamide/0.1% naphthylethylene diamine dihydrochloride/2.5% H3PO4) and incubated at room temperature for 10 min. The optical density (OD) of the wells was determined using a kinetic microplate reader (Molecular Devices) at a test wavelength of 550 nm and a reference wavelength of 650 nm. Nitrite was determined using NaNO2 as a standard. Background nitrite values of media were determined in each case and subtracted from the experimental values. The nitrite concentration was calculated from a standard curve and expressed as nmol per 105 cells for J774 or nmol per well for VSMC.
RNA extractions and RT–PCR
Total RNA was extracted from samples using an updated version of the single-step method of RNA isolation by acid guanidine thiocyanate-phenol-chloroform extraction method described by Chomczynski & Sacchi (1987). J774 and VSMC were treated with the relevant drugs LPS, cyclosporin A and FK506 at varying concentrations (see Results) and were incubated for 8 and 16 h for J774 and VSMC, respectively. RT–PCR was performed using the RNA PCR Core Kit (Perkin Elmer Cetus). First strand cDNA was synthesized using random hexamers and MuLV reverse transcriptase. PCR amplification was performed using specific synthesized primers for the inducible NO synthase 2 (NOS2) and β-actin. The intron spanning primers were: NOS2 forward, 5′-AGA AGC ACA AAG TCA CAG ACA TGG CTT GCC-3′, NOS2 reverse, 5′-TTG GAC CAC TGG ATC CTG CCG ATG CAG CGA-3′ and β-actin forward, 5′-GGG AAT TCA TGG GTC AGA AGG ACT CCT A-3′ and β-actin reverse 5′-GGG ATC CGG GTT CAG GGG GGC CTC GGT-3′. PCR was performed using 330 ng of total RNA per reaction. The PCR amplification reaction consisted of 94°C for 30 s, 63°C for 30 s and 72°C for 60 s for 24–30 cycles, followed by 10 min at 72°C performed in the GeneAmp PCR System 9600 (Perkin Elmer Cetus). PCR was performed in the linear range of the amplification reaction. PCR of β-actin and NOS2 were assessed in the same tube so that equal amounts of cDNA were used for the two sets of primers, to enable a comparison of the intensities of the PCR products. The PCR products were electrophoresed on a 1.5% agarose gel, then visualized and photographed using UV fluorescence. The relative density of bands was quantified with a scanner attached to a computer using MCID image analysis software.
Southern analysis
After electrophoresis of the PCR products on the 1.5% agarose gel, the gel was rinsed in 0.4 M NaOH solution for 20 min. DNA was transferred onto Hybond N+ membrane (Amersham, Sydney, Australia) by capillary transfer and fixed by alkali solution (0.4 M NaOH). The membrane was prehybridized for 10 min at 42°C in Amersham Rapid Hybridization Buffer and then hybridized for 2 h at 42°C with rat NOS2 5′-ATT TGG ACT TGC AAG AGA TAT CCG AGG TGG CCT-3′ and β-actin 5′-TGT GGT GCC AAA TCT TCT CCA TAT CGT CCC AGT-3′ internal oligonucleotides that had been labelled with γ32P-ATP (Amersham). Blots were washed in 2×SSC 0.1% SDS at 55°C four times, exposed to X-ray film and developed.
Data analysis
Each individual experiment was conducted with three or six replicates, and experiments were repeated four to five times with different cell cultures. Arithmetic means are given with s.e.mean. Statistical comparisons with the controls were made by the Student's unpaired t-test followed by a Student–Newman–Keul's post-test. Differences were considered significant when P values of <0.05 were obtained.
Results
Accumulation of nitrite in J774 with stimulation by LPS and rIFNγ
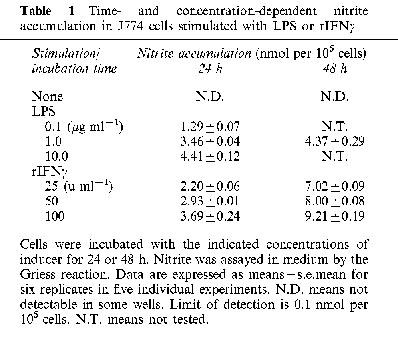
Nitrite levels in control wells without stimulation remained below the level of detection (0.1 nmol per 105 cells) up to 48 h. Nitrite production was not detectable up to 6 h after stimulation with LPS or rIFNγ, but it increased up to 24 h with LPS, and up to 48 h with rIFNγ (Table 1). Nitrite accumulation was not detectable with less than 0.001 μg ml−1 of LPS or 10 u ml−1 of rIFNγ after 24 h incubation. With 48 h incubations in the presence of LPS, some cultures showed reduced respiratory activity by XTT assay, and hence nitrite data were not included in the Table.
Table 1.
