Abstract
Differences in the mechanism of non-adrenergic, non-cholinergic (NANC) inhibitory responses to preganglionic- and post-ganglionic nerve stimulation were investigated in the guinea-pig isolated trachea.
Stimulation of the vagus nerve at frequencies above 4 Hz elicited NANC relaxation of the trachealis muscle. Responses to low frequencies of stimulation (4–8 Hz) were abolished by the nitric oxide (NO) synthase inhibitor L-NOARG (10 μM), while a L-NOARG resistant component was observed at higher stimulus frequencies. The L-NOARG-resistant component of NANC inhibitory responses to higher frequencies of vagus nerve stimulation were significantly attenuated by the proteinase α-chymotrypsin (2 U/ml), suggesting that a neuropeptide such as VIP may contribute to NANC responses.
When postganglionic nerves were stimulated by electrical field stimulation (EFS), responses were readily elicited at frequencies below 4 Hz. Like responses to vagus nerve stimulation, responses to low frequency (<4 Hz) EFS were abolished by L-NOARG while a L-NOARG-resistant component was apparent at higher stimulus frequencies.
The L-NOARG-resistant component of NANC inhibitory responses to EFS was sensitive to α-chymotrypsin only if stimuli were delivered in either long trains at a low frequency (4 Hz for 10–30 s) or short trains of high frequency (16 Hz for 2.5–7.5 s).
Responses to preganglionic nerve stimulation were approximately 35% of the amplitude of responses to EFS in the same preparations.
In conclusion, responses to preganglionic and postganglionic NANC inhibitory nerve stimulation in the guinea-pig trachea differ in maximum amplitude, frequency-response characteristics and the contributions of cotransmitters. We suggest that these differences may be explained by filtering of preganglionic input to postganglionic NANC neurons. These results have implications in all studies where EFS is considered to be representative of physiological stimulation of post-ganglionic nerve stimulation.
Keywords: Non-adrenergic, non-cholinergic (NANC), nitric oxide, neuropeptides
Introduction
The parasympathetic innervation of the guinea-pig trachea can initiate both trachealis muscle contraction and relaxation. Stimulation of the vagus nerve, which carries the parasympathetic preganglionic nerve supply to the trachea, elicits both cholinergic muscarinic contractions and non-adrenergic, non-cholinergic (NANC) relaxations in vivo (Chesrown et al., 1980; Yip et al., 1981). Both the cholinergic and NANC inhibitory responses to vagal stimulation are abolished by ganglionic blockade (Chesrown et al., 1980; Yip et al., 1981), suggesting that the responses depend upon stimulation of postganglionic neuronal cells. Pharmacological studies of the NANC inhibitory response to electrical field stimulation (EFS) of isolated strips of trachea suggest that these responses are mediated by nitric oxide (NO) at low frequencies of stimulation (Tucker et al., 1990; Li & Rand, 1991) and vasoactive intestinal polypeptide (VIP) or a related peptide at higher frequencies (Tucker et al., 1990; Li & Rand, 1991; Ellis & Farmer, 1989a,1989b). The results of these studies should be interpreted cautiously, however, since EFS stimulates all nerve fibres within a preparation regardless of their anatomical origin. Hence, it is not clear whether NO and VIP are released by a single population of nerves. For example, VIP-containing nerve fibres within the trachealis muscle have been shown to originate from sympathetic ganglia in the guinea-pig (Kummer et al., 1992). Therefore, it is possible that NO is released by parasympathetic NANC nerve fibres, whereas VIP is released by a population of sympathetic nerve fibres.
Canning & Undem (1993a,1993b) described an in vitro preparation of the guinea-pig trachea in which NANC responses to vagus nerve stimulation can be reliably elicited with a threshold frequency of 4 Hz. In contrast, NANC responses are elicited by EFS at considerably lower frequencies (Li & Rand, 1991; Ellis & Undem, 1990; Kalenberg & Satchell, 1979). One interpretation of the higher stimulus frequencies required to elicit responses to vagus nerve stimulation is that a neuropeptide such as VIP, rather than NO, is the mediator released, since neuropeptides are generally considered to be released only during high frequency stimulation (Morris & Gibbins, 1992; Lundberg, 1996). However, studies of several other mammalian species indicate that NANC inhibitory responses to EFS in tracheal smooth muscle are mediated exclusively by NO (Fisher et al., 1993; Kannan & Johnson, 1992; Belvisi et al., 1992; Yu et al., 1994). Thus, another interpretation of the high frequency of vagal stimulation required to produce a relaxation is that it results in a lower frequency of discharge in postganglionic neurons. Indeed Canning & Undem (1993a,1993b) hypothesized that filtering of preganglionic input may account for the stimulus frequency-response characteristics that they observed. Therefore, we have characterized the responses to both EFS and preganglionic nerve stimulation in preparations of guinea-pig isolated trachea. The results suggest that EFS and stimulation of the vagus nerve elicit responses which differ in their dependence on co-transmitters and support the concept that preganglionic stimuli are filtered at the ganglionic synapse.
Methods
Animals
Male and female guinea-pigs (short-haired coloured; 250–350 g) were killed by immersion in an 80% CO2-air mixture. All experiments were conducted with the approval of the University of Melbourne Animal Experimentation Ethics Committee.
