Abstract
The cardioprotective effect of N-[(1S, trans)-2-hydroxycyclopentyl]adenosine (GR79236), an adenosine A1 receptor agonist, was compared with that produced by ischaemic preconditioning in an anaesthetized rabbit model of myocardial ischaemia and reperfusion. In addition, we examined the effect of different body core temperatures on GR79236- or ischaemic preconditioning-induced cardioprotection when administered prior to ischaemia, and on cardioprotection induced by GR79236 administered 10 min prior to the onset of reperfusion.
When rabbits were subjected to 30 min occlusion of the left coronary artery, followed by 2 h reperfusion, GR79236 (3×10−8 mol kg−1 i.v. (10.5 μg kg−1 i.v.)) or ischaemic preconditioning (5 min ischaemia followed by 5 min reperfusion), administered or applied 10 min prior to the occlusion, significantly limited the development of infarction. The cardioprotective effect of ischaemic preconditioning was significantly greater than that seen after administration of GR79236. Pre-treatment with the selective adenosine A1 receptor antagonist 8-cyclopentyl-1,3-dipropylxanthine (DPCPX, 3.3×10−6 mol kg−1 (1 mg kg−1 i.v.)), prevented the cardioprotective effect of GR79236, but not that of ischaemic preconditioning.
Maintaining body core temperature at 38.5°C rather than at 37.0°C did not influence infarct size in control groups of rabbits, but reduced the cardioprotective effect of GR79236 when administered 10 min prior to occlusion or 10 min prior to the onset of reperfusion. The cardioprotective effect of ischaemic preconditioning was not temperature-dependent.
In conclusion, myocardial protection conferred by GR79236 in anaesthetized rabbits is mediated via adenosine A1 receptors. Myocardial protection can be conferred when GR79236 is administered before the onset of ischaemia or reperfusion, and is reduced when body core temperature is maintained at 38.5°C rather than at 37.0°C. In contrast, myocardial protection conferred by ischaemic preconditioning is not reduced by adenosine A1 receptor blockade, or by maintaining body core temperature at 38.5°C rather than at 37.0°C. These findings point to distinct differences in the mechanisms of induction of myocardial protection by adenosine A1 receptor agonist and ischaemic preconditioning. They also highlight the need for careful control of body core temperature when investigating the phenomenon of cardioprotection.
Keywords: Myocardial protection, ischaemic preconditioning, GR79236, adenosine A1 receptor agonist
Introduction
Since its first description by Murry et al., 1986, there has been great effort to elucidate the mechanism by which ischaemic preconditioning confers its cardioprotective effect. The phenomenon of myocardial ischaemic preconditioning, whereby a brief period of ischaemia, followed by reperfusion, attenuates the myocardial ischaemia-reperfusion injury associated with a more sustained period of ischaemia, is a powerful protective mechanism which has been demonstrated in all animal models yet studied. Ischaemic preconditioning induces two phases of myocardial protection. The initial, acute, phase of protection described by Murry et al. (1986) lasts 1–2 h (Murry et al., 1991; van Winkle et al., 1991; Li et al., 1992; Miura et al., 1992). This is followed by a delayed phase, or second window, of protection (Marber et al., 1993; Kuzuya et al., 1993), that develops 12–24 h after ischaemic preconditioning, and lasts for at least a further 48 h (Baxter et al., 1997).
Early studies showed that the protective properties of the acute phase of ischaemic preconditioning can be prevented by pre-treatment with non-selective adenosine receptor antagonists (Liu et al., 1991; Downey et al., 1993). Pre-treatment with adenosine or selective A1, but not A2, receptor agonists, mimics the infarction-limiting effect of ischaemic preconditioning (Downey et al., 1993). Taken together, these data suggest a role for adenosine A1 receptors in the process of ischaemic preconditioning, at least in the rabbit, with evidence for a similar role in the dog and pig (Grover et al., 1992; Martin et al., 1993; Auchampach & Gross, 1993; van Winkle et al., 1992). Adenosine receptors have also been implicated in triggering the delayed phase of myocardial protection (Baxter et al., 1994).
N-[(1S, trans)-2-hydroxycyclopentyl]adenosine (GR79236) is a potent and selective agonist at adenosine A1 receptors (Gurden et al., 1993), and induces both the classic, acute protection, and the delayed phase of protection, in a rabbit model of myocardial ischaemia and reperfusion (Travers et al., 1998). GR79236 also reduces infarct size in a pig model of myocardial ischaemia and reperfusion (Louttit et al., 1999). Although GR79236 induces cardioprotection in the rabbit model, we have noted, at times, that the extent of protection seen can be variable, particularly during periods of high ambient temperature. Interestingly, McClanahan et al. (1994) have shown that pentostatin, an inhibitor of adenosine deaminase, reduced infarct size in pigs only when combined with mild hypothermia, suggesting that the cardioprotective effect of adenosine can be influenced by temperature. We therefore investigated whether variations in body core temperature could be responsible for the variation in cardioprotective activity sometimes seen with GR79236 in anaesthetized rabbits. In addition, we investigated whether myocardial protection induced by ischaemic preconditioning, the mechanism which GR79236 is assumed to mimic, is similarly influenced by body core temperature.
The cardioprotective effects of adenosine A1 receptor agonists administered prior to ischaemia are well established. However, the evidence to suggest that they are beneficial when administered during ischaemia, just prior to reperfusion, is controversial. Using an anaesthetized rabbit model, Thornton et al. (1992) were not able to show protection when a 5 min infusion of N6-(phenyl-2R-isopropyl)-adenosine (R-PIA) was administered, commencing 2 min prior to reperfusion. However, using a different A1 receptor agonist, cyclopentyladenosine, infused for 65 min, starting 5 min prior to reperfusion in the anaesthetized rabbit, Norton et al. (1992) did show a reduction in infarct size. In addition, we have recently shown that GR79236 reduces infarct size when administered 10 min prior to the onset of reperfusion, in an anaesthetized pig model of myocardial ischaemic and reperfusion (Louttit et al., 1999). We have therefore investigated the effect of GR79236, administered 10 min prior to the onset of reperfusion, on the development of infarction in the rabbit model, and determined whether any protective activity seen is influenced by temperature.
Methods
Surgical procedures
This research complied with national legislation and with Company policy on the Care and Use of Animals and with related codes of practice.
