Abstract
The modulatory activity of extracellular H+ and Zn2+ was examined on ATP-responses at rat P2X1 (rP2X1) and rat P2X3 (rP2X3) receptors expressed in Xenopus oocytes and studied under voltage-clamp conditions.
Superfused ATP (0.03–30 μM, at pH 7.5) evoked inward currents at rP2X1 receptors (EC50 value, 300±7 nM). ATP potency was reduced 2 fold at pH 6.5, and 6 fold at pH 5.5, without altering the maximum ATP effect. Alkaline conditions (pH 8.0) did not alter ATP activity.
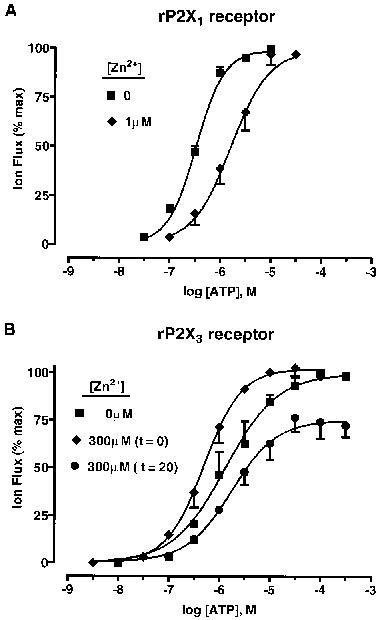
Superfused ATP (0.01–300 μM, at pH 7.5) evoked inward currents at rP2X3 receptors (EC50 value, 1.8±0.3 μM). ATP activity was affected only at pH 5.5, reducing agonist potency 15 fold without altering the maximum ATP effect.
Extracellular Zn2+ inhibited ATP-responses at rP2X1 receptors in a time-dependent manner, a 20 min pre-incubation being optimal (IC50 value, 1.0±0.2 μM). However, the Zn2+ effect was pH-independent, suggesting Zn2+- and H+-inhibition of ATP-responses occur through independent processes.
Extracellular Zn2+ weakly potentiated ATP-responses at rP2X3 receptors (EC50 value, 11±1 μM). The Zn2+ effect was dependent on pre-incubation time and, with 20 min pre-incubation periods, Zn2+ potentiated then inhibited ATP-responses in a concentration-dependent, but pH-independent, manner.
In summary, ATP activity at rP2X1 receptors was decreased by both extracellular H+ and Zn2+ and their effects were additive. ATP activity at rP2X3 receptors was less sensitive to H+-inhibition and, in contrast, was potentiated by Zn2+ in a pH-independent manner. These differential effects may help distinguish P2X1 and P2X3 receptors in whole tissues.
Keywords: P2X receptor, ionotropic receptor, ion channel, ATP, extracellular pH, H+, Zn2+, oocyte
Introduction
Adenosine 5′-triphosphate (ATP) acts as a fast signalling molecule at P2X receptors in central, peripheral and enteric neurons and associated neuro-effector tissues (Burnstock, 1997). Seven P2X receptor subunits (P2X1–7) have been identified thus far, of which P2X1–6 subunit mRNA transcripts and receptor proteins are present in neurons (Humphrey et al., 1998; King, 1998). Homomeric P2X1 and P2X3 receptors examined in the present study are now classified (Group 1) P2X receptors, which are defined as α,β-meATP-sensitive, rapidly-inactivating, suramin-sensitive (Humphrey et al., 1998). However, there is limited scope to distinguish the operational profiles of P2X1 and P2X3 receptors. Previously, we have commented on the effects of H+ ions (i.e., extracellular pH, pHe) and Zn2+ ions on ATP-responses at homomeric P2X2 (Group 2) and P2X4 (Group 3) receptors, at which significant differences have been observed in their modulatory actions (King et al., 1996; 1997; Wildman et al., 1998; 1999b). We have extended our survey of H+ and Zn2+ modulation to include Group 1 P2X receptors, in the hope of discriminating between P2X1 and P2X3 receptors as well as Group 1 from Groups 2 and 3 P2X receptors.
In an earlier study, the amplitude of ATP-responses at P2X1 and P2X3 receptors were shown to be reduced by lowering pHe, although the H+ effect on agonist potency and efficacy was not investigated (Stoop et al., 1997). An inhibitory effect by H+ ions was also observed at P2X4 receptors (Wildman et al., 1999b), which contrasted with a potentiating effect at P2X2 receptors (King et al., 1996; 1997). Thus, the first objective of this study focused on changes in agonist potency and efficacy at rP2X1 and rP2X3 receptors under acidic and alkaline conditions, to see if such changes matched the H+ effect at P2X4 receptors. The second part of the present study focused on Zn2+ modulation of Group 1 P2X receptors, and the dependence of such modulatory effects on time and extracellular pH, in the knowledge that this transition metal also affected ATP-activity at P2X2 and P2X4 receptors in different ways in a time- and pH-dependent manner (see Wildman et al., 1998; 1999b). Finally, we have compared and contrasted the modulatory activity of H+ and Zn2+ ions at rP2X1, rP2X2, rP2X3 and rP2X4 receptors to establish operational profiles distinct for each P2X subunit. Part of this study has been communicated to The Physiological Society (King et al., 1999).