Vagus nerve-trachealis muscle preparations
The trachea, including the larynx, was excised and the surrounding tissues dissected away, with the exception of the vagus nerve and oesophagus. Both sympathetic trunks were removed together with the stellate and superior cervical ganglia (Figure 1). The vagi were tied together just below the nodose ganglia and the superior laryngeal nerves (SLN) and pharyngeal nerves were cut. For recording of trachealis muscle tension, the trachea was opened with a longitudinal incision through the first 12 cartilage rings and stainless steel hooks, attached to cotton threads were inserted into rings six and seven (caudal to the larynx) on either side of the trachealis muscle. Preparations were placed in a 250 ml organ bath filled with Krebs solution (composition (mM): NaCl 133.5, KCl 4.7, NaH2PO4 1.3, NaHCO3 16.3, MgSO4 0.6, CaCl2 2.5 and glucose 7.8) maintained at 37°C and vigorously bubbled with 95% O2/5% CO2. One of the cotton threads was attached to a Grass FT03 force transducer, while the other was tied to an anchor in the bath. The vagi were pulled through a pair of platinum ring electrodes (diameter 1 mm); stimuli were applied in 10 s trains of maximal voltage (80 V) square wave pulses of 1.0 ms duration at a range of frequencies (1–32 Hz) using a Grass S48 stimulator. These stimulation parameters were chosen to ensure that all nerve fibres were stimulated.
Figure 1.
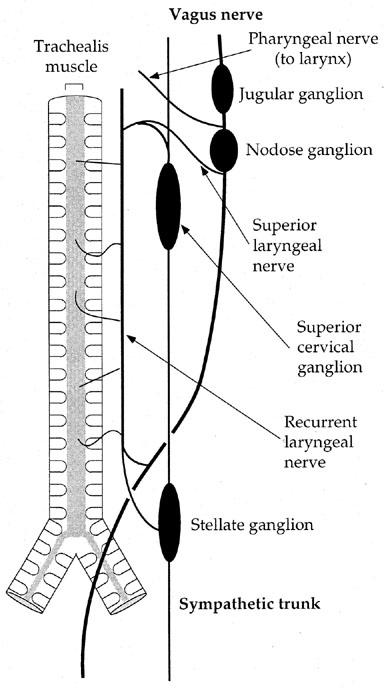
Schematic diagram illustrating the extrinsic nerve pathways to the guinea-pig trachea, viewed from the dorsal aspect. The oesophagus, which contains cell bodies of NANC inhibitory neurons innervating the trachea (Moffatt et al., 1998; Fischer et al., 1998) has been omitted for clarity.
RLN-trachealis muscle preparations
In some experiments the effects of stimulating the recurrent laryngeal nerve were investigated. For this purpose a simple and reliable preparation was developed which could be maintained for several hours in a 50 ml organ bath. The trachea, with the oesophagus attached, was excised and both RLN were isolated close to their origin from the vagus nerve. The rostral eight rings of trachea were prepared for recording as described above and the RLN were dissected free from the trachea/oesophagus tissue mass back to the level of the aortic arch. The trachea and oesophagus posterior to the 12th ring were removed and the preparation was mounted as described above in a 50 ml organ bath. Both RLN were pulled through a 1 mm diameter platinum ring electrode for stimulation as described above.
In some experiments the effects of preganglionic nerve stimulation and electrical field stimulation were compared. In these experiments, the trachea was anchored on a platinum rod, which served as an anchor and as an electrode. A second platinum rod, horizontally oriented and positioned 5 mm from the preparation served as an indifferent electrode for EFS. The stimulus parameters for EFS were: 0.2 ms, submaximal voltage in 10 s trains. In order to record smooth muscle responses a single stainless steel hook was inserted through the ventral cartilage rings. At the end of the experiment preparations were exposed to tetrodotoxin (TTX; 1 μM) in order to verify the neurogenic nature of responses to EFS.
SLN-trachealis muscle preparations
In further experiments preparations were stimulated via the SLN. In these experiments the vagi were isolated and cut as far cranial as possible to the nodose ganglia. The vagus was cut caudal to the nodose ganglion and not included in these preparations. The trachea was then excised from the animal and prepared for recordings of smooth muscle tension as described above for the RLN-trachealis muscle preparation. The surrounding tissues were dissected away leaving the cranial portion of the vagus nerve (with nodose ganglion), SLN and RLN intact. The vagi and nodose ganglion were pulled through an electrode for stimulation as described above. In some experiments, preparations were set up enabling the stimulation of either the RLN or SLN.
EFS strip preparations
Animals were killed as described above and the trachea (without oesophagus) was excised and opened longitudinally through the ventral cartilage rings. The trachea was then cut transversely to produce several strips 3–4 cartilage rings in length. Stainless steel hooks attached to cotton threads were inserted into the cartilage on either side of the trachealis muscle, and each strip was then mounted in a 50 ml organ bath as described above. Parallel platinum ring electrodes were placed around the preparation for stimulation of post-ganglionic nerve fibres by EFS with trains of 0.2 ms pulse duration at submaximal voltage (60–100 V), determined by constructing a voltage-response curve for each preparation. TTX (1 μM) was added at the end of each experiment to verify that responses were of neural origin. If EFS elicited responses to the highest stimulation frequency (32 Hz) in the presence of TTX, the data were discarded.
