Abstract
We investigated the ability of an acute pro-oxidant challenge in vivo to deteriorate glycaemic control and insulin action in the obese Zucker rat, a model of insulin resistance associated with oxidant stress. In obese animals, the daily administration for 1 week of hydroquinone (HQ) in combination with L-buthionine sulphoximine (BSO), elevated fasting plasma glycaemia and insulinaemia and markedly aggravated i.v. glucose-stimulated hyperinsulinaemia without significantly affecting i.v. glucose tolerance, suggesting exacerbated insulin resistance. Intermediate effects on hyperinsulinaemia in obese animals were determined with HQ treatment alone while BSO treatment alone had no effect. In contrast, none of the pro-oxidant treatments affected age-matched, insulin sensitive, lean Zucker rats. Our data therefore demonstrate for the first time, a vulnerability to deterioration in insulin action in an established insulin resistant state following an environmental pro-oxidative insult. This may have relevance in the conversion of insulin resistance syndrome (IRS) to non-insulin dependent diabetes mellitus (NIDDM).
Keywords: Insulin resistance, oxidant stress, obese Zucker rat, NIDDM
Introduction
The ‘prediabetic' obese Zucker rat exhibits many features of IRS (metabolic Syndrome X) (see Reaven, 1995), including fasting hyperinsulinaemia and impaired glucose tolerance (Zucker & Antoniades, 1972). In addition, we have recently demonstrated oxidant stress in the obese Zucker rat (Laight et al., 1998a), also identified in NIDDM (Gopaul et al., 1995), which is thought to contribute to insulin resistance (Paolisso et al., 1994). This notion has been supported by the obese Zucker rat by reports of antioxidant-mediated improvements in insulin action both in vitro (Jacob et al., 1996) and in vivo (Laight et al., 1998b). However it is not known if this insulin resistant, ‘prediabetic' state is susceptible to a further deterioration in glycaemic control or insulin action in response to a pro-oxidant challenge which may facilitate the development of NIDDM. We have now investigated this possibility by treating the obese Zucker rat in vivo with the established pro-oxidant HQ, which induces redox cycles in biological systems (Stenius et al., 1989). HQ was administered either alone or in combination with the glutathione (GSH)-depleting agent BSO (Baruchel et al., 1995), which can amplify HQ-mediated injury (Laight et al., 1999).
Methods
Male, 12-week old obese/lean Zucker rats obtained from Harlan (Bicester, Oxon, U.K.) were treated daily with HQ (50 mg kg−1 i.p.), BSO (50 mg kg−1 i.p.) or HQ+BSO (each at 50 mg kg−1 i.p.) for 7 days as previously described by Laight et al. (1999). Temporal control animals received normal saline (2 ml kg−1 i.p.) daily. Food and water were allows ad libitum. Obese rats and lean littermates were then fasted overnight and anaesthetized with thiopentone sodium (120 mg kg−1 i.p.) to allow an i.v. glucose tolerance test. After 30 min stabilization, a blood sample was collected immediately before the glucose load at t=0, to determine basal values of plasma glucose and insulin. D-glucose was then injected (0.5 g kg−1 i.v.) and venous blood samples collected in EDTA anticoagulant at t=1, 3, 6, 12, and 24 min. Samples were kept on ice before centrifugation to derive plasma (within 30 min) which was stored at −20°C prior to analysis. D-glucose and insulin were determined using a photometric method (Trinder assay, Sigma Chemical Co., Poole, Dorset, U.K.) and by radioimmunoassay (Biogenesis Ltd, Poole, Dorset, U.K.), respectively. Glycosuria was assessed using a Diabur/test 5000 diagnostic indicator strip (Boehringer Mannheim, Lewes, Sussex, U.K.).
Materials
Reagents were obtained from Sigma Chemical Co., Poole, Dorset, U.K.
Statistics
Data are expressed as mean±s.e.mean. Glucose tolerance and glucose-stimulated hyperinsulinaemia were determined as the area under the curve (AUC) for glucose and insulin levels, respectively, between t=0 and 24 min. The difference between the two means was assessed by Student's unpaired, two-tailed t-test. A multicomparison of means was conducted using one way ANOVA followed by Dunnett's test. Statistical significance was accepted at the 5% level.
