Abstract
Adenosine 5′-triphosphate (ATP) is an enteric neurotransmitter which acts at purine receptors on intestinal nerve and muscle. This study set out to shed light on the receptor mechanisms by which exogenous and endogenous ATP influences intestinal peristalsis.
Peristalsis in isolated segments of the guinea-pig small intestine was triggered by a perfusion-induced rise of the intraluminal pressure. Motor changes were quantified by alterations of the peristaltic pressure threshold (PPT) at which propulsive muscle contractions were elicited.
ATP (⩾3 μM) increased PPT and abolished peristalsis at concentrations of 100–300 μM. Adenosine 5′-O-2-thiodiphosphate (ADPβS, 3–100 μM) was more potent, whereas α,β-methylene ATP (α,β-meATP, 3–100 μM) was less potent, than ATP in depressing peristalsis.
8-Phenyltheophylline (10 μM) attenuated the anti-peristaltic effect of 10 and 30 μM ATP but not that of higher ATP concentrations. Apamin (0.5 μM) counteracted the ability of ATP, ADPβS and α,β-meATP to enhance PPT. Suramin (300 μM) and pyridoxal phosphate-6-azophenyl-2′,4′-disulphonic acid (PPADS, 150 μM) antagonized the inhibitory effect of α,β-meATP on peristalsis but did not alter the effect of ATP and ADPβS.
PPADS (50–150 μM) reduced PPT by as much as 50%. This stimulant effect on peristalsis was prevented by suramin (300 μM) but left unaltered by apamin (0.5 μM) and NG-nitro-L-arginine methyl ester (300 μM).
These data show that exogenous and endogenous ATP inhibits intestinal peristalsis via different apamin-sensitive purinoceptor mechanisms. Exogenous ATP depresses peristalsis mostly via suramin- and PPADS-insensitive P2 receptors, whereas endogenous purines act via P2 receptors sensitive to both suramin and PPADS.
Keywords: Apamin, ATP, purinoceptors, suramin, enteric nervous system, intestinal peristalsis
Introduction
Adenosine 5′-triphosphate (ATP) is a non-adrenergic non-cholinergic transmitter of the enteric nervous system (Ralevic & Burnstock, 1998). Following stimulation, varicosities of enteric neurones release ATP (White, 1982; McConalogue et al., 1996) which acts at purinoceptors on intestinal nerve and muscle in a cell-specific manner and thus stimulates or inhibits intestinal motility. For instance, ATP depolarizes S neurones of the submucosal plexus (Barajas-Lopez et al., 1994) and mediates fast excitatory postsynaptic potentials in the myenteric plexus (Galligan & Bertrand, 1994; LePard et al., 1997). This stimulant effect of ATP is brought about by P2 purinoceptors coupled to non-selective cation channels (Evans et al., 1992; Barajas-Lopez et al., 1994; Zhou & Galligan, 1996; Christofi et al., 1997; LePard et al., 1997) and gives rise to cholinergic contractions of the gut (Barthó et al., 1997). To complicate the situation, however, ATP can also inhibit cholinergic neurotransmission in the myenteric and submucosal plexuses through an action on presynaptic P2 receptors (Barajas-Lopez et al., 1995; Kamiji et al., 1995; LePard et al., 1997).
While the effects of purines on enteric neurones have been recognized only recently, ATP has long been considered as a co-transmitter of inhibitory motor neurones causing depression of intestinal motor activity. Thus, ATP produces fast inhibitory junction potentials which lead to relaxation of the circular muscle, an action that is brought about by P2 purinoceptors coupled to apamin-sensitive small conductance Ca2+-dependent K+ channels (Niel et al., 1983; Costa et al., 1986; Crist et al., 1992; Zagorodnyuk & Maggi, 1994). There is good evidence that ATP or a related purine is an inhibitory transmitter in the gut and as such participates in the descending relaxation of the circular muscle in response to distension, an essential component of the intestinal motor pattern of peristalsis (Crist et al., 1992; Lyster et al., 1992; Keef et al., 1993; Waterman & Costa, 1994; Holzer et al., 1997; Barthó et al., 1998). Pharmacological analysis has revealed that the relaxant effect of purines on intestinal muscle is in fact mediated by two distinct subtypes of P2 purinoceptors. One group of receptors is activated by α,β-methylene ATP (α,β-meATP) and inhibited by suramin and pyridoxal phosphate-6-azophenyl-2′,4′-disulphonic acid (PPADS), whereas the other group of receptors is activated by adenosine 5′-O-2-thiodiphosphate (ADPβS) and resistant to suramin and PPADS (Windscheif et al., 1995; Bültmann et al., 1996; Zagorodnyuk & Maggi, 1998). In addition, excitatory P2 receptors causing contraction are also present on the intestinal smooth muscle (Wiklund & Gustafsson, 1988; Kennedy & Humphrey, 1994; Zagorodnyuk & Maggi, 1998).
