Abstract
In primary unpassaged rat brain capillary endothelial cell cultures (RBECs), using reverse-transcriptase PCR with primers specific for P2Y receptor subtypes, we detected mRNA for P2Y2, P2Y4 and P2Y6, but not P2Y1 receptors.
None of the various nucleotides tested reduced forskolin elevated cyclic AMP levels in RBECs. ATP and ATPγS, as well as adenosine, enhanced cyclic AMP accumulation in the presence of forskolin.
Comparison of the concentration response curves to ATPγS with those for ATP and adenosine, at different incubation times, indicated that the response to purine nucleotides was not wholly dependent on conversion to adenosine. Adenosine deaminase abolished the response to adenosine but only reduced the response to ATP by about 50%. These results suggest the participation of a receptor responsive to nucleotides.
Isobutylmethylxanthine and 8-sulphophenyltheophylline prevented the cyclic AMP response, while neither 8-cyclopentyl-1,3-dipropylxanthine nor SCH58261 were effective antagonists. 2-chloradenosine gave a robust response, but neither 2-chloro-N6-cyclopentyladenosine nor CGS 21680 were agonists.
These results show that adenosine and ATP can elevate the cyclic AMP levels of brain endothelial cells by acting on receptors which have a pharmacology apparently distinct from known P2Y and adenosine receptors.
Keywords: P2Y receptors, purinoceptors, brain endothelial cells, cyclic AMP
Introduction
Extracellular nucleotides such as ATP, ADP, UTP and UDP exert diverse effects on cellular function by acting on two subfamilies of P2 receptors, the intrinsic ion channel P2X receptors and the G protein-coupled P2Y receptors (Fredholm et al., 1997). The mammalian P2Y receptor subfamily currently comprises five cloned members (P2Y1, P2Y2, P2Y4, P2Y6 and P2Y1). One of the challenges of this emerging pharmacology is to relate the mechanism of action of individual members to the functional responses of cells through an understanding of the second messenger systems recruited. All of these five receptors have been described as coupled to phosphoinositide-specific phospholipase C (PLC) (Harden et al., 1995; Boarder & Hourani, 1998), and much of their action at different cellular sites has been interpreted as downstream of PLC. The reports of cloned receptors have revealed little evidence for direct regulation of cyclic AMP, with the exception of the P2Y11 receptor which has been shown to couple to stimulation of adenylyl cyclase as well as PLC (Communi et al., 1997). However, there are various reports of agonists at P2Y receptors in native systems eliciting a change in cyclic AMP. Best established is inhibition of cyclic AMP synthesis by P2 receptors in platelets (Hourani & Hall, 1994), hepatocyes (Tomura et al., 1992), C6 glioma cells (Schachter et al., 1996), Schwann cells (Berti-Mattera et al., 1996) and a clonal brain endothelial cell (Webb et al., 1996; Hechler et al., 1998a,Hechler et al., 1998b). The molecular nature of the negative cyclase-coupled receptors is not securely defined. In addition, native systems provide several examples of stimulation of P2Y receptors which result in sensitization of cyclic AMP responses to either other agonists acting at Gs-coupled receptors or to forskolin. Such sensitization may be direct or it may be downstream of PLC and the activation of protein kinase C (Boarder et al., 1988). Following a report of sensitization by extracellular ATP in microvascular endothelial cells (Allsup & Boarder, 1991) evidence was presented which was consistent with a direct sensitization of adenylyl cyclase by P2 receptors (Johnson et al., 1991). This was followed by a reported synergistic action by ATP with forskolin in aortic endothelial cells (Cote et al., 1993).
Regulation of [Ca2+]i and cyclic AMP are central to the control of brain microvascular permeability (Rubin et al., 1991; Abbott & Revest, 1991). Brain capillary endothelial cells form a regulated blood-brain-barrier under the influence of diffusable mediators from astrocytes. The nature and mechanisms of action of these mediators are unclear, but candidates include the nucleotides. In 1991 it was shown that ATP increases [Ca2+]i in rat brain endothelial cells (Revest et al., 1991). Subsequent studies showed that some combination of P2X, P2Y2 and P2Y1-like receptors could be found on the cultured cells (e.g. Nobles et al., 1995). The P2Y1-like receptor was unusual in that it lead to an increase in [Ca2+]i by mobilization of intracellular stores in the apparent absence of a PLC response (Vigne et al., 1994; Albert et al., 1997). In a clonal cell line derived from RBECs (B10) it has also been reported (Webb et al., 1996) that a P2Y receptor is coupled to the inhibition of adenylyl cyclase. We found no evidence of inhibition of adenylyl cyclase by P2Y receptors, but did report a substantial increase in accumulation of cyclic AMP in response to ATP, in the presence of a concentration of forskolin which produced only a small effect on its own (Albert et al., 1997). This led to the studies in the present report investigating the possibility that P2Y receptors can sensitize the adenylyl cyclase response to forskolin in native RBEC cultures.