Time- and concentration-dependent nitrite accumulation in J774 cells stimulated with LPS or rIFNγ
Accumulation of nitrite in VSMC with stimulation by LPS and rIL-1β
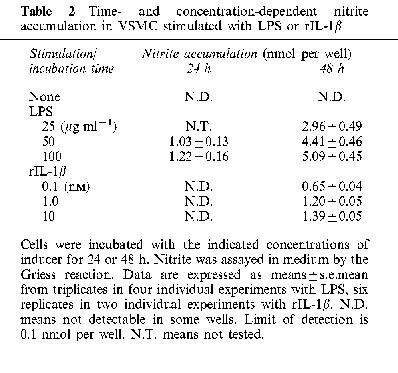
The number of VSMC in each well varied between 1 and 2×105 at the end of the assays as determined by the Trypan blue exclusion test. Nitrite accumulation in control cells without stimulation was not detectable up to 48 h. Production of nitrite remained undetectable up to 12 h but increased thereafter for up to 48 h with stimulation by LPS (Table 2). rIL-1β also induced a concentration-dependent, but much slower accumulation of nitrite to a maximum of 1.4 nmol per well per 48 h at 10 nM (Table 2).
Table 2.
Time- and concentration-dependent nitrite accumulation in VSMC stimulated with LPS or rIL-1β
Effect of cyclosporin A or FK506 co-incubated with inducing agents in J774 cells
Co-incubation of cyclosporin A (1 μM) with LPS (1.0 μg ml−1) or IFNγ (100 u ml−1) reduced nitrite accumulation to about 60% of that with LPS alone and to about 50% of that with IFNγ alone, respectively (Figure 1). A higher concentration of cyclosporin A (10 μM) reduced nitrite accumulation further (to 10% of LPS alone) but this also reduced cell viability below 95% in some cultures that were excluded from analysis. In contrast, FK506 (up to 1 μM) did not significantly alter (less than 10% reduction) nitrite accumulation in LPS- and IFN-treated cells, despite its higher potency as an immunosuppressant. High concentrations of FK506 (10 μM) also reduced cell viability below 95%, suggesting the inhibition of nitrite production at this concentration was related to toxicity. In all cultures analysed, cyclosporin A and FK506 had no significant effect on cell respiration, as measured by XTT (Jost et al., 1992).
Figure 1.
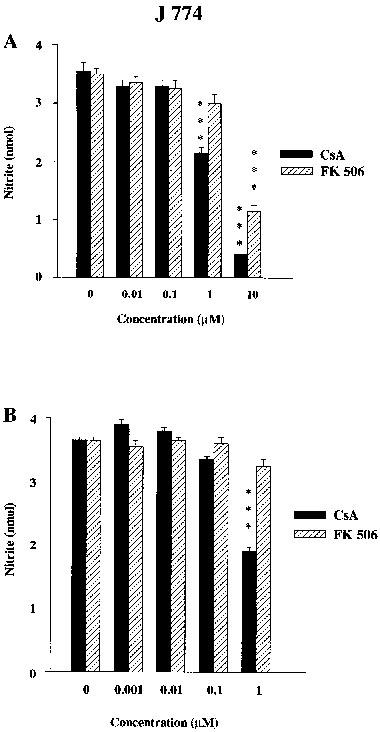
Influence of cyclosporin A (CsA, filled columns) and FK506 (stippled columns) at various concentrations on (A) LPS (1.0 μg ml−1) or (B) IFNγ (100 u ml−1)-stimulated nitrite accumulation in J774 cells. The data shown are means±s.e.mean of six replicates in five different experiments for (A) and four different experiments for (B). ***P<0.001, *P<0.05 compared with respective controls.
Effect of cyclosporin A and FK506 co-incubated with inducing agents in VSMC
Co-incubation of cyclosporin A (1 μM) with LPS (50 μg ml−1) or IL-1β (0.001 μM) reduced nitrite accumulation to about 40% of LPS alone and 55% of that with IL-1β alone (Figure 2). A higher concentration (10 μM) reduced nitrite to 9% of LPS alone, but again in some cultures (excluded) this compromised cell viability. FK506 (up to 1 μM) had no effect on LPS-induced cells, but reduced nitrite accumulation slightly in IL-1β treated cells, without affecting cell respiration (by XTT).
Figure 2.