Results
The body weights of 13-week-old obese Zucker rats were not significantly affected by pro-oxidant treatments (control 432.7±10.7 g; HQ 421.4±14.2 g; BSO 433.2±6.7 g; HQ+BSO 413.5±10.8 g; n=5–6) and were greater (P<0.01) than corresponding lean Zucker rat weights (control 270.0±11.1 g; HQ 260.0±6.6 g; BSO 277.0±10.4 g; HQ+BSO 260.5±4.6 g) (n=6). Similarly, obese plasma cholesterol levels ((mM) control 1.6±0.2; HQ 1.8±0.1; BSO 1.6±0.1; HQ+BSO 1.4±0.1) (n=5–6) and plasma total free fatty acid levels ((mg ml−1) control 1.6±0.1; HQ 1.8±0.1; BSO 1.8±0.1; HQ+BSO 1.9±0.1) (n=5–6) were not affected by pro-oxidant treatments and were greater (P<0.01) than corresponding lean values ((mM) cholesterol: control 0.6±0.03; HQ 0.7±0.1; BSO 0.7±0.1; HQ+BSO 0.7±0.1; total free fatty acids: ((mg ml−1) control 0.8±0.03; HQ 0.6±0.02; BSO 0.8±0.03; HQ+BSO 0.8±0.03) (n=6). Furthermore, plasma markers of organ toxicity including urea, creatinine, creatinine kinase, alanine aminotransferase and pancreatic lipase were not elevated by pro-oxidant treatments in either lean or obese animals (data not shown).
Fasting glycaemia was similar in obese and lean Zucker rats while obese animals showed a relative fasting hyperinsulinaemia (Table 1). HQ +BSO treatment elicited a rise in fasting glycaemia in obese animals accompanied by an elevation in fasting hyperinsulinaemia (Table 1). Furthermore, four out of six obese animals treated with HQ+BSO tested positive for glycosuria (>5% w v−1) after 1 week. Intravenous glucose tolerance was impaired in obese relative to lean Zucker rats and was not altered by pro-oxidant treatments (Table 2). However, i.v. glucose-stimulated insulinaemia, which was greater in obese relative to lean animals, was further markedly elevated by HQ+BSO treatment (Figure 1 and Table 2) in the obese Zucker rat. HQ treatment alone raised i.v. glucose-stimulated insulinaemia to an intermediate level in obese animals, while BSO treatment alone was without effect.
Table 1.
Fasting glycaemia and insulinaemia in 13-week-old lean and obese Zucker rats following a pro-oxidant challenge
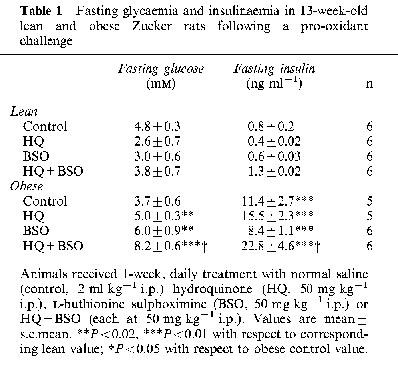
Table 2.
Glucose tolerance and glucose-stimulated insulinaemia following an i.v. glucose load (0.5 g kg−1) in 13-week-old lean and obese Zucker rats following a pro-oxidant challenge

Figure 1.
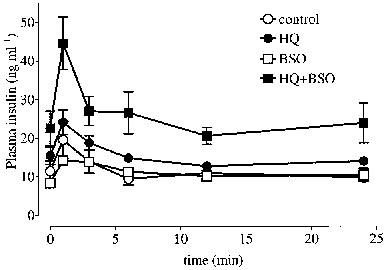
Plasma insulin curves following an i.v. glucose load (0.5 g kg−1) at t=0 min in 13-week-old obese Zucker rats (n=5–6) after 1 week, daily treatment with normal saline (control, 2 ml kg−1 i.p.), hydroquinone (HQ, 50 mg kg−1 i.p.), L-buthionine sulphoximine (BSO, 50 mg kg−1 i.p.) or HQ+BSO (each at 50 mg kg−1 i.p.). Values are mean±s.e.mean. See text for basal values of glucose and insulin.