Methods
Cell culture
The rat brain capillary endothelial cells (RBECs) were prepared and cultured as described in Albert et al. (1997). Briefly, the cell culture was grown out from a purified preparation of rat brain cortical capillaries plated directly into 96-well plates or T25 flasks. The culture medium was MEM-D-Val with 20% foetal calf serum, penicillin (100 iu ml−1), streptomycin (100 μg ml−1), glutamine (27 mg ml−1) and 75 μg ml−1 endothelial cell growth supplement (Sigma E-2759). The preparation was an almost completely homogenous culture of microvascular endothelial cells exhibiting a diffuse immunoreactivity for factor VIII. Cells were used at confluence in 96-well plates for the cyclic AMP assay and mRNA was extracted from confluent T25 flasks for the RT–PCR.
Cyclic AMP assay
For the cyclic AMP assay 96-well multiwells were transferred to a 37°C water bath, growth medium aspirated, and replaced with 50 μl balanced salt solution (BSS, composition in (mM): NaCl 125, KCl 5.4, NaHCO3 16.2, HEPES 30, NaH2PO4 1, MgSO4 0.8, CaCl2 1.8, glucose 5.5, pH 7.4). After 30 min a further 50 μl of BSS was added containing, where appropriate, a 2 fold final concentration of antagonists or inhibitors, followed 10 min later with 20 μl of 6 fold final concentration of agonists and forskolin. The incubation was stopped, after 5 min except where indicated otherwise, by addition of 40 μl of 2 M trichloroacetic acid. Cyclic AMP was measured in these trichloroacetic acid extracts after neutralization with ether or freon:octylamine extraction and addition of NaHCO3, by use of the protein binding assay (Brown et al., 1971).
Reverse transcriptase–polymerase chain reaction (RT–PCR)
The RT–PCR for P2Y1, P2Y2, P2Y4 and P2Y6 mRNA were carried out exactly as described earlier (Harper et al., 1998). Each run included a positive control to which was added 5 ng of plasmid DNA containing the whole coding region of the appropriate P2Y receptor. To check for contamination with genomic DNA each run also included first strand synthesis in the absence of reverse transcriptase.
Statistical analysis
Statistical analyses were with Graph-Pad Prism2 (Graphpad Software Inc., San Diego, CA, U.S.A.).
Materials
Cell culture supplies were from GIBCO (Paisley, Scotland). [3H]-Cyclic AMP was from Amersham, Bucks, U.K. 2MeSATP, CCPA and CGS 21680 were from Research Biochemicals (Semat, Herts, U.K.) SCH 58261 was a kind gift of Scherring and Ro-20 1724 was a kind gift of Roche, Welwyn, Bucks, U.K. Other chemicals were from Fischer Scientific (Loughborough, U.K.) or Sigma (Poole, Dorset, U.K.).
Results
RT–PCR of RBECs
To understand the potential for P2 nucleotide receptor regulation of cyclic AMP in RBECs by the four P2Y receptor subtypes for which sequences were available for the rat, RNA was extracted from 25 cm2 flasks of unpassaged brain endothelial cells, cDNA synthesized and used as template for PCR employing primers designed to specifically amplify regions encoding for rat P2Y1, P2Y2, P2Y4 and P2Y6 receptors. In each case amplification of the brain endothelial cell cDNA template was in parallel with amplification of the cognate receptor DNA sequence in a plasmid containing the whole coding region of each receptor. This provided a positive control for the amplification procedure. Amplification products were detected for P2Y2, P2Y4 and P2Y6 (Figure 1) upon RT–PCR from RNA preparations from three separate cell preparations. These products were cloned and sequenced to confirm their identity. Amplification of the P2Y1 receptor mRNA was not detected.
Figure 1.
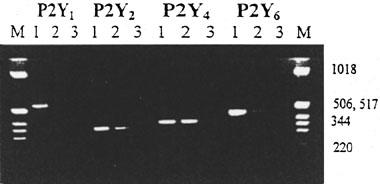
Agarose gel electrophoresis of RT–PCR amplification products from RBECs. Size markers (M) are 1 Kb ladder (Gibco BRL); approximate sizes are indicated. The receptors corresponding to the specific primers used in each amplification are indicated at the top. For each primer set lane 1 is a PCR from 5 ng of plasmid DNA containing the whole coding region of the appropriate P2Y receptor as a positive control, and lanes 2 and 3 used the RBEC cDNA as template in the presence (lane 2) and absence (lane 3) of reverse transcriptase respectively. The Figure is representative of three independent experiments on separate cell preparations.
Purinergic regulation of cyclic AMP in RBECs: effect of forskolin and phosphodiesterase inhibitors
In initial experiments studying cyclic AMP responses to purinergic agonists in RBECs cells were preincubated with IBMX (10 min) followed by 5 min stimulation with nucleotides in the presence of IBMX. Under these conditions the nucleotides had no effect on the levels of cyclic AMP, although forskolin in the presence of IBMX gave a very large increase in cyclic AMP levels (Table 1A). This forskolin and IBMX elevated cyclic AMP accumulation was not affected by 300 μM ATP (not shown). The nucleotides had no effect in the absence of forskolin and IBMX (Table 1A and Figure 2).
Table 1.