Although purines have been found to inhibit propulsive peristalsis (Okwuasaba et al., 1977; Van Nueten et al., 1977) it is not known which of the excitatory and inhibitory effects of purinoceptor activation on intestinal nerve and muscle have a bearing on the action of exogenous and endogenous ATP on propulsive motility. This type of study is important because the complexity of the peristaltic motor circuits in the enteric nervous system is such that drug effects on propulsive motility cannot be predicted from their influence on stationary motor reflexes (Tonini et al., 1996; Holzer et al., 1997). Using the guinea-pig isolated ileum, we hence set out to characterize the receptors that mediate the inhibitory effect of exogenous ATP on peristalsis by using α,β-meATP, ADPβS, suramin, PPADS and apamin and to probe the action of endogenously released purines by testing the peristaltic motor responses to suramin and PPADS.
Methods
Propulsive peristalsis
Adult guinea-pigs (TRIK strain, IEP SAS Dobrá Voda, Bratislava, Slovakia) of either sex and 350–450 g body weight were stunned and bled. The distal small intestine (jejunum and ileum) was excised, flushed of luminal contents and placed, for up to 4 h in Tyrode solution, kept at room temperature and oxygenated with a mixture of 95% O2 and 5% CO2. The composition of the Tyrode solution was (mM): NaCl 136.9, KCl 2.7, CaCl2 1.8, MgCl2 1.0, NaHCO3 11.9, NaH2PO4 0.4, glucose 5.6. For studying peristalsis, the distal small intestine was divided into eight segments, each being approximately 8 cm long. Four intestinal segments were set up in parallel and secured horizontally in organ baths containing 30 ml of Tyrode solution at 37°C. The system for eliciting and recording propulsive peristalsis has previously been described (Holzer et al., 1997; 1998). In brief, prewarmed Tyrode solution was continuously infused into the lumen of the segments at a rate of 0.5 ml min−1. The intraluminal pressure at the aboral end of the segments was measured with a pressure transducer whose signal was, via an analogue/digital converter, fed into a personal computer and recorded and analysed with the software ‘Peristal 1.0' (Heinemann Scientific Software, Graz, Austria).
The fluid passing through the gut lumen was directed into a vertical outlet tubing which ended 4.1 cm above the fluid level in the organ bath. When fluid was infused, the intraluminal pressure rose slowly until it reached a threshold at which peristalsis was triggered (Figure 1; Holzer et al., 1998). The aborally moving wave of peristaltic contraction resulted in a spike-like increase in the intraluminal pressure and caused emptying of the segment. The peristaltic pressure threshold (PPT) was used to quantify drug effects on peristalsis. Inhibition of peristalsis was reflected by an increase in PPT, and abolition of peristalsis manifested itself in a lack of propulsive motility in spite of an intraluminal pressure of 400 Pa as set by the position of the outlet tubing. Although in this case PPT exceeded 400 Pa, abolition of peristalsis was expressed quantitatively by assigning PPT a value of 400 Pa in order to obtain numerical results suitable for further statistical evaluation.
Figure 1.
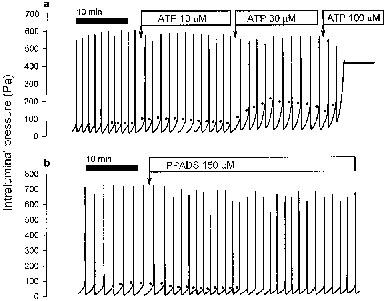
Recordings of the action of ATP (a) and PPADS (b) on peristalsis. The drugs were administered to the organ bath at the indicated concentrations. The ATP-induced inhibition of peristalsis manifested itself in a rise of the peristaltic pressure threshold (PPT, indicated by dots), which at the concentration of 100 μM resulted in complete shutdown of peristalsis. PPADS caused a sustained stimulation of peristalsis as deduced from its effect to lower PPT.
The effectiveness of peristalsis was assessed by regular visual inspection of the preparations and by monitoring the minimum of the intraluminal pressure that was achieved after completion of each peristaltic wave. This residual intraluminal pressure, which normally was about 5 Pa, is a sensitive measure of the emptying capacity of the peristaltic wave (Holzer et al., 1997).
The preparations were allowed to equilibrate in the organ bath for a period of 30 min during which they were kept in a quiescent state. Thereafter the bath fluid was renewed and peristaltic motility initiated by intraluminal perfusion of the segments. After basal peristaltic activity had been recorded for a period of 30 min, the drugs to be tested were administered into the bath, i.e., to the serosal surface of the intestinal segments, at volumes not exceeding 1% of the bath volume. The corresponding vehicle solutions were devoid of any effect.
Three sets of experiments were carried out. Firstly, the concentration-related influence of ATP, α,β-meATP and ADPβS (3–300 μM) on peristalsis was studied, these agonists being administered in a cumulative manner at 15 min intervals (Figures 1 and 2). Secondly, the susceptibility of the peristaltic motor effects of ATP, α,β-meATP and ADPβS (3–300 μM) to a number of drugs was tested, these drugs being administered at appropriate time intervals before exposure to the purinoceptor agonists (Table 1, Figures 2 and 3). Thirdly, the ability of PPADS (50–150 μM) to reduce PPT was examined and the susceptibility of the peristaltic motor effect of PPADS (150 μM) to a number of drugs was analysed, these drugs being administered at appropriate time intervals before exposure to PPADS (Table 2) and the response to PPADS being observed for a period of 30 min. Each substance was tested on at least five segments from five different guinea-pigs.
Figure 2.