Methods
Oocyte preparation
Xenopus laevis frogs were anaesthetized in Tricaine (0.4% w v−1), killed by decapitation, and ovarian lobes removed surgically. Oocytes (stages V and VI) were defolliculated by a 2-step process involving collagenase treatment (Type IA, 2 mg ml−1 in a Ca2+-free Ringer's solution, for 2–3 h) and, thereafter, stripping away the follicular layer with fine forceps. Defolliculated oocytes were stored in Barth's solution (pH 7.5, at 4°C) containing (mM): NaCl 110, KCl 1, NaHCO3 2.4, Tris HCl 7.5, Ca(NO3)2 0.33, CaCl2 0.41, MgSO4 0.82; gentamycin sulphate, 50 μg l−1. Defollicated oocytes were injected cytosolically with either rP2X1 or rP2X3 cRNA (40 nl, 1 μg ml−1), incubated for 48 h at 18°C in Barth's solution then kept at 4°C for up to 12 days until used in electrophysiological experiments.
Electrophysiology
ATP-activated membrane currents (IATP) (Vh=−60 to −90 mV) were recorded from cRNA-injected oocytes using a twin-electrode voltage-clamp amplifier (Axoclamp 2B). The voltage-recording and current-recording microelectrodes (1–5 MΩ tip resistance) were filled with 3.0 M KCl. Oocytes were superfused with Ringer's solution (5 ml min−1, at 18°C) containing (mM): NaCl 110, KCl 2.5, HEPES 5, BaCl2 1.8, adjusted to pH 7.5. Where stated, the pH of the bathing solution was adjusted using either 1.0 N HCl or 1.0 N NaOH to achieve the desired level. Electrophysiological data were stored on a computer using a MP100 WSW interface (Biopac Systems Inc.) and analysed using the software package Acqknowledge III (Biopac).
Solutions
All solutions were nominally Ca2+-free to avoid the activation of an endogenous Ca2+-dependent Cl− current (ICl(Ca)) present in oocytes (Barish, 1983). ATP was prepared in a Ca2+-free Ringer's solution (concentrations as stated in the text) and superfused by a gravity-feed continuous flow system which allowed rapid addition and washout. ATP was added for 120 s or until the current reached a peak, then washed out for a period of 20 min. Data were normalized to the maximum current (Imax) evoked by ATP at pH 7.5, including ATP-responses recorded at other pH levels. The concentration required to evoke 50% of the maximum agonist response (EC50) was taken from Hill plots, constructed using the equation log (I/Imax-I), where I is the current evoked by each concentration of ATP.
The effects of extracellular zinc were investigated on agonist activity in two ways. First, C/R curves for the modulatory activity of Zn2+ ions were constructed using a submaximal concentration of ATP and data were normalized to the amplitude of control ATP-responses at pH 8.0, 7.5, 6.5 and 5.5. These experiments were carried out for different pre-incubation periods for Zn2+ (i.e., 0, 20 and 40 min) prior to the addition of ATP. Second, Zn2+ ions were added to ATP solutions and C/R curves for ATP were constructed and normalized to the maximal ATP effect under Zn2+-free conditions at pH 7.5. In some experiments using rP2X3, Zn2+ was added to the superfusate 20 min prior to the addition of ATP and C/R curves to ATP constructed.
Statistics
Data are presented as mean±s.e.mean of four sets of data from different oocyte batches. Significant differences were determined by either unpaired Student's t-test or one-way analysis of variance (ANOVA) followed by Dunnett's test, using commercially-available software (Instat v2.05a, GraphPad).
Drugs
All common salts and reagents were AnalaR grade (Aldrich Chemicals, U.K.). Adenosine 5′-triphosphate disodium salt (ATP) and zinc chloride were purchased from Sigma Chemical Co. (Poole, Dorset, U.K.). Drugs were dissolved in Ringer's solution with the pH adjusted to match individual experiments.