Cyclic AMP in RBECs: forskolin and PDE inhibitors
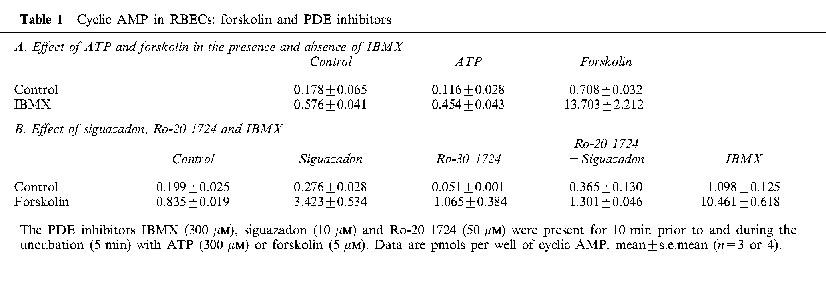
Figure 2.
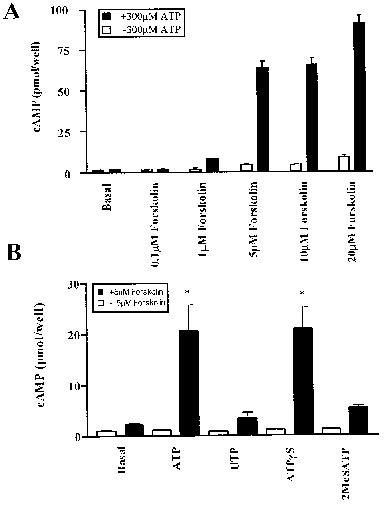
Cyclic AMP levels in RBECs in the presence of forskolin and nucleotide agonists. (A) The effect of increasing concentrations of forskolin in the absence and presence of 300 μM ATP with 5 min incubation times. (B) The effect of agonists at 300 μM in the presence and absence of forskolin at 5 μM with 5 min incubation times. *Significantly different from forskolin alone by ANOVA and Dunnett's post-test. Data are mean±s.e.mean, n=4, in each case.
To see whether the lack of effect of nucleotides on cyclic AMP was related to the presence of IBMX in some of the experiments described above, we sought conditions of phosphodiesterase (PDE) inhibition in the absence of a methylxanthine inhibitor. We tried the PDE 3 inhibitor siguazadon and the PDE 4 inhibitor Ro-20 1724. Siguazadon at 10 μM and Ro-20 1724 at 50 μM, either alone or together, did not lead to the substantial elevation of cyclic AMP levels by forskolin seen with IBMX (Table 1B). The combination of siguazadon and Ro-20 1724 therefore failed to provide suitable conditions for investigating regulation of cyclic AMP synthesis.
We then asked whether nucleotides could reduce forskolin elevated cyclic AMP levels in the absence of PDE inhibition. Figure 2A shows the effect of ATP on the response to increasing concentrations of forskolin. In the absence of PDE inhibition there was a modest increase in response to forskolin. Rather than attenuating this, the presence of ATP led to an unexpected and very substantial potentiation of the response to forskolin at levels of 5 μM and above. This stimulation of cyclic AMP levels by ATP did not occur at 0.1 μM forskolin, and was modest at 1 μM forskolin. Cells stimulated with 300 μM ATP in the presence of 5 μM forskolin for different periods of time showed a clear increase after 1 min, with the accumulation of cyclic AMP increasing in an approximately linear manner for up to 15 min (not shown). Subsequent experiments were with 5 min incubations except where indicated otherwise.
The effect of ATP, UTP, ATPγS and 2MeSATP on cyclic AMP levels in the presence and absence of forskolin are shown in Figure 2B. Responses to 300 μM UTP or 300 μM 2MeSATP in the presence of 5 μM forskolin were small compared to those of 300 μM ATP or ATPγS. All of these agonists elicit Ca2+ responses at these concentrations in these cells (Albert et al., 1997). UDP also failed to give a substantial stimulation in the presence of 5 μM forskolin (data not shown).
Figure 3 shows the effect of increasing concentrations of ATP, UTP and α,β-methylene ATP. Typically the response to ATP shows signs of plateau between 100 and 300 μM (three out of four in this group of experiments). When the results from this series of experiments were pooled, and calculated on the assumption that the response at 300 μM was the maximum tissue response to ATP, then the apparent −log EC50 for ATP was 4.02±0.66 (n=4). From Figure 3 it can also be seen that there was no substantial response to UTP or α,β-methylene ATP at any of the concentrations tested.
Figure 3.
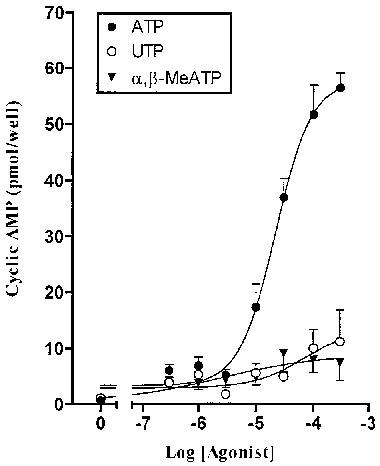
Cyclic AMP levels in RBECs in the presence of 5 μM forskolin stimulated with increasing concentrations of ATP, UTP or α,β-methylene ATP. Data are mean±s.e.mean from a single experiment in quadruplicate representative of several similar experiments. Data pooled across experiments are presented in the text.