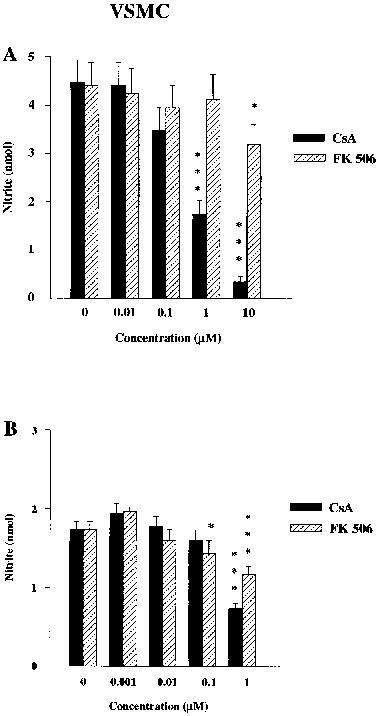
Influence of cyclosporin A (CsA, filled columns) and FK506 (stippled columns) at various concentrations on (A) LPS (50 μg ml−1) or (B) IL-1β (0.001 μM) stimulated nitrite accumulation in VSMC. The data shown are means±s.e.mean of triplicates in four different experiments for (A) and six replicates in three different experiments for (B). ***P<0.001, *P<0.05 compared with respective controls.
Effect of cyclosporin A and FK506 exposure period on nitrite production in J774 cells
To further analyse the mechanisms responsible for suppression of nitrite production the actions of cyclosporin and FK506 were examined at a concentration of 1 μM which did not compromise cell viability in any cultures. J774 cells were incubated with LPS (1.0 μg ml−1) (Figure 3) or IFNγ (100 u ml−1) (Figure 4) for 12 h, washed, and nitrite accumulation measured over a subsequent 24 h period. Whereas exposure to cyclosporin A (1 μM) after incubation with the stimulant had no effect on subsequent nitrite accumulation, co-incubation of cyclosporin A or co-incubation followed by a further period of exposure to cyclosporin A, reduced nitrite accumulation (to 60% and 33% with LPS and IFNγ, respectively). FK506 (1 μM) had no effect on nitrite accumulation with any period of exposure. In contrast, exposure to NMMA (300 μM) after incubation with the stimulant greatly reduced subsequent nitrite accumulation, but co-incubation of NMMA and stimulant did not reduce subsequent nitrite accumulation following washout of both compounds from the cells (Figures 3 and 4). However, in cells stimulated with IFNγ, it was observed that co-incubation with NMMA potentiated subsequent nitrite accumulation following washout of both compounds (Figure 4). This unexpected increase in nitrite after NMMA did not occur following co-incubation of NMMA with LPS as the stimulant. Cell respiration was not affected by these incubations.
Figure 3.
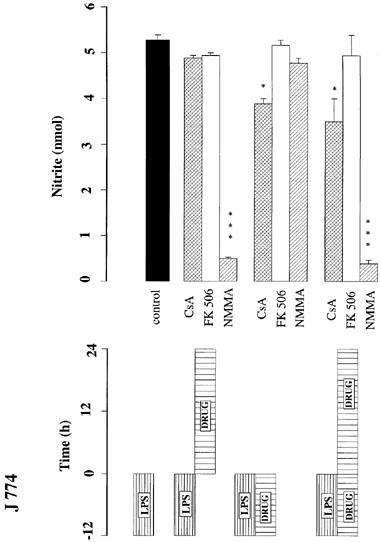
Influence of cyclosporin A (CsA, 1.0 μM), FK506 (1.0 μM) or NMMA (300 μM) on nitrite accumulation (nmol per 105 cells per 24 h) in J774 cells using various incubation regimes, as indicated by the bars in the lower panel. J774 cells were exposed to LPS (1.0 μg ml−1) in the presence or absence of the indicated drugs for 12 h. Cells were washed at least three times with fresh culture medium and incubated for a further 24 h in the presence or absence of the drugs. The data shown are the mean±s.e.mean of triplicates in four different experiments. ***P<0.001, **P<0.05, compared with LPS control.
Figure 4.
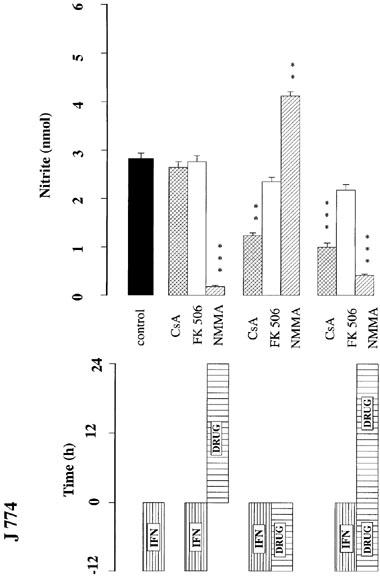
Influence of cyclosporin A (CsA, 1.0 μM), FK506 (1.0 μM) of NMMA (300 μM) on nitrite accumulation (nmol per 105 cells per 24 h) in J774 cells using various incubation regimes, as indicated by the bars in the lower panel. J774 cells were exposed to IFNγ (100 u ml−1) in the presence or absence of the indicated drugs for 12 h. Cells were washed at least three times with fresh culture medium and incubated for a further 24 h in the presence or absence of the drugs. The data shown are the mean±s.e.mean of triplicates in four different experiments. ***P<0.001, **P<0.01 compared with IFNγ control.