Results
Effects of extracellular pH on IATP at rP2X1 and rP2X3 receptor
Superfused ATP (0.03–30 μM) evoked rapidly-activating, rapidly-inactivating inward currents (IATP) in Xenopus oocytes expressing homomeric rP2X1 receptors. The amplitude of the evoked IATP was dependent on extracellular pH (pHe) and decreased as the superfusate was made more acidic (Figure 1A). This decrease was due to a fall in ATP potency, as judged by a change in the EC50 value (see Table 1) without a concomitant change in the maximum ATP effect (see Figure 2A). Thus, ATP potency was reduced 2 fold at pH 6.5 and 6 fold at pH 5.5, whereas alkaline conditions (pH 8.0) had no effect on agonist potency. The modulatory effects of H+ at rP2X1 receptors were reversed upon readjusting the superfusate to pH 7.5.
Figure 1.

Extracellular pH modulates ATP activity at P2X (Group 1) receptors. (A) Whole-cell inward currents evoked by ATP (1 μM), at four levels of extracellular pH (8.0, 7.5, 6.5, 5.5), at rP2X1 receptors expressed in Xenopus oocytes. All records from the same oocyte (Vh=−60 mV). (B) Whole-cell inward currents evoked by ATP (3 μM), at the same pHe levels, at rP2X3 receptors. All records from the same oocyte (Vh=−90 mV).
Table 1.
ATP potency at Group 1 P2X receptors at different pHe levels
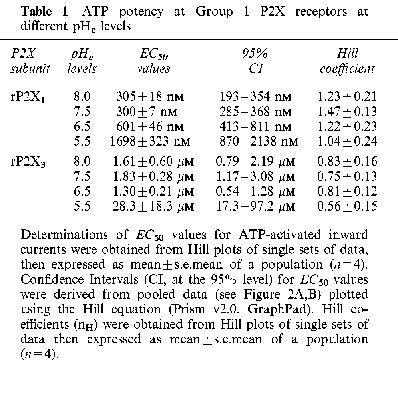
Figure 2.
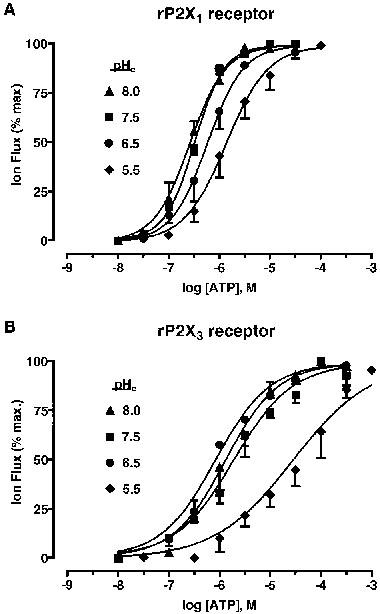
pH-dependent changes in ATP potency at P2X (Group 1) receptors. (A) Concentration/response (C/R) curves for whole-cell inward currents (IATP) evoked by ATP (10 nM–100 μM), at four levels of pHe (8.0, 7.5, 6.5, 5.5), at rP2X1 receptors. The amplitude of IATP was normalized to the maximum ATP effect at pH 7.5. (B) C/R curves for IATP at rP2X3 receptors under the same conditions. Data points are means±s.e.mean, n=4.
Superfused ATP (0.1–300 μM) also evoked rapidly-activating, rapidly-inactivating inward currents (IATP) from Xenopus oocytes expressing homomeric rP2X3 receptors. The IATP of activated rP2X3 receptors was insensitive to small changes to pHe (±1 pH unit), although the amplitude of IATP decreased significantly (P<0.05) at pH 5.5 (Figure 1B). This decrease was due to a fall in ATP potency (∼15 fold decrease) (see Table 1), without a concomitant change in the maximum ATP effect (Figure 2B). The modulatory effects of H+ at rP2X3 receptors were also reversed upon readjusting the superfusate to pH 7.5.
Modulatory effects Zn2+ ions of IATP
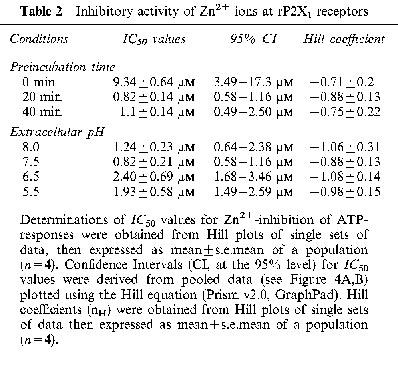
Extracellular Zn2+ (0.01–300 μM) inhibited IATP at rP2X1 receptors, yet potentiated IATP at rP2X3 receptors, in a reversible manner (Figure 3A, B). Zn2+-inhibition of rP2X1 receptors was not only dependent on concentration but also the pre-incubation time for Zn2+ (Figure 4A). Zn2+ was approximately 10 fold more potent, as judged by changes in IC50 values (Table 2), when superfused for 20 min or longer prior to ATP application. The ability of Zn2+ to inhibit IATP at rP2X1 was not altered appreciably over the range of pH 8.0–5.5 (Figure 4B). Since agonist potency at rP2X1 is already reduced as the extracellular solution was made more acidic, Zn2+ and H+-inhibition appear to be additive and to occur by independent processes.