Adenosine gave similar responses to those of ATP and ATPγS in the presence of 5 μM forskolin (Figure 4). Pooled data of paired experiments showed that the responses to 5 min stimulations with 300 μM adenosine or ATPγS were 138±25 and 139±51%, respectively, of that of ATP (no significant difference by paired t-test). The apparent EC50 values for the three agonists were also very similar. This is shown in Figure 4 B,D,F. Apparent −log EC50 values pooled across experiments were 4.26±0.71 (n=4) for ATPγS, and 4.49±0.24 (n=4) for adenosine.
Figure 4.
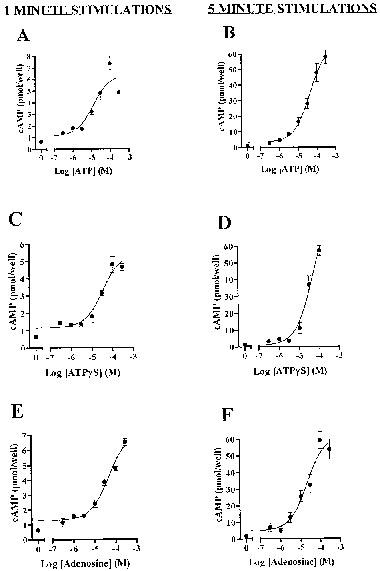
Accumulation of cyclic AMP in RBECs in response to 1 min or 5 min stimulation with agonists in the presence of 5 μM forskolin. Data are mean±s.e.mean from a single experiment in quadruplicate. Data pooled across experiments are presented in the text.
Response to ATP is not exclusively dependent on conversion to adenosine
If the responses to ATP and ATPγS were due solely to breakdown to adenosine we might expect that with shorter incubation times the response to ATPγS would be smaller than that of ATP, and that the size of the responses to ATP and ATPγS would be reduced compared to adenosine. Figure 4 shows a series of experiments in which the responses to the three agonists were compared with 1 min (left panels) and 5 min (right panels) incubation times. In each case the size of the response at 1 min was smaller than that at 5 min, but in each case the size of response at 1 min was the same for all three agonists. There was no change in EC50 resulting from the difference in incubation times.
To further address the question of whether the response to ATP was mediated by conversion to adenosine we used adenosine deaminase to remove adenosine formed during the incubation. The response to 300 μM adenosine was completely eliminated in the presence of 2 units ml−1 of adenosine deaminase (data not shown). Over four experiments the response to 300 μM ATP was reduced to 59.2±11.0% by adenosine deaminase. This is illustrated in Figure 5, which also shows the antagonism by 8-sulphophenyltheophylline (8-SPT) of the response to ATP and of the residual response to ATP in the presence of adenosine deaminase. Both show effectively complete antagonism by 300 μM 8-SPT, with an IC50 similar to that which we see with adenosine, of 10–30 μM.
Figure 5.
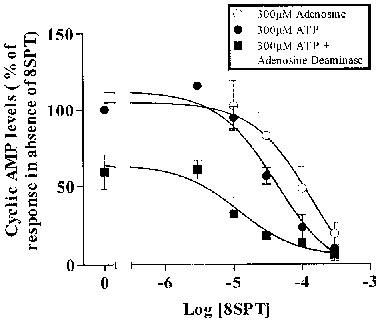
Effect of 8-SPT and adenosine deaminase on the stimulation of cyclic AMP by ATP (300 μM) or adenosine (300 μM) in the presence of 5 μM forskolin. ATP adenosine and ATP plus 2 units ml−1 adenosine deaminase. Data are mean±s.e.mean (n=4).
Effects of adenosine receptor agonists and antagonists
We undertook further studies with agonists and antagonists known to act at adenosine receptors, not only to characterize the response to adenosine but also to see if the pharmacology of the response to ATP was divergent from that of responses to adenosine. Figure 6 shows the effect of stimulation with 2-chloradenosine (agonist at A1, A2A and A2B receptors), the A1 selective agonist CCPA and the A2A selective agonist CGS 21680. While 2-chloradenosine gave a robust response, neither CCPA nor CGS 21680, in a range of concentrations at which they are effective agonists at A1 and A2A receptors, gave any response to all. The non-selective agonist NECA also elicited a response, of similar magnitude to that of ATP, which was maximal at 10 μM (not shown).
Figure 6.
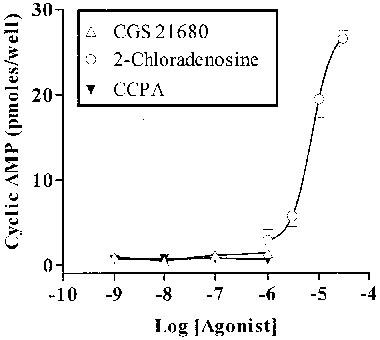
Effect of three adenosine receptor agonists on cyclic AMP accumulation in RBECs in the presence of 5 μM forskolin. CCPA, CGS 21680 and 2-chloradenosine. Data are mean±s.e.mean from a single experiment in quadruplicate (representative of several similar experiments).