Male New Zealand White rabbits (2.4–3.2 kg, Charles River Ltd.) were anaesthetized to effect with pentobarbitone sodium (approximately 45 mg kg−1 i.v.). A tracheotomy was performed and the animal artificially ventilated with room air (ventilation volume 3.5 ml kg−1; ventilation rate of 60 inspirations min−1) supplemented with oxygen, to maintain blood pH and blood gas tensions close to those seen in conscious rabbits (unpublished observations; pH 7.34; PCO2 36.4; PO2 91.6; n=44). A femoral artery was cannulated to allow measurement of arterial pressure from which heart rate was derived, and for the withdrawal of arterial blood for the analysis of blood pH and blood gas tensions. A catheter was placed in the left jugular vein for the administration of anaesthetic, as required, and drugs. The heart was exposed via a left lateral thoracotomy in the 5th intercostal space, and a suture passed under a branch of the left coronary artery to form a snare so that a reversible occlusion could be applied. The thoracotomy was covered, and remained so, with the exception of the time when the snare around the coronary artery was being tightened or released, to minimise heat loss. Rabbit body core temperature was monitored using a thermistor (Digitron, RS Components; calibrated measurement uncertainty±0.3°C) placed in the colon, and carefully maintained (±0.3°C) at either 37.0°C or 38.5°C by manually adjusting the heat setting of a homeothermic blanket control unit.
Experimental design
After a stabilization period of approximately 30 min, an intravenous bolus dose of heparin (250 iu kg−1 i.v.) was administered to prevent thrombus formation during the experimental protocol. Myocardial ischaemia was induced by tightening the snare around the coronary artery for a period of 30 min. Blood flow was restored by release of the snare, and the myocardium reperfused for 2 h. Twenty minutes before the end of the reperfusion period, a second bolus dose of heparin (500 iu kg−1 i.v.) was administered.
Three series of experiments were performed; the experimental protocols are shown in Figure 1. In the first group of experiments (Series 1), the effect of the adenosine A1 receptor antagonist 8-cyclopentyl-1,3-dipropylxanthine (DPCPX, 3.3×10−6 mol kg−1 i.v. (1 mg kg−1 i.v.)), or its vehicle (1 ml kg−1 i.v.), on the cardioprotective effect of GR79236 (3×10−8 mol kg−1 i.v. (10.5 μg kg−1 i.v.)) or ischaemic preconditioning (5 min ischaemia followed by 5 min reperfusion), was investigated in rabbits in which body core temperature was maintained at 37.0±0.3°C. GR79236 or its vehicle (saline; 1 ml i.v.) was administered, or ischaemic preconditioning applied, 10 min prior to coronary artery occlusion. DPCPX or its vehicle was administered 10 min prior to the administration of GR79236, saline, or the onset of ischaemic preconditioning. In a subsequent group of experiments (Series 2), the effect of body core temperature on GR79236- or ischaemic preconditioning-induced cardioprotection was examined. Rabbits were maintained (±0.3°C) at either 37.0 or 38.5°C, and GR79236 or saline, or ischaemic preconditioning, applied 10 min prior to occlusion. Finally, in the third series of experiments (Series 3), GR79236 or saline was administered 10 min prior to the onset of reperfusion in rabbits maintained (±0.3°C) at either 37.0 or 38.5°C.
Figure 1.
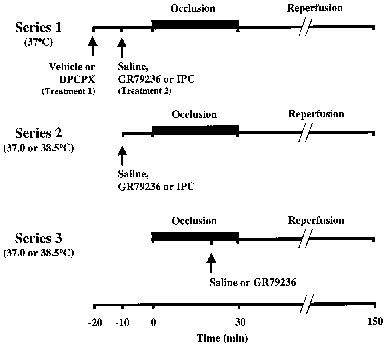
Experimental protocols for Series 1, 2 and 3 experiments. Ischaemic preconditioning (IPC) is 5 min of ischaemia followed by 5 min reperfusion.
All experiments were recorded and analysed using a Modular Instruments Inc. data acquisition system and the Bioreport haemodynamics software package. Drug-induced change in mean arterial pressure (MAP) or heart rate (HR) was expressed as per cent change from the pre-dose value.
At the end of the experimental protocol, hearts were removed and the aorta perfused retrogradely with heparinized saline to flush the coronary vasculature of residual blood. The coronary artery was re-occluded and the coronary vasculature perfused with a suspension of fluorescent Zn/Cd particles (1–10 μm diameter). After the hearts had been frozen, they were cut into 2 mm thick slices parallel with the atrioventricular groove, and the right ventricle discarded. The slices were allowed to thaw and then incubated in a 1% solution of triphenyltetrazolium chloride (TTC) in phosphate buffered saline at 37.0°C for 10 min.
Infarcted myocardium remained unstained by the TTC. Any area of the heart not containing fluorescent particles was defined as the risk zone for infarction. The areas of infarcted tissue, risk zone and total ventricular area was measured using digital planimetry. Infarct size was expressed as a percentage of the risk zone, and the risk zone as a percentage of the total left ventricular area.
Drugs and reagents
GR79236 was synthesized in Medicinal Chemistry, Glaxo Research and Development, dissolved in sterile saline, and administered over a period of 1 min. DPCPX was obtained from Research Biochemicals International (RBI, Sigma-Aldrich, Poole, Dorset), dissolved in 200 μl DMSO and 200 μl 1 M NaOH, and made up to volume using sterile saline (total volume of DMSO and 1 M NaOH used amounted to 6% v v−1). One ml kg−1 i.v. of the DPCPX solution was administered over a period of 2 min. Sodium pentobarbitone (Sagatal) was obtained from Rhone-Merieux (Harlow, Essex), heparin from Leo Pharmaceuticals (Princess Risborough, Bucks). Triphenyltetrazolium chloride and phosphate buffered saline was obtained from Sigma-Aldrich (Poole, Dorset), and zinc-cadmium sulphide fluorescent particles were obtained from Duke Scientific (Christison Ltd., Gateshead, Tyne and Wear), and suspended in saline immediately prior to use.
Statistical analysis
Values shown are arithmetic mean±s.e.mean where n represents the number of observations. The data were analysed using analysis of variance. In the analysis of MAP and HR the data were adjusted to account for the basal measurements. A P value of <0.05 was considered to indicate a statistically significant difference.
Results
Series 1 experiments–effect of DPCPX on cardioprotection conferred by GR79236 or ischaemic preconditioning
Haemodynamics
Mean arterial pressure (MAP) and heart rate (HR) values at the beginning of the experimental protocol, and at time points throughout the experiment, are shown in Table 1. Changes in MAP or HR in response to the different treatments are shown in Figure 2.
Table 1.
Haemodynamic values for Series 1 experiments
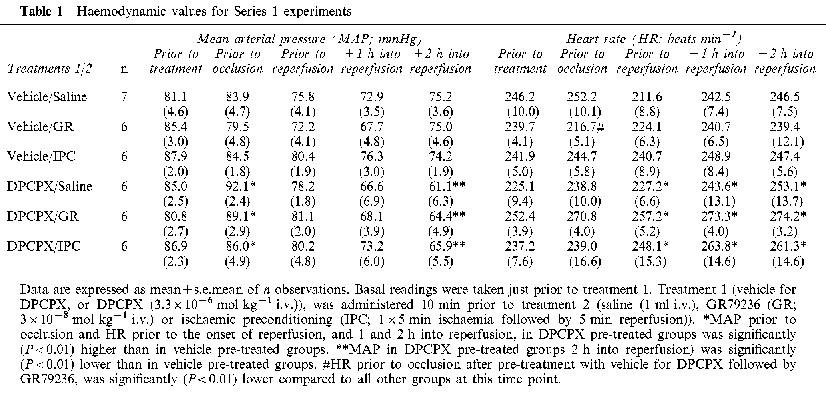
Figure 2.
