Abstract
This study was conducted to assess whether a 21-aminosteroid, U74389G, could prevent the down-regulation of hepatic cytochrome P450 (P450) induced by acute moderate hypoxia or an inflammatory reaction.
The rabbits of two groups (n=6 per group) were subjected to acute moderate hypoxia (PaO2≈35 mmHg), one pre-treated with U74389G (3 mg kg−1 i.v. every 6 h, for 48 h). The rabbits of two other groups received 5 ml of turpentine s.c., one of them being pre-treated with U74389G (3 mg kg−1 i.v. every 6 h, for 72 h). The kinetics of theophylline (2.5 mg kg−1) were assessed to evaluate the activity of the P450. Once the rabbits were sacrificed, the P450 content and the amount of thiobarbituric acid reactive substances (TBARS), a marker of lipid peroxidation, were estimated in the liver.
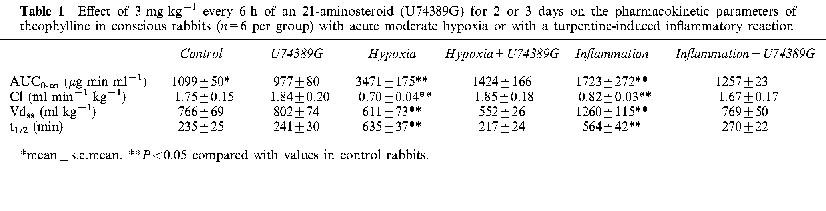
Compared with control rabbits, hypoxia and inflammation increased theophylline plasma concentrations, as a result of a decrease in theophylline systemic clearance (P<0.05). Both experimental conditions reduced hepatic content of P450 by 40–50% (P<0.05) and increased the amount of hepatic TBARS by around 50% (P<0.05). Pre-treatment with U74389G prevented the hypoxia- and inflammation-induced decrease in theophylline systemic clearance, the down-regulation of hepatic P450, and the increase in liver TBARS.
It is concluded that in the rabbit, U74389G prevents hepatic P450 depression produced by acute moderate hypoxia and a turpentine-induced inflammatory reaction, possibly by eliciting a radical quenching antioxidant activity.
Keywords: Hypoxia, inflammation, cytochrome P450, 21-aminosteroids, theophylline, kinetics
Introduction
In patients with complicated acute or chronic obstructive lung disease, the activity of the cytochrome P450 (P450), estimated by the clearance of theophylline, is reduced (Hendeles et al., 1986). In animal models, hypoxia (Letarte & du Souich, 1984) as well as a turpentine-induced inflammatory reaction (Parent et al., 1992; Barakat & du Souich, 1996) reduce the metabolic clearance of theophylline, secondary to a down-regulation of selected apoproteins of the P450 (Kurdi et al., 1999). The mechanism(s) underlying the inflammation- or hypoxia-induced decrease in theophylline clearance remain poorly characterized.
Acute moderate hypoxia in vivo increases hepatic lipid peroxidation, microsomal chemiluminescence and superoxide dismutase activity, while it diminishes hepatic reduced glutathione (GSH) and glutathione peroxidase activity (Proulx & du Souich, 1995a). These changes induced by hypoxia appear to be due to the formation of reactive oxygen intermediates (ROI), such as superoxide anion (O2•−) (Minor et al., 1993), hydrogen peroxide (H2O2) (Matuschak et al., 1996), and nitric oxide (NO•) (Gess et al., 1997). Supporting the involvement of ROI in the down-regulation of the P450, vitamin A (Grover et al., 1985) and α-tocopherol (Lee & Clemens, 1992) prevent the hypoxia-induced decrease in P450.
A turpentine-induced inflammatory reaction causes oxidative stress in the liver characterised by a decrease in activity of enzymatic scavengers and of GSH, and an increase in hepatic xanthine oxidase activity and in the amount of thiobarbituric acid reactive substances (TBARS) (Proulx & du Souich, 1995b). Moreover, the serum of rabbits with a turpentine-induced inflammatory reaction contains mediators capable of decreasing the activity of P450 and of increasing the concentration of TBARS in cultured hepatocytes, phenomena that are negatively associated (El-Kadi et al., 1997). Further supporting the involvement of ROI in the depression of the P450 by an inflammatory reaction is the fact that N-acetylcysteine prevents the down-regulation of the P450 induced by endotoxins (Ghezzi et al., 1985).
21-Aminosteroids are potent inhibitors of lipid peroxidation that can partially protect from an ischaemic lesion (Levitt et al., 1994) or from an inflammatory reaction caused by endotoxins (Zhang et al., 1995). The use of antioxidants in inflammatory diseases has been widely advocated (Means, 1994), however there is no information as to whether antioxidants can prevent the inflammation- or hypoxia-induced damage in hepatic P450. The objectives of the present study were to investigate in vivo whether a 21-aminosteroid (U74389G; Figure 1) could prevent the decrease in hepatic P450 provoked by acute moderate hypoxia or by a turpentine-induced acute inflammatory reaction.
Figure 1.
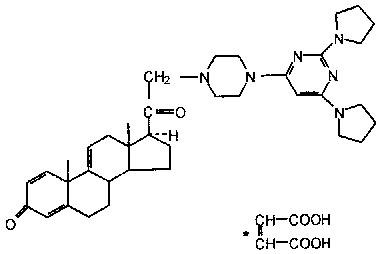
Structure of the 21-aminosteroid, U74389G.