Effect of cyclosporin A and FK506 exposure period on nitrite production in VSMC
Using protocols similar to those above, VSMC were incubated with LPS (50 μg ml−1) for 24 h, washed, and nitrite accumulation measured over a subsequent 48 h period (Figure 5). Exposure to cyclosporin A (1 μM) after incubation with LPS reduced subsequent nitrite accumulation to 46%, whereas co-incubation with cyclosporin A or co-incubation followed by a further period of exposure to cyclosporin A, reduced nitrite accumulation to 26 or 14%, respectively. Similarly, exposure to FK506 (1 μM) after incubation with LPS did not reduce subsequent nitrite accumulation, but co-incubation of FK506 or co-incubation followed by a further period of exposure to FK506, did reduce nitrite accumulation to 38 or 35%, respectively. In contrast, exposure to NMMA (300 μM) after incubation with the stimulant greatly reduced subsequent nitrite accumulation, but co-incubation of NMMA and stimulant did not alter subsequent nitrite accumulation following their washout from the cells (Figure 5). Cell respiration was not affected by these incubations.
Figure 5.
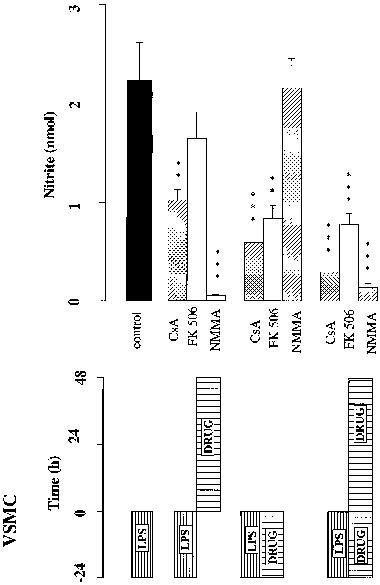
Influence of cyclosporin A (CsA, 1.0 μM), FK506 (1.0 μM) or NMMA (300 μM) on nitrite accumulation (nmol per well per 48 h) in VSMC using various incubation regimes, as indicated by the bars in the lower panel. VSMC were exposed to LPS (50 μg ml1−) in the presence or absence of the indicated drugs for 24 h. Cells were washed at least three times with fresh culture medium and incubated for a further 48 h in the presence or absence of the drugs. The data shown are mean±s.e.mean of triplicates in four different experiments. ***P<0.001, **P<0.01 compared with LPS control.
Effect of cyclosporin A and FK506 on NOS2 mRNA expression
In J774 the effects of co-incubation of increasing amounts of cyclosporin A (0.1–10 μM) with LPS (1.0 μg ml−1) on the mRNA for NOS2 was investigated using RT–PCR in three experiments. In non-induced, control cells no NOS2 mRNA was detected. Following stimulation with LPS, an intense PCR product of NOS2 was observed after 8 h, and with increasing amounts of cyclosporin A the expression of NOS2 message was reduced in a concentration dependent fashion. β-Actin products were equivalent in all incubations, including the highest concentration of cyclosporin.
Rat VSMC that had been treated with LPS (50 μg ml−1), cyclosporin A alone (1 μM) or LPS co-incubated with cyclosporin A (0.1–10 μM) were studied in three experiments. RT–PCR showed no PCR product in control or cyclosporin A-treated cells, whilst LPS induced the expression of NOS2 mRNA. Co-incubation of LPS with cyclosporin A reduced the expression of NOS2 mRNA (Figure 6), but did not affect β-actin transcript. The ratio of NOS2 and β-actin bands were quantitated by scanning densitometry, and these data are also indicated in Figure 6. In contrast, co-incubation of LPS with FK506 (0.1 to 10 μM) did not affect the PCR product. The results from the PCR with cyclosporin A were confirmed by performing Southern blot analysis on the gel. Internally labelled 32P-NOS2 and β-actin oligonucleotides bound cleanly to the NOS2 and β-actin products.
Figure 6.
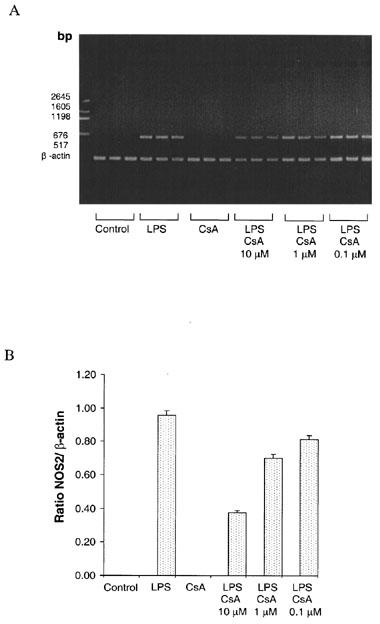
Reverse Transcription Polymerase Chain Reaction (RT–PCR) of mRNA isolated from VSMC after 16 h incubation. All samples containing PCR products were incubated in triplicate. (A) Photograph of an agarose gel in which lanes 1–3 are Controls, lanes 4–6 are samples treated with media containing 50 μg ml-1 LPS, lanes 7–9 samples treated with cyclosporin A (CsA, 1 μM) alone, lanes 10–12 samples co-incubated with LPS and 10 μM CsA, lanes 13–15 LPS and 1 μM CsA and lanes 16–18 LPS and 0.1 μM CsA. Expected PCR product sizes are 636 bp for NOS2 and 212 bp for β-actin. (B) The relative density of bands of PCR products shown as ratios of NOS2 and β-actin products. Results are means±s.e.mean of three experiments.