Effect of DPCPX (3.3×10−6 mol kg−1 i.v.), or its vehicle (1 ml kg−1 i.v.), on mean arterial pressure (MAP) and heart rate (HR), and on peak changes in MAP and HR produced following administration of saline (1 ml i.v.), GR79236 (3×10−8 mol kg−1 i.v.), or application of ischaemic preconditioning (5 min ischaemia followed by 5 min reperfusion). In each pair of histobars, the first bar shows the effect of treatment 1, and the second bar shows the effect of treatment 2. Data are expressed as mean percentage peak change in MAP and HR±s.e.mean (n=6–7). *Pre-treatment with DPCPX produced a significant (P<0.01) increase in MAP and HR compared to pre-treatment with vehicle. #GR79236-induced peak reductions in MAP and HR after pre-treatment with DPCPX are significantly (P<0.01) smaller than after pre-treatment with vehicle.
There were no statistically significant differences in basal MAP or HR values between the groups. Intravenous administration of the vehicle for DPCPX, or the vehicle (saline) for GR79236, had little effect on MAP or HR. In rabbits pre-treated with the vehicle for DPCPX, GR79236 reduced MAP and HR by approximately 15%. The effect on MAP was transient and had returned to basal values just prior to occlusion. However, HR remained significantly (P<0.01) lower, just prior to occlusion, than in all other groups (Table 1). Haemodynamic variations caused by the occlusion-reperfusion protocol prevented us making an assessment of the duration of action of GR79236. However, in previous studies, we have found that GR79236-induced bradycardia lasts for approximately 30 min (unpublished observation). Also in rabbits pre-treated with vehicle for DPCPX, ischaemic preconditioning markedly reduced MAP in most instances. However, this effect was transient, and MAP had returned to basal levels just prior to occlusion. HR was little changed by the ischaemic preconditioning protocol. When compared with the effects of its vehicle, DPCPX caused a significant (P<0.01) increase in MAP and HR, and HR remained significantly (P<0.01) elevated prior to occlusion. However, there were no significant differences in MAP or HR between the groups of saline- or GR79236-treated, or ischaemically preconditioned groups of rabbits (Table 1). Pre-treatment with DPCPX prevented GR79236-induced reductions in MAP and HR, but did not influence haemodynamic changes observed after treatment with saline or during ischaemic preconditioning (Figure 2).
In rabbits pre-treated with the vehicle for DPCPX, MAP tended to decrease, and HR tended to increase, over the course of the experiments, but these effects did not reach statistical significance. In rabbits pre-treated with DPCPX, similar trends were observed, but MAP was significantly (P<0.01) lower 2 h into repefusion, and HR significantly (P<0.01) higher just prior to the onset of reperfusion, and 1 and 2 h into reperfusion, compared to those pre-treated with vehicle for DPCPX.
Infarct size
Left coronary artery occlusion rendered approximately 28% of the left ventricle at risk of infarction. There were no significant differences in risk zone size between the groups (Table 2). DPCPX had little effect on infarct size in its own right. GR79236 produced a significant (P<0.01) limitation of the development of infarction, as did ischaemic preconditioning. The limitation in the development of infarction by ischaemic preconditioning was significantly (P<0.01) greater than that seen after administration of GR79236. Myocardial protection induced by GR79236, but not that by ischaemic preconditioning, was prevented by pre-treatment with DPCPX. These results are shown in Figure 3.
Table 2.
Risk zone size in Series 1, Series 2 and Series 3 experiments
Figure 3.
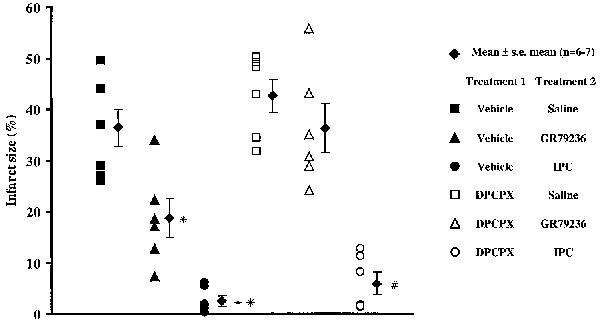
Effect of pre-treatment with DPCPX (3.3×10−6 mol kg−1 i.v.) or its vehicle (1 ml kg−1 i.v.) on the development of infarction after treatment with GR79236 (3×10−8 mol kg−1 i.v.) or ischaemic preconditioning (IPC; 5 min ischaemia, followed by 5 min reperfusion). The filled diamonds show mean infarct size expressed as a percentage of the risk zone, the error bars represent s.e.mean. All other symbols show infarct size for each individual rabbit. *In the presence of the vehicle for DPCPX, GR79236 evoked a significant (<0.01) limitation in the development of infarction compared to saline. **In the presence of vehicle for DPCPX, IPC evoked a significant (P<0.01) limitation in the development of infarction compared to GR79236. #In the presence of DPCPX, IPC evoked a significant (P<0.01) limitation in the development of infarction compared to GR79236 and saline.
Series 2 experiments–influence of body core temperature on cardioprotection induced by GR79236 administered prior to occlusion, or by ischaemic preconditioning
Haemodynamics
MAP and HR values at the beginning of the experimental protocol, and at time points throughout the duration of the experiment, are shown in Table 3. Changes in MAP or HR in response to the different treatments are shown in Figure 4.
Table 3.
Haemodynamic values for Series 2 experiments
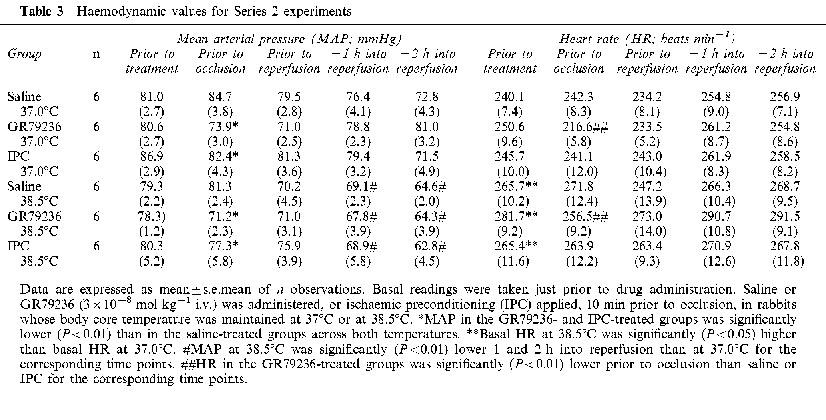
Figure 4.
