Abstract
To investigate the effect of moderate hypoxia alone or combined with an inflammatory reaction or after 3-methylcholanthrene (3MC) pre-treatment on cytochrome P450 (P450), conscious rabbits were exposed for 24 h to a fractional concentration of inspired O2 of 10% (mean PaO2 of 34 mmHg). Hypoxia decreased theophylline metabolic clearance (ClM) from 1.73±0.43 to 1.48±0.13 ml min−1 kg−1 (P<0.05), and reduced (P<0.05) the formation clearance of theophylline metabolites, 3-methylxanthine (3MX), 1-methyluric acid (1MU) and 1,3-dimethyluric acid (1,3DMU). Hypoxia reduced the amount of CYP1A1 and 1A2 but increased CYP3A6 proteins.
Turpentine-induced inflammatory reaction reduced (P<0.05) the formation clearance of 3MX, 1MU, and 1,3DMU, and diminished the amount of CYP1A1, 1A2 and 3A6 proteins. However, when combined with hypoxia, inflammation partially prevented the decrease in ClM, especially by impeding the reduction of 1,3DMU. The amount of CYP1A1 and 1A2 remained reduced but the amount of CYP3A6 protein returned to normal values.
Pre-treatment with 3MC augmented the ClM by 114% (P<0.05) due to the increase in the formation clearance of 3MX, 1MU and 1,3DMU. 3MC treatment increased the amount of CYP1A1 and 1A2 proteins. Pre-treatment with 3MC prevented the hypoxia-induced decrease in amount and activity of the P450.
It is concluded that acute moderate hypoxia and an inflammatory reaction individually reduce the amount and activity of selected apoproteins of the P450. However, the combination of hypoxia and the inflammatory reaction restores P450 activity to near normal values. On the other hand, pre-treatment with 3MC prevents the hypoxia-induced depression of the P450.
Keywords: Hypoxia, inflammation; 3-methylcholanthrene; cytochrome P450; theophylline; kinetics; conscious rabbits
Introduction
Compared with healthy subjects and patients with uncomplicated asthma, in patients with chronic obstructive lung disease (COLD), pulmonary oedema, pulmonary heart disease, or congestive heart failure, the clearance of theophylline can be reduced by as much as 30–60% (Hendeles et al., 1977; Powell et al., 1978). Since patients with these disease states present hypoxia, the decrease in theophylline rate of metabolism has been associated to hypoxia (Jacobs & Senior, 1974). This assumption is supported by in vivo animal studies showing that moderate hypoxia decreases the clearance of theophylline (Letarte & du Souich, 1984).
In man, an inflammatory reaction such as an acute viral respiratory infection enhances theophylline plasma concentrations secondary to a decrease in its clearance (Chang et al., 1978); bacterial pneumonia reduces the clearance of theophylline and of antipyrine (Vozeh et al., 1978; Sonne et al., 1985), and BCG vaccination is able to decrease theophylline clearance (Gray et al., 1983). In animal models, non-infectious inflammatory reactions, such as those induced by the injection of turpentine, carrageenan or Freund's adjuvant, also diminish the rate of biotransformation of xenobiotics (Beck & Whitehouse, 1974; Parent et al., 1992). Although it is accepted that hypoxia and an inflammatory reaction individually depress the activity of the cytochrome P450 (P450) (Richer & Lam, 1993), in patients with advanced COLD, the effect of hypoxia on the P450 remains controversial (Cusack et al., 1986; du Souich et al., 1989), perhaps because the combined effect of hypoxia and an inflammatory reaction, as seen in patients with advanced COLD, on the P450 may not be as predicted from each individual condition.
The aims of the present study were to document in vivo the effect of acute moderate hypoxia alone and combined with an inflammatory reaction, a condition that down-regulates the P450, on hepatic P450, or combined with 3-methylcholanthrene pre-treatment (3MC), a condition that induces selective apoproteins of the P450. The activity of the P450 was assessed by administering theophylline to conscious rabbits and measuring the rate of formation of its metabolites. Theophylline was used as a substrate because its major metabolite, 1,3DMU, results from theophylline 8-hydroxylation catalyzed in man by several P450 apoproteins, such as CYP1A2, 2D6, 2E1, and 3A4. Minor metabolites include 3MX result of a 1-demethylation carried out by CYP1A2, and 1-methylxanthine product of a 3-demethylation catalyzed by CYP1A1 and CYP1A2, which is 8-hydroxylated by xanthine oxidase to yield 1MU (Sarkar & Jackson, 1994; Zhang & Kaminsky 1995). In addition, it was of interest to assess the repercussion of these experimental conditions on the amount of hepatic P450 apoproteins involved in the metabolism of theophylline, essentially CYP1A1 and 1A2, and marginally 3A6, but the latter being very important for the metabolism of numerous drugs.
Methods
Animals
Male New Zealand white rabbits (2.2–3.6 kg) purchased from Les Lapins Léonard Inc. (St-Vincent Mirabel, Québec, Canada) were used throughout the study. They were maintained on Purina pellets and water ad libitum for at least 1 week before any experimental work was undertaken. Before each experiment, a sterile Bardex Foley catheter (No. 8–10) was introduced into the bladder of the animals. To stabilize the physiologic parameters 1 h before the experiment started, the rabbits were placed into restraining cages (Plaslabs, Lansing, MI, U.S.A.), and a catheter (Butterfly 21, Abbott Ireland Ltd) was inserted into the central artery of an ear for blood sampling. A polyethylene tube (PE 50, Intramedic Becton, Dickinson and Company, Parsippany, NJ, U.S.A.) was introduced into a lateral vein of the ear for the infusion of a solution of 0.9% NaCl and 5% glucose (50/50) at a rate of 0.382 ml min−1 to replace basal losses through lungs and kidneys, and blood sampling. The rabbits were conscious throughout the experiment.
Experimental protocol
Effect of hypoxia on theophylline metabolism
To induce the hypoxia, the rabbits were introduced in a plexiglas chamber (0.75×1.20×1.25 m3), where a 10% fractional concentration of inspired O2 (FiO2) was adjusted with an oxygen monitor (OM-15, Sensor Medics Corp., CA, U.S.A.) connected to an electrovalve (Asco Valves, Brantford, Ontario, Canada) that allowed the access of nitrogen into the chamber which displaced the air off. The 10% FiO2 was selected to obtain an arterial partial pressure of O2 (PaO2) of approximately 35 mmHg. Humidity in the chamber was maintained at 50% by recirculating the air through a refrigerating system. The temperature was kept at 22–24°C. All the rabbits had access to Purina Laboratory Chow and water ad libitum for the 48 h that lasted the hypoxia. Control rabbits were also placed into the chamber for the experiments, but breathing room air (FiO2=21%). After 1 h of stabilization, the rabbits were left in the plexiglas chamber breathing room air (n=8) or breathing an atmosphere with a low FiO2 (n=8) for 24 h, and then received 2.5 mg kg−1 of theophylline i.v. Blood samples (1.0 ml) were withdrawn at 0, 60, 90, 120, 125, 180, 240, 300, 360, 390, 420 and 480 min after the injection to assay theophylline in plasma. Urine was collected for 24 h while the rabbits were hypoxic in the chamber, to assay theophylline and its metabolites, e.g. 3-methylxanthine (3MX), 1-methyluric acid (1MU) and 1,3-dimethyluric acid (1,3DMU). Arterial blood samples (0.5 ml) were withdrawn at 0, 120, 360 and 450 min to monitor arterial blood gases and pH with a gas analyser (IL Micro 13-03/213-05, Instrumentation Laboratory, Lexington, MA, U.S.A.).