We investigated the effect of a series of antagonists present for 10 min before and during the incubation with adenosine or ATP (both at 300 μM). The non-selective antagonists IBMX (300 μM) and 8-SPT (Figure 5) were effective at blocking the responses to both adenosine and ATP, as described above. However, the presence of the antagonist DPCPX and the A2A selective antagonist SCH 58261, each at concentrations up to 1 μM, had no effect on the responses to stimulation with 300 μM adenosine or ATP (not shown).
Effect of cyclo-oxygenase inhibition
To determine whether the stimulation of cyclic AMP levels was secondary to the formation of cyclo-oxygenase products such as prostaglandins we stimulated the cells in the presence of 1 μM indomethacin. Results from a representative experiment are shown in Table 2. ATP, ATPγS and adenosine gave substantial stimulations of cyclic AMP accumulation in the presence of 5 μM forskolin. In each case this was unaffected by the presence of indomethacin. Pooled over three separate experiments (each in quadruplicate) with different cell preparations the stimulation by ATP (300 μM) plus forskolin (5 μM) in the presence of 1 μM indomethacin was 96.5±8.4% of the stimulation in the absence of indomethacin.
Table 2.
Effect of indomethathacin on ATP, ATPγS and adenosine stimulation of cyclic AMP accumulation

Discussion
In brain capillary endothelial cells regulation of cyclic AMP level by nucleotides is complicated by the existence of multiple P2 receptors, and by the use of different types of brain capillary cell cultures in different laboratories. Webb et al. (1996), using a clonal cell line (B10) of rat brain capillary endothelial cells, describe an inhibition of cyclic AMP on stimulation of a P2Y receptor. In the unpassaged brain capillary endothelial cultures (RBECs) we have previously reported that 2MeSATP does not reduce cyclic AMP levels which have been raised by forskolin (Albert et al., 1997). This implies that the inhibition of cyclic AMP synthesis by P2Y1 receptors seen in transfected 1321N1 cells (our unpublished data) does not occur in RBEC cultures. The RT–PCR work described here reinforces this view. There was no detectable expression of mRNA for P2Y1 receptors, while sequences encoding P2Y2, P2Y4 and P2Y6 receptors were easily detected. We conclude from this that P2Y1 receptors do not regulate unpassaged cells, and in particular they do not regulate cyclic AMP in these cells, and that the [Ca2+]i response to 2MeSATP and ADP seen in the absence of a detectable phosphoinositide response in these unpassaged cell cultures (Albert et al., 1997) is not at a P2Y1 receptor.
The presence of mRNA encoding P2Y4 and P2Y6 receptors indicate a potential for P2Y mediated, forskolin-dependent, stimulations of cyclic AMP. However, the elevations of cyclic AMP by ATP seen here do not have the characteristics consistent with the currently cloned P2Y receptors. Firstly, stimulation cannot be seen in the presence of IBMX. Secondly, 8-SPT is an effective antagonist. This excludes, not only the P2Y1, P2Y2, P2Y4 and P2Y6 receptors described above, but also the P2Y11 receptor, which appears to be a candidate for an ATP receptor coupled to adenylyl cyclase, since 8-SPT is not an antagonist at P2Y11 receptors (Communi et al., 1997). The failure of either UTP or UDP to stimulate the cyclic AMP response seen with ATP is another indication that neither P2Y2, P2Y4 or P2Y6 are involved. For this reason no further work with antagonists directed at P2 receptors was undertaken.
The observation that both ATP and adenosine are able to stimulate cyclic AMP accumulation raises the possibility that ATP is acting by conversion to adenosine and subsequent stimulation of Gs-coupled A2 receptors. We report here various observations which make this unlikely. Both adenosine and ATPγS had the same potency as ATP. This was true for both 1 min and 5 min stimulations. If the rate of conversion of ATP to adenosine was sufficiently rapid as to be complete in less than 1 min of incubation, then ATP could have the same potency even if it had no action of its own. However, this cannot be true of the ectonucleotide resistant analogue ATPγS, even taking into account that it is not completely resistant to breakdown and that it is not completely pure. Impurities (e.g. ATP/ADP) would have to account for the majority of the compound if this were the explanation. The comparisons of concentration response curve for these three agonists at 1 min and 5 min (Figure 4) are inconsistent with the hypothesis that stimulation by ATPγS is dependent on breakdown to adenosine. The data indicate the presence of a receptor which responds to ATPγS itself, and not just one which responds to adenosine.
The hypothesis that the action of ATP is dependent on conversion to adenosine was further explored with the adenosine deaminase experiments. Removal of adenosine in the bulk phase was complete, as indicated by the elimination of responses to added adenosine, but there was only a partial loss of the response to added ATP. Two hypotheses must be considered. Firstly, that there is a biophase at the cell surface where adenosine formed from ATP reaches a sufficient concentration locally to stimulate adenosine receptors before it is metabolized by adenosine deaminase. This is possible, even though we are working with cell monolayers. Secondly, that ATP is partially converted to adenosine, and that both ATP and adenosine act with forskolin to stimulate the accumulation of cyclic AMP. The conversion of ATP to adenosine then only results in a loss of response when the adenosine is removed by adenosine deaminase.
