Abstract
In the present study, we examined whether deprivation of oestrogens by ovariectomy could modify learning and memory deficits caused by a continuous intracerebroventricular (i.c.v.) infusion of amyloid β-peptide (Aβ), the major constituent of senile plaques in AD.
Neither long-term (3 months) nor short-term (1 month), deprivation of oestrogens by ovariectomy caused a significant impairment in spatial learning and memory in a water maze and spontaneous alternation behaviour in a Y-maze.
A continuous i.c.v. infusion of Aβ-(1-42) caused spatial learning and memory deficits in both ovariectomized and sham-operated rats.
The Aβ-induced working memory deficits were significantly potentiated in ovariectomized rats compared with sham-operated rats when mnemonic ability was examined 3 months after ovariectomy.
These results suggest that long-term deprivation of oestrogens induced by ovariectomy increases susceptibility to memory deficits produced by Aβ-(1-42) in rats.
Keywords: Alzheimer's disease, amyloid β-peptide, oestradiol, menopause, ovariectomy, spatial memory
Introduction
Alzheimer's disease (AD) is the most common cause of a progressive decline of cognitive function in aged humans, and is characterized by the presence of numerous senile plaques and neurofibrillary tangles accompanied by neuronal loss. The senile plaques are composed of amyloid β-peptide (Aβ), a 40–42 amino acid peptide fragment of the β-amyloid precursor protein (APP) (Yankner, 1996; Selkoe, 1996). Transgenic mice which overexpress human APP containing the mutations associated with familial AD develop many of the pathological characterizations associated with AD (Games et al., 1995; Johnson-Wood et al., 1997; Sturchler-Pierrat et al., 1997). In addition, Aβ is cytotoxic to neurons (Yankner et al., 1990) and renders neurons vulnerable to various insults including excitotoxicity (Koh et al., 1990; Mattson et al., 1992).
The prevalence of AD after age 65 is two to three times higher in women than men (Jorm et al., 1987). Several studies have indicated that replacement therapy with oestrogens in postmenopausal women delays the onset and decreases the risk of AD (Fillit et al., 1986; Henderson et al., 1994; Ohkura et al., 1994; Tang et al., 1996; Henderson, 1997), although others failed to show the effects (Brenner et al., 1994). The mechanisms by which oestrogens affect the pathogenic processes in AD are still unknown. It has been demonstrated that oestrogens modulate cholinergic neuronal activity (Dohanich et al., 1982; Singh et al., 1994), monoamine metabolism (Shimizu & Bray, 1993) and the expression of brain-derived neurotrophic factor (BDNF) mRNA in the brain (Singh et al., 1995). Oestrogens have also been shown to attenuate excitotoxicity, oxidative injury and Aβ toxicity (Behl et al., 1995; Goodman et al., 1996), to regulate APP metabolism (Jaffe et al., 1994), and to reduce the neuronal generation of Aβ in vitro (Xu et al., 1998). However, it is not clear whether oestrogens can modulate the effects produced by Aβ in vivo
We have previously demonstrated that a continuous intracerebroventricular (i.c.v.) infusion of Aβ-(1-40) or Aβ-(1-42) in male rats results in learning and memory deficits, suggesting that the accumulation of Aβ in the brain is associated with cognitive impairments in AD (Nitta et al., 1994; 1997; Tanaka et al., 1998; Yamada et al., 1998; 1999a). In male rats treated with Aβ-(1-40), dysfunctions of cholinergic and dopaminergic neuronal systems were observed as evidenced by the decrease in the nicotine- and KCl-induced increase in acetylcholine and dopamine release in vivo, respectively (Itoh et al., 1996). We also observed changes in ciliary neurotrophic factor protein levels in the brain (Yamada et al., 1995) and in the expression of BDNF mRNA in the hippocampus (Yamada et al., 1997), activation of glial cells (Nitta et al., 1997) and a deficiency of long-term potentiation in the CA1 field of the hippocampus in this rat model of AD (Nabeshima & Itoh, 1997). We proposed that oxidative stress is involved in the Aβ-induced learning and memory deficits, since the potent antioxidants idebenone and α-tocopherol prevented these deficits (Yamada et al., 1999b).
With the goal of determining whether oestrogens play a role in the Aβ-induced learning and memory deficits, we investigated the effects of deprivation of oestrogens induced by ovariectomy on Aβ-induced learning and memory deficits in rats. To assess the mnemonic ability in rats, we measured spontaneous alternation behaviour in a Y-maze (Maurice et al., 1994) and reference and working memory in a water maze task (Morris, 1984; Morris et al., 1990). Furthermore, the serum levels of oestradiol and follicle-stimulating hormone (FSH) were measured to determine the effect of ovariectomy and whether the Aβ infusion affected hormone secretion. Since Aβ-(1-42) apparently plays a more important role than Aβ-(1-40) in the pathology of AD (Jarrett & Lansbury, 1993; Iwatsubo et al., 1994), rats were continuously infused with Aβ-(1-42) into the cerebral ventricle in the present study. The continuous i.c.v. infusion of Aβ-(1-42) was started either 1 or 3 months after ovariectomy. In the sham-operated and ovariectomized control groups, Aβ-(40-1), but not Aβ-(1-42), was infused.