Effect of turpentine-induced inflammatory reaction on theophylline metabolism
Control rabbits (n=8) breathing air received 2.5 ml of a solution of sterile saline s.c. in both hind legs, and 48 h later, 2.5 mg kg−1 of theophylline i.v. Blood samples (1.0 ml) were withdrawn at 0, 5, 10, 15, 25, 35, 45, 60, 90, 120, 150, 180, 240, 300, 360, 420 and 480 min to assay theophylline. Urine was collected before and for 24 h after theophylline administration to assay theophylline, 3MX, 1MU and 1,3DMU. Additional blood samples (2.5 ml) were withdrawn prior to the administration of turpentine and 48 h later when theophylline was injected to assay seromucoids. Rectal temperature was measured with an electronic thermometer (Toshiba Digital Clinical Thermometer, Toshiba Glass Co. Ltd) prior to the injection of turpentine and 48 h after. After at least 10 days, the same eight rabbits received s.c. 2.5 ml of turpentine in both hind legs, to produce a chemical abscess with an inflammatory reaction (Ashton et al., 1970); 48 h later, they received 2.5 mg kg−1 of theophylline intravenously, and the protocol described above was applied. The turpentine-induced inflammatory reaction model was used because the serum of rabbits with a turpentine-induced local inflammatory reaction affects hepatic cytochrome P450 in a similar manner as do serum of humans with an upper respiratory viral infection (El-Kadi et al., 1997).
Combined effect of hypoxia and an inflammatory reaction on theophylline metabolism
In preliminary studies, the lowest PaO2 that rabbits with an inflammatory reaction could tolerate was determined to be 55 mmHg. The rabbits (n=6) received turpentine as described above, and 24 h later, were introduced into the plexiglas chamber with a FiO2 set at 12% and 24 h later, the rabbits received 2.5 mg kg−1 of theophylline i.v. To assay theophylline and seromucoids, blood samples and urine were collected as described earlier. To monitor arterial blood gases and pH (IL-Micro 13-03 pH/Blood Gas Analyser; Instrumentation Laboratory, Lexington, MA, U.S.A.), serial blood samples (0.25 ml) were withdrawn prior to and 120, 360 and 450 min after the administration of theophylline.
Combined effect of 3MC and hypoxia on theophylline metabolism
Rabbits were segregated into four groups (7/group): the rabbits of the first and second groups received saline or corn oil s.c., rabbits of the third group received 80 mg kg−1 of 3MC suspended in corn oil s.c., and the rabbits of the fourth group received 80 mg kg−1 of 3MC suspended in corn oil s.c. and 24 h later were subjected to a 10% FiO2. All the rabbits received 2.5 mg kg−1 i.v. of theophylline 48 h after the administration of corn oil or 3MC. To assess theophylline plasma concentrations, blood samples were withdrawn prior to and at 60, 90, 120, 180, 240, 300, 360, 420 and 480 min after its administration. Urine was collected for 24 h to assay theophylline metabolites.
Assays
To assess whether the changes in P450 activity induced by hypoxia and inflammation were secondary to pre- or post-translational changes in P450, the amounts of CYP1A1, 1A2 and 3A6 proteins were quantified by Western blot analysis in microsomal proteins of rabbits exposed to a 10% FiO2 for 24 h (n=3), rabbits with an inflammatory reaction (n=3), and rabbits exposed to the combination of hypoxia and inflammation (n=3), rabbits which received 3MC (n=3) and rabbits which have been exposed to 3MC and hypoxia (n=3). Proteins were separated by polyacrylamide gel electrophoresis (7.5% polyacrylamide) under non-reducing conditions (Smith, 1994). Separated proteins were electrophoretically transferred to a nitro-cellulose membrane using a semidry transfer process (Bio-Rad, Hercules, CA, U.S.A.). CYP1A1 and 1A2 were detected with a polyclonal anti-rabbit CYP1A1 (Oxford Biochemical Research, Oxford, MI, U.S.A.), and visualized with an alkaline phosphatase conjugated secondary antibody using nitro blue tetrazolium as the substrate (Kruger, 1994). CYP3A6 was detected with a monoclonal anti-rat CYP3A1 (Oxford Biochemical Research, Oxford, MI, U.S.A.) using a chemiluminescence reagent (horseradish peroxidase enzyme) conjugated secondary antibody and visualized by autoradiography (Thorpe et al., 1985). The intensities of the bands were measured with a software Alphaimager version 3.24.
Theophylline and metabolites in plasma and urine were assayed by HPLC as described elsewhere (du Souich et al., 1989). The precision of the assay for theophylline concentrations of 3 and 16 μg ml−1 in plasma and urine was 2.1 and 2.3%; the precision for 3MX, 1MU and 1,3DMU at the concentration 16 μg ml−1 in urine was 4.8, 5.7 and 6.7%, respectively. Protein content in the microsomal preparation was determined according the method of Lowry et al. (1951). The precision of the method for an average concentration of 200 μg ml−1 of albumin was 3.4%. Seromucoids were measured as described elsewhere (Parent et al., 1992), and the precision of the assay for an average concentration of 25 mg dl−1 was 5.3%.
Drugs and chemicals
Theophylline and its metabolites, 3MX, 1MU and 1,3DMU, 3MC, nitro blue tetrazolium and other chemicals were purchased from Sigma Chemical Company (St. Louis, MS, U.S.A.). Polyacrilamide was purchased from Bio-Rad Laboratories (Hercules, CA, U.S.A.). The alkaline phosphatase secondary antibody was acquired from Oxford Biochemical Research (Oxford, MI, U.S.A.), and the horseradish peroxidase enzyme was purchased from Amersham Life Sciences (Oakville, Ontario, Canada).
Analysis of data
The area under theophylline plasma concentration-time curve (AUC0→t) from zero to the last concentration measured (Ct) was estimated by means of the trapezoidal method. The AUC0→∞ was obtained by adding to the AUC0→t the value of Ct/z, where z is theophylline rate constant of disposition estimated from the slope of the terminal phase of theophylline plasma concentrations. Predicted apparent volume of distribution of theophylline at steady state (VdSS) was estimated according to noncompartmental analysis based on statistical moment theory (Gibaldi & Perrier, 1982) with the computer program Pharmacokinetic Data Analysis Program included in Lotus 1,2,3, Version 2.2 (Lotus Development Corporation, Cambridge, MD, U.S.A.). The fraction of theophylline metabolic clearance generating each metabolite was calculated as follows:
 |
where ClS, ClR and ClM are theophylline systemic, renal and metabolic clearances, respectively. D is theophylline dose, and XU is the amount of theophylline recovered in urine. The fraction of theophylline undergoing metabolism (FTM) is defined by:
The fraction of theophylline being metabolised to 3MX (F3MXM), assuming that the metabolite is solely eliminated through the kidney, is calculated as follows:
where XU3MX is the amount of 3MX recovered in urine. The formation clearance of 3MX or the fraction of theophylline metabolic clearance yielding 3MX (ClM3MX) is calculated using the following equation:
Similar equations were used to calculate the formation clearance of 1MU and 1,3DMU. For these calculations, the values of plasma concentrations and urinary amounts were expressed in molar concentrations.
Comparison of the results obtained under the various experimental conditions with those in the control group was carried out using a one way analysis of variance (ANOVA) followed by Newman-Keuls post hoc test (Winer, 1971). The minimal level of significance was P<0.05. All results are presented as mean±standard error (s.e.).