Methods
Male New-Zealand rabbits (Ferme Cunicole, Les Lapins Léonard, Mirabel, Canada) weighing 2.0–2.2 kg were used throughout the study. The rabbits were kept in well ventilated cages and were fed with dry food and water ad libitum for at least 7 days for acclimatization before being included in the experiments. The rabbits were segregated in six groups, the first group (n=6) being control. The second group (n=6) was used to assess the effect of U74389G on theophylline clearance. Groups 3 (n=6) and 4 (n=6) were used to document the effect of hypoxia on theophylline kinetics in absence and in presence of U74389G, respectively. Groups 5 (n=6) and 6 (n=6) were used to assess the effect of an inflammatory reaction on theophylline kinetics in absence and in presence of U74389G, respectively.
Experimental protocol
At time 0, the rabbits of the six experimental groups received in a lateral vein of an ear 2.5 mg kg−1 of theophylline dissolved in sodium chloride (NaCl) 0.9%. Blood samples were withdrawn prior to and at 5, 10, 15, 20, 30, 60, 120, 180, 240, 300, 360, 420 and 480 min, after the injection of theophylline, through a catheter (Butterfly-21, Abbot Ireland, Sligo, Ireland) inserted in the central artery of an ear.
Acute moderate hypoxia was induced by placing the rabbits in a plexiglass chamber (0.75×1.20×1.25 m3) where the fractional concentration of inspired O2 (FiO2) was 10%, regulated with an oxygen monitor (OM-15, Sensor Medics Corp., CA, USA) connected to an electrovalve (Asco Valves, Brantford, Ontario, Canada) that allowed the access of nitrogen. The 10% FiO2 was chosen to obtain an arterial partial pressure of O2 (PaO2) of around 35 mmHg. Humidity in the chamber was maintained at 50% by the re-circulation of the air through a refrigerating system. The temperature was kept at 22–24°C. The rabbits were placed in the chamber 24 h prior, and for the 8 h the kinetics of theophylline lasted. All animals had free access to Purina Laboratory Chow and water during the 32 h of the experiment. Arterial blood samples were drawn at different times to control blood gases and pH (1312 pH/Blood Gas Analyzer, Instrumentation Laboratory, Lexington, MA, U.S.A.).
The inflammatory reaction was induced locally by injecting turpentine (2.5 ml) s.c. at two distinct sites on the back of the rabbits (Parent et al., 1992). The kinetics of theophylline were assessed 48 h later. To assess the severity of the inflammation, rectal temperature was measured with an electronic thermometer (model 2013A; The Lumiscope Company Inc., NJ, U.S.A.), and seromucoids were isolated as described elsewhere (Parent et al., 1992) before and 48 h later, at the peak of the inflammatory reaction.
To discount an effect of U74389G on theophylline kinetics, U74389G dissolved in NaCl 0.9% was injected intravenously (3 mg kg−1), every 6 h for 48 h, before the kinetics of theophylline were assessed. In hypoxic rabbits, U74389G was administered 24 h before hypoxia was induced, and during the 24 h period of hypoxia. Rabbits with an inflammatory reaction received 3 mg kg−1 of U74389G every 6 h for 72 h, starting 24 h before the administration of turpentine. Theophylline in plasma was assayed by high performance liquid chromatography (Letarte & du Souich, 1984).
Rabbits of all groups were sacrificed 8 h after the administration of theophylline, and the liver was removed to assess the amount of P450 and TBARS, as a marker of lipid peroxidation. An aliquot of the liver was used to obtain a 17% (w v−1) homogenate in 0.25 M sucrose solution, which was centrifuged at 600×g for 8 min, and the resulting supernatant at 12,000×g for 10 min. The supernatant of the latter centrifugation was re-centrifuged with 8 mM CaCl2 at 27,000×g for 15 min. The ensuing supernatant was collected and stored at −80°C, and the pellet was re-suspended in 0.15 M KCl solution and re-centrifuged at 27,000×g for 15 min (Cinti et al., 1972). The pellet was isolated and covered with ice-cold 0.25 M sucrose solution, and stored at −80°C. The amount of TBARS formed during hypoxia or the inflammatory reaction was assessed in the supernatant by means of the thiobarbituric acid reaction (Ohkawa et al., 1979). The amount of hepatic P450 was measured in the pellet according to the method described by Omura & Sato (1964). Protein content in the hepatic supernatant and microsomal fractions (pellet) was measured using the method of Lowry et al. (1951).
Drugs and chemicals
Theophylline and other chemicals were purchased from Sigma Chemical Company (St. Louis, MO, U.S.A.). The 21-aminosteroid, U74389G (21-[4-(2,6-di-l-pyrrolidinyl-4-pyrimidinyl)-1-piperazinyl]-pregna-1,4,9(11)-triene-3,20-dione (2)-2-butenedioate; C37H50N6O2•C4H4O4), graciously provided by Pharmacia-Upjohn Company (Kalamazoo, Michigan), is a 16-desmethylated derivative of tirilazad mesylate (Figure 1).
Analysis of data
Theophylline terminal rate constant of disposition (z), terminal half-life (t½), area under its plasma concentration-time curve (AUC0–∞), systemic clearance (Cl) and predicted apparent volume of distribution at steady state (VdSS) were estimated according to non-compartmental analysis based on statistical moment theory (Gibaldi & Perrier, 1982) with the computer program Pharmacokinetic Data Analysis Program included in Lotus 1,2,3, Version 2.2 (Lotus Development Corporation, Cambridge, MD, U.S.A.).