Experimental procedures: nerve-trachealis preparations
Indomethacin (3 μM) was always present in the Krebs solution in order to prevent a decline in neural responses over time (Undem et al., 1990) and to prevent indirect effects by cyclo-oxygenase products. Unless otherwise stated, experiments were conducted in the presence of cholinergic (atropine, 1 μM) and adrenergic (bretylium 1–10 μM or propranolol 1 μM, as stated) antagonists. All preparations were contracted to approximately 80% of maximum with prostaglandin F2α (PGF2α; 1 μM) in order to study inhibitory effects of nerve stimulation. The possible involvement of NO in the NANC responses to nerve stimulation was investigated using the NOS inhibitor NG-nitro-L-arginine (L-NOARG) which was added to the bath after a series of responses at 4–32 Hz had been elicited at 10 min intervals. A bath concentration of 10 μM of L-NOARG was found to be maximally effective. After 30 min a second series of responses was elicited. The protease α-chymotrypsin was used to investigate the possible involvement of a neuropeptide in responses resistant to L-NOARG. This protease has been previously shown to inhibit responses to applied VIP and EFS of the guinea-pig trachea (Tucker et al., 1990; Li & Rand, 1991; Ellis & Farmer, 1989a,1989b). The concentration of α-chymotrypsin (2 U/ml) used presently was found to be maximally effective after 30 min in preliminary experiments. The relatively short reliable life of the vagus nerve-trachea preparation necessitated that these experiments be conducted on separate preparations. Preparations were stimulated at 32 Hz to establish the maximal control relaxation and L-NOARG (10 μM) was then added to the bath. After 30 min a series of responses was elicited (as above) and the preparation washed. L-NOARG and α-chymotrypsin (2 U/ml) were then added to the bath and a second series of responses were elicited 30 min later.
In some of the RLN-trachea preparations the possible contribution of capsaicin-sensitive sensory nerves to NANC inhibitory response to extrinsic nerve stimulation was investigated. After a control stimulus, the preparations were exposed to capsaicin at a concentration of 3 μM for 60 min followed by several washes over a 30 min period before further stimulations.
Experimental procedures: EFS preparations
Experiments were always conducted in the presence of indomethacin, atropine and bretylium as described above. After equilibration each strip was exposed to capsaicin (3 μM) for 60 min and thoroughly washed in order to deplete c-fibre afferents of excitatory peptide neurotransmitters. After tension returned to control levels, the preparations were contracted with PGF2α (1 μM) and a control EFS-response curve (see below) was constructed. The preparations were then exposed to L-NOARG (10 μM) and stimulated at 16 Hz every 10 min until the inhibitory effect of the drug had stabilized (20–30 min) after which a second EFS-response curve was constructed. α-chymotrypsin (2 U/ml) was then added to the bath and, again after the effect of the drug had stabilized, a third EFS-response curve was constructed. In preliminary experiments, we added α-chymotrypsin cumulatively up to a concentration of 10 U/ml and observed no additional inhibitory effect at concentrations above 1 U/ml. Most preparations maintained a stable level of tone throughout the experimental period; preparations were discarded if tone decreased more than approximately 30% of the initial contraction to PGF2α.
EFS was delivered according to two regimes. In the first series of experiments, preparations were stimulated with 10 s trains of stimuli at 1, 2, 4, 8, 16 and 32 Hz (i.e. fixed duration). In the second series of experiments, preparations were stimulated at 4 and 16 Hz in trains of 40, 120 and 240 pulses (i.e. fixed pulse number).
Analysis and statistics
In preliminary experiments a 32 Hz stimulus was found to elicit a maximum response in nerve-trachea preparations; responses are therefore expressed as a percentage of this maximum for each preparation. In EFS preparations, responses are expressed as a percentage of the induced tone. All data are expressed as mean±s.e.mean. A standard Student's t-test was used to establish the statistical significance of differences; P<0.05 was accepted as significant.
Sources of drugs
Atropine sulphate, bretylium tosylate, D-tubocurarine chloride, hexamethonium bromide, propranolol hydrochloride, L-NOARG, α-chymotrypsin (type II, bovine pancreas), tetrodotoxin and indomethacin were purchased from Sigma. PGF2α was purchased from Upjohn. All drugs were dissolved as stock concentrations (1–10 mM) in distilled water with the exception of capsaicin (DMSO) and indomethacin (absolute ethanol).
Results
Vagus nerve-trachealis muscle preparations
In the presence of atropine (1 μM) and bretylium (1 μM) and after contracture with PGF2α (1 μM), vagal stimulation elicited frequency-dependent NANC relaxations (Figure 2a). The threshold frequency required to elicit appreciable NANC relaxation was 4 Hz and maximal relaxation was observed at 32 Hz (Figure 2a). Responses to 32 Hz stimulation were attenuated by D-tubocurarine (10 μM) to 17±4% (n=5) of control responses, indicating that preganglionic nerve fibres were stimulated by vagus nerve stimulation.
Figure 2.