Results
Effect of hypoxia on P450 activity and apoproteins
Exposure of the rabbits to a 10% FiO2 atmosphere established a very stable hypoxemia, with a PaO2 ranging from 33.2±1.2 (mean±s.e.mean) to 34.5±1.6 mmHg; PaCO2 decreased from 20.4±0.9 to 12.9±0.5 mmHg, with no repercussions on arterial pH, i.e. before hypoxia it was 7.526±0.105 and remained fairly constant throughout the study at 7.566±0.009. Compared with animals breathing air, hypoxia increased theophylline plasma concentrations in the terminal phase and as a consequence, theophylline AUC0-∞ increased by almost 20% (Table 1). The AUC0-∞ was enhanced because hypoxia decreased theophylline ClM, as reflected by the decrease in the formation clearance of the three metabolites (Figure 1). Compared with rabbits breathing air, hypoxia reduced the amount of CYP1A1 and 1A2 proteins, but increased that of 3A6 (Figure 2).
Table 1.
Influence of hypoxia (PaO2=33.3±1.2 mmHg) on the kinetic parameters of theophylline injected intravenously (2.5 mm kg−1) to conscious rabbits (n=8/group)
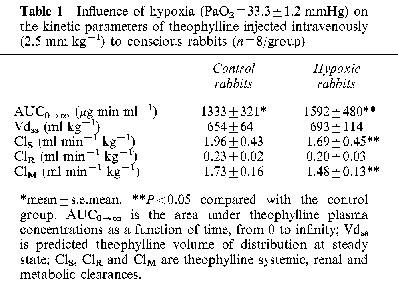
Figure 1.
Fraction of theophylline metabolic clearance generating 3-methylxanthine (3MX), 1-methyluric acid (1MU) and 1,3-dimethyluric acid (1,3DMU) following the intravenous administration of 2.5 mg kg−1 of theophylline to control conscious rabbits, or rabbits with acute moderate hypoxia. Vertical bars are s.e.mean. *P<0.05 compared with the control.
Figure 2.
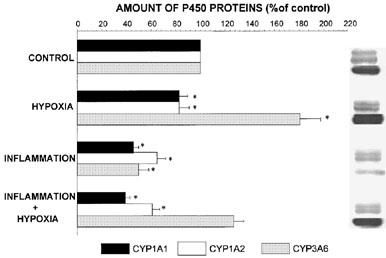
Relative amounts and bands of hepatic CYP1A1, 1A2 and 3A6 immunoreactive proteins assessed by Western immunoblot analysis in control rabbits, rabbits subjected to hypoxia (PaO2≈35 mmHg) for 24 h, rabbits with a turpentine-induced inflammatory reaction, and rabbits with hypoxia and a turpentine-induced inflammatory reaction. Vertical bars are s.e.mean. *P<0.05 compared with control.
Effect of turpentine-induced inflammatory reaction on P450 activity and apoproteins
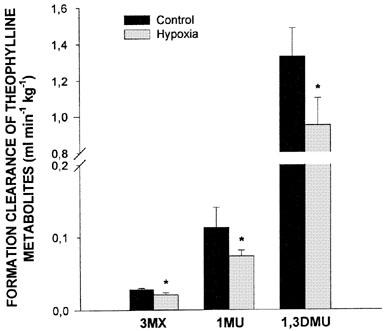
Forty eight hours after turpentine administration, a marked inflammatory reaction with a granuloma was apparent at the site of the injection. Rectal temperature increased from 39.1±0.2 to 40.2±0.2°C (P<0.05), as well as the seromucoids from 35.9±5.7 to 82.5±12.1 mg dl−1 (P<0.05). The inflammatory reaction increased theophylline terminal phase plasma concentrations (Figure 3), and consequently theophylline AUC0-∞ (P<0.05) (Table 2). The increase in AUC0-∞ was secondary to a reduction in theophylline ClM. The inflammatory reaction decreased (P<0.05) the formation clearance of 3MX and 1MU by 95 and 63%, respectively. Even if the amount of 1,3DMU collected in urine diminished by 36%, this difference did not reach statistical significance (Figure 4). Compared with control rabbits, the turpentine-induced inflammatory reaction reduced the amount of CYP1A1, 1A2 and 3A6 proteins by almost 50% (Figure 2). Renal clearance of theophylline was not altered by the inflammatory reaction (Table 2).
Figure 3.
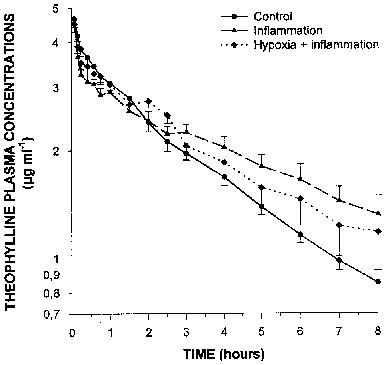
Plasma theophylline concentrations as a function of time following the intravenous administration of 2.5 mg kg−1 to control conscious rabbits, to rabbits with a turpentine-induced inflammatory reaction, and rabbits with an inflammatory reaction and acute moderate hypoxia (PaO2≈55 mmHg) for 24 h. Vertical bars are s.e.mean.
Table 2.
Effect of a turpentine-induced inflammatory reaction alone or associated with hypoxia on the kinetics of intravenous theophylline (2.5 mg kg−1) in conscious rabbits
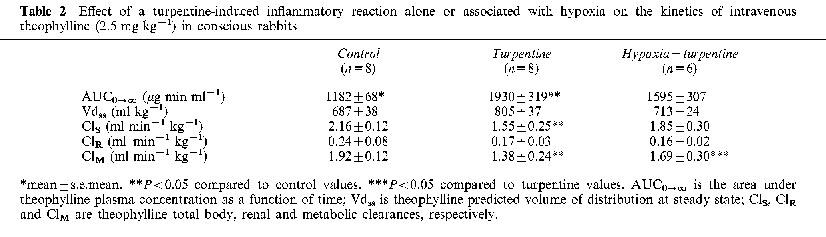
Figure 4.
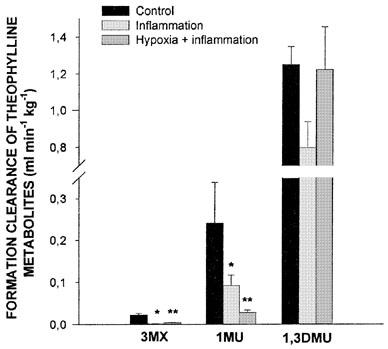
Fraction of theophylline metabolic clearance generating 3-methylxanthine (3MX), 1-methyluric acid (1MU) and 1,3-dimethyluric acid (1,3DMU) following the intravenous administration of 2.5 mg kg−1 of theophylline to controls conscious rabbits, rabbits with a turpentine-induced inflammatory reaction, and rabbits with an inflammatory reaction and acute moderate hypoxia (PaO2≈55 mmHg) for 24 h. Vertical bars are s.e.mean. *, **P<0.05 compared with the control or with inflammation alone, respectively.