The comparison of the results of the various experimental groups and control group was carried out using a one-way analysis of variance for parallel groups. All pairwise multiple comparison procedures were conducted using the Student-Newman-Keuls method. The significance criteria was established at P<0.05. All results are presented as mean±standard error (s.e.mean).
Results
In control rabbits, breathing air and without an inflammatory reaction, the administration of U74389G for 2 days did not modify theophylline systemic clearance and volume of distribution (Table 1). On the other hand, in control rabbits, the amount of hepatic P450 was 1.021±0.059 nmol mg−1 protein, a value that was not altered by the administration of U74389G, i.e. 1.017±0.81 nmol mg−1 protein. Similarly, compared with controls, the administration of U74389G did not modify the amount of TBARS in liver, i.e. 0.289±0.015 vs 0.269±0.009 nmol mg−1 protein.
Table 1.
Effect of 3 mg kg−1 every 6 h of an 21-aminosteroid (U74389G) for 2 or 3 days on the pharmacokinetic parameters of theophylline in conscious rabbits (n=6 per group) with acute moderate hypoxia or with a turpentine-induced inflammatory reaction
Effect of U74389G on hypoxia-induced decrease in hepatic P450
In rabbits breathing room air, mean arterial PaO2 was 90±3 mmHg, and in those exposed to a 10% FiO2, average PaO2 was decreased to 35±2 mmHg (P<0.05). Arterial PaCO2 and pH were not affected by the experimental condition, i.e. 24±1 mmHg and 7.50±0.01 in hypoxic rabbits, and 25±1 mmHg and 7.49±0.02 in control rabbits, respectively.
Hypoxia diminished considerably the rate of decay of theophylline plasma concentrations. As a consequence, in hypoxic rabbits, theophylline AUC0-∞ was three fold greater than that in control rabbits (P<0.05) (Table 1); the increase in AUC0-∞ was secondary to a reduction in theophylline clearance. Theophylline volume of distribution was not affected by hypoxia. After 24 h of hypoxia, average amount of total hepatic P450 was 30% smaller (P<0.05) than in control animals (Figure 2). On the other hand, compared with control rabbits, hepatic TBARS more than doubled (P<0.05) in hypoxic rabbits (Figure 3).
Figure 2.
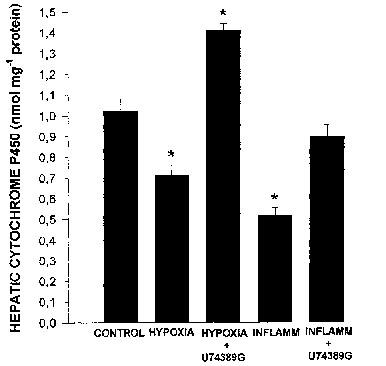
Effect of acute moderate hypoxia and of turpentine-induced inflammatory reaction (INFLAMM) on hepatic cytochrome P450 of rabbits pre-treated with 3 mg kg−1 of a 21-aminosteroid (U74389G) or saline every 6 h for 2 or 3 days, respectively. Vertical bars are s.e.mean. *P<0.05 compared with the control.
Figure 3.

Effect of acute moderate hypoxia and of turpentine-induced inflammatory reaction (INFLAMM) on hepatic lipid peroxidation, as assessed by the measure of the amount of thiobarbituric acid reactive substances, in rabbits pre-treated with 3 mg kg−1 of a 21-aminosteroid (U74389G) or saline every 6 h for 2 or 3 days, respectively. Vertical bars are s.e.mean. *P<0.05 compared with the control.
Pre-treatment with U74389G prevented the hypoxia-induced decrease in theophylline clearance (Table 1), and as a consequence, theophylline plasma concentrations were close to control values, as was theophylline AUC0-∞. In hypoxic animals pre-treated with U74389G, the amount of total hepatic P450 was 40% greater than in control rabbits (P<0.05), i.e. U74389G not only hampered hypoxia-induced down-regulation of P450 but increased it (Figure 2). In addition, the administration of U74389G hindered the hypoxia-induced increase in hepatic TBARS (Figure 3).
Effect of U74389G on the inflammation-induced decrease in hepatic P450
Rectal temperature increased from basal values of 38.6±0.1°C to 40.8±0.1°C (P<0.01) 48 h after the s.c. administration of turpentine. Before the injection of turpentine, baseline concentrations of seromucoids were 43±3 mg dl−1, and increased to 189±11 mg dl−1 (P<0.001) 48 h after the injection of turpentine.
In rabbits with the inflammatory reaction, the rate of decline of theophylline plasma concentrations was decreased; as a consequence, compared with control rabbits, theophylline AUC0-∞ increased by 57% (P<0.05) (Table 1). The AUC0-∞ was enhanced because of a 55% decrease in the systemic clearance of theophylline (P<0.05). The inflammatory reaction increased theophylline apparent volume of distribution by 65% (P<0.05). In the rabbits with the inflammatory reaction, the amount of total hepatic P450 was almost 50% smaller (P<0.05) than in controls (Figure 2), and the amount of TBARS in the liver was enhanced by 44% (P<0.05) (Figure 3).
Pre-treatment of rabbits with an inflammatory reaction with U74389G prevented the decrease in theophylline systemic clearance (Table 1). As a consequence, theophylline plasma concentrations were similar to those observed in control rabbits. Pre-treatment with U74389G averted the decrease in total hepatic P450 (Figure 2), and in addition, precluded the increase in hepatic TBARS (Figure 3).