Haemodynamic effect of saline (1 ml i.v.) GR79236 (3×10−8 mol kg−1 i.v.) or ischaemic preconditioning (5 min ischaemia followed by 5 min reperfusion) administered prior to occlusion, and of saline (1 ml i.v.) or GR79236 (3×10−8 mol kg−1 i.v.) administered 10 min prior to the onset of reperfusion, in rabbits whose body core temperature was maintained at 37.0°C or at 38.5°C. Data are expressed as mean percentage peak change in MAP or HR±s.e.mean (n=6).
There were no significant differences in basal MAP values between the groups. Basal HR was significantly (P<0.05) higher in the groups of rabbits in which body temperature was maintained at 38.5°C compared to those kept at 37.0°C (Table 3). Intravenous administration of GR79236 reduced MAP and HR, whereas ischaemic preconditioning produced transient reductions in MAP, but had little (<10%) effect on HR (Figure 4). These effects on MAP and HR were not significantly influenced by body temperature. MAP was still significantly (P<0.01) lower just prior to occlusion after treatment with GR79236 or after ischaemic preconditioning than in vehicle-treated groups of rabbits, and HR was significantly (P<0.01) lower in GR79236-treated groups of rabbits than in those that received vehicle or ischaemic preconditioning (Table 3). MAP tended to decline over the course of the experiment in all groups, and was significantly (P<0.01) lower 1 and 2 h into reperfusion in rabbits where body temperature was maintained at 38.5°C, compared with that seen in those maintained at 37.0°C.
Infarct size
Occlusion of the left coronary artery for 30 min rendered approximately 30% of the left ventricle at risk of infarction. There were no significant differences in risk zone size between the groups (Table 2). Infarct size was not significantly different in saline-treated rabbits where body temperature was maintained at 37.0 or at 38.5°C. When body temperature was maintained at 37.0°C, GR79236 significantly (P<0.05) limited the development of infarction. However, when body temperature was maintained at 38.5°C, infarct size was not significantly different from that in the saline-treated control groups of rabbits at this temperature (Figure 5). In contrast to the cardioprotective effect of GR79236, limitation of infarct development conferred by ischaemic preconditioning was unaffected by temperature. Ischaemic preconditioning-induced protection was significantly (P<0.05) greater than that induced by GR79236 at both temperatures tested (Figure 5).
Figure 5.
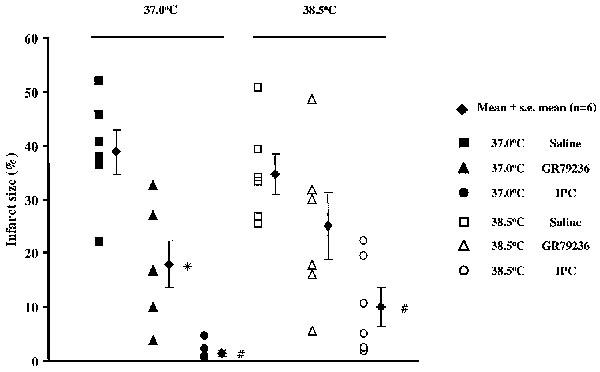
Effect of saline (1 ml i.v.), GR79236 (3×10−8 mol kg−1 i.v.), or ischaemic preconditioning (IPC; 5 min ischaemia followed by 5 min reperfusion) on the development of infarction when administered or applied 10 min prior to occlusion, and the influence of maintaining body core temperature at 37.0°C or at 38.5°C. The filled diamonds show mean infarct size expressed as a percentage of the risk zone, the error bars represent s.e.mean. All other symbols show infarct size for each individual rabbit. *At 37°C GR79236 evoked a significant (P<0.05) limitation of the development of infarction compared to saline. #IPC evoked a significant (P<0.05) limitation of the development of infarction compared to GR79236 at the corresponding temperature.
Series 3 experiments–cardioprotective effect of GR79236 administered prior to reperfusion and the influence of temperature
Haemodynamics
MAP and HR values at the beginning of the experimental protocol, and at time points throughout the duration of the experiment, are shown in Table 4. Changes in MAP or HR in response to the different treatments are shown in Figure 4.
Table 4.
Haemodynamic values for Series 3 experiments
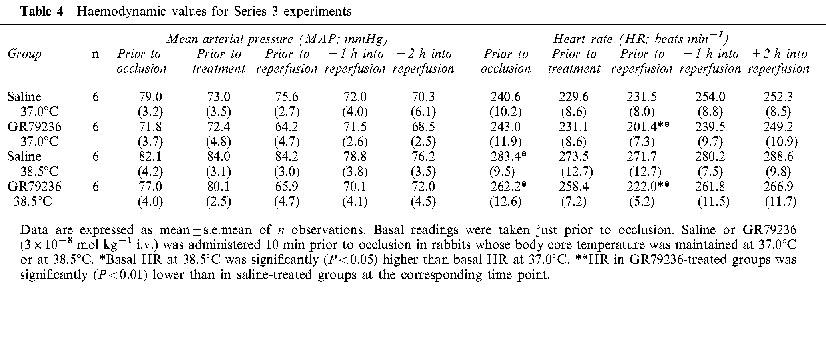
There were no significant differences in basal MAP values between the groups. Basal HR values were significantly (P<0.05) higher when body temperature was maintained at 38.5 rather than at 37.0°C (Table 4). Intravenous administration of GR79236 reduced MAP and HR, but these changes were not significantly influenced by body temperature. The reduction in MAP caused by GR79236 returned to basal levels just prior to reperfusion. However, HR remained significantly (P<0.01) lower just prior to reperfusion, than that seen in saline-treated groups of rabbits.
Infarct size
Occlusion of the left coronary artery for 30 min rendered approximately 30% of the left ventricle at risk of infarction. There were no significant differences in risk zone size between the groups (Table 2). In groups of rabbits treated with saline 10 min prior to the onset of reperfusion, development of infarction was similar whether body temperature was maintained at either 37.0 or 38.5°C. When body temperature was maintained at 37.0°C, GR79236 significantly (P<0.01) limited infarct development, but this effect was lost when body temperature was maintained at 38.5°C (Figure 6).
Figure 6.
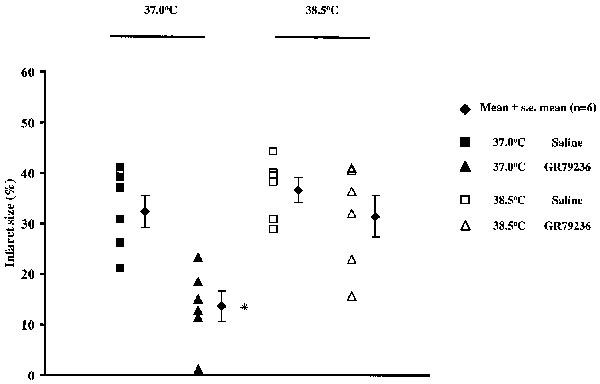
Effect of saline (1 ml i.v.) or GR79236 (3×10−8 mol kg−1 i.v.) on the development of infarction, administered 10 min prior to the onset of reperfusion, and the influence of maintaining body core temperature at 37.0°C or at 38.5°C. The filled diamonds show mean infarct size, the error bars represent s.e.mean. All other symbols show infarct size for each individual rabbit. *At 37.0°C GR79236 evoked a significant (P<0.01) limitation in the development of infarction compared to all other treatments.