(a) Effect of ATP to increase the peristaltic pressure threshold (PPT) as observed in the presence of vehicle or 8-phenyltheophylline (8-PT; 10 μM) which were administered 15 min before the ATP concentration-response curve was recorded. (b) Comparison of the effects of ATP, ADPβS and α,β-meATP to increase PPT. The concentration-response curves were recorded in a cumulative manner, and the graph shows peak changes in PPT which occurred within 15 min after exposure to each purine concentration. The values represent means±s.e.mean, n⩾6. *P<0.05 versus vehicle (a; two sample t-test) or ATP (b; one way analysis of variance).
Table 1.
Effect of various drugs on the peristaltic pressure threshold (PPT)

Figure 3.

Effects of suramin plus PPADS, apamin and L-NAME on the concentration-dependent action of ATP (a), ADPβS (b) and α,β-meATP (c) to increase the peristaltic pressure threshold (PPT). The concentration-response curves were recorded in a cumulative manner, and the graph shows peak changes in PPT which occurred within 15 min after exposure to each purine concentration. Vehicle, suramin (300 μM) plus PPADS (150 μM), apamin (0.5 μM) and L-NAME (300 μM) were administered 30 min before exposure to the purinoceptor agonists. The values represent means±s.e.mean, n⩾6. *P<0.05 versus vehicle (one way analysis of variance).
Table 2.
Effect of various drugs on the effect of PPADS to lower the peristaltic pressure threshold (PPT)

Drugs and solutions
Adenosine 5′-O-2-thiodiphosphate (ADPβS), adenosine 5′-triphosphate (ATP), α,β-methylene ATP (α,β-meATP), apamin (1 mM), atropine (1 mM), hexamethonium (10 mM), naloxone (1 mM) and NG-nitro-L-arginine methyl ester (L-NAME; 30 mM) were purchased from Sigma (Vienna, Austria) and dissolved in Tyrode solution at the indicated concentrations. Pyridoxal phosphate-6-azophenyl-2′,4′-disulphonic acid (PPADS; 10 mM) and suramin (30 mM) were obtained from Research Biochemicals International (RBI, Natick, MA, U.S.A.) and likewise dissolved in Tyrode solution. 8-Phenyltheophylline (8-PT; Sigma; 1 mM) was dissolved in 1% dimethyl sulphoxide, 1% NaOH (1 M) and 98% distilled water (per cent by volume). The concentrations referred to in brackets indicate the stock solutions which were diluted with Tyrode solution before use.
Data calculation and statistics
The PPT of three consecutive peristaltic contractions was averaged to determine the baseline values recorded immediately before administration of a drug. The same procedure was applied to calculate the peak values of drug-induced changes in PPT, unless peristalsis was abolished in which case PPT was assigned a value of 400 Pa. Quantitative data are presented as means±s.e.mean of n experiments, n referring to the number of guinea-pigs used in the test. The results were evaluated statistically with the paired or two-sample Student's t-test or one way analysis of variance. A probability value P<0.05 was regarded as significant.
Results
Effects of ATP, ADPβS and α,β-meATP on peristalsis
The PPT at baseline ranged from 60–90 Pa (Table 1, Figures 1 and 2). Administration of ATP (3–300 μM) to the organ bath increased PPT (Figure 1a) in a concentration-related manner (Figure 2). In addition, ATP attenuated the effectiveness of peristalsis, which was reflected by a decrease in the amplitude of the peristaltic waves and an increase in the residual intraluminal pressure (Figure 1a). The inhibitory effect of ATP on peristalsis took some 5–10 min to reach a maximum (Figure 1a), whereafter PPT begun to return very slowly to the baseline level. For this reason it was possible to record the concentration-response relationship for ATP in a cumulative manner, i.e., by exposing the preparations to increasing concentrations of the purine at 15 min intervals when the preceding response had peaked (Figures 1a and 2). At concentrations of 100–300 μM, ATP abolished peristaltic motility (Figures 1a and 2). The anti-peristaltic effect of 10 and 30 μM ATP, but not of higher concentrations of the purine, was slightly but significantly attenuated after a 15 min presence of 10 μM 8-phenyltheophylline (Figure 2a) which per se had no influence on peristalsis (Table 1).
The inhibitory effect of ATP on peristalsis was mimicked by ADPβS (3–100 μM) which turned out to be more potent than ATP (Figure 2b). Another P2 receptor agonist, α,β-meATP (10–100 μM), was also able to moderately increase PPT but was less potent than ATP (Figure 2b). Concentrations of α,β-meATP higher than 100 μM were not affordable. There were also qualitative differences between the peristaltic motor actions of the three purinoceptor agonists inasmuch as ADPβS caused a brief decrease followed by a prolonged increase in PPT (data not shown). Such an initial stimulant effect, which involved only 1–2 peristaltic contractions, was not consistently seen with α,β-meATP and ATP.
Subsequently it was investigated whether apamin (0.5 μM; Waterman & Costa, 1994; Holzer et al., 1997), suramin (300 μM; Galligan & Bertrand, 1994; Bültmann et al., 1996) and PPADS (50–150 μM; Windscheif et al., 1995; Bültmann et al., 1996) would influence the ability of ATP, ADPβS and α,β-meATP to enhance PPT (i.e., to inhibit peristalsis). As reported previously (Holzer et al., 1997), apamin caused a transient reduction of PPT on its own, an effect that after a 30 min exposure to the drug had largely gone although it was still statistically significant (Table 1). Figure 3a–c shows that apamin attenuated the anti-peristaltic action of ATP and ADPβS and suppressed that of α,β-meATP.