Discussion
The present findings extend our preliminary observations (Akita et al., 1994) that the immunosuppressant drug cyclosporin A blocks the production of NO, which is induced by many cytokines including interleukin-2 (Moncada et al., 1991). We have now demonstrated using both time course experiments on nitrite production and RT–PCR that cyclosporin A blocks the transcription of mRNA for NOS2 in J774 and VSMC. In contrast, FK506, a related immunosuppressant drug considered like cyclosporin A to act upon T-cell function through suppression of calcineurin (Liu, 1993), is a weaker suppressor of NO production, although it is a more potent suppressor of interleukin-2 gene expression. This indicates that these two compounds act through different mechanisms to suppress NO production.
NO induction plays an important role in host immune defence mechanisms. NO is an effector of the tumoricidal (Hibbs et al., 1987; Stuehr & Nathan, 1989) and the microbiocidal (Langermans et al., 1992; Green et al., 1993; Summersgill et al., 1992; Bermudez, 1993) activities of murine macrophages. Several cytokines and growth factors have been reported to block the induction of NOS2, including IL-4 (Liew et al., 1991; Oswald et al., 1992), IL-10 (Gazzinelli et al., 1992), TGF-β family (Ding et al., 1990; Junquero et al., 1992), EGF (Heck et al., 1992), macrophage-deactivating factor (Ding et al., 1990) and platelet-derived growth factor (Scott-Burden et al., 1992). NOS2 expression is also suppressed by glucocorticoids and lipocortin-1 (Di Rosa et al., 1990; Wu et al., 1995), as well as anti-inflammatory drugs such as antagonists of platelet-activating factor (Szabo et al., 1993; Arthur et al., 1995; Yin et al., 1998). The mechanisms by which these cytokines and drugs suppress NOS2 expression are multiple, but these have not been studied systematically. We report here that induction of NOS2 by LPS or cytokines is inhibited by therapeutic levels of cyclosporin A (up to 1.0 μM) in J774 cells and VSMC. Despite its higher potency as an immunosuppressant (Kino et al., 1987; Tocci et al., 1989; Yoshimura et al., 1989), FK506 was weaker than cyclosporin A in suppressing NO synthesis and did not affect NOS2 transcription. It is therefore clear that the inhibitory effects of the immunosuppressants on NO production result not only from suppression of LPS-induced cytokine induction, but are also exerted at the level of NOS2 gene expression for cyclosporin, and post-transcriptionally in the case of FK506.
Neither compound appeared to affect NOS2 catalytic activity, as shown by the contrasting effects of exposing the cells to cyclosporin A or FK506 with those of NMMA given together or given after incubation with the inducing agents. Cyclosporin A inhibits the expression of NOS2, indicated by the RT–PCR and the inhibition of nitrite accumulation when co-incubated with the inducing agent and washed from the medium, but not (at least for J774 cells) when incubated after enzyme induction has begun. In contrast, NMMA, which blocks NOS enzyme catalytic activity competitively and reversibly, does not inhibit nitrite accumulation when co-incubated with the inducer then washed from the medium, but it virtually abolishes nitrite accumulation when incubated after inducer has been removed but enzyme induction has occurred. The unexpected increase in nitrite production that occurred following incubation with IFN-γ and NMMA raises the possibility that NO produced during the induction phase in some way acts in a negative feedback manner to suppress the expression of NOS2 or other genes involved in NO production (e.g. those replenishing essential cofactors, Gross & Levi, 1992). Once NMMA is removed from the medium these ‘super-induced' enzymes may be allowed to express full activity.
Notwithstanding these observations, it has been found in neural tissues that FK506 is capable of blocking NOS-1 catalytic activity, albeit indirectly (Dawson et al., 1993). These authors reported that FK506 blocks dephosphorylation of the NOS1 isoform, this being a post-translational modification of the protein that is necessary for full expression of its activity. If such an effect also occurs with NOS2 activity, this post-translational event might contribute to the suppression of nitrite production by FK506 and cyclosporin A seen in VSMC when post-incubated after LPS is removed from the cells.