Methods
Animals
The rats used in the present study were females of the Wistar strain (7 weeks old; Charles River Japan Inc., Yokohama, Japan) weighing 180±5 g at the beginning of the experiments. They were housed in groups of two or three in a temperature- and light-controlled room (23°C; 12 h light cycle starting at 09 00 h) and had free access to food and water, except during the behavioural experiments.
All experiments were performed in accordance with the Guidelines for Animal Experiments of the Nagoya University School of Medicine, the Guiding Principles for the Care and Use of Laboratory Animals approved by the Japanese Pharmacological Society, and the National Institutes of Health Guide for the Care and Use of Laboratory Animals.
Experimental design
Female rats were anaesthetized with pentobarbital (50 mg kg−1, i.p.), and underwent a bilateral ovariectomy or sham operation. The continuous i.c.v. infusion of Aβ was started either 1 or 3 months after these operations. The treatment groups generated were as follows: the sham-operated rats with an Aβ-(40-1) infusion [sham-Aβ-(40-1)] as a control group, sham-operated rats with an Aβ-(1-42) infusion [sham-Aβ-(1-42)], ovariectomized rats with an Aβ-(40-1) infusion [OVX-Aβ-(40-1)] as ovariectomized control rats, and the ovariectomized rats with an Aβ-(1-42) infusion [OVX-Aβ-(1-42)]. Accordingly, two batches of the four treatment groups, each consisting of 8–10 rats, were prepared. One batch of animals began i.c.v. Aβ infusion 1 month after ovariectomy, and the remainder were infused with Aβ i.c.v. 3 months after the ovariectomy. The behavioural study was started on day 7 after the start of the Aβ infusion, and the behavioural tests were carried out as follows; the locomotor activity test on day 7, Y-maze test on day 8, and water-maze test on days 9–16 after the start of the Aβ infusion.
Aβ infusion
Rats were anaesthetized with pentobarbital (50 mg kg−1, i.p.) and placed in a stereotaxic apparatus 1 or 3 months after the surgery for ovariectomy. The infusion cannula connected to a mini-osmotic pump (flow rate, 0.5 μl h−1; total capacity, 200 μl; Alzet 2002; Alza, Palo Alto, CA, U.S.A.) which was filled with either Aβ-(1-42) or Aβ-(40-1) was implanted into the right ventricle (A: −0.3, L: 1.2, V: 4.5) according to the atlas of Paxinos & Watson (1986). The pump was placed subcutaneously in the neck of rat. The continuous i.c.v. infusion of Aβ at a dose of 300 pmol day−1 was maintained for at least 14 days. We have previously confirmed that the vehicle by itself has no effect on learning behaviour at this flow rate (Nitta et al., 1994; 1997).
Measurement of locomotor activity
Locomotor activity was measured on day 7 after the start of the Aβ infusion. The experimental apparatus consisted of a locomotor cage (25×42×20 cm), with photobeams placed 2 cm above the floor at 1-inch intervals along two sides of the cage (Colombus Instruments, U.S.A.). Locomotor activity was measured during a 10 min period (Fuji et al., 1993).
Y-maze task
The Y-maze task was carried out as described previously (Maurice et al., 1994) on day 8 after the start of the Aβ infusion. The experimental apparatus consisted of a black-painted Y-maze made of plywood. Each arm of the Y-maze was 35 cm long, 25 cm high and 10 cm wide and positioned at an equal angle (labelled A, B, and C). Each rat was placed at the end of one arm and allowed to move freely through the maze during an 8 min session. The sequence of arm entries was recorded manually (i.e., ACBCAB, etc.). A spontaneous alternation behaviour, which is regarded as a measure of spatial memory (Maurice et al., 1994; Yamada et al., 1996), was defined as the entry into all three arms on consecutive choices in overlapping triplet sets (i.e., ACB, CBC, BCA, CBA). The per cent spontaneous alternation behaviour was calculated as the ratio of actual to possible alternations (defined as the total number of arm entries −2)×100.
Water maze task
The water maze task was carried out from days 9–16 after the start of the Aβ infusion. The experimental apparatus consisted of a circular water tank (140 cm in diameter and 45 cm high). A transparent platform (10 cm in diameter and 25 cm high) was set inside the tank, which was filled, to a height of 27 cm, with water at approximately 23°C; the surface of the platform was 2 cm below the surface of the water. The pool was located in a large test room, in which there were many cues external to the maze (e.g., pictures, lamps, etc.). The position of the cues remained unchanged throughout the water maze task (Nitta et al., 1994).
Reference memory test (Morris, 1984; Nitta et al., 1994): For each training trial, the rat was put into the pool at one of five starting positions, the sequence of the positions being selected randomly. The platform was located in a constant position throughout the test period in the middle of one quadrant, equidistant from the center and edge of the pool. In each training session, the latency to escape onto the hidden platform was recorded. If the rat found the platform, it was allowed to remain there for 15 s and was then returned to its home cage. If the rat was unable to find the platform within 90 s, it was put on the platform for 15 s, and then the training was terminated and a maximum score of 90 s was assigned. The path taken by the rat was recorded automatically using a video image motion analyzer (Neuroscience Inc., Tokyo, Japan), and then the swim distance and swim speed were analysed. Training was conducted for 5 consecutive days, twice a day, from days 9–13 after the start of the Aβ infusion.
Probe test (Morris, 1984; Nitta et al., 1994): Immediately after the tenth training trial on day 13 after the start of the Aβ infusion, the platform was removed from the pool and the animals were tested in a 90 s spatial probe trial. The time spent in the platform-quadrant where the platform had been located during the training was measured.