Discussion
The present results show that in vivo acute moderate hypoxia and an inflammatory reaction reduce the clearance of theophylline, decrease the amount of total hepatic P450 and increase the amount of hepatic TBARS. Pre-treatment of the rabbits with a 21-aminosteroid, U74389G, prevents the decrease in total hepatic P450 and in theophylline clearance, as well as the increase in TBARS. Moreover, U74389G not only prevents the hypoxia-induced decrease in amount of total P450 but increases it, an effect that is not due to enzyme induction, since control experiments reveal that U74389G does not increase the amount of total P450. In vivo, moderate hypoxia reduces the amounts of CYP1A1 and 1A2 proteins but increases that of CYP3A6, and as a result the clearance of theophylline is decreased (Kurdi et al., 1999). Therefore, the results of the actual study suggest that U74389G does impede the hypoxia-induced down-regulation of selected apoproteins of hepatic P450, but does not prevent the induction of other isoforms of P450, indicating that different mechanisms underline the effect of hypoxia on the different P450 apoproteins.
The decreases in P450 are closely associated to the increase in hepatic TBARS (r=0.7628; Figure 4). Since ROI can down-regulate multiple apoproteins of P450 (Karuzina & Archakov, 1994), the protective effect of U74389G may be secondary to the inhibition of ROI and/or factors that induce the formation of ROI. 21-Aminosteroids are lipophilic compounds with an anti-lipid peroxidation effect that is rather complex, thought to be due to a vitamin E-like membrane antioxidant action (Braughler et al., 1987), to the ability to scavenge ROI (Braughler et al., 1987; Althaus et al., 1993; Braughler & Pregenzer, 1989), and to the potential to chelate iron or change the redox properties of iron, in which case it will inhibit or terminate the initiation of oxidative reactions (Ryan & Petry, 1993). 21-Aminosteroids scavenge O2•− (Fabian et al., 1998), H2O2 (Horwitz et al., 1996), hydroxyl radicals (Khalil et al., 1998). NO• (Fernandez Rodriguez et al., 1997) and peroxynitrite (Fici et al., 1997). In addition, 21-aminosteroids are strong inhibitors of the activated superoxide-generating NADPH oxidase system of neutrophils (Thomas et al., 1993). Finally, 21-aminosteroids elicit a membrane-stabilising effect (Wang et al., 1996), and block neutrophil infiltration (Palma-Vargas et al., 1996). All these effects may have contributed to the present results.
Figure 4.
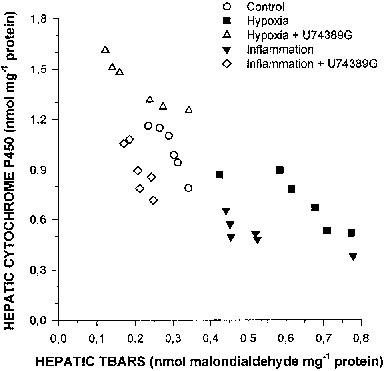
Changes in the amount of total hepatic cytochrome P450 as a function of the amount of hepatic thiobarbituric acid reactive substances (TBARS) in control rabbits, rabbits with acute moderate hypoxia pre-treated or not with 3 mg kg−1 every 6 h of a 21-aminosteroid (U74389G), and rabbits with a turpentine-induced acute inflammatory reaction pre-treated or not with 3 mg kg−1 every 6 h of a 21-aminosteroid (U74389G).
Hypoxic conditions increase the formation of ROI in many tissues including the liver (Proulx & du Souich, 1995a; El-Bassiouni et al., 1998), and since pre-treatment with vitamin A (Grover et al., 1985) or with a α-tocopherol (Lee & Clemens, 1992) prevents or diminishes hypoxia- or anoxia-reoxygenation-induced down-regulation of hepatic P450, we may postulate that U74389G prevents hypoxia-induced P450 down-regulation by scavenging ROI. Alternatively, post-haemorrhage ischaemia, ischaemia-reperfusion, and anoxia/hypoxia increase the transcription of cytokines (Helfman & Falanga, 1993), as depicted by the overexpression of IL-1, IL-2, TNF-α, and IFN-γ (Serrick et al., 1994), as well as the formation of cyclo-oxygenase (Nakhostine & Lamontagne, 1994) and lipoxygenase by-products (Kuzuya et al., 1993). Cytokines such as TNF-α, IFN-γ, IL-1 and IL-6 induce the production of ROI (Ghezzi et al., 1985; Adamson & Billings, 1992; Feng et al., 1995), as well as the formation of NO• in hepatocytes (Curran et al., 1990; Spitzer, 1994). 21-Aminosteroids inhibit the release of TNF-α, IL-2, IL-6, and IFN-γ (Salahudeen et al., 1996) by depressing the levels of mRNA encoding for these proteins (Shenkar & Abraham, 1995). Therefore, another potential mechanism of action of U74389G could be the inhibition of the release of cytokines, and consequently prevention of formation of ROI and of the down-regulation of the P450.