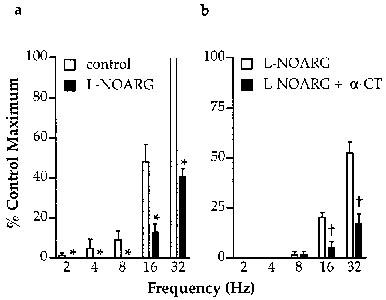
Frequency-dependence of NANC inhibitory responses to vagus nerve stimulation (80 V, 1.0 ms, 10 s trains) and the effect of NOS inhibition with L-NOARG (10 μM) and cleavage of neuropeptides by α-chymotrypsin (α-CT; 2 U/ml). All experiments were conducted on PGF2α-contracted preparations in the presence of indomethacin (3 μM), bretylium (1 μM) and atropine (1 μM). Note that in experiments on vagus nerve-trachealis preparations effects of L-NOARG (a) and L-NOARG+α-chymotrypsin (b) were examined in separate preparations. All points represent the results of six experiments. *Indicates significant inhibition of responses by L-NOARG (P<0.05, paired t-test); †Indicates significant effect of α-chymotrypsin (P<0.05, paired t-test).
L-NOARG (10 μM) abolished responses to vagus nerve stimulation at 4 and 8 Hz and significantly attenuated responses to higher stimulus frequencies (Figure 2a). Increased concentrations of L-NOARG (up to 100 μM) were without additional effect (data not shown). In separate experiments, the effect of α-chymotrypsin in combination with L-NOARG was investigated. The L-NOARG-resistant component of responses to high frequency stimulation were significantly reduced by α-chymotrypsin (2 U/ml) to less than 50% of their pre-treatment amplitudes (Figure 2b).
EFS preparations: fixed train length
In PGF2α-contracted ring preparations of trachea, EFS elicited NANC relaxations. The frequency-response characteristics of these responses was markedly different to those elicited by vagus nerve stimulation as responses were readily elicited at stimulus frequencies below 4 Hz (Figure 3). Responses to low frequency EFS (<2 Hz) were abolished by L-NOARG (10 μM) while a L-NOARG-resistant component was apparent at higher stimulus frequencies (Figure 3). However, unlike responses to vagus nerve stimulation, α-chymotrypsin significantly reduced responses to EFS only at 4 Hz (Figure 3).
Figure 3.
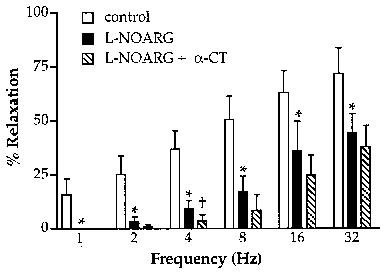
Frequency-dependence of NANC inhibitory responses to EFS (submaximal voltage, 0.2 ms, 10 s trains) and the effect of NOS inhibition with L-NOARG (10 μM) and cleavage of neuropeptides by α-chymotrypsin (α-CT; 2 U/ml). All experiments were conducted on PGF2α-contracted preparations in the presence of indomethacin (3 μM), bretylium (1 μM) and atropine (1 μM). All points represent the results of six experiments. *Indicates significant inhibition of responses by L-NOARG (P<0.05, paired t-test); †indicates significant effect of α-chymotrypsin (P<0.05, paired t-test).
EFS preparations: variable train length
EFS of preparations in trains of 40, 120 and 240 pulses produced responses of similar amplitude at 4 and 16 Hz (Figure 4a), although the time courses of the responses were different. Following 240 pulses at 4 Hz, responses returned to 50% of baseline after 2.62±0.12 min, whilst responses to the same number of pulses at 16 Hz took 3.16±0.16 min (P<0.05, n=8). Although the attenuation of responses by L-NOARG was similar between the two stimulus frequencies (Figure 4b,c), the sensitivity of the remaining responses to α-chymotrypsin was different. Thus all L-NOARG-resistant responses to EFS at 4 Hz were significantly attenuated by α-chymotrypsin, whereas only the responses to shorter train lengths (40 and 120 pulses) were reduced at 16 Hz (Figure 4b,c). There was no difference in the kinetics of L-NOARG-resistant responses to 16 Hz trains of EFS after the addition of α-chymotrypsin.
Figure 4.
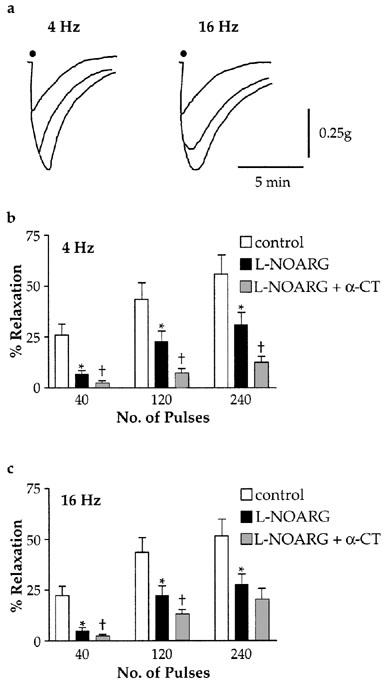
Effect of EFS (submaximal voltage, 0.2 ms) train length on the sensitivity of NANC inhibitory responses to L-NOARG (10 μM) and α-chymotrypsin (2 U/ml). (a) Overlaid representative tracings of responses from a single preparation, showing that although the amplitude of responses to increasing numbers of pulses (40, 120 and 240) was similar between 4 and 16 Hz, responses to 16 Hz EFS were more prolonged. (b,c) Grouped data showing the inhibitory effect of L-NOARG and L-NOARG+α-chymotrypsin on EFS-induced responses. All experiments were conducted on PGF2α-contracted preparations in the presence of indomethacin (3 μM), bretylium (1 μM) and atropine (1 μM). All points represent results from eight experiments. *Indicates significant inhibition of responses by L-NOARG (P<0.05, paired t-test); †indicates significant effect of α-chymotrypsin (P<0.05, paired t-test).