Figure 3.
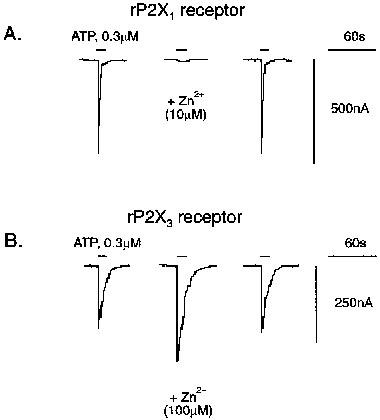
Zn2+ ions modulate ATP activity at P2X (Group 1) receptors. (A) Whole-cell inward currents evoked by ATP (0.3 μM, approx. EC50) before, during and after washout of Zn2+ (10 μM; 20 min pre-incubation; at pH 7.5) at rP2X1 receptors. All records from the same oocyte (Vh=−60 mV). (B) Whole-cell inward currents to ATP (0.3 μM, approx. EC20) before, during and after washout of Zn2+ (100 μM; 0 min pre-incubation; at pH 7.5). All records from the same oocyte (Vh=−60 mV). Note that the Zn2+ effect at rP2X1 and rP2X3 receptors reversed on washout (20 min).
Figure 4.

Zn2+-inhibition of ATP-responses at rP2X1 receptors. (A) Inhibition curves for Zn2+ modulation of whole-cell inward currents (IATP) evoked by ATP (0.3 μM, approx. EC50), where Zn2+ was applied over three pre-incubation periods (0, 20 and 40 min), at pH 7.5. (B) Inhibition curves for Zn2+ against IATP, at four different levels of pHe (8.0, 7.5, 6.5, 5.5). Each concentration of Zn2+ was applied 20 min prior to and during ATP superfusion. Data points are mean±s.e.mean, n=4.
Table 2.
Inhibitory activity of Zn2+ ions at rP2X1 receptors
Zn2+-potentiation of IATP at rP2X3 receptors was dependent on both concentration and the pre-incubation time for Zn2+. When applied simultaneously with ATP, Zn2+ (0.1–300 μM) potentiated IATP by approximately 50% above control responses in a manner described by a sigmoid C/R curve (EC50, 10.9±0.74 μM, n=4) (Figure 5A). However, the effects of Zn2+ were more complex when applied prior to ATP, with a 20 min pre-incubation period for Zn2+ proving optimal. Under these circumstances, Zn2+ showed a greater degree of potentiation, which was then followed by inhibition, in a manner best described by a bell-shaped C/R curve (Figure 5A). The ability of Zn2+ to potentiate IATP at rP2X3 receptors (where Zn2+ was applied simultaneously with ATP) was not altered appreciably over the range pH 8.0–5.5 (Figure 5B).
Figure 5.
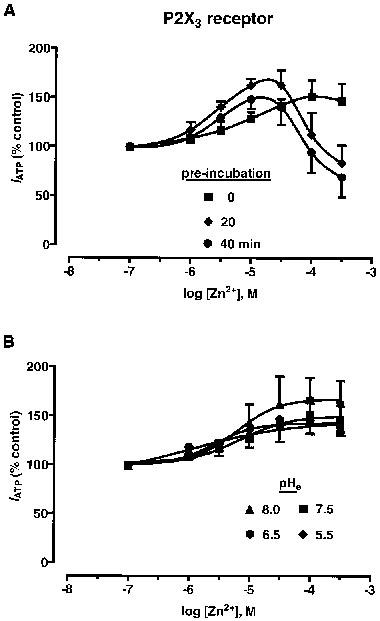
Zn2+-modulation of ATP-responses at rP2X3 receptors. (A) Concentration/response (C/R) curves for Zn2+ modulation of whole-cell inward currents (IATP) to ATP (0.3 μM, approx. EC20), where Zn2+ was applied over three pre-incubation periods (0, 20, 40 min), at pH 7.5. (B) C/R curves for Zn2+ against IATP, at four different levels of pHe (8.0, 7.5, 6.5, 5.5). Each concentration of Zn2+ was applied simultaneously with ATP. Data points are means ±s.e.mean, n=4.