A repeated acquisition test (Morris et al., 1990; Yamada et al., 1994b) was conducted, to assess working memory, for 3 consecutive days from days 14–16 after the start of the Aβ infusion, and consisted of five trials (one session) per day. The working memory test was procedurally similar to the standard training for the water maze test, except that the platform location was changed in each session. Since the platform position was changed daily, the working memory component was evaluated by this task. For each trial, the rat was put into the pool at one of five starting positions, the sequence of the positions being selected randomly. The first trial of each session is an informative sample trial in which the rat is allowed to swim to the platform in its new location and to remain there for 15 s. The rat was then placed in a home cage for an intertrial interval of 1 min. The platform remained in the same location throughout the remaining four trials of the day. Spatial working memory was assessed as the mean performance in the second trials of 3 consecutive days from days 14–16 after the start of the Aβ infusion.
Measurement of serum hormone levels
The rats were killed by decapitation after the behavioural studies on day 18 after the start of Aβ infusion to collect blood samples collected. The serum levels of oestradiol and FSH were measured by radioimmunoassay (SRL Ltd., Tokyo, Japan) to determine the effect of ovariectomy and whether the continuous i.c.v. infusion of Aβ affected hormone secretion.
Drugs
Aβ-(1-42) and Aβ-(40-1) were obtained from Bachem (Torrance, CA, U.S.A.), and dissolved in 35% acetonitrile containing 0.1% trifluoroacetic acid.
Statistical analysis
The results are expressed as mean±s.e.mean. The significance of differences in the data was determined by one-way analysis of variance (ANOVA) followed by Bonferroni's multiple comparison test. A two-way ANOVA was also conducted for analysing data of the water maze. Fisher's PLSD test for multi-group comparisons was used for a post hoc analysis. A P value less than 0.05 was regarded as significant.
Results
Effects of continuous i.c.v. infusion of Aβ at 1 month after an ovariectomy
In the first series of experiments, the continuous i.c.v. infusion of Aβ was started 1 month after the ovariectomy. Locomotor activity in these rats was measured on day 7 after the start of the Aβ infusion. There was no significant difference in locomotor activity among the sham-Aβ-(40-1) (n=9), sham-Aβ-(1-42) (n=10), OVX-Aβ-(40-1) (n=9) and OVX-Aβ-(1-42) (n=10) groups [F(3,34)=2.8768, P>0.05]. The activity counts during a 10 min period in these groups of animals were 2244±180, 2514±183, 1999±170 and 1795±206, respectively.
Figure 1 shows the effects of Aβ at 1 month after an ovariectomy on the performance of the rat in the Y-maze task. There was a significant group effect on spontaneous alternation behaviour [F(3,34)=11.989, P<0.0001] (Figure 1A). The post hoc analysis revealed that the frequency of spontaneous alternation behaviour in the sham-Aβ-(1-42) rats was significantly less than that in the sham-Aβ-(40-1) rats (P<0.001). A significant reduction of the alternation behaviour caused by Aβ-(1-42) was also observed in the ovariectomized rats (P<0.01). Ovariectomy had no effect on the spontaneous alternation behaviour and failed to affect the Aβ-(1-42)-induced impairment of the alternation behaviour (Figure 1A). Since there was no significant difference in the number of arm entries of the four groups of animals [F(3,34)=0.2256, P>0.05] (Figure 1B), the observed changes in spontaneous alternation behaviour are not due to locomotor deficits.
Figure 1.
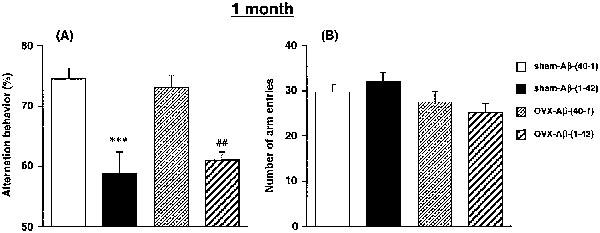
Effects of a continuous i.c.v. infusion of Aβ on spontaneous alternation behaviour in the Y-maze in sham-operated and ovariectomized female rats. The continuous i.c.v. infusion of Aβ was started 1 month after ovariectomy. Spontaneous alternation behaviour (A) and the number of arm entries (B) during an 8-min session in the Y-maze task were measured on day 8 after the start of the Aβ infusion. Values indicate means±s.e.mean (n=9–10). ***P<0.001 vs sham-AB-(40-1). ##P<0.01 vs OVX-Aβ-(40-1).
The effects of Aβ at 1 month after an ovariectomy on the performance of the rat in the water maze task are illustrated in Figure 2. Figure 2A shows the changes in escape latency onto the hidden platform in training trials in each group of rats. The two-way ANOVA with all treatment groups revealed significant main effects of group [F(3,340)=11.493, P<0.0001] and training [F(9,340)=55.874, P<0.0001], but no significant group by trial interactions [F(27, 340)=0.707, P>0.05]. The post hoc analysis indicated that the performance of the sham-Aβ-(1-42) rats was significantly impaired compared with that of the sham-Aβ-(40-1) control rats (P<0.0001). A significant difference in performance was also observed between the OVX-Aβ-(1-42) and OVX-Aβ-(40-1) groups. However, ovariectomy by itself failed to affect the performance of both the Aβ-(40-1) and Aβ-(1-42)-treated groups.