SLN- and RLN-trachealis muscle preparations
Because we were concerned that the differences between our EFS and vagal stimulation studies might be due to damage to nerve pathways during dissection or the difference in preparation time, we devised a smaller and less time consuming preparation allowing pre-ganglionic nerve stimulation. Stimulation of the RLN in this preparation caused relaxation of the trachealis, whereas stimulation of the SLN caused relaxation in only one of twelve preparations (Figure 5a). Stimulation of the SLN in two cases elicited small NANC contractions (Figure 5a). These findings suggest that the entire inhibitory input to the trachea is carried in the RLN.
Figure 5.
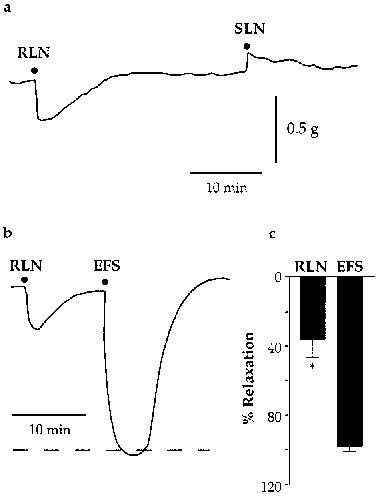
(a) Sample tracing of a preparation prepared for both SLN and RLN stimulation (80 V, 32 Hz, 0.2 ms, 10 s). The preparation was contracted with 1 μM PGF2α in the presence of indomethacin (3 μM), bretylium (1 μM) and atropine (1 μM). Note that while RLN stimulation causes NANC inhibitory responses, in this preparation SLN stimulation caused only a small NANC contraction. Representative of 12 similar experiments. (b) Sample tracing illustrating that NANC inhibitory responses elicited by RLN stimulation (80 V, 32 Hz, 0.2 ms, 10 s) are appreciably smaller than those elicited by EFS (submaximal voltage, 32 Hz, 0.2 ms, 10 s) in the same preparation. (c) Grouped data from experiments comparing RLN stimulation and EFS (n=5). *Indicates significant difference (P<0.05, paired t-test).
The frequency response-characteristics of NANC responses to RLN stimulation in these preparations were the same as those observed in the vagus nerve-trachealis preparation above (data not shown). In five experiments the effects of EFS and RLN stimulation were compared. In all preparations the responses elicited by EFS were of significantly greater amplitude than those elicited by RLN stimulation (Figure 5b,c). Furthermore, EFS elicited responses at stimulus frequencies less than 4 Hz whilst similar stimulation of the RLN was without effect (n=4; data not shown).
Discussion
The present study provides evidence that both NO and a neuropeptide are released by postganglionic NANC nerve fibres within the guinea-pig trachealis muscle in response to preganglionic nerve stimulation. There are clear qualitative and quantitative differences, however, between the responses elicited by postganglionic (EFS) and preganglionic stimulation.
NANC responses to vagus nerve stimulation at 4–8 Hz were completely abolished by L-NOARG in the present experiments, indicating that NO is probably the sole mediator of these responses. When higher stimulus frequencies were used, however, between 25–50% of the response to vagal stimulation was resistant to L-NOARG. The L-NOARG-resistant responses were substantially attenuated by α-chymotrypsin, suggesting the involvement of a peptide in this component of the responses. Hence, as in other tissues innervated by NANC inhibitory neurons, NO and a neuropeptide are released following preganglionic nerve stimulation, but the peptide is released only during higher frequency stimulation (Lundberg, 1996).