NO has anti-proliferative effects on VSMC (Garg & Hassid, 1989) and T cells (Isobe & Nakashima, 1992), and reported side effects of these immunosuppressants include hypertension and nephrotoxicity (Mason, 1989). The weaker effect of FK506 on NO production in J774 and VSMC might confer an advantage over cyclosporin A in clinical immunosuppression, considering that NO production is ‘antihypertensive' and that NOS2 might help support the renal circulation after transplantation. On the other hand, it has been proposed by Burkart et al., (1992) that this action of cyclosporin could be used to advantage, since cyclosporin A suppresses cytotoxicity of rat peritoneal macrophages in pancreatic islet cells by inhibiting the release of NO. They suggested cyclosporin A could be useful for managing acute-onset type 1 diabetes by inhibiting NO production by macrophages.
In summary, the results reported here indicate that cyclosporin A acts to inhibit transcription of NOS2 in J774 and VSMC. In contrast, FK506 is a much weaker suppressor of NO production, and it acts post-transcriptionally. Suppression of NO production by cyclosporin A may contribute to the vascular and renal effects associated with cyclosporin A therapy, or help preserve cellular function in allografts.
Acknowledgments
Supported by the NH & MRC of Australia. We are indebted to Dr I. Campbell (Royal Melbourne Hospital), Dr T. Mandel (Walter and Eliza Hall Institute), and Fujisawa Pharmaceutical Co. Ltd. for their kind gift of drugs. The author thanks Dr T. Tanigawa and K. Yamashita (Walter and Eliza Hall Institute) for useful discussion, and Professor M. Ogawa (Kumamoto University Medical School) for providing the opportunity for Dr Akita to undertake this work.
Abbreviations
- LPS
lipopolysaccharide
- NMMA
N-monomethyl L-arginine
- NOS2
NO synthase type 2
- rIFNγ
recombinant murine interferon-γ
- rIL-1β
recombinant murine interleukin-1β
- VSMC
vascular smooth muscle cells
References
- AKITA K., DUSTING G.J., HICKEY H. Suppression of nitric oxide production by Cyclosporin A and FK 506 in rat vascular smooth muscle cells. Clin. Exp. Pharmacol. Physiol. 1994;21:231–233. doi: 10.1111/j.1440-1681.1994.tb02503.x. [DOI] [PubMed] [Google Scholar]
- ANONYMOUS. Randomised trial comparing tacrolimus (FK506) and cyclosporin in prevention of liver allograft rejection. European FK506 Multicentre Liver Study Group [see comments] Lancet. 1994;344:423–428. [PubMed] [Google Scholar]
- ARTHUR J.F., SHAHIN S., DUSTING G.J. PAF antagonists block induction of nitric oxide synthase in cultured macrophages and vascular smooth muscle cells. Clin. Exp. Pharmacol. Physiol. 1995;22:452–454. doi: 10.1111/j.1440-1681.1995.tb02041.x. [DOI] [PubMed] [Google Scholar]
- BERMUDEZ L.E. Differential mechanisms of intracellular killing of Mycobacterium avium and Listeria monocytogenes by activated human and murine macrophages: the role of nitric oxide. Clin. Exp. Immunol. 1993;91:277–281. doi: 10.1111/j.1365-2249.1993.tb05895.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BURKART V., IMAI Y., KALLMANN B., KOLB H. Cyclosporin A protects pancreatic islet cells from nitric oxide-dependent macrophage cytotoxicity. FEBS lett. 1992;313:56–58. doi: 10.1016/0014-5793(92)81183-m. [DOI] [PubMed] [Google Scholar]
- CHOMCZYNSKI P., SACCHI N. Single-step method of RNA isolation by acid guanidine thiocyanate-phenol-chloroform extraction. Anal. Biochem. 1987;162:156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- DAWSON T.M., STEINER J.P., DAWSON V.L., DINERMAN J.L., UHL J.R., SNYDER S.H. Immunosuppressant FK506 enhances phosphorylation of nitric oxide synthase and protects against glutamate neurotoxicity. Proc. Natl. Acad. Sci. U.S.A. 1993;90:9808–9812. [Google Scholar]
- DI ROSA M., RADOMSKI M., CARNUCCIO R., MONCADA S. Glucocorticoids inhibit the induction of nitric oxide synthase in macrophages. Biochem. Biophys. Res. Commun. 1990;172:1246–1252. doi: 10.1016/0006-291x(90)91583-e. [DOI] [PubMed] [Google Scholar]