Figure 2.
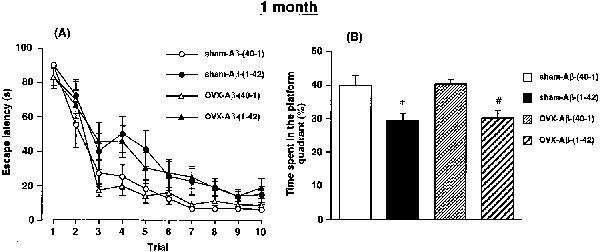
Effects of a continuous i.c.v. infusion of Aβ on performance in the training trials (A) and in the probe trial (B) of the water maze task in sham-operated and ovariectomized female rats. The continuous i.c.v. infusion of Aβ was started 1 month after ovariectomy. The training trials were carried out on days 9–13 after the start of the Aβ infusion. The probe trial was carried out on day 13 after the start of the Aβ infusion, immediately after the tenth training trial. Values indicate means±s.e.mean (n=9–10). *P<0.05 vs sham-Aβ-(40-1). #P<0.05 vs OVX-Aβ-(40-1).
A 90 s probe trial was carried out on day 13 after the start of the Aβ infusion, following the tenth training trial, to examine whether the rats had learned the position of the platform (Figure 2B). There was a significant group effect on the time spent in the platform-quadrant where the platform had been located during the training trials [F(3,34)=6.3644, P=0.0015]. The sham-Aβ-(1-42) rats spent less time in the platform-quadrant than the corresponding Aβ-(40-1)-treated controls (P<0.05). A significant decrease of time spent in the platform-quadrant was also observed in the OVX-Aβ-(1-42) rats, compared with the OVX-Aβ-(40-1) rats. However, ovariectomy by itself did not affect the bias in either the Aβ-(40-1) or Aβ-(1-42)-treated rats.
Effects of continuous i.c.v. infusion of Aβ at 3 months after an ovariectomy
In the second series of experiments, the continuous i.c.v. infusion of Aβ was started 3 months after the ovariectomy. Locomotor activity was measured on day 7 after the start of the Aβ infusion. There was no significant difference in locomotor activity among the sham-Aβ-(40-1) (n=8), sham-Aβ-(1-42) (n=8), OVX-Aβ-(40-1) (n=8), and OVX-Aβ-(1-42) (n=9) groups [F(3,29)=0.3378, P>0.05]. The activity counts during a 10 min period in these groups of animals were 1746±210, 1909±211, 1570±237 and 1763±265, respectively.
The effects of Aβ at 3 months after an ovariectomy on the performance of the rat in the Y-maze task were measured on day 8 after the start of the Aβ infusion. The spontaneous alternation behaviour in the sham-Aβ-(40-1) (n=8), sham-Aβ-(1-42) (n=8), OVX-Aβ-(40-1) (n=8) and OVX-Aβ-(1-42) (n=9) groups was 74.4±3.2, 61.7±3.5, 74.1±5.9 and 60.9±3.6%, respectively, whereas the number of arm entries during an 8 min session in these groups was 22.1±2.3, 24.5±1.8, 17.1±1.8 and 18.4±1.8, respectively. There was a significant group effect on spontaneous alternation behaviour [F(3,29)=3.2383, P=0.036] and the number of arm entries [F(3,29)=3.0569, P=0.044]. A similar magnitude of reduction of the alternation behaviour was observed in the sham-Aβ-(1-42) and OVX-Aβ-(1-42) rats.
The effects of continuous i.c.v. infusion of Aβ at 3 months after the ovariectomy on the water maze performance of the rats in the reference memory test are illustrated in Figure 3. The changes in escape latency onto the hidden platform in training trials in each group of rats are shown in Figure 3A. The two-way ANOVA with all treatment groups revealed significant main effects of group [F(3,290)=7.216, P<0.001] and training [F(9,290)=29.097, P<0.0001], but no significant group by trial interactions [F(27,290)=0.690, P>0.05]. The post hoc analysis indicated that the performance of the OVX-Aβ-(40-1) rats did not differ significantly from that of the sham-Aβ-(40-1) control rats (P=0.1256), indicating that the ovariectomy by itself had no effect on the escape latency. The performance of the sham-Aβ-(1-42) and OVX-Aβ-(1-42) rats was significantly impaired, compared with that in the sham-Aβ-(40-1) control group (P<0.001). The performance of the OVX-Aβ-(1-42) rats was impaired compared with that of the OVX-Aβ-(40-1) rats, but the difference was not significant (P=0.061). There was no difference in escape latency between the sham-Aβ-(1-42) and OVX-Aβ-(1-42) rats, indicating that deprivation of oestrogens for 3 months had no effect on Aβ-induced spatial memory impairment.
Figure 3.