Effect of Zn2+ on ATP potency and efficacy at Group 1 P2X receptors
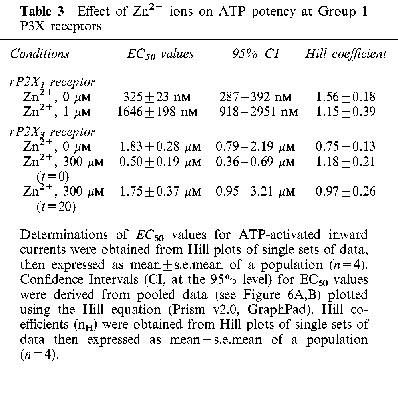
ATP potency and efficacy at rP2X1 and rP2X3 receptors were reassessed in the presence of Zn2+ to help explain its modulatory effects. For rP2X1, Zn2+ was applied for 20 min prior to ATP and at a concentration matching its IC50 value (1 μM). Under these conditions, ATP potency was reduced by 5 fold without changing the maximum ATP effect (Figure 6A). EC50 values and Hill coefficients are given in Table 3. For rP2X3, Zn2+ was applied either simultaneously with ATP (0 min) or prior to ATP (20 min) at a concentration (300 μM) which gave maximal potentiation and inhibition for the appropriate pre-incubation period. Zn2+-potentiation (0 min pre-incubation) was due to a 3–4 fold increase in ATP potency, without any change in agonist efficacy (Figure 6B). Zn2+ inhibition (with 20 min pre-incubation) was due to a 30±5% (n=3) decrease in the maximum ATP effect (decreased agonist efficacy), without any change in agonist potency (Figure 6B). EC50 values and Hill coefficients are given in Table 3.
Figure 6.
Zn2+ modulation of agonist potency and efficacy at P2X (Group 1) receptors. (A) concentration/response (C/R) curves for whole-cell inward currents to ATP (30 nM–30 μM) at rP2X1 receptors, in the absence and present of Zn2+ (1 μM), at pH 7.5. Zn2+ was applied for 20 min prior to each concentration of ATP. (B) C/R curves for whole-cell inward currents to ATP (3 nM–300 μM) at rP2X3 receptors, in the absence and presence of Zn2+ (300 μM) at pH 7.5. Zn2+ was applied either simultaneously with ATP (t=0) or 20 min prior to ATP (t=20). Data points are means±s.e.mean, n=4.
Table 3.
Effect of Zn2+ ions on ATP potency at Group 1 P3X receptors
Discussion
H+ modulation of P2X (Group 1) receptors
The two members of Group 1 (α,β-meATP-sensitive, fast-inactivating, suramin-sensitive) P2X receptors were affected in a similar manner by acidification of the extracellular solution. Agonist potency was decreased at both rP2X1 and rP2X3 receptors, without a concomitant change in agonist efficacy, and this effect was reversed by restoring [H+]o to pH 7.5. Although the H+ effect was broadly similar for these two recombinant P2X receptors, there were subtle differences in the way this was manifested. There was a gradual decrease in agonist potency at rP2X1 receptors over the range of pH 7.5–5.5, and the H+ effect was rather modest (a 6 fold increase in the EC50 value for ATP at pH 5.5). In contrast, agonist potency at rP2X3 receptors was altered only at pH 5.5, at which point the H+ effect was more substantial (a 15 fold increase in the EC50 value for ATP).
The H+ effect at Group 1 P2X receptors is different from the modulatory activity of H+ ions at rP2X2 (Group 2) and rP2X4 (Group 3) receptors. H+ ions potentiate ATP-responses at rP2X2 receptors, by increasing agonist potency without changing efficacy, showing maximum activity at levels as low as pH 5.5 (King et al., 1996; 1997, Stoop et al., 1997). However, it was also reported that highly acidic conditions (<pH 5.0) result in a progressive loss in agonist efficacy at rP2X2 receptors (Stoop & Quayle, 1998). The H+ effect at rP2X4 is characterized by a progressive decrease in agonist potency over the range of pH 7.5–5.5, and a loss of agonist efficacy at pH 5.5 (Wildman et al., 1999b). To some extent, an H+ effect has also been observed at rP2X7 (Group 4) receptors at which acidic conditions (pH 7.5–5.5) progressively inhibited membrane current (through the P2Z ion-channel) and YO-PRO uptake (through the P2Z-activated pore) (Virginio et al., 1997). However, there is no information on changes in agonist potency and efficacy to explain the H+-inhibition of ATP-responses at rP2X7 receptors.
The H+ effect is potentially important for P2X receptor signalling for two reasons. First, large pH shifts (2–3 pH units) occur with the localized metabolic acidosis associated with bone fracture (pH 4.7), ischaemia (pH 5.7), inflammation (pH 5.4), epileptic seizures and injuries related to CNS degenerative changes (DeSalles et al., 1987; Chesler, 1990; Ransom & Philbin, 1992; Steen et al., 1992). Second, transient acidic shifts also occur during CNS neurotransmission (Krishtal et al., 1987; Chesler, 1990; Rose & Dietmer, 1995; Yanovsky et al., 1995), a phenomenon due to the way transmitters are packaged in vesicles. Vacuolar H+-ATPase (V-ATPase) establishes a H+-gradient across the wall of synaptic vesicles, rendering the intravesicular space acidic (pH 5.5) (Cidon & Sihra, 1989). The resultant electrochemical gradient for H+ ions assists in concentrating neurotransmitters inside storage vesicles (for a review, see: Moriyama et al., 1992). The acidic shift associated with neurotransmission may have a significant bearing on the sensitization of postjunctional P2X receptors activated by purinergic nerves, particularly during high frequency transmission where significant accretion of vesicular H+ will occur at the synapse. Little is known, however, about the pH-dependence of purinergic transmission in smooth muscles where P2X1 receptors are concentrated. Similarly, the effect of localized acidosis has not been investigated for P2X3 receptor-mediated currents in sensory nerve endings.