Endotoxins, sepsis, and acute inflammatory reactions down-regulate hepatic P450 apoproteins through transcriptional and post-transcriptional mechanisms, and cytokines and arachidonic acid metabolites are presumably involved as mediators in the cascade of events leading to the depression of the P450 (Morgan, 1997). In the inflammatory reaction, ROI are involved as second messengers and mediators of tissue damage (Winrow et al., 1993). In a model of sepsis induced by the injection of salmonella enteritidis endotoxin, pseudomona aeruginosa and bacterial lipopolysaccharide, the aminosteroids tirilazad mesylate and U74389U do not prevent the increase in TNF-α plasma concentrations (Liu et al., 1994; Loegering et al., 1995; Nakayama et al., 1998), and U74389G has no effect on mitogen-induced TNF-α and IL-6 (Buttgereit et al., 1995). On the other hand, U74389G prevents the release of TNF-α and IL-6 from macrophages of rats with an experimental pneumococcal meningitis (Lorenzl et al., 1995); another 21-aminosteroid, U64500A, reduces the production of IL-1β by monocytes stimulated by myelin (Fisher et al., 1993), and in vivo tirilazad mesylate prevents the release of TNF-α, prostacyclin, and thromboxane B2 in neonatal calves with endotoxaemia secondary to the injection of Escherichia coli lipopolysaccharide (Semrad et al., 1993). This discrepancy between reports does not allow us to postulate that U74389G prevents the P450 down-regulation induced by the inflammatory reaction by inhibiting the release of the cytokines. Alternative mechanisms of action of U74389G include a reduction in ROI, a decreased uptake of C5a, a potent chemoattractant and stimulant of mediators release by polymorphonuclear cells (Hetland et al., 1994), and a diminished production of leukotriene B4 (Gadaleta et al., 1994).
Despite the fact that the down-regulation of the P450 induced by acute moderate hypoxia or by a turpentine-induced inflammatory reaction is completely prevented by U74389G, the mechanism of action is not necessarily the same for both experimental conditions. Effectively, as demonstrated by Kurdi et al. (1999), hypoxia down-regulated CYP1A1 and 1A2 proteins but induced CYP3A6, and the inflammatory reaction depressed all three apoproteins, implying that the mechanism of action of these experimental conditions is not the same, even if in both cases ROI may directly or indirectly be involved in the P450 down-regulation as supported by the present study. The differences can be multiple, such as source and species of ROI, signalling pathways, counter-regulatory mechanisms and serum mediators.
Acknowledgments
This study was supported by the Medical Research Council of Canada (Grant No. MT-14478). The authors are grateful for the excellent technical assistance of Ms Hélène Maurice and Mrs Lucie Héroux. The authors thank the Pharmacia Upjohn Company, Kalamazoo, Michigan, for providing the U74389G, and a fellowship to support Dr Ahmed Galal.
Abbreviations
- AUC0□ – ∞
area under theophylline plasma concentration-time curve from 0 to ∞
- Cl
theophylline systemic clearance
- COLD
chronic obstructive lung disease
- FiO2
fractional concentration of inspired O2
- GSH
reduced glutathione
- H2O2
hydrogen peroxide
- NO•
nitric oxide
- O2•−
superoxide anion
- P450
cytochrome P450
- PaCO2
arterial partial pressure of CO2
- PaO2
arterial partial pressure of O2
- ROI
reactive oxygen intermediate
- t½
theophylline half-life
- VdSS
predicted theophylline volume of distribution at steady state
- z
terminal rate constant of disposition
References
- ADAMSON G.M., BILLINGS R.E. Tumor necrosis factor induced oxidative stress in isolated mouse hepatocytes. Arch. Biochem. Biophys. 1992;294:223–229. doi: 10.1016/0003-9861(92)90161-o. [DOI] [PubMed] [Google Scholar]
- ALTHAUS J.S., ANDRUS P.K., WILLIAMS C.W., VOIGTLANDER P.F., VON, CAZERS A.R., HALL E.D. The use of salicylate hydroxylation to detect hydroxyl radical generation in ischemic and traumatic brain injury. Reversal by tirilazad mesylate (U-74006F) Mol. Chem. Neuropath. 1993;20:147–162. doi: 10.1007/BF02815368. [DOI] [PubMed] [Google Scholar]
- BARAKAT M., DU SOUICH P. Effect of nifedipine on the elimination of theophylline in the rabbit subjected to hypoxia or to an inflammatory reaction. J. Pharm. Pharmacol. 1996;48:906–910. doi: 10.1111/j.2042-7158.1996.tb05999.x. [DOI] [PubMed] [Google Scholar]