The P2 receptor antagonists suramin and PPADS lowered PPT to a significant extent, an effect that was sustained for at least 30 min (Figure 1b, Tables 1 and 2). After 30 min of exposure, neither suramin nor PPADS altered the inhibitory effect of ATP on peristalsis (n=6 for each antagonist, data not shown). Combined administration of suramin and PPADS caused a sustained reduction of PPT, that was indistinguishable from that of either drug alone (Tables 1 and 2). Exposure to suramin plus PPADS failed, however, to influence the ability of ATP (Figure 3a) and ADPβS (Figure 3b) to enhance PPT, whereas the weak inhibitory action of α,β-meATP on peristalsis was prevented by suramin plus PPADS (Figure 3c).
The nitric oxide (NO) synthase inhibitor NG-nitro-L-arginine methyl ester (L-NAME, 300 μM; Waterman & Costa, 1994) caused a transient reduction of PPT (Holzer et al., 1997), a response that after 30 min had largely waned although it was still statistically significant (Table 1). L-NAME was without effect on the ability of ADPβS (Figure 3b) and α,β-meATP (Figure 3c) to enhance PPT.
Effect of PPADS on peristalsis
PPADS (50–150 μM) lowered PPT in a concentration-related manner (Table 2), which was associated with an increase in the frequency of peristaltic contractions (Figure 1b), a parameter that under the current experimental conditions is determined by the PPT and the compliance of the intestinal wall. The simultaneous decrease in the amplitude of the peristaltic waves was most likely a sequel of the frequency rise induced by the drug, given that an increase in peristaltic frequency due to doubling of the luminal perfusion rate attenuated the amplitude of the peristaltic waves to a similar extent (n=5; data not shown). PPADS's stimulant effect on peristalsis was quick in onset, reached a maximum within 5 min and was sustained for at least 30 min (Figure 1b).
Further experiments were carried out to examine whether the pro-peristaltic action of PPADS would be influenced by a 30-min exposure to apamin, suramin or L-NAME. In assessing their effects it need be considered that these drugs per se decreased PPT to various degrees (Tables 1 and 2). Suramin (300 μM) was as effective as PPADS (150 μM) in lowering PPT and prevented PPADS from causing a further fall of PPT (Table 2). Apamin (0.5 μM) and L-NAME (300 μM) reduced PPT to a lesser degree than suramin and did not halt PPADS to lower PPT to a level of 40–45 Pa as was the case in the presence of vehicle (Table 2). Expressed as a percentage of the PPT recorded before exposure to PPADS, the action of PPADS to decrease PPT was eliminated by suramin and attenuated by apamin and L-NAME (Table 2). It should not go unnoticed that combined exposure to apamin or L-NAME and PPADS made the segments appear hyperexcitable and often disturbed the regular pattern of peristalsis.
In order to examine whether cholinergic neurones contribute to the pro-peristaltic action of PPADS, the facilitatory influence of this P2 receptor antagonist on peristalsis was tested in the presence of atropine (1 μM) plus naloxone (0.5 μM) or hexamethonium (100 μM) plus naloxone. The additional use of naloxone was necessary because atropine and hexamethonium alone suppressed peristaltic motor activity which, however, was largely restored when the opioid receptor antagonist was added in the continued presence of atropine or hexamethonium (Table 1). Table 2 documents that neither atropine plus naloxone nor hexamethonium plus naloxone altered PPADS's (150 μM) ability to lower PPT to about 50% of the level recorded before exposure to PPADS.
Discussion
Aims and limitations of the study
ATP affects the activity of intestinal nerve and muscle (see Introduction). Since little is known as to whether these actions have a bearing on the regulation of propulsive motility, we examined the effect of exogenous and endogenous purines on peristaltic motor activity in the guinea-pig isolated small intestine. The first objective was to describe the action of purines on peristalsis, because drug effects on this propagated motor pattern cannot be deduced from their influence on standing motor reflexes (Tonini et al., 1996; Holzer et al., 1997). The second aim was to characterize the receptors involved in the peristaltic motor actions of purines, and the third goal was to seek pharmacological evidence for an involvement of endogenous purines in peristaltic motor regulation. In interpreting the data it needs to be kept in mind that the study of drug effects on peristalsis is complicated by the multiplicity of sites at which drugs can interfere with the neural control and muscular effector systems of propulsive motility. Any inference of possible sites of action can only be drawn in analogy with known sites of drug actions in intestinal nerve-muscle preparations.