Zn2+ modulation of P2X (Group 1) receptors
The Zn2+ effect, in contrast to the above H+ effect, was dissimilar at rP2X1 and rP2X3 receptors–inhibition and potentiation, respectively. The inhibitory action of Zn2+ at rP2X1 receptors was characterized by a fall in agonist potency without any change in agonist efficacy. The immediacy of Zn2+-inhibition, where Zn2+ was applied simultaneously with ATP, indicated an extracellular locus for its site of action. However, the time-dependency and increased potency of Zn2+-inhibition at rP2X1 receptors also pointed to the involvement of a second intracellular site of action. Time-dependent Zn2+-inhibition of ATP-responses was also seen at rP2X2 receptors (Wildman et al., 1998) and, to some extent, at rP2X3 receptors (present study).
The actions of Zn2+ at rP2X3 receptors were complex, yet bore a similarity to its actions at rP2X2 receptors (Wildman et al., 1998). When applied simultaneously with ATP, the main Zn2+ effect was potentiation of ATP-responses at rP2X3 receptors. The same effect occurs at rP2X2 receptors, although the magnitude of the Zn2+ effect is much greater. With Zn2+-preincubation, the Zn2+ effect at rP2X3 receptors was characterized by a concentration-dependent potentiation followed by inhibition; the same occurs at rP2X2 receptors. Potentiation was due to an increase in agonist potency at rP2X3 receptors, whereas inhibition was primarily due to a decrease in agonist efficacy (by 30%). Zn2+-inhibition at rP2X2 receptors is characterized by a 50% loss of agonist efficacy and a decrease in agonist potency (Wildman et al., 1998). The observed Zn2+-inhibition (and Zn2+-potentiation) at P2X receptors expressed in oocytes is not due to alterations in ecto-ATPase activity since the enzyme is absent in defolliculated cells (Ziganshin et al., 1995).
The Zn2+ effect at P2X (Group 1) receptors is important for two reasons. First, it now provides a simple means to distinguish rP2X1 receptors from rP2X3 receptors when, presently, there are no P2X subunit-selective agonists and antagonists. Second, Zn2+ ions are taken up and concentrated in synaptic vesicles of defined subsets of central neurons (for a review, see: Smart et al., 1994), although the accumulation of Zn2+ in peripheral nerves has not yet been studied. It is not known if vesicular Zn2+, like vesicular H+, acts as an agent for packaging of neurotransmitters and/or serves as a neuromodulator of receptor function. The Zn2+ content of some synaptic boutons is as high as 200–300 μM, and the vesicular concentration may be higher still (Smart et al., 1994). The plasma Zn2+ concentration in rat is 21 μM, although much (∼99%) is bound to seral proteins (Walker & Kelleher, 1978). The impact of either vesicular-released or extracellular-fluid Zn2+ on purinergic signalling has not been assessed in tissues where P2X1 and P2X3 subunits are concentrated.
H+ and Zn2+ modulation of recombinant rP2X1–4 receptors
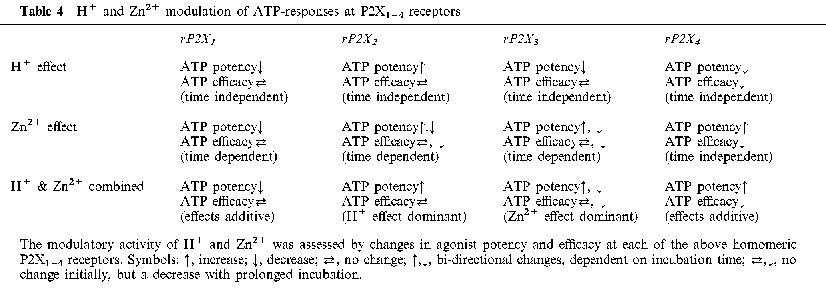
This present study of Group 1 P2X subunits represents a part of an extended survey of H+ and Zn2+ modulation of P2X subunits known to be associated with neurotransmission. We have already commented at length on the modulatory activity of H+ and Zn2+ at rP2X2 and rP2X4 receptors (King, 1998; King et al., 1996; 1997; Wildman et al., 1998; 1999b). The modulatory activity of H+ and Zn2+ ions is important because of their presence in synaptic vesicles and likelihood they are co-released with ATP during purinergic transmission. The outcome of the present study, when taken with our past results, shows a spectrum of H+ and Zn2+ effects at homomeric P2X1–4 receptors. Table 4 summarizes the available information on these effects and establishes an operational profile for each P2X subunit. Such information on the H+ and Zn2+ effects, together with distinct agonist profiles for such agents as the naturally-occurring diadenosine polyphosphates series (Brown et al., 1999; Wildman et al., 1999a), may be helpful in the future to identify the presence of P2X subunits in native P2X receptors at a time when P2X subunit-selective antagonists are not available.