If both adenosine and ATP act at cell surface receptors to stimulate cyclic AMP then the pharmacology of the adenosine portion of the stimulation does not clearly indicate one of the described adenosine receptors (Alexander & Peters, 1998). A1 receptors are described as Gi/o coupled to inhibition of adenylyl cyclase, and are also excluded because CCPA does not act as an agonist and DPCPX does not act as an antagonist. A2 receptors are described as coupled to the stimulation of adenylyl cyclase via Gs. A2A receptors are excluded because the selective agonist CGS 21680 does not elicit a response and the responses to ATP and adenosine are not antagonized by SCH58261. Neither is the response blocked by DPCPX. This is an A2B antagonist with a pA2 of around seven at cloned and transfected A2B receptors (Alexander et al., 1996), so under conditions used in these studies DPCPX would be expected to be an effective antagonist at A2B receptors. However, these data do not completely exclude a contribution from A2B receptors.
We conclude that the stimulation by ATP of cyclic AMP levels in RBECs is not at known P2Y receptors, and neither is it likely to be completely dependent on conversion to adenosine and action at known P1 receptors. The answer could lie in the form of separate receptors for adenosine and ATP, although the results here are inconsistent with a contribution from known P2 receptors. If two receptors are responsible, there is the potential for complex interactions between the receptors and their signalling pathways in the generation of the data reported here. An alternative explanation is that the response is at a common receptor for ATP and adenosine, as proposed for presynaptic regulation of noradrenaline release from peripheral nerve terminals (Shinozuka et al., 1988; Kurz et al., 1993; Smith et al., 1997) and stimulation of adenylyl cyclase in NG108-15 cells (Matsuoka et al., 1995).
Conigrave et al. (1998) have recently reported evidence for a novel cyclic AMP-linked P2 receptor which responds to ATP but not UTP. However, this response appears distinct from that described in the present paper in several respects. For example, adenosine is a weak agonist, and the response is enhanced by IBMX and insensitive to 8-SPT. A better fit with the present series of observations is the forskolin enhanced cyclic AMP response to ATP of bovine aortic endothelial cells (Cote et al., 1993), where adenosine is an effective agonist, 2MeSATP and UTP are ineffective, and ATPγS gives the same response as ATP. This implies that an atypical cyclic AMP elevating response to ATP may occur in diverse endothelial cells.
With respect to the mechanisms by which cyclic AMP is elevated in RBECs on stimulation with ATP, the most likely explanation is augmentation of a Gs coupled response by forskolin similar to that reported earlier (Cote et al., 1993, and references therein). An effect via elevated [Ca2+]i is unlikely, since both UTP and 2MeSATP elevate [Ca2+]i but do not effect cyclic AMP. A further possibility is that phospholipase A2 is activated by these agonists, leading to the prostaglandin-dependent stimulation of cyclic AMP. Such a mechanism for nucleotide receptor regulation of cyclic AMP has been described by Post et al. (1996), who show that indomethacin can block this paracrine pathway for stimulation of adenylyl cyclase. The responses to ATP and adenosine in the brain capillary endothelial cells are seen in the presence of indomethacin, indicating that this is not the pathway for stimulation of cyclic AMP accumulation in these experiments.
In this paper we have shown that the cyclic AMP levels of rat brain capillary endothelial cells, maintained in culture without passage, are regulated by extracellular adenosine and ATP. ATP probably acts in part by conversion to adenosine, but experiments with ATPγS provide evidence that a receptor for nucleotides also contributes to these results. Both agonists act on receptors which have a pharmacology which is distinct from those previously cloned and characterized. The nature of the receptors responsible will be the subject of further investigations. In situ these cells form the blood-brain-barrier by a process maintained by adjacent astrocytes and involving the regulation of cyclic AMP levels. It has previously been shown that the 1321N1 astrocytoma cell line releases ATP in a regulated manner into the medium (Lazarowski et al., 1995). The results presented here provide further information on the way in which nucleotides released by astrocytes could contribute to the regulation of the blood-brain-barrier. A further understanding of these events will require clarification of the receptors involved and study of how the cyclic AMP cascade interacts with other cell signalling pathways, such as the p42 and p44 mitogen activated protein kinases, which are also regulated by P2Y receptors in these endothelial cells (Albert et al., 1997).
Acknowledgments
We thank The Wellcome Trust and The Medical Research Council for financial support.