In contrast to vagus nerve stimulation, EFS elicited responses which had different frequency-response characteristics and sensitivity to L-NOARG and α-chymotrypsin. Thus, as in previous studies (Li & Rand, 1991; Ellis & Undem, 1990; Kalenberg & Satchell, 1979), NANC inhibitory responses to EFS of the guinea-pig isolated trachea could be elicited at frequencies lower than 1 Hz and were abolished by L-NOARG only at low stimulus frequencies (1–2 Hz). Furthermore, when stimuli were delivered in 10 s trains (as in vagus nerve-trachea preparations), α-chymotrypsin had no significant effect on the L-NOARG resistant component of responses to EFS. This discrepancy between the effect of α-chymotrypsin on vagus nerve stimulation- and EFS-induced responses was investigated by applying EFS in trains of different lengths at two frequencies. The results of these studies suggest that two L-NOARG-resistant transmitters may participate in NANC responses to EFS, and that only one mediator is sensitive to cleavage by α-chymotrypsin. Thus, although responses to EFS at 4 and 16 Hz were of identical amplitude when similar numbers of pulses were delivered, both the time courses of the responses (significantly longer duration at 16 Hz) and sensitivity to α-chymotrypsin (less marked at 16 Hz) were different. These findings are supported by previous studies of the pharmacology of NANC responses to EFS in this preparation. For example, Ellis & Farmer (1989a,1989b) and Li & Rand (1991) showed that the response to 4 Hz could be attenuated by VIP antisera or α-chymotrypsin using EFS of the guinea-pig trachea delivered in 30 s trains. However, in a similar study Tanihata & Uchiyama (1996) observed no significant effect of α-chymotrypsin when using 5 s trains of EFS. Together, these results suggest that to elicit NANC responses that have an α-chymotrypsin-sensitive component EFS must be delivered at either low frequency in long trains (>10 s) or high frequency in short trains (<5 s). A L-NOARG and α-chymotrypsin-resistant component to NANC nerve stimulation was also observed in the rat gastric fundus and is thought to represent the contribution of a purine to NANC neurotransmission (D'Amato et al., 1992). The third mediator of NANC responses to EFS in this preparation may be adenosine (Coleman & Levy, 1974; Piper & Hollingsworth, 1996), although we have not examined this possibility in the present studies.
There are several possible explanations for the different responses produced by vagus nerve stimulation and EFS. Firstly, the larger responses to EFS might represent artefacts of this sometimes capricious stimulation method (Keef et al., 1991). Indeed, intense EFS has been shown to release prostanoids in a TTX-resistant manner in the guinea-pig trachea (Fernandes et al., 1994). However, we were careful to avoid TTX-resistant relaxations in the present study. Although we cannot definitively rule out other artefacts such as effects on muscle electrophysiology, we do not consider it likely that the obvious differences in the responses elicited by preganglionic nerve stimulation and EFS are due to differences in the method of stimulation per se. Secondly, it is possible that during the dissection process, crucial, but not grossly obvious preganglionic pathways may be damaged. In an attempt to eliminate this possibility, a simpler preparation of the trachea-oesophagus complex was developed to allow stimulation of the RLN, hence avoiding any possible branching in the more cranial pathways. This preparation, however, had the same frequency response characteristics as the vagus nerve-trachealis muscle preparation. Furthermore, there is evidence to suggest that the frequency-response characteristics reported here may mirror the in vivo situation. Yip et al. (1981) found that vagal stimulation at 1–3 Hz required a stimulus duration of 60 s before NANC relaxation was obtained. This observation indicates that in vivo, a 10 s stimulus train at low frequency (as used in this study) would not elicit a NANC response. Hence the failure of low frequency vagal stimuli to elicit responses is probably not the result of damage to nerve pathways in establishing an in vitro preparation.
A third possibility is that not all of the preganglionic fibres to the trachea are carried in the RLN or that NANC inhibitory fibres project to the trachea from a non-vagal source. The most obvious alternative route to the trachea from the vagus is the SLN. It was found that stimulation of the SLN pathway, however, rarely evoked NANC inhibitory responses. Hence, in the present studies, the vast majority of all NANC input to the trachea is carried in the RLN and would appear to have a vagal origin. This contrasts with the findings of Canning & Undem (1994a) who were able to consistently elicit responses by stimulating the SLN. The reason for this difference is not clear but may be related to the differences in the strains of guinea-pigs used. In this study, short-haired coloured guinea-pigs were used, whereas Dunkin-Hartley guinea-pigs were used by Canning & Undem (1994a).
A fourth possibility is that preganglionic input is filtered in some way during ganglionic transmission (Canning & Undem, 1993a,1993b). There is electrophysiological evidence that filtering of preganglionic input does occur in airway ganglia (Canning & Undem, 1994b; Dey, 1994). Single preganglionic stimuli do not readily elicit action potentials in some airway postganglionic neurons. Rather, preganglionic stimuli elicit excitatory post-synaptic potentials in some postganglionic neurons, several of which must summate in order to generate an action potential (Canning & Undem, 1994b; Dey, 1994). The differences in the pharmacology of NANC inhibitory responses to EFS and preganglionic nerve stimulation provide some evidence that preganglionic input to NANC inhibitory neurons may be filtered. By estimation from the results of the EFS experiments, it would appear that the maximal preganglionic stimulus frequency (32 Hz) is filtered to produce a shorter train of action potentials with a lower frequency. Thus, an EFS stimulus producing a response matching the pharmacological characteristics and amplitude of maximal vagus nerve stimulation-evoked responses (32 Hz, 10 s) would lie between 4–16 Hz and have a duration of approximately 5 s. This extrapolation suggests that approximately two to four preganglionic action potentials produce a single action potential in the post ganglionic neuron. If ganglionic filtering accounts for the present results, it may indicate that the NANC inhibitory innervation does not contribute to resting tone and is activated only by strong reflex input. Furthermore, since the NANC postganglionic neurons are located within the myenteric plexus of the oesophagus in the guinea-pig they may be involved in a more complex neural circuit than a simple preganglionic-postganglionic neuron relay (Moffatt et al., 1998; Fischer et al., 1998). The oesophageal myenteric plexus of the guinea-pig contains some 1300 neurons per square centimetre (Furness & Costa, 1987) and the functions of these neurons are enigmatic since the musculature of the oesophagus is entirely striated in this species.