- BRAUGHLER J.M., PREGENZER J.F. The 21-aminosteroid inhibitors of lipid peroxidation: reactions with lipid peroxyl and phenoxyl radicals. Free Radic. Biol. Med. 1989;7:125–130. doi: 10.1016/0891-5849(89)90003-8. [DOI] [PubMed] [Google Scholar]
- BRAUGHLER J.M., PREGENZER J.F., CHASE R.L. Novel 21-amino steroids as potent inhibitors of iron-dependent lipid peroxidation. J. Biol. Chem. 1987;262:10438–10440. [PubMed] [Google Scholar]
- BUTTGEREIT F., BRINK I., THIELE B., BURMESTER G.R., HIEPE F., HALL E.D. Effects of methylprednisolone and 21-aminosteroids on mitogen-induced interleukin-6 and tumor necrosis factor-alpha production in human peripheral blood mononuclear cells. J. Pharmacol. Exp. Ther. 1995;275:850–853. [PubMed] [Google Scholar]
- CINTI D.L., MOLDEUS P., SCHENKMAN J.B. Kinetic parameters of drug-metabolizing enzymes in Ca2+-sedimented microsomes from rat liver. Biochem. Pharmacol. 1972;21:3249–3256. doi: 10.1016/0006-2952(72)90089-5. [DOI] [PubMed] [Google Scholar]
- CURRAN R.D., BILLIAR T.R., STUEHR M.D.D., OCHOA J.B., HARBRECHT B.G., FLINT S.G., SIMMONS R.L. Multiple cytokines are required to induce hepatocyte nitric oxide production and inhibit total protein synthesis. Ann. Surg. 1990;212:462–471. doi: 10.1097/00000658-199010000-00009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- EL-BASSIOUNI E.A., ABO-OLLO M.M., HELMY M.H., ISMAEL S., RAMADAN M.I. Changes in the defense against free radicals in the liver and plasma of the dog during hypoxia and/or halothane anaesthesia. Toxicology. 1998;128:25–34. doi: 10.1016/s0300-483x(98)00045-6. [DOI] [PubMed] [Google Scholar]
- EL-KADI A.O.S., MAURICE H., ONG H., DU SOUICH P. Down-regulation of the hepatic cytochrome P450 by an acute inflammatory reaction: implication of mediators in human and animal serum in the liver. Br. J. Pharmacol. 1997;121:1164–1170. doi: 10.1038/sj.bjp.0701232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FABIAN R.H., DEWITT D.S., KENT T.A. The 21-aminosteroid U-74389G reduces cerebral superoxide anion concentration following fluid percussion injury of the brain. J. Neurotrauma. 1998;15:433–440. doi: 10.1089/neu.1998.15.433. [DOI] [PubMed] [Google Scholar]
- FENG W., RIVARD J.J., GANSER J.A., LEBIEN T.W., NATH K.A., MUELLER D.L., BEHRENS T.W. Bcl-xL rescues WEHI 231 B lymphocytes from oxidant-mediated death following diverse apoptotic stimuli. J. Immunol. 1995;155:66–75. [PubMed] [Google Scholar]
- FERNANDEZ RODRIGUEZ M.P., BELMONTE A., MEIZOSO M.J., GARCIA-NOVIO M., GARCIA-IGLESIAS E. Effect of tirilazad on brain nitric oxide synthase activity during cerebral ischemia in rats. Pharmacology. 1997;54:108–112. doi: 10.1159/000139476. [DOI] [PubMed] [Google Scholar]
- FICI G.J., ALTHAUS J.S., VOIGTLANDER P.F. VON. Effects of lazaroids and a peroxynitrite scavenger in a cell model of peroxynitrite toxicity. Free Rad. Biol. Med. 1997;22:223–228. doi: 10.1016/s0891-5849(96)00296-1. [DOI] [PubMed] [Google Scholar]
- FISHER M., PLANTE G.M., DOYLE E.M. Inhibition of inflammatory cell-mediated myelin oxidation and interleukin-1 beta generation by a 21-aminosteroid, U74500A. J. Neurol. Sci. 1993;119:189–194. doi: 10.1016/0022-510x(93)90133-j. [DOI] [PubMed] [Google Scholar]
- GADALETA D., VERMA M., DAVIS J.M. Inhibition of neutrophil leukotriene generation by the 21-aminosteroid, U-74389F. J. Surg. Res. 1994;57:233–237. doi: 10.1006/jsre.1994.1137. [DOI] [PubMed] [Google Scholar]
- GIBALDI M., PERRIER D.Noncompartmental analysis based on statistical moment theory Pharmacokinetics 1982New York: Marcel Dekker, Inc; 409–417.ed. Swarbrick J. [Google Scholar]
- GESS B., SCHRICKER K., PFEIFER M., KURTZ A. Acute hypoxia upregulates NOS gene expression in rats. Am. J. Physiol. 1997;273:R905–R910. doi: 10.1152/ajpregu.1997.273.3.R905. [DOI] [PubMed] [Google Scholar]
- GHEZZI P., BIANCHI M., GIANERA L., LANDOLFO S., SALMONA M. Role of reactive oxygen intermediates in the interferon-mediated depression of hepatic drug metabolism and protective effect of N-acetylcysteine in mice. Cancer Res. 1985;45:3444–3447. [PubMed] [Google Scholar]
- GROVER S.K., SRIVASTAVA K.K., SINGH V.S., MISRA U.K. Effect of vitamin A on hepatic microsomal drug metabolising enzymes activity in rats exposed to acute hypoxia. Int. J. Vitam. Nutr. Res. 1985;55:391–393. [PubMed] [Google Scholar]
- HELFMAN T., FALANGA V. Gene expression in low oxygen tension. Am. J. Med. Sci. 1993;306:37–41. doi: 10.1097/00000441-199307000-00010. [DOI] [PubMed] [Google Scholar]