Discussion
Cardioprotective effect of GR79236–comparison with ischaemic preconditioning
In keeping with the current literature describing myocardial protection induced by adenosine A1 receptor agonists (see Introduction), we have shown that GR79236 (Gurden et al., 1993), an adenosine A1 receptor agonist, has cardioprotective properties in an anaesthetized rabbit model of myocardial ischaemia and reperfusion (Travers et al., 1998). In the present study, when administered 10 min prior to occlusion, GR79236 (3×10−8 mol kg−1 i.v.) significantly limited the development of infarction, compared to the vehicle-treated control group. Similarly, and as described in the literature, ischaemic preconditioning (one 5 min episode of ischaemia followed by 5 min reperfusion), also markedly limited the development of infarction in this model. This reduction in the development of infarction with ischaemic preconditioning was significantly greater than that conferred by GR79236, suggesting that adenosine A1 receptor activation might not be the only pathway through which ischaemic preconditioning can confer myocardial protection in this model. The lesser degree of protection conferred by GR79236 is unlikely to reflect the dose used; previous (unpublished) experiments using a range of doses of GR79236 revealed that the cardioprotective effect of 1 or 3×10−8 mol kg−1 i.v. are approximately equal. Thus, the maximum protective effect of A1 receptor stimulation seems to be less than that of ischaemic preconditioning. In keeping with activation of adenosine A1 receptors, GR79236 reduced heart rate (HR), and this was associated with a fall in mean arterial pressure (MAP). The effect on HR had not fully recovered to baseline values at the time of occlusion but, in previous (unpublished) studies, we have found that the haemodynamic effects of a single bolus dose of GR79236 (3×10−8 mol kg−1 i.v.) in the anaesthetized rabbit last approximately 30 min. It seems unlikely that these haemodynamic events are responsible for the protective properties of GR79236. Changes in MAP or HR (Ytrehus et al., 1994), or rate pressure product (Chien et al., 1994), were found not to significantly influence infarct size in the rabbit. In addition, the adenosine A1 receptor agonist R-PIA, significantly limited the development of infarction even when the bradycardia and severe hypotension that it normally produced were prevented by pacing (Tsuchida et al., 1992). In contrast, the adenosine A2 receptor agonist, 2-[4-(2-Carboxyethyl)phenethyl-amino]-5′-N-ethylcarboxamido adenosine (CGS 21680) elicited similar reductions in blood pressure to R-PIA, but failed to limit infarct development in the anaesthetized rabbit (Thornton et al., 1992). Furthermore, GR79236-induced cardioprotection occurs independently of changes in HR and MAP (Louttit et al., 1999), albeit in the pig, and GR79236-induced myocardial protection in the rabbit is similar, and maximal, for doses of 1 or 3×10−8 mol kg−1 i.v. (unpublished observations), whereas the higher dose of GR79236 produces a more marked bradycardia. Thus, the protection conferred occurs in the face of differing degrees of change in MAP and HR. For the same reasons, and since there was little difference between control infarct size at 37.0 or at 38.5°C, the differences seen in basal HR (Tables 3 & 4) are also unlikely to account for the cardioprotective properties of this drug.
Involvement of adenosine A1 receptors in myocardial protective mechanisms
Whilst having little effect on infarct size in its own right, the adenosine A1 receptor antagonist DPCPX (3.3×10−6 mol kg−1 i.v.) abolished GR79236-induced cardioprotective and haemodynamic effects, thus confirming a role for adenosine A1 receptors in the mechanism of action of GR79236. The protective effect of ischaemic preconditioning can be prevented with the non-selective adenosine receptor agonist 8-(p-sulphophenyl)theophylline (8-SPT) in anaesthetized rabbits (Liu et al., 1991; Downey et al., 1993; and our unpublished observations). However, in the present study, DPCPX did not block the cardioprotective effect of ischaemic preconditioning. This might seem surprising because DPCPX does block cardioprotection evoked by ischaemic preconditioning in the pig (Schwarz et al., 1991; Louttit et al., 1999), ferret (Gomoll, 1996) and dog (Auchampach & Gross, 1993). The present results suggest that in the rabbit heart, ischaemic preconditioning appears to be able to confer protection via an adenosine A1 receptor-independent route. Indeed, Downey et al. (1993) have reported that DPCPX does not block protection conferred by ischaemic preconditioning in the rabbit heart in situ (although no data were presented to support this observation); similar findings have been made in rabbit isolated hearts (Liu et al., 1994; Lasley and Mentzer, 1995), and cardiac myocytes preconditioned by simulated ischaemia (Armstrong & Ganote, 1994). In contrast, Rice et al. (1996) and Wang et al. (1997) reported that DPCPX reduced, but did not abolish, cardiomyocyte protection afforded by simulated ischaemic preconditioning. Taken together, these findings suggest that stimulation of adenosine A1 receptors does not play a predominant role in the protection conferred by ischaemic preconditioning in the rabbit heart. Thus, the pathway through which ischaemic preconditioning is mediated might be different depending on the species studied. Recently, a novel adenosine A1-like receptor, insensitive to DPCPX, and termed the A3 receptor, has been cloned and characterized (Zhou et al., 1992). A definitive role for this receptor as a possible route through which ischaemic preconditioning might be mediated has been precluded by a lack of selective agonists and antagonists. However, cautious interpretation of experiments using the pharmacological tools available, has shown that protection can be induced in rabbit isolated cardiomyocytes (Armstrong & Ganote, 1994), rabbit isolated hearts (Tracey et al., 1997) and rabbit heart in situ (Smith et al., 1996), via an adenosine receptor with properties consistent with those of the adenosine A3 receptor. It is even conceivable that a considerable degree of redundancy exists in the mechanism(s) through which ischaemic preconditioning confers protection in the rabbit heart. Thus, stimulation of either adenosine A1 or A3 receptors might maximally protect the myocytes, so that even after blockade of a single population (e.g. A1 receptors), the overall extent of the protection remains undiminished.