Receptors mediating the anti-peristaltic action of exogenous purines: ADPβS-sensitive but suramin/PPADS-insensitive inhibitory P2 receptors on the circular muscle
Exogenous ATP was found to enhance the pressure threshold of distension-induced peristalsis in a concentration-dependent manner, so that at high purine concentrations peristaltic motor activity was completely suppressed and the intestinal musculature failed to contract. The anti-peristaltic action of low ATP concentrations appears to involve adenosine (P1) receptors, because it was attenuated by an effective concentration of the adenosine receptor antagonist 8-PT (10 μM; Kamiji et al., 1995). It is conceivable that ATP was rapidly degraded to adenosine (Katsuragi et al., 1993) which is known to suppress peristalsis in an 8-PT-sensitive manner (Hancock & Coupar, 1995) or that ATP and its breakdown product adenosine 5′-diphosphate were themselves able to activate adenosine receptors (Wiklund & Gustafsson, 1988). Since, however, the overall shape of the concentration-response curve for ATP was little changed by 8-PT it would appear that the major part of ATP's anti-peristaltic action was mediated by P2 receptors. This inference is supported by the data obtained with P2 receptor agonists and antagonists.
The potency of ATP in depressing peristalsis was surpassed by ADPβS while α,β-meATP was only poorly active. Given that ADPβS is a relatively selective P2Y receptor agonist while α,β-meATP is a preferred ligand of P2X receptors (Ralevic & Burnstock, 1998), it would seem that exogenous ATP inhibits peristalsis primarily via interaction with a P2Y purinoceptor subtype. It need be realized, though, that a conclusive differentiation of P2X and P2Y purinoceptors by the P2 receptor agonists α,β-meATP and ADPβS and the P2 receptor antagonists suramin and PPADS is not possible (Kennedy & Humphrey, 1994; Ralevic & Burnstock, 1998; Zagorodnyuk & Maggi, 1998).
Previous work has shown that α,β-meATP and ADPβS activate two distinct P2 receptors which in the guinea-pig colon mediate relaxation of the circular muscle (Zagorodnyuk & Maggi, 1998). Both receptor mechanisms are sensitive to apamin, yet the relaxant responses caused by ADPβS are resistant to suramin and PPADS whereas those caused by α,β-meATP are blocked by the two P2 receptor antagonists (Zagorodnyuk & Maggi, 1998). These findings are paralleled by the currently observed actions of ADPβS and α,β-meATP on peristalsis in the guinea-pig small intestine. Since, however, the activity of α,β-meATP to inhibit peristalsis in a suramin-/PPADS-sensitive manner was very weak, it follows that peristaltic motor inhibition caused by exogenous ATP is predominantly brought about by ADPβS-sensitive but suramin/PPADS-resistant P2 receptors on the circular muscle. Such a muscular site of action is in keeping with the observation that the effect of ADPβS and α,β-meATP to inhibit peristalsis was left unchanged by L-NAME which prevents the formation of NO in inhibitory motor neurones und thus eliminates a component of inhibitory neuro-muscular transmission (Crist et al., 1992; Lyster et al., 1992; Keef et al., 1993; Waterman & Costa, 1994). Further consistent with a direct action on the muscle is the ability of apamin to prevent the anti-peristaltic effect of ATP, ADPβS and α,β-meATP, because both types of inhibitory P2 receptors on the circular muscle utilize an apamin-sensitive transduction mechanism (Zagorodnyuk & Maggi, 1998).
Receptors mediating the anti-peristaltic action of endogenous purines: α,β-meATP-, suramin- and PPADS-sensitive inhibitory P2 receptors on the circular muscle
Although suramin and PPADS failed to prevent the peristaltic motor inhibition caused by exogenous ATP, they stimulated peristaltic motor activity in a persistent manner. Since the currently employed concentrations of suramin (300 μM; Galligan & Bertrand, 1994; Bültmann et al., 1996) and PPADS (50–150 μM; Windscheif et al., 1995; Bültmann et al., 1996) are thought to be effective and selective in their pharmacological action, it would appear that these P2 receptor antagonists unmask an anti-peristaltic action of endogenous purines, which is mediated by receptors different from those activated by exogenous ATP. The suramin/PPADS-sensitive receptors stimulated by endogenous purines are probably identical with those stimulated by exogenous α,β-meATP. An analogous observation has been made with regard to purine-induced hyperpolarization of the guinea-pig colon circular muscle (Zagorodnyuk & Maggi, 1998). A muscular site of action by which suramin and PPADS stimulate peristalsis can be deduced from the observation that, in the presence of apamin and the NO synthase inhibitor L-NAME, PPADS lowered PPT to the same level as in their absence. Although this finding is somewhat difficult to interprete because apamin and L-NAME decreased PPT on their own, it would appear that PPADS, apamin and L-NAME all act on a similar target to lower PPT, i.e., by blocking the NO-mediated slow component and the purine-mediated apamin-sensitive fast component of neuro-muscular transmission from inhibitory motor neurones (Niel et al., 1983; Costa et al., 1986; Crist et al., 1992; Lyster et al., 1992; Keef et al., 1993; Waterman & Costa, 1994; Zagorodnyuk & Maggi, 1994).
Possible reasons why exogenous and endogenous purines activate different P2 purinoceptors within the peristaltic circuitry
The differential activation of two P2 receptor types by endogenous and exogenous ATP can be explained in more than one way. For instance, the location of P2 receptors sensitive to α,β-meATP, suramin and PPADS may be restricted to neuro-muscular junctions whereas the ADPβS-sensitive but suramin/PPADS-resistant receptors are located extrajunctionally (Zagorodnyuk & Maggi, 1998). Another possibility is that structural or enzymatic barriers prevent exogenous ATP and ADPβS from reaching P2 receptors that are sensitive to α,β-meATP, suramin and PPADS.