- HENDELES L., MASSANARI M., WEINBERGER M.Theophylline Applied pharmacokinetics. Principles of therapeutic drug monitoring 1986Spokane, WA: Applied Therapeutics, Inc; 1105–1118.eds. Evans, W.E., Schentag, J.J. & Jusko, W.J. [Google Scholar]
- HETLAND G., DEL ZOPPO G.L., MORI E., THOMAS W.S., HUGLI T.E. Uptake of C5a polymorphonuclear leukocytes (PMNs) after focal cerebral ischemia. I. Effect of tirilazad mesylate intervention on C5a uptake by PMNs. Immunopharmacol. 1994;27:191–198. doi: 10.1016/0162-3109(94)90015-9. [DOI] [PubMed] [Google Scholar]
- HORWITZ L.D., WALLNER J.S., DECKER D.E., BUXSER S.E. Efficacy of lipid soluble, membrane-protective agents against hydrogen peroxide cytotoxicity in cardiac myocytes. Free Rad. Biol. Med. 1996;21:743–753. doi: 10.1016/0891-5849(96)00177-3. [DOI] [PubMed] [Google Scholar]
- KARUZINA I.I., ARCHAKOV A.I. The oxidative inactivation of cytochrome P450 in monooxygenase reactions. Free Rad. Biol. Med. 1994;16:73–97. doi: 10.1016/0891-5849(94)90245-3. [DOI] [PubMed] [Google Scholar]
- KHALIL A., FORTUN A., HEBERT S., JAY-GERIN J.P., EL ABBOUYI A., WALLACH J., FULOP T., JR Novel 21-aminosteroid U-74389G inhibits low-density lipoprotein peroxidation induced by .OH and O2-. free radicals. Life Sci. 1998;63:769–779. doi: 10.1016/s0024-3205(98)00332-4. [DOI] [PubMed] [Google Scholar]
- KURDI J., MAURICE H., EL-KADI A.O.S., ONG H., DALKARA S., BÉLANGER P.M., DU SOUICH P. Effect of hypoxia alone or combined with inflammation and 3-methylcholanthrene on hepatic cytochrome P450 in conscious rabbits. Br. J. Pharmacol. 1999;12:365–373. doi: 10.1038/sj.bjp.0702795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KUZUYA T., HOSHIDA S., KIM Y., OE H., HORI M., KAMADA T., TADA M. Free radical generation coupled with arachidonate lipoxygenase reaction relates to reoxygenation induced myocardial cell injury. Cardiovasc. Res. 1993;27:1056–1060. doi: 10.1093/cvr/27.6.1056. [DOI] [PubMed] [Google Scholar]
- LEE S.M., CLEMENS M.G. Effect of alpha-tocopherol on hepatic mixed function oxidases in hepatic ischemia/referfusion. Hepatology. 1992;15:276–281. doi: 10.1002/hep.1840150217. [DOI] [PubMed] [Google Scholar]
- LETARTE L., DU SOUICH P. Influence of hypercapnia and/or hypoxemia and metabolic acidosis on theophylline kinetics in conscious rabbits. Am. Rev. Respir. Dis. 1984;129:762–766. doi: 10.1164/arrd.1984.129.5.762. [DOI] [PubMed] [Google Scholar]
- LEVITT M.A., SIEVERS R.E., WOLFE C.L. Reduction of infarct size during myocardial ischemia and reperfusion by lazaroid U-74500A, a nonglucocorticoid 21-aminosteroid. J. Cardiovasc. Pharmacol. 1994;23:136–140. doi: 10.1097/00005344-199401000-00019. [DOI] [PubMed] [Google Scholar]
- LIU P., VONDERFECHT S.L., MCGUIRE G.M., FISHER M.A., FARHOOD A., JAESCHKE H. The 21-aminosteroid tirilazad mesylate protects against endotoxin shock and acute liver failure in rats. J. Pharmacol. Exp. Ther. 1994;271:438–445. [PubMed] [Google Scholar]
- LOEGERING D.J., RICHARD C.A., LEAHY K.P., DAVISON C.B. The antioxidant, U74389, ameliorates the depression of vascular reactivity caused by lipopolysaccharide. Life Sci. 1995;57:PL321–326. doi: 10.1016/0024-3205(95)02172-f. [DOI] [PubMed] [Google Scholar]
- LORENZL S., KOEDEL U., FREI K., BERNATOWICZ A., FONTANA A., PFISTER H.W. Protective effect of a 21-aminosteroid during experimental pneumococcal meningitis. J. Infect. Dis. 1995;172:113–118. doi: 10.1093/infdis/172.1.113. [DOI] [PubMed] [Google Scholar]
- LOWRY O.H., ROSEBROUGH N.J., FARR A.L., RANDALL R.J. Protein measurement with the folin phenol reagent. J. Biol. Chem. 1951;193:265–275. [PubMed] [Google Scholar]
- MATUSCHAK G.M., JOHANNS C.A., CHEN Z., GAYNOR J., LECHNER A.J. Brief hypoxic stress down-regulates E. coli-induced IL-1α and IL-1β gene expression in perfused liver. Am. J. Physiol. 1996;271:R1311–R1318. doi: 10.1152/ajpregu.1996.271.5.R1311. [DOI] [PubMed] [Google Scholar]
- MEANS E.D. 21-Aminosteroids (‘lazaroids') Adv. Exp. Med. Biol. 1994;366:307–312. [PubMed] [Google Scholar]
- MINOR T., ISSELHARD W., BERGHAUS K. Parenchymal and vascular endothelial cell injury in the hypoxic and reperfused rat liver. Evidence for superoxide anion generation by perfusion with ferricytochrome c. Biomed. Pharmacother. 1993;47:213–218. doi: 10.1016/0753-3322(93)90059-t. [DOI] [PubMed] [Google Scholar]
- MORGAN E.T. Regulation of cytochromes P450 during inflammation and infection. Drug. Metab. Rev. 1997;29:1129–1188. doi: 10.3109/03602539709002246. [DOI] [PubMed] [Google Scholar]