Combined effect of hypoxia and an inflammatory reaction on P450 activity and apoproteins
Rectal temperature as well as seromucoids were increased in rabbits with the inflammatory reaction, i.e. from 38.8±0.3 to 40.1±0.1°C (P<0.05), and from 23.6±8.4 to 51.6±5.9 mg dl−1 (P<0.05), respectively. Before the induction of hypoxia, PaO2 was 89±5 mmHg, and when theophylline was injected, the PaO2 was 54±1 mmHg; arterial pH remained rather stable all along the experiment (7.488±0.011) as did the PaCO2 (17.0±0.6 mmHg). In hypoxic rabbits with an inflammatory reaction (Figure 3), theophylline plasma concentrations and AUC0-∞ were only slighly increased (P>0.05) (Table 2). Compared with rabbits with a turpentine-induced inflammatory reaction, theophylline metabolic clearance was increased in rabbits with combined hypoxia and inflammation. Compared with control rabbits, in hypoxic rabbits with an inflammatory reaction, the formation clearance of 3MX and of 1MU decreased by 83 and 88%, respectively (P<0.05), and that of 1,3DMU was not modified (≈5%) (Figure 4). In rabbits with combined hypoxia and inflammation, CYP1A1 and 1A2 proteins were about 50% the values observed in control rabbits, although the amount of CYP3A6 protein remained slightly greater than that observed in controls (Figure 2).
Combined effect of 3MC and hypoxia on P450 activity and apoproteins
Compared with the results obtained in rabbits receiving saline s.c., corn oil did not modify the kinetics of theophylline. In rabbits receiving 3MC, theophylline plasma concentrations and theophylline AUC0-∞ were lower than in controls (Table 3) due to an increase in theophylline ClM. The administration of 3MC enhanced the rate of production of all three metabolites (Figure 5). Hypoxia did not reverse the enzyme-induction produced by 3MC, i.e. theophylline AUC0-∞ was similar to that observed when the rabbits were exposed only to 3MC (Table 3). In rabbits receiving 3MC and subjected to hypoxia, theophylline ClM was increased by 112%, and as a consequence, the formation clearance of all three metabolites was enhanced (Figure 5). Relative to the amount of CYP1A1 and 1A2 proteins in control rabbits, 3MC increased the amount of these proteins by around 23 and 20% (P<0.05), respectively, and the combination of 3MC and hypoxia enhanced the amount of these apoproteins by 47 and 25%, respectively. On the other hand, 3MC and the combination of 3MC and hypoxia tended (P>0.05) to reduce the amount of CYP3A6 protein to 88 and 74% the values observed in controls, respectively (Figure 6).
Table 3.
Influence of 3-methylcholanthrene (3MC) (80 mg kg−1) alone or combined to hypoxia on the kinetic parameters of intravenous theophylline (2.5 mg kg−1) in conscious rabbits (n=7/group)
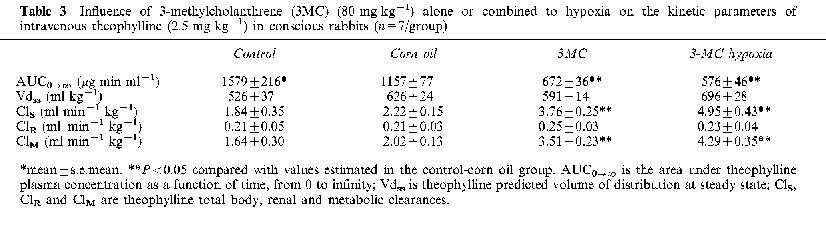
Figure 5.
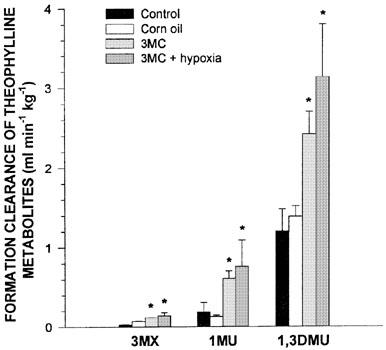
Fraction of theophylline metabolic clearance generating 3-methylxanthine (3MX), 1-methyluric acid (1MU) and 1,3-dimethyluric acid (1,3DMU) following the intravenous administration of 2.5 mg kg−1 of theophylline to control conscious rabbits, to control rabbits receiving corn oil s.c., to rabbits receiving 3-methylcholanthrene (3MC) (80 mg kg−1 s.c.) or to rabbits receiving 3MC and subjected to acute moderate hypoxia (PaO2≈35 mmHg) for 24 h. Vertical bars are s.e.mean. *P<0.05 compared with the control or with 3MC alone, respectively.
Figure 6.
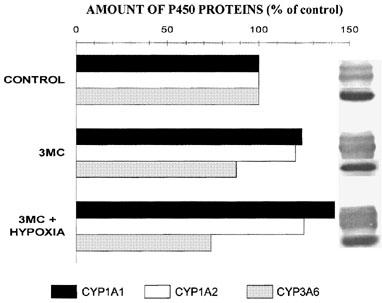
Relative amounts and bands of hepatic CYP1A1, 1A2 and 3A6 immunoreactive proteins assessed by Western immunoblot analysis in control rabbits, and rabbits that received 3-methylcholanthrene (3MC) alone (80 mg kg−1) or combined with hypoxia (PaO2≈35 mmHg) for 24 h.
Discussion
Moderate hypoxia reduced the formation clearance of 3MX, 1MU and 1,3DMU, and decreased the amount of CYP1A1 and 1A2 proteins, but increased CYP3A6 protein, implying that in the rabbit, CYP3A6 has a minor role in the formation of 1,3DMU, and that CYP1A1 and 1A2, as well as other P450 apoproteins, are the main source of 1,3DMU. Similar conclusion has been reached concerning the relative importance of CYP1A1, 1A2 and 3A4 in the metabolism of theophylline in humans (Tjia et al., 1996). In addition, these results show that hypoxia affects selectively some P450 apoproteins. Several explanations may be invoked to understand the hypoxia-induced down-regulation of CYP1A1 and 1A2. Under hypoxic conditions, the organism reduces energy turnover and improves energetic efficiency, and to this purpose, ATP turnover is depressed (Hayashi et al., 1997), and ATP-demanding processes, such as protein synthesis and degradation are diminished (Hochachka et al., 1997). Taking into account the turnover of CYP1A1 and 1A2 proteins, with half-lives of 16 and 10 h (Shiraki & Guengerich, 1984), a decrease in the expression of these proteins is compatible with the results reported in the present study.
In vivo, hypoxia induces a time-dependent decrease in reduced glutathione (GSH) and other cellular antioxidants, and an increase in lipid peroxidation end-products in the liver (El-Bassiouni et al., 1998) as a result of the formation of reactive oxygen intermediates (ROI), such as superoxide anion (O2•−) (Minor et al., 1993; Caraceni et al., 1995), hydrogen peroxide (H2O2) (Matuschak et al., 1996), and nitric oxide (NO•) (Gess et al., 1997). Theoretically, ROI could degrade P450 apoproteins directly as suggested by the fact that H2O2 formed in the hemoprotein active centre may interact with the enzyme associated Fe2+ leading to heme destruction and enzyme inactivation (Karuzina & Archakoz, 1994). Supporting a direct role of ROI in the P450 depression, is the fact that ROI generator systems are able to reduce the activity of the P450 (Khatsenko et al., 1993; Proulx & du Souich, 1995a). On the other hand, O2•− and H2O2 can depress the expression of selected apoproteins of hepatic P450 (Flowers & Miles, 1991; Barker et al., 1994). NO• can inactivate reversibly (Kim et al., 1995) and irreversibly P450 apoproteins (Minamiyama et al., 1997), and can depress the mRNA encoding for p450 apoproteins (Khatsenko & Kikkawa, 1997). The fact that a single dose of 10000 IU of vitamin A prevents the effect of hypoxia on the P450 further supports the hypothesis that ROI are involved in the hypoxia-induced P450 down-regulation (Grover et al., 1985).