Purinoceptors with a minor role in the peristaltic circuitry
Purines can inhibit the release of enteric acetylcholine (Barajas-Lopez et al., 1995; Kamiji et al., 1995; LePard et al., 1997), which makes it conceivable that purinoceptor antagonists facilitate peristalsis by enforcing cholinergic transmission in the gut. Such an action, however, cannot account for the properistaltic effect of PPADS, which was preserved in the presence of atropine or hexamethonium plus naloxone. Under these conditions, acetylcholine can no longer act as the major excitatory transmitter of enteric neurones, and peristalsis is driven by non-cholinergic excitatory co-transmitters among which tachykinins play a prominent role (Holzer et al., 1998).
Since PPADS failed to inhibit peristaltic motility in the presence of atropine or hexamethonium, it can also be ruled out that ATP or a related purine substitutes for acetylcholine when the cholinergic junctions of the enteric circuits subserving peristalsis are blocked. Such a possibility appeared conceivable because purines acting via suramin/PPADS-sensitive P2 receptors can mediate fast synaptic transmission in the myenteric plexus (Galligan & Bertrand, 1994; LePard et al., 1997) and cause intestinal contractions both via activation of cholinergic neurones (Barthó et al., 1997) and a direct action on the muscle (Kennedy & Humphrey, 1994; Zagorodnyuk & Maggi, 1998). Exogenous ADPβS was indeed able to stimulate peristalsis, an effect that was short and transient and quickly superseded by the drug's inhibitory motor action. Since the excitatory P2 receptors in the myenteric plexus are particularly sensitive to α,β-meATP (LePard et al., 1997), while those on the muscle are preferentially activated by ADPβS, it would follow that the stimulant effect of ADPβS on peristalsis arises from activation of excitatory P2 receptors in the muscle layer.
Physiological implications of purines in peristaltic motor regulation
The pro-peristaltic action of suramin and PPADS is likely to reflect the participation of endogenous purines in neuro-muscular transmission from the inhibitory motor neurones subserving peristalsis. The finding that PPADS failed to surpass the suramin-induced facilitation of peristalsis shows that both suramin and PPADS occlude the same population of P2 receptors. Being a co-transmitter of inhibitory motor neurones, ATP is thought to contribute to descending muscle relaxation in response to distension, an essential component of propulsive motility (Crist et al., 1992; Lyster et al., 1992; Keef et al., 1993; Waterman et al., 1994; Holzer et al., 1997). If so, blockade of PPADS-sensitive P2 receptors is expected to cause a partial interruption of inhibitory neuro-muscular transmission, which in turn results in peristaltic motor stimulation. It needs to be considered, though, that progressive interruption of inhibitory neuro-muscular transmission by combined exposure to PPADS+apamin, PPADS+L-NAME (this study) or apamin+L-NAME (Waterman & Costa, 1994; Holzer et al., 1997) disturbs the regular pattern of peristaltic acitivity, which attests to the essential role of inhibitory motor neurones in the coordination of propulsion.
Another issue of physiological relevance relates to the question as to how muscular receptors activated by endogenous or exogenous purines can influence PPT. Two possibilities are conceivable. Firstly, the purine-induced rise of PPT may result from the depressant effect of purine-induced muscle relaxation on the discharge of intrinsic sensory neurones subserving peristalsis (Kunze et al., 1998). Secondly, the rise of PPT may be a consequence of the compromised ability of the muscle to contract in the presence of exogenous purines. Opposite actions of the purinoceptor antagonists suramin and PPADS are expected to decrease PPT.
Conclusions
Exogenous and endogenous purines inhibit intestinal peristalsis via different apamin-sensitive P2 purinoceptor mechanisms. Exogenous ATP depresses peristalsis mostly via ADPβS-sensitive but suramin-/PPADS-resistant P2 receptors, whereas endogenous purines act via P2 receptors that are sensitive to α,β-meATP, suramin and PPADS. Purines thus seem to play a physiological role in the regulation of propulsive motility in the gut, and drugs affecting purinergic transmission are likely to have a profound impact on peristaltic motor performance.
Acknowledgments
This study was supported by the Austrian Research Foundation FWF (grant P11834-MED), an Austrian Exchange Programme (ÖAD) fellowship to A.Shahbazian and the Hungarian Research Grants OTKA T-020277 and T-026463. The authors thank Evelyn Painsipp and Milana Jocic for their excellent technical assistance.