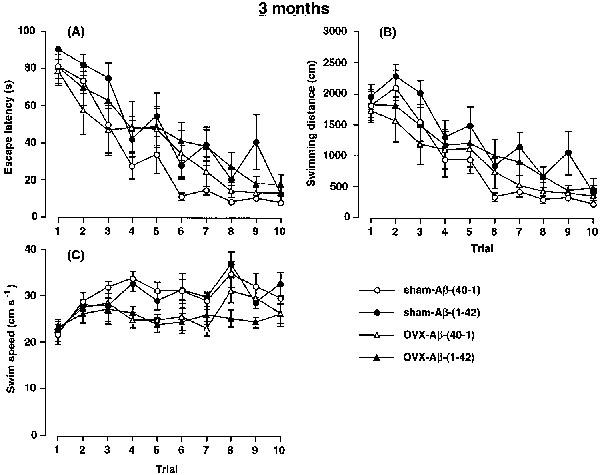
Effects of a continuous i.c.v. infusion of Aβ on escape latency (A), swimming distance (B) and swimming speed (C) in the training trials of the water maze task in sham-operated and ovariectomized female rats. The continuous i.c.v. infusion of Aβ was started 3 months after the ovariectomy. The training trials were carried out on days 9–13 after the start of the Aβ infusion. Values indicate means±s.e.mean (n=8–9).
The performance in the water maze reference memory test was also analysed in terms of swimming distance (Figure 3B) and swimming speed (Figure 3C), because the swimming ability of the rat may have been altered by the ovariectomy. Consistent with the changes in escape latency, there were significant main effects of group [F(3,290)=7.606, P<0.0001] and training [F(9,290)=25.662, P<0.0001], but not of group by trial interactions [F(27,290)=0.594, P>0.05] on the swimming distance. The post hoc analysis indicated that there was no significant difference in performance between the OVX-Aβ-(40-1) and sham-Aβ-(40-1) rats, indicating that ovariectomy by itself had no effect on the performance. The performance in the sham-Aβ-(1-42) rats was significantly impaired, compared with that in the sham-Aβ-(40-1) control group (P<0.001), indicating that Aβ-(1-42) impaired spatial reference memory formation in normal female rats. The OVX-Aβ-(1-42) rats showed an impairment of performance compared with the sham-Aβ-(40-1) (P=0.037) and OVX-Aβ-(40-1) rats (P=0.056).
Swimming speed of the rat was calculated based on the escape latency and swimming distance, and the changes over the ten training trials are shown in Figure 3C. There were significant main effects of group [F(3,290)=16.571, P<0.0001] and training [F(9,290)=5.737, P<0.0001], but not of group by trial interactions [F(27,290)=1.174, P>0.05] in swimming speed. The swimming speeds of the OVX-Aβ-(40-1) and OVX-Aβ-(1-42) rats were significantly slower than those of the sham-Aβ-(40-1) and sham-Aβ-(1-42) rats (P<0.001).
Performance in the working memory (repeated acquisition) test is shown in Figure 4. In terms of escape latency (Figure 4A), the two-way ANOVA with all treatment groups revealed significant main effects of group [F(3,145)=3.957, P=0.0095], training [F(4,145)=93.921, P<0.0001] and group by trial interactions [F(12,145)=2.350, P=0.0087]. The post-hoc analysis revealed that the performance of the OVX-Aβ-(40-1) rats did not differ significantly from that of the sham-Aβ-(40-1) control group (P=0.7911). There was also no significant difference in performance between the sham-Aβ-(40-1) and sham-Aβ-(1-42) rats (P=0.9942). The results suggest that the sham-Aβ-(1-42) rats learned the new position of the platform as quickly as the sham-Aβ-(40-1) rats did, following the standard water maze training for 5 days. In contrast, a significant difference in performance between the OVX-Aβ-(40-1) and OVX-Aβ-(1-42) rats was evident (P=0.0038). The performance of the OVX-Aβ-(1-42) rats was also significantly impaired compared with that of the sham-Aβ-(1-42) rats (P=0.0086), suggesting that ovariectomy potentiates the Aβ-(1-42)-induced impairment of spatial learning and memory. Similar changes were observed in swimming distance (Figure 4B). The two-way ANOVA revealed significant main effects of group [F(3,145)=2.830, P=0.0406], training [F(4,145) =109.908, P<0.0001] and group by trial interactions [F(12,145)=2.458, P=0.0060]. The post hoc analysis revealed a significant difference in performance between the OVX-Aβ-(40-1) and OVX-Aβ-(1-42) rats (P=0.0061).
Figure 4.
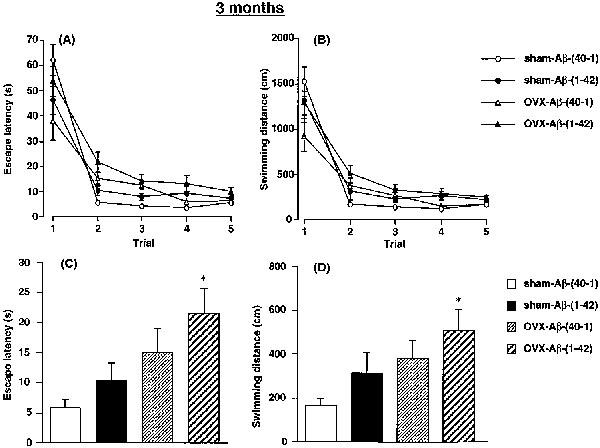
Effects of a continuous i.c.v. infusion of Aβ on performance in the working memory test of the water maze task in sham-operated and ovariectomized female rats. The continuous i.c.v. infusion of Aβ was started 3 months after the ovariectomy. The working memory test (five trials per day) was carried out on days 14–16 after the start of the Aβ infusion. Changes in the mean escape latency and swimming distance for 3 days during five trials are shown in (A) and (B), respectively. The mean escape latency and swimming distance in the test trials (second trial) for 3 days are shown in (C) and (D), respectively. Values indicate means±s.e.mean (n=8–9). *P<0.05 vs sham-Aβ-(40-1).