Moderate hypoxia for 24 h increases hepatic CYP3A6, a result that is in agreement with the report of Matsubayashi et al. (1997) showing that hypoxia enhances the rate of metabolism of bromocriptine, a substrate of CYP3A subfamily. Taking into account that CYP3A subfamily catalyses the formation of NO• (Kuo et al., 1995), and that hypoxia stimulates the formation of NO• (Gess et al., 1997) in the liver and in peripheral blood vessels (Fujimoto & Itoh, 1997), we might speculate that in response to hypoxia, the expression of CYP3A subfamily is enhanced to increase the blood flow to selected territories. Further studies must be conducted to confirm such hypothesis.
It has been shown that hypoxia enhances the concentration of circulating cytokines by increasing the outflow of TNF-α, IL-1α, and IL-1β from the liver (Wibbenmeyer et al., 1995; Matuschak et al., 1996), and TNF-α and IL-1α from the lung (Matuschak et al., 1998). In vivo, despite the fact that IL-1β, TNF-α, IL-6 and IFN-γ elicit a differential effect on P450 apoproteins, they depress CYP1A1 and 1A2 (Morgan, 1997). On the other hand, in rabbit's cultured hepatocytes, IL-1β and IL-2 reduce induced CYP3A6, and INF-γ is able to decrease the amounts of constitutive and induced CYP3A6 protein (Calleja et al., 1998), suggesting that these cytokines may not intervene in the hypoxia-induced increase in CYP3A6 protein. Since cytokines induce the production of ROI (Ghezzi et al., 1985; Feng et al., 1995) and the formation of NO• in hepatocytes (Spitzer, 1994), it is tempting to postulate that hypoxia-induced cytokines are the mediators leading to the down-regulation of CYP1A1 and 1A2.
Turpentine-induced inflammatory reaction reduced theophylline ClM secondary to the down-regulation of CYP1A1, 1A2 and 3A6. The exact mechanism underlying the turpentine-induced P450 down-regulation is unknown but it is probably associated to a pre-translational mechanism (Morgan, 1989). The P450 depression induced by the inflammatory reaction is mediated by serum factors (El-Kadi et al., 1997; El-Kadi & du Souich, 1998) assumed to be cytokines (Morgan, 1997). As discussed above, cytokines can trigger the production of ROI which may down-regulate P450 apoproteins. Supporting such hypothesis, the turpentine-induced inflammatory reaction is associated with an intrahepatic increase in lipid peroxidation, decrease in catalase, superoxide dismutase and glutathione peroxidase activities, and a reduction in the levels of GSH (Proulx & du Souich, 1995b).
The combination of hypoxia and the inflammatory reaction does not elicit an additive effect on the P450, but rather the effect of each experimental condition is reduced. Since the formation of 1,3DMU is not affected and CYP1A1 and 1A2 proteins are depressed, we may assume that the combination of hypoxia and inflammation protects or even increases other P450 apoproteins implicated in the formation of 1,3DMU. Several mechanisms may be invoked to explain the decrease in effect of each experimental condition. On the one hand, T-cells are necessary for the P450 down-regulation induced by an inflammatory reaction (Topfer et al., 1995), and hypoxia inhibits T-cell function (Meehan, 1987). In addition, hypoxia down-regulates E. coli-induced TNF-α, IL-1α and IL-1β hepatic genes, and so decreases the expression of TNF-α, IL-1α and IL-1β proteins (Wibbenmeyer et al., 1995; Matuschak et al., 1996). On the other hand, hypoxia prevents the pneumonia-induced activation of lipid peroxidation in liver mitochondria (Semenov & Iarosh, 1991), and attenuates the H2O2-induced mechanical and metabolic tissue damage (Hara & Abiko, 1995). Therefore, hypoxia may not only reduce the plasma mediators required to down-regulate the P450, but may also diminish intracellular events leading to the P450 down-regulation triggered by the inflammatory reaction. On the other hand, whenever hypoxia-induced CYP3A6 is involved in the formation of NO• (Kuo et al., 1995) and NO• is involved in the hypoxia-induced P450 depression (Minamiyama et al., 1997; Khatsenko & Kikkawa, 1997), the inflammatory reaction, by decreasing CYP3A6, should limit the effect of hypoxia. Further studies are required to understand the exact mechanism involved in the hypoxia-inflammation interaction.
3MC increases the amount of CYP1A1 and 1A2 proteins and prevents hypoxia-induced down-regulation of the P450. These results agree with reports showing that the P450 down-regulation induced by endotoxins, carrageenan and INF-γ, IL-1α, IL-1β, and IL-6 is prevented by the administration of 3MC (Ishikawa et al., 1992; Clark et al., 1995). Several explanations, not mutually exclusive, could be put forward to understand the present results. In the liver acinus, constitutive P450 apoproteins are essentially concentrated in the perivenous zone, where tissue oxygen tension is around 4%, with decreasing concentrations of P450 in midzonal and periportal zones, where tissue oxygen reaches tensions of 13% (Oinonen & Lindros, 1998). Endotoxins and cytokines produce a preferential perivenous cell injury (Ohno & Maier, 1995), where the production of ROI is greater than in periportal zones (Kukielka & Cederbaum, 1995). We might postulate that hypoxia affects more importantly P450 apoproteins in the perivenous zone that in the periportal zone. On the other hand, 3MC produces a marked increase of CYP1A1 and 1A2, and a 10% decrease of other P450 apoproteins in perivenous hepatocytes (Tanaka et al., 1997). Since in the present study, 3MC was injected 24 h before the rabbits were exposed to hypoxia, we may speculate that the induction produced in the perivenous zone masks any deleterious effect of hypoxia. Alternatively, 3MC depresses CYP2B1, 2C11 and 2E1 and oxidase activity of hepatic P450 (Sakai et al., 1992), apoproteins known to catalyse H2O2 and other ROI and to induce lipid peroxidation (Sakai et al., 1992; Ohmori et al., 1993); as a consequence, the ability of hypoxia to generate ROI and to depress CYP1A1 and 1A2 would be attenuated. Finally, 3MC may stabilize selected apoproteins because of its tight binding to them (Huang et al., 1986).
In conclusion, in vivo acute moderate hypoxia reduces the amount of CYP1A1 and 1A2 proteins, although it increases that of CYP3A6. On the other hand, a turpentine-induced inflammatory reaction decreases the amount of CYP1A1, 1A2 and 3A6 proteins. The combination of hypoxia and an inflammatory reaction elicits a very limited effect on the in vivo metabolism of theophylline, despite the fact that the amounts of CYP1A1 and 1A2 proteins remain comparable to that measured during the inflammatory reaction alone. From a practical point of view, the present results may explain why in patients with advanced complicated COLD, i.e. with hypoxia and chronic bronchial inflammatory reactions, the metabolism of theophylline is scarcely affected by the disease, and therapy with oxygen does not enhance theophylline metabolism (Cusack et al., 1986; du Souich et al., 1989). Indeed such extrapolation to human pathology must be done with caution because of species, pathology, and P450 apoprotein specificity of the cytokines. The pre-treatment of the rabbits with 3MC increased CYP1A1 and 1A2 preventing the down-regulation of these apoproteins induced by hypoxia.