Effect of temperature on myocardial protective mechanisms
McClanahan et al. (1994) showed in anaesthetized pigs, that pentostatin, an agent that would be expected to increase the concentration, and prolong the presence, of endogenous adenosine in the myocardium, reduced infarct size when body temperature was maintained at 35.0°C. However, when body temperature was maintained at 37.0°C, the protective effect of pentostatin was lost. Thus, in their studies, temperature alone was shown to influence the effect of an agent that confers cardioprotection, presumably indirectly via adenosine receptors, and this effect occurred over a temperature range that did not influence infarct size in vehicle-treated pigs. Previously, we have found that the cardioprotective effect of GR79236 in rabbits can be variable, particularly under conditions of high ambient temperature. Those experiments were carried out using a homeothermic blanket control system, with the thermistor placed in the oesophagus, to control body temperature. However, when we measured rabbit body core temperature using a thermometer placed in the colon, via the rectum, we found that it was approximately 1–1.5°C greater than that measured simultaneously from the oesophageal probe, and that this difference could vary on a day-to-day basis. We cannot account for this observation, but it is possible that the air moving in and out of the trachea produces a cooling effect on the thermistor in the oesophagus, resulting in greater heat input from the blanket. We therefore carried out studies to investigate whether differences in body core temperature could influence the cardioprotective effect of GR79236 because, in the light of the experiments carried out by McClanahan et al. (1994), small variations in body temperature within an experimental group might explain the variable results we obtained with GR79236. Subsequently, we used a calibrated Digitron temperature probe placed in the colon, via the rectum, and adjusted the homeothermic blanket control unit manually, so that rabbit body core temperature was maintained within ±0.3°C of the desired temperature.
In vehicle-treated control groups of rabbits, infarct sizes were similar irrespective of whether body temperature was maintained at 37.0 or at 38.5°C (Figure 5). GR79236 administered 10 min prior to occlusion significantly limited the development of infarction compared to control groups of rabbits maintained at 37.0°C, but when the drug was administered to rabbits maintained at 38.5°C, the cardioprotective effect of GR79236 became less consistent, and mean infarct size was not significantly different from that seen in the control group at this temperature. Ischaemic preconditioning was also markedly protective, but in contrast to the cardioprotective effect of GR79236, was not significantly influenced by temperature. However, the limitation of infarct development in ischaemically preconditioned rabbits was less consistent when the experiment was carried out at 38.5°C (Figure 5), so perhaps a further increase in body temperature might render ischaemic preconditioning less effective. This observation may lend support to the concept that more than one receptor mediates protection conferred by ischaemic preconditioning in the rabbit, but with only one of the receptors (A1) being influenced by temperature. Indeed, Stojanov & Proctor (1990) and Broadley et al. (1985), have shown that temperature-induced variability in tissue responsiveness to adenosine analogues is linked to activation of adenosine A1, but not A2, receptors. Thus, it is possible that coupling of GR79236 to the adenosine A1 receptor, and/or subsequent signal transmission, is altered with an elevation of body temperature. We therefore compared the magnitude of GR79236-induced haemodynamic changes at 37.0°C with those seen at 38.5°C, and found GR79236-induced changes in MAP or HR were not different at the two temperatures (Figure 4). Similarly, in studies in which GR79236 was administered prior to reperfusion (Series 3), there were no significant differences between reductions in MAP and HR at the two temperatures (Figure 4). Thus, in the present study, we found no convincing evidence to support the concept that signal transduction via adenosine A1 receptors is modulated by temperature.
Whether or not adenosine A1 receptor agonists can protect the myocardium when administered during (rather than before) ischaemia, is controversial (see Introduction). When GR79236 was administered 10 min prior to the onset of reperfusion in rabbits in which body temperature was maintained at 37.0°C, the development of infarction was limited by a similar extent to that seen when GR79236 was administered prior to coronary artery occlusion at this temperature (Figure 6). This suggests that most of the damage to the myocardium occurs during the last 10 min of ischaemia, and/or during reperfusion. When GR79236 was administered 10 min prior to the onset of reperfusion in rabbits where body temperature was maintained at 38.5°C, the cardioprotective effect of GR79236 was lost (Figure 6). Thus, treatment with an adenosine A1 receptor agonist prior to ischaemia, or just prior to reperfusion, is equally protective and, in both cases, the protective effect is less when body temperature is maintained at 38.5°C rather than at 37.0°C. This might explain why Baxter et al. (personal communication), were unable to limit the development of infarction by administration of GR79236 just prior to the onset of reperfusion, since in their studies, body core temperature was maintained between 38.0 and 39.0°C.
There is an increasing amount of evidence in the literature to show that development of infarction is markedly temperature-sensitive. Chien et al. (1994) measured infarct size in 18 rabbits in which body temperature was maintained at a given level within a range of 35–42°C. The relationship between infarct size and temperature was such that for every 1°C increase in body temperature, there was a 10% increase in infarct size. Hale & Kloner (1997) made a similar observation in rabbit hearts, by inducing regional hypothermia of the risk zone. In the anaesthetized pig, over a temperature range of 35–39°C, there is a 20% increase in infarct size with each 1°C increase in body temperature (Dunker et al., 1996). It might not be surprising that we did not see a difference in infarct size in control rabbits maintained at 37.0 or at 38.5°C, because of comparatively smaller difference between these temperatures and those evaluated by other authors, and the relatively small numbers of animals used. Nevertheless, it is established that if the severity of an ischaemic insult is increased by prolonging the period of the sustained occlusion, the protection conferred by ischaemic preconditioning is lost (van den Doel et al., 1998). Thus, there becomes a time during the infarction-inducing process beyond which ischaemic preconditioning is unable to protect the myocardium from ischaemia and reperfusion-induced injury. It is therefore tempting to hypothesise that an increase in body temperature may impair cardioprotective mechanisms, and thus make it more difficult for GR79236 to confer myocardial protection. What these mechanisms might be remains unknown. However, glucose utilization and lactate production in non-ischaemic or ischaemic hearts has been shown to be increased at higher temperatures suggesting a greater metabolic demand (Ichihara et al., 1981). The damage induced when oxygen or calcium was re-introduced after anoxic or calcium-free perfusion (oxygen and calcium paradoxes, respectively), was highly temperature-dependent in rat isolated hearts (Hearse et al., 1978). In both of these paradoxes, cellular damage increased sharply over a temperature range of 33–36°C. Thus, increasing the temperature at which these investigations are carried out, promotes processes that might be associated with ischaemia reperfusion-induced damage, and this may make it more difficult to protect against ischaemia and reperfusion injury.
In conclusion, the present study has shown that the adenosine A1 receptor agonist GR79236 reduces infarct size in an anaesthetized rabbit model of myocardial ischaemia and reperfusion, an effect mediated by adenosine A1 receptors. The protective effect of ischaemic preconditioning in the rabbit seems not, however, to be attributable to activation of adenosine A1 receptors. The body temperature at which experiments are carried out can markedly influence the extent of protection afforded by GR79236, but not that of ischaemic preconditioning, over a temperature range that does not significantly influence infarct size in its own right. Thus, even small differences in body temperature might be expected to induce variability within an experimental group and significantly influence the outcome of the study. For this reason, it is essential when carrying out studies of this nature, to maintain body core temperature within a very strictly predetermined range. In addition, our results confirm that administration of an adenosine A1 receptor agonist can reduce infarct size when administered just prior to the onset of reperfusion, suggesting that the damage against which GR79236 protects occurs mainly during reperfusion.
Acknowledgments
We would like to thank Mark Lennon for his considerable efforts, and rapid responses, to our requests for statistical analyses and advice.