When the escape latencies of the sample trials (first trials) for 3 consecutive days in the working memory test were compared, there was no significant difference in escape latency [F(3,29)=2.1045, >>P=0.1214] (Figure 4A) or swimming distance [F(3,29)=2.3201, P=0.0961] (Figure 4B), suggesting that no significant difference exists in procedural (reference) memory among the five treatment groups. In contrast, when performance in the second trials for 3 days in the working memory test was analysed as a measure of spatial working memory, there were significant differences in escape latency [F(3,29)=4.3107, P=0.0124] (Figure 4C) and swimming distance [F(3,29)=3.1949, P=0.0381] (Figure 4D). The post-hoc analysis revealed that performance of either sham-Aβ-(1-42) or OVX-Aβ-(40-1) rats did not differ significantly from that of the sham-Aβ-(40-1) control group (P>0.05), suggesting that either Aβ-(1-42) infusion or ovariectomy alone has no effect on working memory. However, both the escape latency (t=3.4337, P<0.05) and swimming distance (t=3.0444, P<0.05) in the OVX-Aβ-(1-42) rats was significantly longer than those in the sham-Aβ-(40-1) rats, suggesting an interaction of Aβ-(1-42) infusion and ovariectomy in modulating working memory. There was no significant difference in performance between the sham-Aβ-(1-42) and OVX-Aβ-(1-42) rats or the OVX-Aβ-(40-1) and OVX-Aβ-(1-42) rats (P>0.05) (Figure 4C,D).
Table 1 shows the body weights and serum oestradiol and FSH levels on day 18 after the start of the Aβ infusion, which was started 3 months after the ovariectomy. The OVX-Aβ-(40-1) and OVX-Aβ-(1-42) rats were significantly heavier than the sham-Aβ-(40-1) rats. The serum oestradiol levels were significantly reduced in the OVX-Aβ-(40-1) and OVX-Aβ-(1-42) rats as compared with the sham-Aβ-(40-1) rats. Inversely, the FSH levels were significantly increased in the ovariectomized rats. There were no differences in the body weight or serum oestradiol and FSH levels between the OVX-Aβ-(40-1) and OVX-Aβ-(1-42) rats and between the sham-Aβ-(40-1) and sham-Aβ-(1-42) rats.
Table 1.
Body weight and serum levels of oestradiol and FSH in the sham-operated and ovariectomized rats continuously infused with Aβ
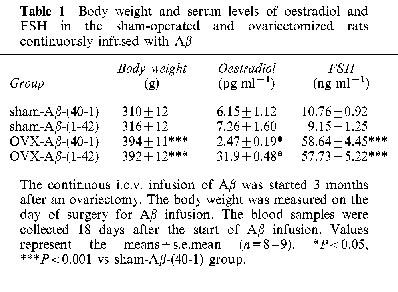
Similarly, when Aβ infusion was started 1 month after the ovariectomy, we observed a significant increase in the body weights (P<0.01) and a decrease in the serum oestradiol levels (P<0.01) in the OVX-Aβ-(40-1) and OVX-Aβ-(1-42) as compared with the sham-Aβ-(40-1) rats. The continuous i.c.v. Aβ-(1-42) infusion had no effect on the body weights and serum oestradiol levels in either sham-operated or ovariectomized rats (data not shown).
Discussion
In the present study, we found that a continuous i.c.v. infusion of Aβ-(1-42) in young female rats causes learning and memory deficits, as evidenced by the impairment of performance in the Y-maze and water maze tasks. The Aβ-(1-42) infusion in female rats impaired reference memory in the water maze and spontaneous alternation behaviour in the Y-maze, but did not affect working memory. Our previous studies have shown that the same treatment with Aβ-(1-40) (Nitta et al., 1994; 1997; Tanaka et al., 1998; Yamada et al., 1998) or Aβ-(1-42) (Yamada et al., 1999a,1999b) at the same dose (300 pmol day−1) in male rats produces a similar degree of behavioural impairment although Aβ-(1-42) caused deficits in both references and working memory (Yamada et al., 1999a,1999b). Therefore, it is likely that there are no major gender differences in rats in susceptibility to Aβ-(1-42) in vivo.
It was previously demonstrated that oestrogen deprivation caused by ovariectomy in young female rats does not markedly affect spatial memory in the Morris water maze task, although it produces a marked impairment of nonspatial active avoidance learning and retention (Singh et al., 1994). In contrast, a recent study by Daniel et al. (1997) showed that ovariectomy produced a significant impairment of spatial working memory in a radial arm maze test. We found in the present study that long-term (3 months) deprivation of oestrogens by ovariectomy tended to impair the performance of rats in the repeated acquisition test of water maze although the same treatment had little effect on performance in the standard water maze acquisition test. Accordingly, we assume that spatial working memory, but not reference memory, may be susceptible to the long-term deprivation of oestrogens. Since short-term (1 month) deprivation of oestrogens by ovariectomy did not affect the performance in water maze nor spontaneous alternation behaviour in Y-maze, it is plausible that fluctuating levels of oestrogens across the oestrous cycle may have little behavioural significance in female rats.