- DING A., NATHAN C.F., GRAYCAR J., DERYNCK R., STUEHR D.J., SRIMAL S. Macrophage deactivating factor and transforming growth factor-β1, -β2, -β3 inhibit induction of macrophage nitrogen oxide synthesis by IFN-γ. J. Immunol. 1990;145:940–944. [PubMed] [Google Scholar]
- GARG U.C., HASSID A. Nitric oxide-generating vasodilators and 8-bromo-cyclic guanosine monophosphate inhibit mitogenesis and proliferation of cultured rat vascular smooth muscle cells. J. Clin. Invest. 1989;83:1774–1777. doi: 10.1172/JCI114081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GAZZINELLI R.T., OSWALD L.P., JAMES S.L., SHER A. IL-10 inhibits parasite killing and nitrogen oxide production by IFN-γ-activated macrophages. J. Immunol. 1992;148:1792–1796. [PubMed] [Google Scholar]
- GREEN S.J., NACY C.A., SCHREIBER R.D., GRANGER D.L., CRAWFORD R.M., MELTZER M.S., FORTIER A.H. Neutralization of gamma interferon and tumor necrosis factor alpha blocks in vivo synthesis of nitrogen oxides from l-arginine and protection against Fracisella tularensis infection in Mycobacterium bovis BCG-treated mice. Infect. Immun. 1993;61:689–698. doi: 10.1128/iai.61.2.689-698.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GROSS S.S., LEVI R. Tetrahydrobiopterin synthesis. An absolute requirement for cytokin-induced nitric oxide generation by vascular smooth muscle. J. Biol. Chem. 1992;267:25772–25779. [PubMed] [Google Scholar]
- HECK D.E., LASKIN D.L., GARDNER C.R., LASKIN J.D. Epidermal growth factor suppresses nitric oxide and hydrogen peroxide production by keratinocytes. J. Biol. Chem. 1992;267:21277–21280. [PubMed] [Google Scholar]
- HIBBS J.B., JR, VARIN Z., TAINTOR R.R. L-arginine is required for expression of the activated macrophage effector mechanism causing selective metabolic inhibition in target cells. J. Immunol. 1987;138:550–565. [PubMed] [Google Scholar]
- ISOBE K., NAKASHIMA I. Feedback suppression of Staphylococcal enterotoxin-stimulated T-lymphocyte proliferation by macrophages through inductive nitrite oxide synthesis. Infect. Immun. 1992;60:4832–4837. doi: 10.1128/iai.60.11.4832-4837.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- JOST L.M., KIRKWOOD J.M., WHITESIDE T.L. Improved short- and long-term XTT-based colorimetric cellular cytotoxicity assay for melanoma and other tumor cells. J. Immunol. Meth. 1992;147:153–165. doi: 10.1016/s0022-1759(12)80003-2. [DOI] [PubMed] [Google Scholar]
- JUNQUERO D.C., SCOTT-BURDEN T., SCHINI V.B., VANHOUTTE P.M. Inhibition of cytokine-induction by transforming growth factor-β1 in human smooth muscle cells. J. Physiology. 1992;454:451–465. doi: 10.1113/jphysiol.1992.sp019273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KINO T., HATANAKA H., MIYATA S., INAMURA N., NISHIYAMA M., YAJIMA T., GOTO T., OKUHARA M., KOHSAKA M.H., AOKI H., OCHIAI T. FK-506, a novel immunosuppressant isolated from a streptomyces, 2 immunosuppressive effect of FK-506 in vitro. J. Antibiot. 1987;60:1256–1265. doi: 10.7164/antibiotics.40.1256. [DOI] [PubMed] [Google Scholar]
- LANGERMANS A.M., VAN DER HULST M.E.B., NIBBERING P.H., HIEMSTRA P.S., FRANSEN L., FURTH R.V. IFN-γ-induced L-arginine-dependent toxoplasmastatic activity by endogenous tumor necrosis factor α. J. Immunol. 1992;148:568–574. [PubMed] [Google Scholar]
- LANGREHR J.M., MURASE N., MARKUS P.M., CAI X., NEUHAUS P., SCHRAUT W.R.L., SIMMONS R.L., HOFFMAN R.A. Nitric oxide production in host-versus-graft and graft-versus-host reaction in the rat. J. Clin. Invest. 1992;90:679–683. doi: 10.1172/JCI115911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LIEW F.Y., LI Y., STEVERN A., MILLOTT S., SCHMIDT J., SALTER M., MONCADA S. A possible novel pathway of regulation by murine T helper type-2 (Th2) cells of a Th1 activity via the modulation of the induction of nitric oxide synthase on macrophages. Eur. J. Immunol. 1991;21:2489–2494. doi: 10.1002/eji.1830211027. [DOI] [PubMed] [Google Scholar]
- LIU J. FK506 and cyclosporin: molecular probes for studying intracellular signal transduction. Trends Pharmacol. Sci. 1993;14:182–188. doi: 10.1016/0165-6147(93)90206-y. [DOI] [PubMed] [Google Scholar]