Abbreviations
- DPCPX
8-cyclopentyl-1,3-dipropylxanthine
- GR79236
(N-[(1S, trans)-2-hydroxycyclopentyl]adenosine
- HR
heart rate
- IPC
ischaemic preconditioning
- R-PIA
R(−)N6-2-phenylisopropyl adenosine
- TTC
triphenyltetrazolium chloride
References
- ARMSTRONG S., GANOTE C.E. Adenosine receptor specificity in preconditioning of isolated rabbit cardiomyocytes: evidence of A3 receptor involvement. Cardiovasc. Res. 1994;28:1049–1056. doi: 10.1093/cvr/28.7.1049. [DOI] [PubMed] [Google Scholar]
- AUCHAMPACH J.A., GROSS G.J. Adenosine A1 receptors, KATP channels and ischaemic preconditioning in dogs. Am. J. Physiol. 1993;264:H1327–H1336. doi: 10.1152/ajpheart.1993.264.5.H1327. [DOI] [PubMed] [Google Scholar]
- BAXTER G.F., GOMA F.M., YELLON D.M. Characterisation of the infarct-limiting effect of delayed preconditioning: time course and dose-dependency studies in the rabbit myocardium. Basic Res. Cardiol. 1997;92:159–167. doi: 10.1007/BF00788633. [DOI] [PubMed] [Google Scholar]
- BAXTER G.F., MARBER M.S., PATEL V.C., YELLON D.M. Adenosine receptor involvement in a delayed phase of myocardial protection 24 hours after ischaemic preconditioning. Circulation. 1994;90:2993–3000. doi: 10.1161/01.cir.90.6.2993. [DOI] [PubMed] [Google Scholar]
- BROADLEY K.J., BROOME S., PATON D.M. Hypothermia-induced supersensitivity to adenosine for responses mediated via A1 receptors but not A2 receptors. Br. J. Pharmacol. 1985;84:407–415. doi: 10.1111/j.1476-5381.1985.tb12924.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CHIEN G.L., WOLFF R.A., DAVIS R.F., VAN WINKLE D.M. "Normothermic range" temperature affects myocardial infarct size. Cardiovasc. Res. 1994;28:1014–1017. doi: 10.1093/cvr/28.7.1014. [DOI] [PubMed] [Google Scholar]
- DOWNEY J.M., LIU G.S., THORNTON J.D. Adenosine and the anti-infarct effects of preconditioning. Cardiovasc. Res. 1993;27:3–8. doi: 10.1093/cvr/27.1.3. [DOI] [PubMed] [Google Scholar]
- DUNKER D.J., KLASSEN C.L., ISHIBASHI Y., HERRLINGER S.A., PAVEK T.J., BACHE R.J. Effect of temperature on myocardial infarction in swine. Am. J. Physiol. 1996;270:H1189–H1199. doi: 10.1152/ajpheart.1996.270.4.H1189. [DOI] [PubMed] [Google Scholar]
- GOMOLL A.W. Cardioprotection associated with preconditioning in the anaesthetised ferret. Basic Res. Cardiology. 1996;91:433–443. doi: 10.1007/BF00788724. [DOI] [PubMed] [Google Scholar]
- GROVER G.J., SLEPH P.G., DZWONCZYK S. Role of myocardial ATP-sensitive potassium channels in mediating preconditioning in the dog heart and their possible interaction with adenosine A1 receptors. Circulation. 1992;86:1310–1316. doi: 10.1161/01.cir.86.4.1310. [DOI] [PubMed] [Google Scholar]
- GURDEN M.F., COATES J., ELLIS F., EVANS B., FOSTER M., HORNBY E., KENNEDY I., MARTIN D.P., STRONG P., VARDEY C.J., WHEELDON A. Functional characterisation of three adenosine receptor types. Br. J. Pharmacol. 1993;109:693–698. doi: 10.1111/j.1476-5381.1993.tb13629.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HALE S.L., KLONER R.A. Myocardial temperature in acute myocardial infarction: protection with mild regional hypothermia. Am. J. Physiol. 1997;273:H220–H227. doi: 10.1152/ajpheart.1997.273.1.H220. [DOI] [PubMed] [Google Scholar]
- HEARSE D.J., HUMPHREY S.M., BULLOCK G.R. The oxygen paradox and the calcium paradox: Two facets of the same problem. J. Mol. Cell. Cardiol. 1978;10:641–668. doi: 10.1016/s0022-2828(78)80004-2. [DOI] [PubMed] [Google Scholar]
- ICHIHARA K., ROBISHAW J.D., VARY T.C., NEELY J.R. Protection of ischaemic myocardium from metabolic products. Acta Med. Scand. 1981;651 Suppl. I:13–18. doi: 10.1111/j.0954-6820.1981.tb03627.x. [DOI] [PubMed] [Google Scholar]
- KUZUYA T., HOSHIDA S., YAMASHITA N., FUGI H., OE H., HORI M., KAMADA T., TADA M. Delayed effects of sublethal ischaemia on the acquisition of tolerance to ischaemia. Circ. Res. 1993;72:1293–1299. doi: 10.1161/01.res.72.6.1293. [DOI] [PubMed] [Google Scholar]
- LASLEY R.D., MENTZER R.M., JR Preconditioning and its potential role in myocardial protection during cardiac surgery. J. Card. Surg. 1995;10:349–353. doi: 10.1111/j.1540-8191.1995.tb00622.x. [DOI] [PubMed] [Google Scholar]
- LI Y., WHITTAKER P., KLONER R.A. The transient nature of the effects of ischaemic preconditioning on myocardial infarct size and ventricular arrhythmia. Am. Heart J. 1992;123:346–353. doi: 10.1016/0002-8703(92)90645-c. [DOI] [PubMed] [Google Scholar]
- LIU G.S., RICHARDS S.C., OLSSON R.A., MULLANE K., WALSH R.S., DOWNEY J.M. Evidence that the adenosine A3 receptor may mediate the protection afforded by preconditioning in the isolated rabbit heart. Cardiovasc. Res. 1994;28:1057–1061. doi: 10.1093/cvr/28.7.1057. [DOI] [PubMed] [Google Scholar]
- LIU G.S., THORNTON J., VAN WINKLE D.M., STANLEY A.W.H., OLSSON R.A., DOWNEY J.M. Protection against infarction afforded by preconditioning is mediated by A1 adenosine receptors in the rabbit heart. Circulation. 1991;84:350–356. doi: 10.1161/01.cir.84.1.350. [DOI] [PubMed] [Google Scholar]
- LOUTTIT J.B., HUNT A.E.E., MAXWELL M.P., DREW G.M. The time-course of cardioprotection induced by GR79236, a selective adenosine A1 receptor agonist, in myocardial ischaemia-reperfusion injury in the pig. J. Cardiovasc. Pharmacol. 1999;33:285–291. doi: 10.1097/00005344-199902000-00016. [DOI] [PubMed] [Google Scholar]