The most important findings in the present study are that the Aβ-(1-42)-induced working memory deficits in the water maze were potentiated by ovariectomy in the rats after a long-term (3 months), but not a short-term (1 month) deprivation of oestrogens. These results suggest that long-term deprivation of oestrogens induced by ovariectomy, combined with Aβ-(1-42) infusion, produces deficits in working memory tasks in the rodent which are not produced by either Aβ-(1-42) infusion of ovariectomy alone. It is unlikely that susceptibility to Aβ-induced memory deficits is affected by changes in circulating levels of oestrogens since the effects of ovariectomy were only detected with long-term hormone deprivation.
There was no significant difference in exploratory activity between the sham-operated and ovariectomized rats in this study, but a significant reduction of swimming speed was evident in the ovariectomized rats compared with the sham-operated rats. It could be thus speculated that the observed alterations in the water maze may be due to a mere impairment of swimming ability, but not cognitive functions. However, this is unlikely because there was no difference at all in swimming speed between the OVX-Aβ-(40-1) and OVX-Aβ-(1-42) rats. In addition, in the repeated acquisition working memory test, no significant difference in either the escape latency or swimming distance at the first sample trials for 3 days was observed among the four treatment groups. Accordingly, the marked increases in escape latency and swimming distance at the second test trials are not due to an impairment of swimming ability. Rather, it is highly likely that the deprivation of oestrogens caused by ovariectomy potentiated the Aβ-(1-42)-induced spatial working memory deficits.
We confirmed that ovariectomy significantly reduced the serum oestradiol levels and that there were no differences in the body weight or serum oestradiol and FSH levels between the OVX-Aβ-(40-1) and OVX-Aβ-(1-42) rats and between the sham-Aβ-(40-1) and sham-Aβ-(1-42) rats. Therefore, it is unlikely that the observed potentiation of the Aβ-(1-42)-induced impairment of working memory caused by ovariectomy in the repeated acquisition water maze test is due to the different levels of serum oestrogens.
The mechanisms by which long-term deprivation of oestrogens modulate the Aβ-(1-42)-induced learning and memory deficits in female rats remain obscure. It was demonstrated that oestrogens modulate cholinergic neuronal activity (Dohanich et al., 1982; Singh et al., 1994), monoamine metabolism (Shimizu & Bray, 1993) and the expression of BDNF mRNA (Singh et al., 1995). Oestrogens were also shown in vitro to attenuate excitotoxicity, oxidative injury and Aβ toxicity (Behl et al., 1995; Goodman et al., 1996), to regulate the metabolism of β-amyloid precursor protein (Jaffe et al., 1994), and to reduce the neuronal generation of Aβ (Xu et al., 1998). These effects of oestrogens may be involved in its modulator role in the Aβ-(1-42)-induced learning and memory deficits.
We assume that cholinergic deficits produced by long-term deprivation of oestrogens may play a most important role in ovariectomy-induced potentiation of Aβ-(1-42)-induced working memory deficits. For example, it has been demonstrated that long-term, but not short-term loss of ovarian function produces deficits in choline acetyltransferase and trkA expression in the medial septum and nucleus basalis magnocellularis (Gibbs, 1998). On the other hand, Aβ has been shown to inhibit acetylcholine release and choline uptake in hippocampal slices (Kar et al., 1998). We have demonstrated that a continuous i.c.v. infusion of Aβ-(1-40) produces a marked reduction of nicotine- and/or KCl-induced stimulation of acetylcholine release in the hippocampus/cerebral cortex in vivo (Itoh et al., 1996). Taken together, these results suggest a mechanism by which Aβ could exacerbate cholinergic deficits produced by the long-term deprivation of oestrogens induced by ovariectomy.
We believe that learning and memory in the rodent should be assessed by diverse tasks in which different motivation is involved and different skill is required for better performance. Furthermore, since Aβ was continuously infused into the brain, its neurotoxicity is considered to be dependent on the infusion period. Therefore, all the rats were subjected to all the behavioural tests in the present study. On the other hand, since it has been reported that stress can impair spatial memory (Selden et al., 1990; Diamond et al., 1996), it is possible that the prior experience of the rat may influence performance and thus distort the interpretation of the data obtained. To exclude the possibility, further studies may be necessary.
Clinical studies have indicated that replacement therapy with oestrogens in post-menopausal women delays the onset and decreases the risk of AD (Henderson et al., 1994; Ohkura et al., 1994; Tang et al., 1996). To investigate the beneficial effects of oestrogens in AD, we should examine the effects of oestrogen replacement therapy on the Aβ-(1-42)-induced learning and memory deficits in ovariectomized female rats. Our preliminary experiment shows that the oestrogen replacement therapy in the ovariectomized rats, which was carried out by implanting an 17-β-oestradiol-containing hydroxyapatite disk (500 μg oestradiol per disk, Yamamura et al., 1995) subcutaneously, has some beneficial effects on performance in the repeated acquisition water maze test (unpublished observation).
In conclusion, long-term deprivation of oestrogens induced by ovariectomy, combined with Aβ-(1-42) infusion, produces deficits in working memory tasks in the rodent which are not produced by either Aβ-(1-42) infusion or ovariectomy alone. Such conditions may contribute to cognitive decline in post-menopausal women with AD.