Abbreviations
- α,β-meATP
α,β-methylene adenosine 5′-triphosphate
- ADPβS
adenosine 5′-O-2-thiodiphosphate
- ATP
adenosine 5′-triphosphate
- L-NAME
NG-nitro-L-arginine methyl ester
- PPADS
pyridoxal phosphate-6-azophenyl-2′,4′-disulphonic acid
- PPT
peristaltic pressure threshold
- 8-PT
8-phenyltheophylline
References
- BARAJAS-LOPEZ C., ESPINOSA-LUNA R., GERZANICH V. ATP closes a potassium and opens a cationic conductance through different receptors in neurons of guinea-pig submucous plexus. J. Pharmacol. Exp. Ther. 1994;268:1396–1402. [PubMed] [Google Scholar]
- BARAJAS-LOPEZ C., MULLER M.J., PRIETO-GOMEZ B., ESPINOSA-LUNA R. ATP inhibits the synaptic release of acetylcholine in submucosal neurons. J. Pharmacol. Exp. Ther. 1995;274:1238–1245. [PubMed] [Google Scholar]
- BARTHÓ L., LÉNÁRD L., MAGGI C.A. Evidence for the involvement of P2-purinoceptors in the cholinergic contraction of the guinea-pig ileum. Br. J. Pharmacol. 1997;121:1507–1508. doi: 10.1038/sj.bjp.0701350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BARTHÓ L., LÉNÁRD L., SZIGETI R. Nitric oxide and ATP co-mediate the NANC relaxant response in the guinea-pig taenia caeci. Naunyn-Schmiedeberg's Arch. Pharmacol. 1998;358:496–499. doi: 10.1007/pl00005283. [DOI] [PubMed] [Google Scholar]
- BÜLTMANN R., DUDECK O., STARKE K. Evaluation of P2 receptor antagonists at two relaxation-mediating P2 receptors in guinea-pig taenia coli. Naunyn-Schmiedeberg's Arch. Pharmacol. 1996;353:445–451. doi: 10.1007/BF00261442. [DOI] [PubMed] [Google Scholar]
- CHRISTOFI F.L., GUAN Z., WOOD J.D., BAIDAN L.V., STOKES B.T. Purinergic Ca2+ signaling in myenteric neurons via P2 purinoceptors. Am. J. Physiol. 1997;273:G463–G473. doi: 10.1152/ajpgi.1997.272.3.G463. [DOI] [PubMed] [Google Scholar]
- COSTA M., FURNESS J.B., HUMPHREYS C.M.S. Apamin distinguishes two types of relaxation mediated by enteric nerves in the guinea-pig gastrointestinal tract. Naunyn-Schmiedeberg's Arch. Pharmacol. 1986;332:79–88. doi: 10.1007/BF00633202. [DOI] [PubMed] [Google Scholar]
- CRIST J.R., HE X.D., GOYAL R.K. Both ATP and the peptide VIP are inhibitory neurotransmitters in guinea-pig ileum circular muscle. J. Physiol. (London) 1992;447:119–131. doi: 10.1113/jphysiol.1992.sp018994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- EVANS R.J., DERKACH V., SURPRENANT A. ATP mediates fast synaptic transmission in mammalian neurons. Nature. 1992;357:503–505. doi: 10.1038/357503a0. [DOI] [PubMed] [Google Scholar]
- GALLIGAN J.J., BERTRAND P.P. ATP mediates fast synaptic potentials in enteric neurons. J. Neurosci. 1994;14:7563–7571. doi: 10.1523/JNEUROSCI.14-12-07563.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HANCOCK D.L., COUPAR I.M. Functional characterization of the adenosine receptor mediating inhibition of peristalsis in the rat jejunum. Br. J. Pharmacol. 1995;115:739–744. doi: 10.1111/j.1476-5381.1995.tb14995.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HOLZER P., LIPPE I.T., HEINEMANN A., BARTHÓ L. Tachykinin NK1 and NK2 receptor-mediated control of peristaltic propulsion in the guinea-pig small intestine in vitro. Neuropharmacology. 1998;37:131–138. doi: 10.1016/s0028-3908(97)00195-0. [DOI] [PubMed] [Google Scholar]
- HOLZER P., LIPPE I.T., LOTFI TABRIZI A., LÉNÁRD L., BARTHÓ L. Dual excitatory and inhibitory effect of nitric oxide on peristalsis in the guinea pig intestine. J. Pharmacol. Exp. Ther. 1997;280:154–161. [PubMed] [Google Scholar]
- KAMIJI T., MORITA K., KATAYAMA Y. ATP regulates synaptic transmission by pre- and postsynaptic mechanisms in guinea-pig myenteric neurons. Neuroscience. 1995;59:165–174. doi: 10.1016/0306-4522(94)90107-4. [DOI] [PubMed] [Google Scholar]
- KATSURAGI T., SHIRAKABE K., SOEJIMA O., TOKUNAGA T., MATSUO K., SATO C., FURUKAWA T. Possible transsynaptic cholinergic neuromodulation by ATP released from ileal longitudinal muscles of guinea pigs. Life Sci. 1993;53:911–918. doi: 10.1016/0024-3205(93)90443-7. [DOI] [PubMed] [Google Scholar]
- KEEF K.D., DU C., WARD S.M., MCGREGOR B., SANDERS K.M. Enteric inhibitory neural regulation of human colonic circular muscle: role of nitric oxide. Gastroenterology. 1993;105:1009–1016. doi: 10.1016/0016-5085(93)90943-7. [DOI] [PubMed] [Google Scholar]
- KENNEDY I., HUMPHREY P.P.A. Evidence for the presence of two types of P2 purinoceptor in the guinea-pig ileal longitudinal smooth muscle preparation. Eur. J. Pharmacol. 1994;261:273–280. doi: 10.1016/0014-2999(94)90117-1. [DOI] [PubMed] [Google Scholar]