Acknowledgments
This work was supported by the Medical Research Council of Canada (Grant # MT-14478).
Abbreviations
- AUC0→t
area under theophylline plasma concentration-time curve from 0 to t
- AUC0→∞
area under theophylline plasma concentration-time curve from 0 to ∞
- ClM
theophylline metabolic clearance
- ClM3MX
fraction of theophylline metabolic clearance yielding 3MX
- ClR
theophylline renal clearance
- ClS
theophylline systemic clearance
- COLD
chronic obstructive lung disease
- 1,3DMU
1,3-dimethyluric acid
- FiO2
fractional concentration of inspired O2
- F3MXM
fraction of theophylline being metabolized to 3MX
- FIM
fraction of theophylline undergoing metabolism
- GSH
reduced glutathione
- H2O2
hydrogen peroxide
- 3MC
3-methylcholanthrene
- 1MU
1-methyluric acid
- 3MX
3-methylxanthine
- NO•
nitric oxide
- O2•−
superoxide anion
- P450
cytochrome P450
- PaCO2
arterial partial pressure of CO2
- PaO2
arterial partial pressure of O2
- ROI
reactive oxygen intermediates
- VdSS
predicted theophylline volume of distribution at steady state
- XU3MX
amount of 3MX recovered in urine
- z
terminal rate constant of disposition
References
- ASHTON F.W., JAMIESON J.C., FRIESEN A.D. Studies on the effect of inflammation on rat serum proteins. Can. J. Biochem. 1970;48:841–850. doi: 10.1139/o70-133. [DOI] [PubMed] [Google Scholar]
- BARKER C.W., FAGAN J.B., PASCO D.S. Down-regulation of P4501A1 and P4501A2 mRNA expression in isolated hepatocytes by oxidative stress. J. Biol. Chem. 1994;269:3985–3990. [PubMed] [Google Scholar]
- BECK F.J., WHITEHOUSE M.W. Impaired drug metabolism in rats associated with acute inflammation: a possible assay for anti-injury agents. Proc. Soc. Exp. Biol. Med. 1974;145:135–140. doi: 10.3181/00379727-145-37763. [DOI] [PubMed] [Google Scholar]
- CALLEJA C., EECKHOUTTE C., DACASTO M., LARRIEU G., DUPUY J., PINEAU T., GALTIER P. Comparative effects of cytokines on constitutive and inducible expression of the gene encoding for the cytochrome P450 3A6 isoenzyme in cultured rabbit hepatocytes: consequences on progesterone 6β-hydroxylation. Biochem. Pharmacol. 1998;56:1279–1285. doi: 10.1016/s0006-2952(98)00178-6. [DOI] [PubMed] [Google Scholar]
- CARACENI P., RYU H.S., VAN THIEL D.H., BORLE A.B. Source of oxygen free radicals produced by rat hepatocytes during postanoxic reoxygenation. Biochim. Biophys. Acta. 1995;1268:249–254. doi: 10.1016/0167-4889(95)00077-6. [DOI] [PubMed] [Google Scholar]
- CHANG K.C., LAUER B.A., BELL T.D. Altered theophylline pharmacokinetics during an acute respiratory viral illness. Lancet. 1978;1:1132–1133. doi: 10.1016/S0140-6736(78)90305-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CLARK M.A., BING B.A., GOTTSCHALL P.E., WILLIAMS J.F. Differential effect of cytokines on the phenobarbital or 3-methylcholanthrene induction of P450 mediated monooxygenase activity in cultured rat hepatocytes. Biochem. Pharmacol. 1995;49:97–104. doi: 10.1016/0006-2952(94)00438-r. [DOI] [PubMed] [Google Scholar]
- CUSACK B.J., CROWLEY J.J., MERCER G.D., HARAN N.B., VESTAL R.E. Theophylline clearance in patients with severe chronic obstructive pulmonary disease receiving supplemental oxygen and the effect of acute hypoxemia. Am. Rev. Respir. Dis. 1986;133:1110–1114. doi: 10.1164/arrd.1986.133.6.1110. [DOI] [PubMed] [Google Scholar]
- DU SOUICH P., HOEN B., SAUNIER C., HARTEMANN D., SAUTEGEAU A., CORNETTE A., DELORME N., POLU J.M., SADOUL P. Theophylline disposition in patients with COLD with and without hypoxemia. Chest. 1989;95:1028–1032. doi: 10.1378/chest.95.5.1028. [DOI] [PubMed] [Google Scholar]
- EL-BASSIOUNI E.A., ABO-OLLO M.M., HELMY M.H., ISMAEL S., RAMADAN M.I. Changes in the defense against free radicals in the liver and plasma of the dog during hypoxia and/or halothane anaesthesia. Toxicology. 1998;128:25–34. doi: 10.1016/s0300-483x(98)00045-6. [DOI] [PubMed] [Google Scholar]
- EL-KADI A.O.S., DU SOUICH P. Depression of the hepatic cytochrome P450 by an acute inflammatory reaction: characterization of the nature of mediators in human and rabbit serum, and in the liver. Life Sci. 1998;63:1361–1370. doi: 10.1016/s0024-3205(98)00400-7. [DOI] [PubMed] [Google Scholar]
- EL-KADI A.O.S., MAURICE H., ONG H., DU SOUICH P. Down-regulation of the hepatic cytochrome P450 by an acute inflammatory reaction: implication of mediators in human and animal serum in the liver. Br. J. Pharmacol. 1997;121:1164–1170. doi: 10.1038/sj.bjp.0701232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FENG L., XIA Y., GARCIA G.A., HWANG D., WILSON C.B. Involvement of reactive oxygen intermediates in cyclooxygenase-2 expression induced by interleukin-1, tumor necrosis factor-α, and lipopolysaccharide. J. Clin. Invest. 1995;95:1669–1675. doi: 10.1172/JCI117842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FLOWERS N.L., MILES P.R. Alterations of pulmonary benzo[a]pyrene metabolism by reactive oxygen metabolites. Toxicology. 1991;68:259–274. doi: 10.1016/0300-483x(91)90074-b. [DOI] [PubMed] [Google Scholar]
- FUJIMOTO S., ITOH T. Role of nitric oxide and nitric oxide-independent relaxing factor in contraction and relaxation of rabbit blood vessels. Eur. J. Pharmacol. 1997;330:177–184. doi: 10.1016/s0014-2999(97)00180-5. [DOI] [PubMed] [Google Scholar]
- GESS B., SCHRICKER K., PFEIFER M., KURTZ A. Acute hypoxia upregulates NOS gene expression in rats. Am. J. Physiol. 1997;273:R905–R910. doi: 10.1152/ajpregu.1997.273.3.R905. [DOI] [PubMed] [Google Scholar]
- GHEZZI P., BIANCHI M., GIANERA L., LANDOLFO S., SALMONA M. Role of reactive oxygen intermediates in the interferon-mediated depression of hepatic drug metabolism and protective effect of N-acetylcysteine in mice. Cancer Res. 1985;45:3444–3447. [PubMed] [Google Scholar]
- GIBALDI KM., PERRIER D.Noncompartmental analysis based on statistical moment theory Pharmacokinetics 1982Marcel Dekker, Inc: New York; 409–417.In: Swarbrick J (ed) [Google Scholar]