In conclusion, the results of the present study indicate that vagal stimulation produces NANC inhibitory responses in the guinea-pig trachea, via a pathway through the RLN. These responses have a different sensitivity to L-NOARG and α-chymotrypsin compared to those produced by EFS. The frequency-response characteristics and maximum amplitude of responses to preganglionic nerve stimulation and EFS are also markedly different. The reason for these differences may be related to filtering of preganglionic input to postganglionic neurons during ganglionic transmission. These findings have important implications regarding the likely NANC neurotransmitters released during autonomic reflexes in the airways.
Acknowledgments
This study was supported by the Australian Research Council. We thank Dr T. Cocks for his comments on the manuscript.
Abbreviations
- EFS
electrical field stimulation
- L-NOARG
NG-nitro-L-arginine
- NO
nitric oxide
- RLN
recurrent laryngeal nerve
- SLN
superior laryngeal nerve
- TTX
tetrodotoxin
- VIP
vasoactive intestinal polypeptide
References
- BELVISI M.G., STRETTON C.D., MIURA M., VERLEDEN G.M., TADJKARIMI S., YACOUB M.H., BARNES P.J. Inhibitory NANC nerves in human tracheal smooth muscle: a quest for the neurotransmitter. J. Appl. Physiol. 1992;73:2505–2510. doi: 10.1152/jappl.1992.73.6.2505. [DOI] [PubMed] [Google Scholar]
- CANNING B.J., UNDEM B.J. Relaxant innervation of the guinea-pig trachealis: demonstration of capsaicin-sensitive and -insensitive vagal pathways. J. Physiol. 1993a;460:719–739. doi: 10.1113/jphysiol.1993.sp019496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CANNING B.J., UNDEM B.J. Evidence that distinct neural pathways mediate parasympathetic contractions and relaxations of the guinea-pig trachealis. J. Physiol. 1993b;471:25–40. doi: 10.1113/jphysiol.1993.sp019889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CANNING B.J., UNDEM B.J. Evidence that antidromically stimulated vagal afferents activate inhibitory neurones innervating guinea-pig trachealis. J. Physiol. 1994a;480:613–625. doi: 10.1113/jphysiol.1994.sp020388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CANNING B.J., UNDEM B.J.Parasympathetic innervation of airways smooth muscle Airways Smooth Muscle: Structure, Innervation and Neurotransmission 1994bBirkhäuser Verlag: Basel, Switzerland; 43–78.In: D. Raeburn and M.A. Giembycz [Google Scholar]
- CHESROWN S.E., VENIGOPALAN C.S., GOLD W.M., DRAZEN J.M. In vivo demonstration of nonadrenergic inhibitory innervation of the guinea pig trachea. J. Clin. Invest. 1980;65:314–320. doi: 10.1172/JCI109674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- COLEMAN R.A., LEVY G.P. A non-adrenergic inhibitory nervous pathway in guinea-pig trachea. Br. J. Pharmacol. 1974;52:167–174. doi: 10.1111/j.1476-5381.1974.tb09697.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D'AMATO M., CURRÒ D., MONTUSCHI P. Evidence for dual components in the non-adrenergic non-cholinergic relaxation in the rat gastric fundus: role of endogenous nitric oxide and vasoactive intestinal polypeptide. J. Auton. Nerv. Syst. 1992;37:175–186. doi: 10.1016/0165-1838(92)90039-j. [DOI] [PubMed] [Google Scholar]
- DEY R.D.Airways ganglia Airways Smooth Muscle: Structure, Innervation and Neurotramission 1994Birkhäuser Verlag: Basel, Switzerland; 79–101.In: D. Raeburn & M.A. Giembycz [Google Scholar]
- ELLIS J.L., FARMER S.G. The effects of vasoactive intestinal polypeptide (VIP) antagonists, and VIP and peptide histidine isoleucine antisera on non-adrenergic, non-cholinergic relaxations of tracheal smooth muscle. Br. J. Pharmacol. 1989a;96:513–520. doi: 10.1111/j.1476-5381.1989.tb11847.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ELLIS J.L., FARMER S.G. Effects of peptidases on non-adrenergic, non-cholinergic inhibitory responses of tracheal smooth muscle: a comparison with effects on VIP- and PHI-induced relaxation. Br. J. Pharmacol. 1989b;96:521–526. doi: 10.1111/j.1476-5381.1989.tb11848.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ELLIS J.L., UNDEM B.J. Non-adrenergic, non-cholinergic contractions in the electrically field stimulated guinea-pig trachea. Br. J. Pharmacol. 1990;101:875–880. doi: 10.1111/j.1476-5381.1990.tb14174.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FERNANDES L.B., HUBBARD W.C., UNDEM B.J. Release of inflammatory mediators from guinea-pig trachea by electrical field stimulation: lack of neuronal involvement. J. Pharmacol. Exp. Ther. 1994;270:1166–1170. [PubMed] [Google Scholar]