- NAKAYAMA M., HASEGAWA N., OKA Y., LUTZKE B., MCCALL J.M., RAFFIN T.A. Effects of the lazaroid, tirilazad mesylate, on sepsis-induced acute lung injury in minipigs. Crit. Care Med. 1998;26:538–547. doi: 10.1097/00003246-199803000-00029. [DOI] [PubMed] [Google Scholar]
- NAKHOSTINE N., LAMONTAGNE D. Contribution of prostaglandins in hypoxia-induced vasodilation in isolated rabbit hearts. Relation to adenosine and KATP channels. Pflügers Arch. 1994;428:526–532. doi: 10.1007/BF00374574. [DOI] [PubMed] [Google Scholar]
- OHKAWA H., OHISHI N., YAGI K. Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal. Biochem. 1979;95:351–358. doi: 10.1016/0003-2697(79)90738-3. [DOI] [PubMed] [Google Scholar]
- OMURA T., SATO R. The carbon monoxide-binding pigment of liver microsomes. 1. Evidence for its hemoprotein nature. J. Biol. Chem. 1964;239:2370–2378. [PubMed] [Google Scholar]
- PALMA-VARGAS J.M., TOLEDO A.H., GARCIA-CRIADO F.J., MISAWA K., LOPEZ-NEBLINA F., TOLEDO-PEREYRA L.H. 21-Aminosteroids block neutrophil infiltration and provide liver protection independent of NO2-/NO3- levels. J. Surg. Res. 1996;66:131–137. doi: 10.1006/jsre.1996.0384. [DOI] [PubMed] [Google Scholar]
- PARENT C., BÉLANGER P.M., JUTRAS L., DU SOUICH P. Effect of inflammation on the rabbit hepatic cytochrome P-450 isoenzymes. Alterations in the kinetics and dynamics of tolbutamide. J. Pharmacol. Exp. Ther. 1992;261:780–787. [PubMed] [Google Scholar]
- PROULX M., DU SOUICH P. Acute moderate hypoxia in conscious rabbits: effect on hepatic cytochrome P450 and on reactive species. J. Pharm. Pharmacol. 1995a;47:392–397. doi: 10.1111/j.2042-7158.1995.tb05817.x. [DOI] [PubMed] [Google Scholar]
- PROULX M., DU SOUICH P. Inflammation-induced decrease in cytochrome P450 in conscious rabbits is accompanied by an increase in hepatic oxidative stress. Res. Commun. Mol. Pathol. Pharmacol. 1995b;87:221–236. [PubMed] [Google Scholar]
- RYAN T.P., PETRY T.W. The effects of 21-aminosteroids on the redox status of iron in solution. Arch. Biochem. Biophys. 1993;300:699–704. doi: 10.1006/abbi.1993.1097. [DOI] [PubMed] [Google Scholar]
- SALAHUDEEN A., WANG C., MCDANIEL O., LAGOO-DENADYALAN S., BIGLER S., BARBER H. Antioxidant lazaroid U-74006F improves renal function and reduces the expression of cytokines, inducible nitric oxide synthase, and MHC antigens in a syngeneic renal transplant model. Partial support for the response-to-injury hypothesis. Transplantation. 1996;62:1628–1633. doi: 10.1097/00007890-199612150-00017. [DOI] [PubMed] [Google Scholar]
- SEMRAD S.D., ROSE M.L., ADAMS J.L. Effect of tirilazad mesylate (U74006F) on eicosanoid and tumor necrosis factor generation in healthy and endotoxemic neonatal calves. Cir. Shock. 1993;40:235–242. [PubMed] [Google Scholar]
- SERRICK C., ADOUMIE R., GIAID A., SHENNIB H. The early release of interleukin-2, tumor necrosis factor-alpha and interferon-gamma after ischemia reperfusion injury in the lung allograft. Transplantation. 1994;58:1158–1162. [PubMed] [Google Scholar]
- SHENKAR R., ABRAHAM E. Effects of treatment with the 21-aminosteroid, U7438F, on pulmonary cytokine expression following hemorrhage and resuscitation. Crit. Care Med. 1995;23:132–139. doi: 10.1097/00003246-199501000-00022. [DOI] [PubMed] [Google Scholar]
- SPITZER J.A. Cytokine stimulation of nitric oxide formation and differential regulation in hepatocytes and nonparenchymal cells of endotoxemic rats. Hepatology. 1994;19:217–228. [PubMed] [Google Scholar]
- THOMAS P.D., MAO G.D., RABINOVITCH A., POZNANSKY M.J. Inhibition of superoxide-generating NADPH oxidase of human neutrophils by lazaroids (21-aminosteroids and 2-methylaminochromans) Biochem. Pharmacol. 1993;45:241–251. doi: 10.1016/0006-2952(93)90398-g. [DOI] [PubMed] [Google Scholar]
- WANG Y., MATHEWS W.R., GUIDO D.M., JAESCHKE H. The 21-aminosteroid tirilazad mesylate protects against liver via membrane stabilization not inhibition of lipid peroxidation. J. Pharmacol. Exp. Ther. 1996;277:714–720. [PubMed] [Google Scholar]
- WINROW V.R., WINYARD P.G., MORRIS C.J., BLAKE D.R. Free radicals in inflammation: second messengers and mediators of tissue destruction. Br. Med. Bull. 1993;49:506–522. doi: 10.1093/oxfordjournals.bmb.a072627. [DOI] [PubMed] [Google Scholar]
- ZHANG H., SPAPEN H., MANIKIS P., ROGIERS P., METZ G., BUURMAN W.A., VINCENT J.L. Tirilazad mesylate (U-74006F) inhibits effects of endotoxin in dogs. Am. J. Physiol. 1995;268:H1847–H1855. doi: 10.1152/ajpheart.1995.268.5.H1847. [DOI] [PubMed] [Google Scholar]