HQ+BSO treatment was associated with a reduction in the hepatic GSH : GSSG ratio from 84.6±3.4 to 57.5±7.7 (n=5–6) (P<0.01) in lean animals and from 74.8±4.5 to 62.1±4.7 (n=5) (P<0.01) in the obese Zucker rat. Furthermore, the plasma level of 8-epi-PGF2α, which was elevated in obese (5.5±1.0 ng ml−1) (n=5) relative to lean (2.7±0.3 ng ml−1) (n=5) animals (P<0.05), tended to be raised following HQ+BSO treatment to 7.0±2 ng ml−1 (n=5) (P>0.05), while the lean value was unchanged (2.5±0.5 ng ml−1) (n=6) (P<0.01 with respect to corresponding obese value).
Discussion
HQ+BSO treatment in rats in vivo has previously been shown to lead to a model of oxidant stress and endothelial dysfunction (Laight et al., 1999). In that study as in the present study, low dose BSO while without major effects per se (see Cuzzocrea et al., 1998) could be shown to augment the pro-oxidant effects of HQ. This is consistent with a greater pro-oxidative challenge when tissue redox cycling activity is combined with a reduction in thiol-dependent antioxidant defence (see Stenius et al., 1989; Baruchel et al., 1995; Laight et al., 1999).
HQ+BSO treatment in the obese Zucker rat resulted in elevations both in fasting and i.v. glucose-stimulated hyperinsulinaemia. Since fasting insulinaemia correlates well with measures of insulin sensitivity (see Reaven, 1995), our data are therefore consistent with a pro-oxidant-mediated worsening of in vivo insulin resistance in the obese Zucker rat. This is supported by the observation that glucose-stimulated hyperinsulinaemia in obese animals following HQ+BSO treatment was associated with an unaltered i.v. glucose tolerance; which likely reflects at least in part, the compensatory nature of the insulin hyper-secretory response.
Furthermore, the development of a fasting hyperglycaemic state and glycosuria in the obese Zucker rat treated with HQ+BSO is of particular interest as it marks the transition from impaired glucose tolerance towards NIDDM following pro-oxidant challenge. The absence of such effects in insulin sensitive, lean Zucker rats following the HQ+BSO regimen, may highlight a metabolic vulnerability to pro-oxidative insult in established insulin resistant states exemplified by the obese Zucker rat. Indeed, this notion is supported by the observation that while the HQ+BSO-mediated fall in hepatic GSH : GSSG ratio, a measure of pro-oxidative insult (Mårtensson et al., 1991), was comparable in lean and obese animals, only the obese Zucker rat responded with an apparent rise in the lipid peroxidation product, 8-epi-PGF2α (Gopaul et al., 1995). This metabolic vulnerability conceivably reflects the ongoing contribution of oxidant stress to poor insulin action in insulin resistance syndrome (see Paolisso et al., 1994; Paolisso & Guigliano, 1996; Laight et al., 1998b), which in the obese Zucker rat is evidenced by the elevated basal plasma level of 8-epi-PGF2α (Gopaul et al., 1995; Laight et al., 1998a).
In conclusion, our data demonstrate a metabolic deterioration in the obese Zucker rat in response to an acute pro-oxidant challenge. This now suggests that established insulin resistant states are susceptible to an additional deterioration in insulin action following environmental exposure to a pro-oxidative insult, which may be sufficient to provoke the onset of NIDDM.
Acknowledgments
This work was supported by Lipha s.a., Lyon, France.