- FISCHER A., CANNING B.J., UNDEM B.J., KUMMER W. Evidence for an esophageal origin of VIP-IR and NO synthase-IR nerves innervating the guinea-pig trachealis: a retrograde neuronal tracing and immunohistochemical analysis. J. Comp. Neurol. 1998;394:326–334. [PubMed] [Google Scholar]
- FISHER J.T., ANDERSON J.W., WALDRON M.A. Nonadrenergic noncholinergic neurotransmitter of feline trachealis: VIP or nitric oxide. J. Appl. Physiol. 1993;74:31–39. doi: 10.1152/jappl.1993.74.1.31. [DOI] [PubMed] [Google Scholar]
- FURNESS J.B., COSTA M. The Enteric Nervous System 1987Churchill Livingstone: Edinburgh; 290 pp [Google Scholar]
- KALENBERG S., SATCHELL D.G. The inhibitory innervation of the guinea-pig trachea: a study of its adrenergic and non-adrenergic components. Clin. Exp. Pharmacol. Physiol. 1979;6:549–559. doi: 10.1111/j.1440-1681.1979.tb00038.x. [DOI] [PubMed] [Google Scholar]
- KANNAN M.S., JOHNSON D.E. Nitric oxide mediates the neural nonadrenergic, noncholinergic relaxation of pig tracheal smooth muscle. Am. J. Physiol. 1992;262:L511–L514. doi: 10.1152/ajplung.1992.262.4.L511. [DOI] [PubMed] [Google Scholar]
- KEEF K.D., HOTTENSTEIN O.D., MEEHAN A.G., ANTHONY T.L., KREULEN D.L. Comparison of neurotransmission with nerve trunk and transmural field stimulation in guinea-pig mesenteric artery. J. Physiol. (Lond.) 1991;441:367–383. doi: 10.1113/jphysiol.1991.sp018756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KUMMER W., FISCHER A., KURROWSKI R., HEYM C. The sensory and sympathetic innervation of guinea-pig lung and trachea as studied by retrograde neuronal tracing and double-labelling immunohistochemistry. Neuroscience. 1992;49:715–737. doi: 10.1016/0306-4522(92)90239-x. [DOI] [PubMed] [Google Scholar]
- LI C.G., RAND M.J. Evidence that part of the NANC relaxant response of guinea-pig trachea to electrical field stimulation is mediated by nitric oxide. Br. J. Pharmacol. 1991;102:91–94. doi: 10.1111/j.1476-5381.1991.tb12137.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LUNDBERG J.M. Pharmacology of cotransmission in the autonomic nervous system: integrative aspects on amines, neuropeptides, adenosine triphosphate, amino acids and nitric oxide. Pharmacol. Rev. 1996;48:113–178. [PubMed] [Google Scholar]
- MOFFATT J.D., DUMSDAY B., MCLEAN J.R. Non-adrenergic, non-cholinergic neurons innervating the guinea-pig trachea are located in the oesophagus: evidence from retrograde neuronal tracing. Neurosci. Lett. 1998;248:37–40. doi: 10.1016/s0304-3940(98)00321-8. [DOI] [PubMed] [Google Scholar]
- MORRIS J.L., GIBBINS I.L.Co-transmission and neuromodulation Autonomic Neuroeffector Mechanisms 1992Harwood Academic Publishers: Chur, Switzerland; 33–119.In: G. Burnstock and C.H. Hoyle (eds) [Google Scholar]
- PIPER A.S., HOLLINGSWORTH M. ATP and β,γ-methylene ATP produce relaxation of guinea-pig isolated trachealis via actions at P1 purinoceptors. Eur. J. Pharmacol. 1996;307:183–189. doi: 10.1016/0014-2999(96)00237-3. [DOI] [PubMed] [Google Scholar]
- TANIHATA S., UCHIYAMA T. Role of nitric oxide in nonadrenergic, non cholinergic relaxation of whole tracheal tube preparations isolated from guinea-pigs. Gen. Pharmacol. 1996;27:827–832. doi: 10.1016/0306-3623(95)02083-7. [DOI] [PubMed] [Google Scholar]
- TUCKER J.F., BRAVE S.R., CHARALAMBOUS L., HOBBS A.J., GIBSON A. L-NG-nitro arginine inhibits non-adrenergic, non-cholinergic relaxations of guinea-pig isolated tracheal smooth muscle. Br. J. Pharmacol. 1990;100:663–664. doi: 10.1111/j.1476-5381.1990.tb14072.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- UNDEM B.J., MYERS A.C., BARTHLOW H., WEINREICH D. Vagal innervation of guinea pig bronchial smooth muscle. J. Appl. Physiol. 1990;69:1336–1346. doi: 10.1152/jappl.1990.69.4.1336. [DOI] [PubMed] [Google Scholar]
- YIP P., PALOMBINI B., COBURN R.F. Inhibitory innervation to the guinea pig trachealis muscle. J. Appl. Physiol. 1981;50:374–382. doi: 10.1152/jappl.1981.50.2.374. [DOI] [PubMed] [Google Scholar]
- YU M., WANG Z., ROBINSON N.E., LEBLANC P.H. Inhibitory nerve distribution and mediation of NANC relaxation by nitric oxide horse airways. J. Appl. Physiol. 1994;76:339–344. doi: 10.1152/jappl.1994.76.1.339. [DOI] [PubMed] [Google Scholar]