- MASON J. Pathophysiology and toxicology of cyclosporin in humans and animals. Pharmacol. Rev. 1989;41:423–434. [PubMed] [Google Scholar]
- MONCADA S., PALMER R.M.J., HIGGS E.A. Nitric Oxide: Physiology, Pathophysiology, and Pharmacology. Pharmacol. Rev. 1991;43:109–142. [PubMed] [Google Scholar]
- OELLERICH M., ARMSTRONG V.W., SCHÜTZ E., SHAW L.M. Therapeutic drug monitoring of cyclosporine and tacrolimus. Update on Lake Louise Consensus Conference on cyclosporin and tacrolimus. Clin. Biochem. 1998;5:309–316. doi: 10.1016/s0009-9120(98)00049-6. [DOI] [PubMed] [Google Scholar]
- OSWALD L.P., GAZZINELLI R.T., SHER A., JAMES S.L. IL-10 synergizes with IL-4 and transforming growth factor-b to inhibit macrophage cytotoxic activity. J. Immunol. 1992;148:3578–3582. [PubMed] [Google Scholar]
- ROEHM N.W., RODGERS G.H., HATFIELD S.M., GLASEBROOK A.L. An improved colorimetric assay for cell proliferation and viability utilizing the tetrazolium salt XTT. J. Immunol. Meth. 1991;142:257–265. doi: 10.1016/0022-1759(91)90114-u. [DOI] [PubMed] [Google Scholar]
- SCOTT-BURDEN T., SCHINI V.B., ELIZONDO E., JUNQUERO D.C., VANHOUTTE P.M. Platelet-derived growth factor suppresses and fibroblast growth factor enhances cytokine-induced production of nitric oxide by cultured smooth muscle cells. Circ. Res. 1992;71:1088–1100. doi: 10.1161/01.res.71.5.1088. [DOI] [PubMed] [Google Scholar]
- STUEHR D.J., NATHAN C.F. Nitric oxide a macrophage product responsible for cytostasis and respiratory inhibition in tumor target cells. J. Exp. Med. 1989;169:1543–1555. doi: 10.1084/jem.169.5.1543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SUMMERSGILL J.T., POWELL L.A., BUSTER B.L., MILLER R.D., RAMIREZ J.A. Killing of Legionell pneumophila by nitric oxide in γ-interferon-activated macrophages. J. Leukocyte Biol. 1992;52:625–629. doi: 10.1002/jlb.52.6.625. [DOI] [PubMed] [Google Scholar]
- SZABO C., WU C.C., MITCHELL J.A., GROSS S.S., THIEMERMANN C., VANE J.R. Platelet-activating factor contributes to the induction of nitric oxide synthase by bacterial lipopolysaccharide. Circ. Research. 1993;73:991–999. doi: 10.1161/01.res.73.6.991. [DOI] [PubMed] [Google Scholar]
- TOCCI M.J., MATKOVICH D.A., COLLIER K.A., KWOK P., DUMONT F., LIN S., DEGUDICIBUS S., SIEKIERKA J.J., CHIN J., HUTCHINSON N.I. The immunosuppressant FK506 selectively inhibits expression of early T cell activation genes. J. Immunol. 1989;143:718–726. [PubMed] [Google Scholar]
- WINLAW D.S., SCHYVENS C.G., SMYTHE G.A., DU Z.-Y., RAINER S.P., KEOGH A.M., MUNDY J.A., LORD R.S., SPRATT P.M., MACDONALD P.S. Urinary nitrate excretion is a non-invasive indicator of acute cardiac allograft rejection and nitric oxide production in the rat. Transplantation. 1994;58:1031–1036. doi: 10.1097/00007890-199411150-00010. [DOI] [PubMed] [Google Scholar]
- WU C.C., CROXTALL J.D., PERRETTI M., BRYANT C.E., THIEMERMANN C., FLOWER R.J., VANE J.R. Lipocortin-1 mediates the inhibition by dexamethasone of the induction by endotoxin of nitric oxide synthase in the rat. Proc. Natl. Acad. Sci. U.S.A. 1995;92:3473–3477. doi: 10.1073/pnas.92.8.3473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- YANG X., CHOWDHURY N., BRETT J., MARBOE C., SCIACCA R.R., MICHLER R.E. Induction of myocardial nitric oxide synthase by cardiac allograft rejection. J. Clin. Invest. 1994;94:714–721. doi: 10.1172/JCI117390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- YIN Z.-L., HICKEY H., DUSTING G.J. A platelet-activating factor (PAF) antagonist (WEB 2170) preserves endothelium-dependent vasodilatation and prevents neo-intima development in rabbit carotid arteries. J. Vasc. Res. 1998;35:156–164. doi: 10.1159/000025579. [DOI] [PubMed] [Google Scholar]
- YOSHIMURA N., MATSUI S., HAMASHIMA T., OKA T. Effect of a new immunosuppressive agent, FK506, on human lymphocyte responses in vitro. 1 inhibition of expression of alloantigen-activated suppressor cells, as well as induction of alloreactivity. Transplantation. 1989;47:351–356. doi: 10.1097/00007890-198902000-00034. [DOI] [PubMed] [Google Scholar]