Abbreviations
- ATPγS
adenosine 5′-O-(3-thiotriphosphate)
- [Ca2+]i
cytosolic Ca2+
- CCPA
2-chloro-N6-cyclopentyladenosine
- DPCPX
8-cyclopentyl-1,3-dipropylxanthine
- IBMX
isobutylmethylxanthine
- 2MeSATP
2-methylthio ATP
- PDE
phosphodiesterase
- PLC
phospholipase C
- RBECs
rat brain capillary endothelial cell cultures
- RT–PCR
reverse transcriptase-polymerase chain reaction
- 8-SPT
8-sulphophenyltheophylline
References
- ABBOTT N.J., REVEST P.A. Control of brain endothelial permeability. Cerebrovasc. Brain. Metab. Rev. 1991;31:1–34. [PubMed] [Google Scholar]
- ALBERT J.L., BOYLE J.P., ROBERTS J.A., CHALLISS R.A.J.C., GUBBY S.E., BOARDER M.R. Regulation of brain capillary endothelial cells by P2Y receptors coupled to Ca2+, phospholipase C and mitogen activated protein kinase. Br. J. Pharmacol. 1997;122:935–941. doi: 10.1038/sj.bjp.0701453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ALEXANDER S.P.H., COOPER J., SHINE J., HILL S. Characterisation of the human brain putative A2B adenosine receptor expressed in Chinese hamster ovary (CHO.A2B4) cells. Br. J. Pharmacol. 1996;119:1286–1290. doi: 10.1111/j.1476-5381.1996.tb16035.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ALEXANDER S.P.H., PETERS J.A. 1998 Receptor & Ion Channel Nomenclature Supplement. Trends Pharmacol. Sci. 1998. pp. 1–98.
- ALLSUP D.J., BOARDER M.R. Comparison of P2 purinergic receptors of aortic endothelial cells with those of adrenal medulla: evidence for heterogeneity of receptor subtype and of inositol phosphate response. Mol. Pharmacol. 1991;38:84–91. [PubMed] [Google Scholar]
- BERTI-MATTERA L.N., WILKINS P.L., MADHUM Z., SUCHOVSKY D. P2-purinergic receptors regulate phospholipase C and adenylyl cyclase activities in immortalised Schwann cells. Biochem. J. 1996;314:555–561. doi: 10.1042/bj3140555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BOARDER M.R., HOURANI S.M.O. The regulation of vascular function by P2 receptors: multiple sites and multiple receptors. Trends Pharmacol. Sci. 1998;19:99–107. doi: 10.1016/s0165-6147(98)01170-5. [DOI] [PubMed] [Google Scholar]
- BOARDER M.R., PLEVIN R., MARRIOTT D.B. Angiotensin II potentiates prostaglandin stimulation of cyclic AMP levels in intact bovine adrenal medulla cells but not adenylate cyclase in permeabilised cells. J. Biol. Chem. 1988;263:11273–11284. [PubMed] [Google Scholar]
- BROWN B.L., ALBANO J.D., EKINS R.P., SGHERZI A.M., TAMPION J.W. A simple and sensitive saturation assay method for the measurement of adenosine 3′,5′-cyclic monophosphate. Biochem. J. 1971;121:561–562. doi: 10.1042/bj1210561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- COMMUNI D., GOVAERTS C., PARMEMTIER M., BOYNAEMS J. Cloning of a human purinergic P2Y receptor coupled to phospholipase C and adenylyl cyclase. J. Biol. Chem. 1997;272:31969–31973. doi: 10.1074/jbc.272.51.31969. [DOI] [PubMed] [Google Scholar]
- CONIGRAVE A.D., LEE J.Y., VAN DER WEYDEN L., JIANG L., WARD P., TASEVSKI V., LUTTRELL B.M., MORRIS M.B. Pharmacological profile of a novel cyclic AMP-linked P2 receptor on undifferentiated HL-60 leukemia cells. Br. J. Pharmacol. 1998;124:1580–1585. doi: 10.1038/sj.bjp.0701985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- COTE S., VAN SANDE J., BOEYNAEMS J.-M. Enhancement of endothelial cAMP accumulation by adenine nucleotides: role of methylxanthine-sensitive sites. Am. J. Physiol. 1993;264:H1498–H1503. doi: 10.1152/ajpheart.1993.264.5.H1498. [DOI] [PubMed] [Google Scholar]
- FREDHOLM B.B., ABBRACCHIO M.P., BURNSTOCK G., DUBYAK G.R., HARDEN T.K., JACOBSEN K.A., SCHWABE U., WILLIAMS M. Towards a revised nomenclature of P1 and P2 receptors. Trends Pharmacol. Sci. 1997;18:79–82. doi: 10.1016/s0165-6147(96)01038-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HARDEN T.K., BOYER J.L., NICHOLAS R.A. P2-purinergic receptors; subtype associated signalling responses and structure. Annu. Rev. Pharmacol. Toxicol. 1995;35:541–579. doi: 10.1146/annurev.pa.35.040195.002545. [DOI] [PubMed] [Google Scholar]
- HARPER S., WEBB T.E., CHARLTON S.J., NG L.L., BOARDER M.R. Evidence that P2Y4 nucleotide receptors are involved in the regulation of rat aortic smooth muscle cells by UTP and ATP. Br. J. Pharmacol. 1998;124:703–710. doi: 10.1038/sj.bjp.0701895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HECHLER B., LEON C., VIAL C., VIGNE P., FRELIN C., CAZENAVE J.P., GACHET C. The P2Y1 receptor is necessary for adenosine 5′-diphosphate-induced platelet aggregation. Blood. 1998a;92:152–159. [PubMed] [Google Scholar]