- GRAY J.D., RENTON K.W., HUNG O.R. Depression of theophylline elimination following BCG vaccination. Br. J. Clin. Pharmacol. 1983;16:735–737. doi: 10.1111/j.1365-2125.1983.tb02253.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GROVER S.K., SRIVASTAVA K.K., SINGH V.S., MISRA U.K. Effect of vitamin A on hepatic microsomal drug metabolising enzymes activity in rats exposed to acute hypoxia. Int. J. Vitam. Nutr. Res. 1985;55:391–393. [PubMed] [Google Scholar]
- HARA A., ABIKO Y. Protective effect of hypoxia on mechanical and metabolic changes induced by hydrogen peroxide in rat hearts. Am. J. Physiol. 1995;268:H614–H620. doi: 10.1152/ajpheart.1995.268.2.H614. [DOI] [PubMed] [Google Scholar]
- HAYASHI K., OCHIAI T., ISHINODA Y., OKAMOTO T., MARAYUMA T., TSUDA K., TSUBOUCHI H. Relationship between cellular ATP content and cellular functions of primary cultured rat hepatocytes in hypoxia. J. Gastroenterol. Hepatol. 1997;12:249–256. doi: 10.1111/j.1440-1746.1997.tb00417.x. [DOI] [PubMed] [Google Scholar]
- HENDELES L., BIGHLEY L., RICHARDSON R.H., HEPLER D.D., CARMICHAEL J. Frequent toxicity from IV aminophylline infusion in critically ill patients. Drug Int. Clin. Pharmacol. 1977;11:2–18. doi: 10.1345/aph.140027. [DOI] [PubMed] [Google Scholar]
- HOCHACHKA P.W., LAND S.C., BUCK L.T. Oxygen sensing and signal transduction in metabolic defense against hypoxia: lessons from vertebrate facultative anaerobes. Comp. Biochem. Physiol., Part A. Physiol. 1997;118:23–29. doi: 10.1016/s0300-9629(96)00372-6. [DOI] [PubMed] [Google Scholar]
- HUANG Y.Y., HARA T., SLIGAR S., COON M.J., KIMURA T. Thermodynamic properties of oxidation-reduction reactions of bacterial, microsomal and mitochondrial cytochromes P-450: an entropy-enthalpy compensation effect. Biochemistry. 1986;25:1390–1394. doi: 10.1021/bi00354a030. [DOI] [PubMed] [Google Scholar]
- ISHIKAWA M., SASAKI K., TAKAYANAGI Y., SASAKI K. Effect of carrageenan-induced inflammation on the induction of hepatic microsomal enzymes by phenobarbital and benzo[α]pyrene in male rats. J. Pharmacobiodyn. 1992;15:139–146. doi: 10.1248/bpb1978.15.139. [DOI] [PubMed] [Google Scholar]
- JACOBS M.H., SENIOR R.M. Theophylline toxicity due to impaired theophylline degradation. Am. Rev. Respir. Dis. 1974;110:342–345. doi: 10.1164/arrd.1974.110.3.342. [DOI] [PubMed] [Google Scholar]
- KARUZINA I.I., ARCHAKOV A.I. The oxidative inactivation of cytochrome P450 in monooxygenase reactions. Free Rad. Biol. Med. 1994;16:73–97. doi: 10.1016/0891-5849(94)90245-3. [DOI] [PubMed] [Google Scholar]
- KHATSENKO O.G., GROSS S.S., RIFKIND A.B., VANE J.R. Nitric oxide is a mediator of the decrease in cytochrome P450-dependent metabolism caused by immunostimulants. Proc. Natl. Acad. Sci. U.S.A. 1993;90:11147–11151. doi: 10.1073/pnas.90.23.11147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KHATSENKO O., KIKKAWA Y. Nitric oxide differentially affects constitutive cytochrome P450 isoforms in rat liver. J. Pharmacol. Exp. Ther. 1997;280:1463–1470. [PubMed] [Google Scholar]
- KIM Y.M., BERGONIA H.A., MULLER C., PITT B.R., WATKINS W.D., LANCASTER J.R., JR Loss and degradation of enzyme-bound heme induced by cellular nitric oxide synthesis. J. Biol. Chem. 1995;270:5710–5713. doi: 10.1074/jbc.270.11.5710. [DOI] [PubMed] [Google Scholar]
- KRUGER N.J.Detection of polypeptides on immunoblots using secondary antibodies or protein A Methods of molecular biology 199432Humana Press, Totowa, NJ; 215–226.In: Walker J.M. (ed) [DOI] [PubMed] [Google Scholar]
- KUKIELKA E., CEDERBAUM A.I. Increased production of hydroxyl radical by pericentral microsomes compared to periportal microsomes after pyrazole induction of cytochrome P4502E1. Biochem. Biophy. Res. Commun. 1995;215:698–705. doi: 10.1006/bbrc.1995.2520. [DOI] [PubMed] [Google Scholar]
- KUO P.C., ABE K.Y., DAFOE D.C. Cytochrome P450IIIA activity and cytokine-mediated synthesis of nitric oxide. Surgery. 1995;118:310–317. doi: 10.1016/s0039-6060(05)80339-3. [DOI] [PubMed] [Google Scholar]
- LETARTE L., DU SOUICH P. Influence of hypercapnia and/or hypoxemia and metabolic acidosis on theophylline kinetics in the conscious rabbit. Am. Rev. Respir. Dis. 1984;129:762–766. doi: 10.1164/arrd.1984.129.5.762. [DOI] [PubMed] [Google Scholar]
- LOWRY O.H., ROSEBROUGH N.J., FARR A.L., RANDALL R.J. Protein measurement with the folin phenol reagent. J. Biol. Chem. 1951;193:265–275. [PubMed] [Google Scholar]
- MATSUBAYASHI K., MATSUMOTO H., FUKUI Y. Contribution of cytochrome P450 3A pathway to bromocriptine metabolism and effects of ferrous iron and hypoxia-re-oxygenation on its elimination in the perfused rat liver. J. Pharm. Pharmacol. 1997;49:551–557. doi: 10.1111/j.2042-7158.1997.tb06840.x. [DOI] [PubMed] [Google Scholar]
- MATUSCHAK G.M., JOHANNS C.A., CHEN Z., GAYNOR J., LECHNER A.J. Brief hypoxic stress down-regulates E. coli-induced IL-1α and IL-1β gene expression in perfused liver. Am. J. Physiol. 1996;271:R1311–R1318. doi: 10.1152/ajpregu.1996.271.5.R1311. [DOI] [PubMed] [Google Scholar]
- MATUSCHAK G.M., MUÑOZ C.F., JOHANNS C.A., RAHMAN R., LECHNER A.J. Upregulation of postbacteremic TNF-alpha and IL-1 alpha gene expression by alveolar hypoxia/reoxygenation in perfused rat lungs. Am. J. Respir. Crit. Care Med. 1998;157:629–637. doi: 10.1164/ajrccm.157.2.9707120. [DOI] [PubMed] [Google Scholar]
- MEEHAN R.T. Immune suppression at high altitude. Ann. Emerg. Med. 1987;16:974–979. doi: 10.1016/s0196-0644(87)80743-6. [DOI] [PubMed] [Google Scholar]
- MINAMIYAMA Y., TAKEMURA S., IMAOKA S., FUNAE Y., TANIMOTO Y., INOUE M. Irreversible inhibition of cytochrome P450 by nitric oxide. J. Pharmacol. Exp. Ther. 1997;283:1479–1485. [PubMed] [Google Scholar]