Table 4.
H+ and Zn2+ modulation of ATP-responses at P2X1–4 receptors
Acknowledgments
We thank the British Heart Foundation and Roche Bioscience (Palo Alto, U.S.A.) for their financial support. Dr G.N. Buell (formerly Glaxo, Geneva) generously provided the cDNA encoding the rat P2X1 receptor. Professor J.N. Wood (UCL, London) provided the cDNA encoding the rat P2X3 receptor.
Abbreviations
- ATP
adenosine 5′-triphosphate
- α,β-meATP
α,β-methylene ATP
- cRNA
capped ribonucleic acid
- DRG
dorsal root ganglia
- EC50
concentration causing 50% of the maximum agonist response
- IC50
concentration inhibiting an agonist response by 50%
- IATP
ATP-activated membrane current
- 2-MeSATP
2-methylthio-ATP
- mRNA
messenger ribonucleic acid
- nH
Hill coefficient
- pHe
extracellular pH
- PPADS
pyridoxal-α5-phosphate-6-azophenyl-2′,4′-disulphonic acid
- TNP-ATP
trinitrophenyl ATP
- Vh
holding potential
- Zn2+
zinc ions
References
- BARISH M. A transient calcium-dependent chloride current in the immature Xenopus oocyte. J. Physiol. 1983;342:309–325. doi: 10.1113/jphysiol.1983.sp014852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BROWN S.G., WILDMAN S.S., KING B.F., BURNSTOCK G. Diadenosine polyphosphates as pharmacological tools to identify P2X1,2,3,4 subunits. Br. J. Pharmacol. 1999;126:24P. [Google Scholar]
- BURNSTOCK G. The past, present and future of purine nucleotides as signalling molecules. Neuropharmacol. 1997;36:1127–1139. doi: 10.1016/s0028-3908(97)00125-1. [DOI] [PubMed] [Google Scholar]
- CHESLER M. The regulation and modulation of pH in the nervous system. Prog. Neurobiol. 1990;34:401–427. doi: 10.1016/0301-0082(90)90034-e. [DOI] [PubMed] [Google Scholar]
- CIDON S., SIHRA T.S. Characterisation of H+-ATPase in rat brain synaptic vesicles: coupling to L-glutamate transport. J. Biol. Chem. 1989;264:8281–8289. [PubMed] [Google Scholar]
- DESALLES A.A., KONTOS H.A., WARD J.B., MARMAROU A., BECKER D.P. Brain tissue pH in severely head-injured patients: a report of 3 cases. Neurosurgery. 1987;20:297–301. doi: 10.1227/00006123-198702000-00017. [DOI] [PubMed] [Google Scholar]
- HUMPHREY P.P.A., KHAKH B.S., KENNEDY C., KING B.F., BURNSTOCK G. Nucleotide receptors: P2X receptors. IUPHAR Compendium of Receptor Characterization and Classification, IUPHAR Media. 1998. pp. 195–208.