Abbreviations
- BSO
buthionine sulphoximine
- GSH
reduced glutathione
- GSSG
oxidized glutathione
- HQ
hydroquinone
- IRS
insulin resistance syndrome
- NIDDM
non-insulin dependent diabetes mellitus
References
- BARUCHEL S., WANG T., FARAH R., JAMALI M., BATIST G. In vivo selective modulation of tissue glutathione in a rat mammary carcinoma model. Biochem. Pharmacol. 1995;50:1505–1508. doi: 10.1016/0006-2952(95)02063-2. [DOI] [PubMed] [Google Scholar]
- CUZZOCREA S., ZINGARELLI B., C'CONNOR M., SALZMAN A.L., SZABO C. Effect of L-buthionine-(S,R)-sulfoximine, an inhibitor of γ-glutamylcysteine synthetase on peroxynitrite- and endotoxic shock-induced vascular failure. Br. J. Pharmacol. 1998;123:525–537. doi: 10.1038/sj.bjp.0701612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GOPAUL N.K., ÄNGGÅRD E.E., MALLET A.I., BETTERIDGE D.J., WOLFF S.P., NOUROOZ-ZADEH J. Plasma 8-epi-PGF2α levels are elevated in individuals with non-insulin dependent diabetes mellitus. FEBS Lett. 1995;368:225–229. doi: 10.1016/0014-5793(95)00649-t. [DOI] [PubMed] [Google Scholar]
- JACOB S., STREEPER R.S., FOGT D.L., HOKAMA J.Y., TRITSCHLER H.J., DIETZE G.J., HENRIKSEN E.J. The antioxidant α-lipoic acid enhances insulin-stimulated glucose metabolism in insulin-resistant rat skeletal muscle. Diabetes. 1996;45:1024–1029. doi: 10.2337/diab.45.8.1024. [DOI] [PubMed] [Google Scholar]
- LAIGHT D.W., DESAI K.M., GOPAUL N.K., ÄNGGÅRD E.E., CARRIER M.J. Dietary vitamin E reduces oxidant stress and improves insulin action in the obese Zucker rat in vivo. Br. J. Pharmacol. 1998b;125:P114. doi: 10.1038/sj.bjp.0702132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LAIGHT D.W., GUNNARSSON P.T., KAW A.V., ÄNGGÅRD E.E., CARRIER M.J. Physiological microassay of plasma total antioxidant status in a model of endothelial dysfunction in the rat following experimental oxidant stress in vivo. Environ. Toxicol. Pharmacol. 1999;7:27–31. doi: 10.1016/s1382-6689(98)00046-5. [DOI] [PubMed] [Google Scholar]
- LAIGHT W., KENGATHARAN K.M., GOPAUL N.K., ÄNGGÅRD E.E., CARRIER M.J. Investigation of oxidant stress and vasodepression to glyceryl trinitrate in the obese Zucker rat in vivo. Br. J. Pharmacol. 1998a;125:895–901. doi: 10.1038/sj.bjp.0702132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MÅRTENSSON J., JAIN A., STOLE E., FRAYER W., AULD P.A.M., MEISTER A. Inhibition of glutathione synthesis in the newborn rat: A model for endogenously produced oxidative stress. Proc. Natl. Acad. Sci. U.S.A. 1991;88:9360–9364. doi: 10.1073/pnas.88.20.9360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PAOLISSO G., D'AMORE A., VOLPE C., BALBI V., SACCOMANNO F., GALZERANO D., GIUGLIANO D., VARRICCHIO M., D'ONOFRIO F. Evidence for a relationship between oxidative stress and insulin action in non-insulin-dependent (type II) diabetic patients. Metab. 1994;43:1426–1429. doi: 10.1016/0026-0495(94)90039-6. [DOI] [PubMed] [Google Scholar]
- PAOLISSO G., GUIGLIANO D. Oxidative stress and insulin action: is there a relationship. Diabetologia. 1996;39:357–363. doi: 10.1007/BF00418354. [DOI] [PubMed] [Google Scholar]
- REAVEN G. Pathophysiology of insulin resistance in human disease. Physiol. Rev. 1995;75:473–486. doi: 10.1152/physrev.1995.75.3.473. [DOI] [PubMed] [Google Scholar]
- STENIUS U., WARHOLM M., RANNUG A., WALLES S., LUNDBERG I., HÖGBERG J. The role of GSH depletion and toxicity in hydroquinone-induced development of enzyme-altered foci. Carcinogenesis. 1989;10:593–599. doi: 10.1093/carcin/10.3.593. [DOI] [PubMed] [Google Scholar]
- ZUCKER L.M., ANTONIADES H.N. Insulin and obesity in the Zucker genetically obese rat ‘fatty'. Endocrinol. 1972;90:1320–1330. doi: 10.1210/endo-90-5-1320. [DOI] [PubMed] [Google Scholar]