- MINOR T., ISSELHARD W., BERGHAUS K. Parenchymal and vascular endothelial cell injury in the hypoxic and reperfused rat liver. Evidence for superoxide anion generation by perfusion with ferricytochrome c. Biomed. Pharmacother. 1993;47:213–218. doi: 10.1016/0753-3322(93)90059-t. [DOI] [PubMed] [Google Scholar]
- MORGAN E.T. Suppression of constitutive cytochrome P450 gene expression in liver of rats undergoing an acute response to endotoxin. Mol. Pharmacol. 1989;36:699–707. [PubMed] [Google Scholar]
- MORGAN E.T. Regulation of cytochromes P450 during inflammation and infection. Drug. Metab. Rev. 1997;29:1129–1188. doi: 10.3109/03602539709002246. [DOI] [PubMed] [Google Scholar]
- OHMORI S., MISAIZU T., NAKAMURA T., TAKANO N., KITAGAWA H., KITADA M. Differential role in lipid peroxidation between rat P450 1A1 and P450 1A2. Biochem. Pharmacol. 1993;46:55–60. doi: 10.1016/0006-2952(93)90347-y. [DOI] [PubMed] [Google Scholar]
- OHNO K., MAIER P. Tumor necrosis factor alpha differentially modulates the cellular response of rat hepatocytes in periportal- and pericentral-equivalent cultures. Eur. J. Pharmacol. 1995;292:205–214. doi: 10.1016/0926-6917(95)90024-1. [DOI] [PubMed] [Google Scholar]
- OINONEN T., LINDROS K.O. Zonation of hepatic cytochrome P-450 expression and regulation. Biochem. J. 1998;329:17–35. doi: 10.1042/bj3290017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PARENT C., BÉLANGER P.M., JUTRAS L., DU SOUICH P. Effect of inflammation on the rabbit hepatic cytochrome P-450 isoenzymes. Alterations in the kinetics and dynamics of tolbutamide. J. Pharmacol. Exp. Ther. 1992;261:780–787. [PubMed] [Google Scholar]
- POWELL J.R., VOZEH S., HOPEWELL P., COSTELLO J., SCHEINER L.B., RIEGELMAN S. Theophylline disposition in acutely ill hospitalized patients. The effect of smoking, heart failure, severe airway obstruction and pneumonia. Am. Rev. Respir. Dis. 1978;118:229–238. doi: 10.1164/arrd.1978.118.2.229. [DOI] [PubMed] [Google Scholar]
- PROULX M., DU SOUICH P. Acute moderate hypoxia in conscious rabbits: effect on hepatic cytochrome P450 and on reactive species. J. Pharm. Pharmacol. 1995a;47:392–397. doi: 10.1111/j.2042-7158.1995.tb05817.x. [DOI] [PubMed] [Google Scholar]
- PROULX M., DU SOUICH P. Inflammation-induced decrease in cytochrome P450 in conscious rabbits is accompanied by an increase in hepatic oxidative stress. Res. Commun. Mol. Pathol. Pharmacol. 1995b;87:221–236. [PubMed] [Google Scholar]
- RICHER M., LAM Y.W.F. Hypoxia, arterial pH and theophylline disposition. Clin. Pharmacokin. 1993;24:283–299. doi: 10.2165/00003088-199325040-00004. [DOI] [PubMed] [Google Scholar]
- SAKAI H., PARK S.S., KIKKAWA Y. Differential oxidase activity of hepatic and pulmonary microsomal cytochrome P-450 isozymes after treatment with cytochrome inducers. Biochem. Biophys. Res. Commun. 1992;187:1262–1269. doi: 10.1016/0006-291x(92)90439-r. [DOI] [PubMed] [Google Scholar]
- SARKAR M.A., JACKSON B.J. Theophylline demethylations as probes for P4501A1 and P4501A2. Drug. Metab. Dispos. 1994;22:827–834. [PubMed] [Google Scholar]
- SEMENOV V.L., IAROSH A.M. The effect of hypoxia on oxidative phosphorylation and lipid peroxidation in rat liver mitochondria upon lung inflammation. Ukr. Biokhim. Zh. 1991;63:95–101. [PubMed] [Google Scholar]
- SHIRAKI H., GUENGERICH F.P. Turnover of membrane proteins: kinetics of induction and degradation of seven forms of rat liver microsomal P-450, NADPH-cytochrome P-450 reductase, and epoxide hydrolase. Arch. Biochem. Biophys. 1984;235:86–96. doi: 10.1016/0003-9861(84)90257-1. [DOI] [PubMed] [Google Scholar]
- SMITH B.J.SDS polyacrylamide gel electrophoresis of proteins Methods of Molecular Biology 199432Humana Press: Totowa, NJ; 23–34.In: Walker JM (ed) [DOI] [PubMed] [Google Scholar]
- SONNE J., DØSSING M., LOFT S., ANDREASEN P.B. Antipyrine clearance in pneumonia. Clin. Pharmacol. Ther. 1985;37:701–704. doi: 10.1038/clpt.1985.117. [DOI] [PubMed] [Google Scholar]
- SPITZER J.A. Cytokine stimulation of nitric oxide formation and differential regulation in hepatocytes and nonparenchymal cells of endotoxemic rats. Hepatology. 1994;19:217–228. [PubMed] [Google Scholar]
- TANAKA T., WATANABE J., ASAKA Y., OGAWA R., KANAMURA S. Quantitative analysis of endoplasmic reticulum and cytochrome P450 in hepatocytes from rats injected with methylcholanthrene. Eur. J. Cell. Biol. 1997;74:20–30. [PubMed] [Google Scholar]
- THORPE G.H.G., KRICKA L.J., MOSELEY S.B., WHITEHEAD T.P. Phenols as enhancers of the chemiluminescent horesradish peroxidase-luminol-hydrogen peroxide reaction: application in luminescence-monitored enzyme immunoassays. Clin. Chem. 1985;31:1335–1341. [PubMed] [Google Scholar]
- TJIA J.F., COLBERT J., BACK D.J. Theophylline metabolism in human liver microsomes: inhibition studies. J. Pharmacol. Exp. Ther. 1996;276:912–917. [PubMed] [Google Scholar]
- TOPFER F., LENTON L.M., BYGRAVE F.L., BEHM C.A. Importance of T-cell-dependent inflammatory reactions in the decline of microsomal cytochrome P450 concentration in the livers of rats infected with fasciola hepatica. Int. J. Parasitol. 1995;25:1259–1262. doi: 10.1016/0020-7519(95)00052-4. [DOI] [PubMed] [Google Scholar]
- VOZEH S., POWELL J.R., RIEGELMAN S., COSTELLO J.F., SCHEINER L.B., HOPEWELL P. Changes in theophylline clearance during acute illness. J. Am. Med. Ass. 1978;240:1882–1884. [PubMed] [Google Scholar]
- WIBBENMEYER L.A., LECHNER A.J., MUÑOZ C.F., MATUSCHAK G.M. Down-regulation of E. coli-induced TNF-α expression in perfused liver by hypoxia-reoxygenation. Am. J. Physiol. 1995;268:G311–G319. doi: 10.1152/ajpgi.1995.268.2.G311. [DOI] [PubMed] [Google Scholar]
- WINER B.J. Statistical Principles in Experimental Design. McGraw-Hill: New York; 1971. pp. 149–257. [Google Scholar]
- ZHANG Z.Y., KAMINSKY L.S. Characterisation of human cytochromes P450 involved in theophylline 8-hydroxylation. Biochem. Pharmacol. 1995;50:205–211. doi: 10.1016/0006-2952(95)00120-o. [DOI] [PubMed] [Google Scholar]