- KING B.F.Molecular Biology of P2X Purinoceptors Cardiovascular Biology of Purines 1998Kluwer Academic Publications, Massachusetts; 159–186.Burnstock, G. Dobson Jr. J.G., Liang B.T. & Linden J. (eds)Ch. 10 [Google Scholar]
- KING B.F., WILDMAN S.S., BURNSTOCK G. Modulatory activity of extracellular H+ and Zn2+ on ATP-responses at recombinant rP2X1 and rP2X3 receptors. J. Physiol. 1999. pp. 518P–30P. [DOI] [PMC free article] [PubMed]
- KING B.F., WILDMAN S.S., ZIGANSHINA L.E., PINTOR J., BURNSTOCK G. Effects of extracellular pH on agonism and antagonism at a recombinant P2X2 receptor. Br. J. Pharmacol. 1997;121:1445–1453. doi: 10.1038/sj.bjp.0701286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KING B.F., ZIGANSHINA L.E., PINTOR J., BURNSTOCK G. Full sensitivity of P2X2 purinoceptor to ATP revealed by changing extracellular pH. Br. J. Pharmacol. 1996;117:1371–1373. doi: 10.1111/j.1476-5381.1996.tb15293.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KRISHTAL O.A., OSIPCHUCK Y.V., SHELEST T.N., SMIRNOFF S.V. Rapid extracellular pH transients related to synaptic transmission in rat hippocampal slices. Brain Res. 1987;436:352–356. doi: 10.1016/0006-8993(87)91678-7. [DOI] [PubMed] [Google Scholar]
- MORIYAMA Y., MAEDA M., FUTAI M. The role of V-ATPase in neuronal and endocrine systems. J. Exp. Biol. 1992;172:171–178. doi: 10.1242/jeb.172.1.171. [DOI] [PubMed] [Google Scholar]
- RANSOM B.R., PHILBIND M. , JR Anoxia-induced extracellular ionic changes in the CNS white matter: the role of glial cells. Can. J. Physiol. Pharmacol. 1992;70:S181–S189. doi: 10.1139/y92-261. [DOI] [PubMed] [Google Scholar]
- ROSE C.R., DEITMER J.W. Stimulus-evoked changes of extra- and intracellular pH in the leech central nervous system. II. Mechanisms and maintenance of pH homeostasis. J. Neurophysiol. 1995;73:132–140. doi: 10.1152/jn.1995.73.1.132. [DOI] [PubMed] [Google Scholar]
- SMART T.G., XIE X., KRISHEK B.J. Modulation of inhibitory and excitatory amino acid receptor ion channels by zinc. Prog. Neurobiol. 1994;42:393–441. doi: 10.1016/0301-0082(94)90082-5. [DOI] [PubMed] [Google Scholar]
- STEEN K.H., REEH P.W., ANTON F., HANDWERKER H.O. Protons selectively induce lasting excitation and sensitisation to mechanical stimulation of nociception in rat skin, in vitro. J. Neurosci. 1992;12:86–95. doi: 10.1523/JNEUROSCI.12-01-00086.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- STOOP R., QUAYLE J.M. Fading and rebound of P2X2 currents at millimolar ATP concentrations caused by low pH. Br. J. Pharmacol. 1998;125:235–237. doi: 10.1038/sj.bjp.0702056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- STOOP R., SURPRENANT A., NORTH R.A. Different sensitivities to pH of ATP-induced currents at four cloned P2X receptors. J. Neurophysiol. 1997;78:1837–1840. doi: 10.1152/jn.1997.78.4.1837. [DOI] [PubMed] [Google Scholar]
- VIRGINIO C., CHURCH D., NORTH R.A., SURPRENANT A. Effects of divalent cations, protons and calmidazolium at the rat P2X7 receptor. Neuropharmacology. 1997;36:1285–1294. doi: 10.1016/s0028-3908(97)00141-x. [DOI] [PubMed] [Google Scholar]
- WALKER B.E., KELLEHER J. Plasma whole blood and urine zinc in the assessment of zinc deficiency in the rat. J. Nutrition. 1978;108:1702–1707. doi: 10.1093/jn/108.10.1702. [DOI] [PubMed] [Google Scholar]
- WILDMAN S.S., BROWN S.G., KING B.F., BURNSTOCK G. Selectivity of diadenosine polyphosphates for rat P2X receptor subunits. Eur. J. Pharmacol. 1999a;367:119–123. doi: 10.1016/s0014-2999(98)00976-5. [DOI] [PubMed] [Google Scholar]
- WILDMAN S.S., KING B.F., BURNSTOCK G. Zn2+ modulation of ATP-responses at recombinant P2X2 receptors and its dependence on extracellular pH. Br. J. Pharmacol. 1998;123:1214–1220. doi: 10.1038/sj.bjp.0701717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WILDMAN S.S., KING B.F., BURNSTOCK G. Modulation of ATP-responses at recombinant rP2X4 receptors by extracellular pH and Zn2+ Br. J. Pharmacol. 1999b;126:762–768. doi: 10.1038/sj.bjp.0702325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- YANOVSKY Y., REYMANN K., HAAS H.L. pH-dependent facilitation of synaptic transmission by histamine in the CA1 region of mouse hippocampus. Eur. J. Neurosci. 1995;7:2017–2020. doi: 10.1111/j.1460-9568.1995.tb00624.x. [DOI] [PubMed] [Google Scholar]
- ZIGANSHIN A.U., ZIGANSHINA L.E., KING B.F., BURNSTOCK G. Characteristics of ecto-ATPase of Xenopus oocytes and the inhibitory actions of suramin on ATP breakdown. Pflügers Archiv (Mol. Cell. Physiol) 1995;429:412–418. doi: 10.1007/BF00374157. [DOI] [PubMed] [Google Scholar]