- MARBER M.S., LATCHMAN D.S., WALKER J.M., YELLON D.M. Cardiac stress protein elevation 24 h after brief ischaemia or heat stress is associated with resistance to myocardial infarction. Circulation. 1993;88:1264–1272. doi: 10.1161/01.cir.88.3.1264. [DOI] [PubMed] [Google Scholar]
- MARTIN B.J., MCCLANAHAN T.B., HAMILTON H.W., GALLAGHER K.P. PD126,280 [Endo (S) Norbornyl-adenosine], a highly selective adenosine receptor agonist, reduces infarct size in swine. Circulation. 1993;88 Suppl. I:I-430. [Google Scholar]
- MCCLANAHAN T.B., MERTZ T.E., MARTIN B.J., GALLAGHER K.P. Pentostatin reduces infarct size in pigs only when combined with mild hypothermia. Circulation. 1994;90:I-478. [Google Scholar]
- MIURA T., ADACHI T., OGAWA T., IWAMOTO T., TSUCHIDA A., IIMURA O. Myocardial infarct size-limiting effect of ischaemic preconditioning: its natural decay and the effect of repetitive preconditioning. Cardiovasc. Pathol. 1992;1:147–154. doi: 10.1016/1054-8807(92)90018-J. [DOI] [PubMed] [Google Scholar]
- MURRY C.E., JENNINGS R.B., REIMER K.A. Preconditioning with ischaemia: a delay of lethal cell injury in ischaemic myocardium. Circulation. 1986;74:1124–1136. doi: 10.1161/01.cir.74.5.1124. [DOI] [PubMed] [Google Scholar]
- MURRY C.E., RICHARD V.J., JENNINGS R.B., REIMER K.A. Myocardial protection is lost before contractile function recovers from ischaemic preconditioning. Am. J. Physiol. 1991;260:H796–H804. doi: 10.1152/ajpheart.1991.260.3.H796. [DOI] [PubMed] [Google Scholar]
- NORTON E.D., JACKSON E.K., TURNER M.B., VIRMANI R., FORMAN M.B. The effects of intravenous infusions of selective adenosine A1 receptor and A2 receptor agonists on myocardial reperfusion injury. American Heart J. 1992;123:332–338. doi: 10.1016/0002-8703(92)90643-a. [DOI] [PubMed] [Google Scholar]
- RICE P.J., ARMSTRONG S.C., GANOTE C.E. Concentration-response relationships for adenosine agonists during preconditioning of rabbit cardiomyocytes. J. Mol. Cell. Cardiol. 1996;28:1355–1365. doi: 10.1006/jmcc.1996.0126. [DOI] [PubMed] [Google Scholar]
- SCHWARZ E.R., MOHRI M., SACK S., ARRAS M. The role of adenosine and its A1 receptor in ischaemic preconditioning. Circulation. 1991;84 Suppl. II:II-191. [Google Scholar]
- SMITH A.H., BANGERTER F.W., MACANDREW J.T., TRACEY W.R., HILL R.J., KENNEDY S.P., MASAMUNE H., WESTER R.T., BUCHHOLZ R.A.IBMECA, a selective rabbit adenosine A3 receptor agonist, pharmacologically preconditions without haemodynamic effects, in situ Circulation 199694Abstract 3218 [Google Scholar]
- STOJANOV I, PROCTOR K.G. Temperature-sensitive adenosine-mediated vasoconstriction in skin microcirculation. J. Pharmacology and Experimental Therapeutics. 1990;253:1083–1089. [PubMed] [Google Scholar]
- THORNTON J.D., LIU G.S., OLSSON R.A., DOWNEY J.M. Intravenous pre-treatment with A1-selective adenosine analogues protects the heart against infarction. Circulation. 1992;85:659–665. doi: 10.1161/01.cir.85.2.659. [DOI] [PubMed] [Google Scholar]
- TRACEY W.R., MAGEE W., MASAMUNE H., KENNEDY S.P., KNIGHT D.R., BUCHHOLZ R.A., HILL R.J. Selective adenosine A3 receptor stimulation reduces ischaemic myocardial injury in the rabbit heart. Cardiovasc. Res. 1997;33:410–415. doi: 10.1016/s0008-6363(96)00240-4. [DOI] [PubMed] [Google Scholar]
- TRAVERS A., MIDDLEMISS D., LOUTTIT J.B. Cardioprotection after repeated dosing with GR79236, an adenosine A1 receptor agonist. Br. J. Pharmacol. 1998;124:102P. [Google Scholar]
- TSUCHIDA A., MIURA T., MIKI T., SHIMAMOTO K., IIMURA O. Role of adenosine receptor activation in myocardial infarct size limitation by ischaemic preconditioning. Cardiovasc. Res. 1992;26:456–461. doi: 10.1093/cvr/26.5.456. [DOI] [PubMed] [Google Scholar]
- VAN DEN DOEL M.A., GHO B.C.G., DUVAL S.Y., SCHOEMAKER R.G., DUNKER D.J., VERDOUW P.D. Hypothermia extends the cardioprotection by ischaemic preconditioning to coronary artery occlusions of longer duration. Cardiovasc. Res. 1998;37:76–81. doi: 10.1016/s0008-6363(97)00222-8. [DOI] [PubMed] [Google Scholar]
- VAN WINKLE D.M., CHEIN G.L., WOLFF R.A., SOIFER B.E., DAVIS R.F. Intracoronary infusion of R-phenylisopropyl adenosine prior to ischaemia/reperfusion reduces myocardial infarct size in swine. Circulation. 1992;86 Suppl. 1:I-213. [Google Scholar]
- VAN WINKLE D.M., THORNTON J.D., DOWNEY D.M., DOWNEY J.M. The natural history of preconditioning: cardioprotection depends on duration of transient ischaemia and time to subsequent ischaemia. Coronary Artery Disease. 1991;2:613–619. [Google Scholar]
- WANG J., DRAKE L., SAJJADI F., FIRESTEIN G.S., MULLANE K.M., BULLOUGH D.A. Dual activation of adenosine A1 and A3 receptors mediates preconditioning of isolated cardiac myocytes. Eur. J. Pharmacol. 1997;320:241–248. doi: 10.1016/s0014-2999(96)00901-6. [DOI] [PubMed] [Google Scholar]
- YTREHUS K., LIU Y., TSUCHIDA A., MIURA T., LIU G.S., YANG X., HERBERT D., COHEN M.V., DOWNEY J.M. Rat and rabbit heart infarction: effects of anaesthesia, perfusate, risk zone, and method of infarct sizing. Am. J. Physiol. 1994;267:H2383–H2390. doi: 10.1152/ajpheart.1994.267.6.H2383. [DOI] [PubMed] [Google Scholar]
- ZHOU Q.Y., LI C., OLAH M.E., JOHNSON R.A., STILES G.L., CIVELLI O. Molecular cloning and characterisation of an adenosine receptor: the A3 adenosine receptor. Proc. Natl. Acad. Sci. U.S.A. 1992;89:7432–7436. doi: 10.1073/pnas.89.16.7432. [DOI] [PMC free article] [PubMed] [Google Scholar]