- HECHLER B., VIGNE P., LEON C., BREITTMAYER J.-P., GACHET C., FRELIN C. ATP derivatives are antagonists of the P2Y1 receptor: similarities to the platelet ADP receptor. Mol. Pharmacol. 1998b;53:727–733. [PubMed] [Google Scholar]
- HOURANI S.M.O., HALL D.A. Receptors for ADP on human blood platelets. Trends Pharmacol. Sci. 1994;15:103–108. doi: 10.1016/0165-6147(94)90045-0. [DOI] [PubMed] [Google Scholar]
- JOHNSON J.A., FRIEDMAN J., HALLIGAN R.D., BIRNMAUMER M., CLARK R.B. Sensitization of adenylyl cyclase by P2 purinergic and M5 muscarinic receptor agonists in L cells. Mol. Pharmacol. 1991;39:539–546. [PubMed] [Google Scholar]
- KURZ K., VON KUGELGEN I., STARKE K. Prejunctional modulation of release in mouse and rat vas deferens: contribution of P1 and P2 receptors. Br. J. Pharmacol. 1993;110:1465–1472. doi: 10.1111/j.1476-5381.1993.tb13986.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LAZAROWSKI E.R., WATT W.C., STUTTS M.J., BOUCHER R.C., HARDEN T.K. Pharmacological selectivity of the cloned human P2U-purinoceptor: potent activation by diadenosine tetraphosphate. Br. J. Pharmacol. 1995;116:1619–1627. doi: 10.1111/j.1476-5381.1995.tb16382.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MATSUOKA I., ZHOU Q., ISHIMOTO H., NAKINISHI H. Extracellular ATP stimulates adenylyl cyclases and phospholipase C through distinct purinoceptors in NG108-15 cells. Mol. Pharmacol. 1995;47:855–862. [PubMed] [Google Scholar]
- NOBLES M., REVEST P.A., COURAUD P.-O., ABBOTT N.J. Characterisation of nucleotide receptors that cause elevation of cytoplasmic calcium in immortalised rat brain endothelial cells (RBE4) and in primary cultures. Br. J. Pharmacol. 1995;115:1245–1252. doi: 10.1111/j.1476-5381.1995.tb15032.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- POST S.R., JACOBSEN J.P., INSEL P.A. P2 purinergic agonists enhance cyclic AMP production in Madin-Darby canine kidney epithelial cells via an autocrine/paracrine mechanism. J. Biol. Chem. 1996;271:2029–2032. doi: 10.1074/jbc.271.4.2029. [DOI] [PubMed] [Google Scholar]
- REVEST P.A., ABBOTT N.J., GILLESPIE J.I. Receptor mediated changes in intracellular Ca2+ in cultured rat brain endothelial cells. Brain Res. 1991;549:159–161. doi: 10.1016/0006-8993(91)90614-2. [DOI] [PubMed] [Google Scholar]
- RUBIN L.L, , HALL D.E., PORTER S., BARBU K., CANNON C., HORNER H.C., JANATPOUR M., LIAW C.W., MANNING K., MORALES J., TANNER L.I., TOMASELLI K.J., BARD F. A cell culture model of the blood-brain-barrier. J. Cell Biol. 1991;115:1725–1735. doi: 10.1083/jcb.115.6.1725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SCHACHTER J.B., LI Q., BOYER J.L., NICHOLAS R.A., HARDEN T.K. Second messenger cascade specificity and pharmacological selectivity of the human P2Y1 purinoceptor. Br. J. Pharmacol. 1996;118:167–173. doi: 10.1111/j.1476-5381.1996.tb15381.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SHINOZUKA K., BJUR R.A., WESTFALL D.P. Characterisation of prejunctional purinoceptors on adrenergic nerves of the rat caudal artery. N.S. Archives Pharmacol. 1988;338:221–227. doi: 10.1007/BF00173391. [DOI] [PubMed] [Google Scholar]
- SMITH A.D., CHEEK D.J., BUXTON I.L.O., WESTFALL D.P. Competition of adenine nucleotides for a 1,3-[3H]-dipropyl-8-cyclopentylxanthine binding site in rat vas deferens. Clin. Exper. Pharm. Physiol. 1997;B24B:492–497. doi: 10.1111/j.1440-1681.1997.tb01233.x. [DOI] [PubMed] [Google Scholar]
- TOMURA H., OKAJIMA F., KONDO Y. Discrimination between two types of P2 purinoceptors by suramin in rat hepatocytes. Eur. J. Pharmacol. 1992;226:363–365. doi: 10.1016/0922-4106(92)90054-y. [DOI] [PubMed] [Google Scholar]
- VIGNE P., FEOLDE E., BREITTMAYER J.P., FRELIN C. Characterisation of the effects of 2-methylthio-ATP and 2-chloro-ATP on brain capillary endothelial cells. Similarities to ADP and differences from ATP. Br. J. Pharmacol. 1994;112:775–780. doi: 10.1111/j.1476-5381.1994.tb13146.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WEBB T.E., FEOLDE E., VIGNE P., NEARY J.T., RUNBERG A., FRELIN C., BARNARD E.A. The P2Y purinoceptor in rat brain microvascular endothelial cells couple to inhibition of adenylate cyclase. Br. J. Pharmacol. 1996;119:1385–1392. doi: 10.1111/j.1476-5381.1996.tb16050.x. [DOI] [PMC free article] [PubMed] [Google Scholar]