- KUNZE W.A., FURNESS J.B., BERTRAND P.P., BORNSTEIN J.C. Intracellular recording from myenteric neurones of the guinea-pig ileum that respond to stretch. J. Physiol. (London) 1998;506:827–842. doi: 10.1111/j.1469-7793.1998.827bv.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LEPARD K.J., MESSORI E., GALLIGAN J.J. Purinergic fast excitatory postsynaptic potentials in myenteric neurons of guinea pig: distribution and pharmacology. Gastroenterology. 1997;113:1522–1534. doi: 10.1053/gast.1997.v113.pm9352854. [DOI] [PubMed] [Google Scholar]
- LYSTER D.J.K., BYWATER R.A.R., TAYLOR G.S., WATSON M.J. Effects of a nitric oxide synthase inhibitor on non-cholinergic junction potentials in the circular muscle of the guinea pig ileum. J. Auton. Nerv. Syst. 1992;41:187–196. doi: 10.1016/0165-1838(92)90058-o. [DOI] [PubMed] [Google Scholar]
- MCCONALOGUE K., TODOROV L., FURNESS J.B., WESTFALL D.P. Direct measurement of the release of ATP and its major metabolites from the nerve fibres of the guinea-pig taenia coli. Clin. Exp. Pharmacol. Physiol. 1996;23:807–812. doi: 10.1111/j.1440-1681.1996.tb01184.x. [DOI] [PubMed] [Google Scholar]
- NIEL J.P., BYWATER R.A.R., TAYLOR G.S. Apamin-resistant post-stimulus hyperpolarization in the circular muscle of the guinea-pig ileum. J. Auton. Nerv. Syst. 1983;9:565–569. doi: 10.1016/0165-1838(83)90014-0. [DOI] [PubMed] [Google Scholar]
- OKWUASABA F.K., HAMILTON J.T., COOK M.A. Antagonism by the methylxanthines of purine nucleotide- and dipyridamole-induced inhibition of peristaltic activity of the guinea pig ileum. Eur. J. Pharmacol. 1977;43:181–194. doi: 10.1016/0014-2999(77)90130-3. [DOI] [PubMed] [Google Scholar]
- RALEVIC V., BURNSTOCK G. Receptors for purines and pyrimidines. Pharmacol. Rev. 1998;50:413–492. [PubMed] [Google Scholar]
- TONINI M., COSTA M., BROOKES S.J.H., HUMPHREYS C.M.S. Dissociation of the ascending excitatory reflex from peristalsis in the guinea-pig small intestine. Neuroscience. 1996;73:287–297. doi: 10.1016/0306-4522(96)00040-1. [DOI] [PubMed] [Google Scholar]
- VAN NUETEN J.M., FONTAINE J., HELSEN L., JANSSEN P.A. Inhibition by purines of peristaltic activity in the guinea-pig ileum. Arch. Int. Pharmacodyn. Ther. 1977;227:168–170. [PubMed] [Google Scholar]
- WATERMAN S.A., COSTA M. The role of enteric inhibitory motoneurons in peristalsis in the isolated guinea-pig small intestine. J. Physiol. (London) 1994;477:459–468. doi: 10.1113/jphysiol.1994.sp020207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WATERMAN S.A., TONINI M., COSTA M. The role of ascending excitatory and descending inhibitory pathways in peristalsis in the isolated guinea-pig small intestine. J. Physiol. (London) 1994;481:223–232. doi: 10.1113/jphysiol.1994.sp020433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WHITE T.D. Release of ATP from isolated myenteric varicosities by nicotinic agonists. Eur. J. Pharmacol. 1982;79:333–334. doi: 10.1016/0014-2999(82)90643-4. [DOI] [PubMed] [Google Scholar]
- WIKLUND N.P., GUSTAFSSON L.E. Indications for P2-purinoceptor subtypes in guinea-pig smooth muscle. Eur. J. Pharmacol. 1988;148:361–370. doi: 10.1016/0014-2999(88)90114-8. [DOI] [PubMed] [Google Scholar]
- WINDSCHEIF U., PFAFF O., ZIGANSHIN A.U., HOYLE C.H.V., BÄUMERT H.G., MUTSCHLER E., BURNSTOCK G., LAMBRECHT G. Inhibitory action of PPADS on relaxant responses to adenine nucleotides or electrical stimulation in guinea-pig taenia coli and rat duodenum. Br. J. Pharmacol. 1995;115:1509–1517. doi: 10.1111/j.1476-5381.1995.tb16644.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ZAGORODNYUK V., MAGGI C.A. Electrophysiological evidence for different release mechanism of ATP and NO as inhibitory NANC transmitters in guinea-pig colon. Br. J. Pharmacol. 1994;112:1077–1082. doi: 10.1111/j.1476-5381.1994.tb13193.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ZAGORODNYUK V., MAGGI C.A. Pharmacological evidence for the existence of multiple P2 receptors in the circular muscle of guinea-pig colon. Br. J. Pharmacol. 1998;123:122–128. doi: 10.1038/sj.bjp.0701558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ZHOU X., GALLIGAN J.J. P2X purinoceptors in cultured myenteric neurons of guinea-pig small intestine. J. Physiol. (London) 1996;496:719–729. doi: 10.1113/jphysiol.1996.sp021722. [DOI] [PMC free article] [PubMed] [Google Scholar]