Acknowledgments
This study was supported in part by Grants-in-Aid for Science Research from the Ministry of Education, Science, Sports and Culture of Japan (No. 07557009, 08457027, 10897005 and 97450), an SRF Grant for Biomedical Research, grants from the Suzuken Memorial Foundation and the Research Foundation for Pharmaceutical Sciences, and by a COE Grant.
Abbreviations
- Aβ
amyloid β-peptide
- AD
Alzheimer's disease
- ANOVA
one-way analysis of variance
- APP
β-amyloid precursor protein
- BDNF
brain-derived neurotrophic factor
- FSH
follicle-stimulating hormone
- i.c.v.
intracerebroventricular
- OVX
ovariectomy
References
- BEHL C., WIDMANN M., TRAPP T., HOLSBOER F. 17-βEstradiol protect neurons from oxidative stress-induced cell death in vitro. Biochem. Biophys. Res. Commun. 1995;216:473–482. doi: 10.1006/bbrc.1995.2647. [DOI] [PubMed] [Google Scholar]
- BRENNER D.E., KUKULL W.A., STERGACHIS A., VAN BELLE G., BOWEN J.D., MCCORMICK W.C., TERI L., LARSON E.B. Postmenopausal estrogen replacement therapy and risk of Alzheimer's disease: a population-based case-control study. Am. J. Epidemiol. 1994;140:262–267. doi: 10.1093/oxfordjournals.aje.a117245. [DOI] [PubMed] [Google Scholar]
- DANIEL J.M., FADER A.J., SPENCER A.L., DOHANICH G.P. Estrogen enhances performance of female rats during acquisition of a radial arm maze. Horm. Behav. 1997;32:217–225. doi: 10.1006/hbeh.1997.1433. [DOI] [PubMed] [Google Scholar]
- DIAMOND D.M, , FLESHNER M., INGERSOLL N., ROSE G.M. Psychological stress impairs spatial working memory: relevance to electrophysiological studies of hippocampal function. Behav. Neurosci. 1996;110:661–672. doi: 10.1037//0735-7044.110.4.661. [DOI] [PubMed] [Google Scholar]
- DOHANICH G.P., WITCHER J.A., WEAVER D.R., CLEMENS L.G. Alteration of muscarinic binding in specific brain areas following estrogen treatment. Brain Res. 1982;241:347–350. doi: 10.1016/0006-8993(82)91075-7. [DOI] [PubMed] [Google Scholar]
- FILLIT H., WEINREB H., CHOLST I., LUINE V., MCEWEN B., AMADOR B., ZABRISKIE J. Observations in a preliminary open trial of estradiol therapy for senile dementia-Alzheimer's type. Psychoneuroendocrinology. 1986;11:337–345. doi: 10.1016/0306-4530(86)90019-3. [DOI] [PubMed] [Google Scholar]
- FUJI K., HIRAMATSU M., KAMEYAMA T., NABESHIMA T. Effects of repeated administration of propentofylline on memory impairment produced by basal forebrain lesion in rats. Eur. J. Pharmacol. 1993;236:411–417. doi: 10.1016/0014-2999(93)90479-2. [DOI] [PubMed] [Google Scholar]
- GAMES D., ADAMS D., ALESSANDRINI R., BARBOUR R., BERTHELETTE P., BLACKWELL C., CARR T., CLEMENS J., DONALDSON T., GILLESPIE F., GUIDO T., HAGOPIAN S., JOHNSON-WOOD K., KHAN K., LEE M., LEIBOWITZ P., LIEBERBURG I., LITTLE S., MASLIAH E., MCCONLOGUE L., MONTOYA-ZAVALA M., MUCKE L., PAGANINI L., PENNIMAN E., ROWER M., SCHENK D., SEUBERT D., SNYDER B., SORIANO F., TAN H., VITALE J., WADSWORTH S., WOLOZIN B., ZHAO J. Alzheimer-type neuropathology in transgenic mice overexpressing V717Fβ-amyloid precursor protein. Nature. 1995;373:523–527. doi: 10.1038/373523a0. [DOI] [PubMed] [Google Scholar]
- GIBBS R.B. Impairment of basal forebrain cholinergic neurons associated with aging and long-term loss of ovarian function. Exp. Neurol. 1998;151:289–302. doi: 10.1006/exnr.1998.6789. [DOI] [PubMed] [Google Scholar]
- GOODMAN Y., BRUCE A.J., CHENG B., MATTSON M.P. Estrogens attenuate and corticosterone exacerbates excitotoxicity, oxidative injury, and amyloid β-peptide toxicity in hippocampal neurons. J. Neurochem. 1996;66:1836–1844. doi: 10.1046/j.1471-4159.1996.66051836.x. [DOI] [PubMed] [Google Scholar]
- HENDERSON V.W. The epidemiology of estrogen replacement therapy and Alzheimer's disease. Neurology. 1997;48 Suppl.7:S27–S35. doi: 10.1212/wnl.48.5_suppl_7.27s. [DOI] [PubMed] [Google Scholar]
- HENDERSON V.W., PAGANINI-HILL A., EMANUEL C.K., DUNN M.E., BUCKWALTER J.G. Estrogen replacement therapy in older women: comparisons between Alzheimer's disease case and nondemented control subjects. Arch Neurol. 1994;51:896–900. doi: 10.1001/archneur.1994.00540210068014. [DOI] [PubMed] [Google Scholar]
- ITOH A., NITTA A., NADAI M., NISHIMURA M., HIROSE M., HASEGAWA T., NABESHIMA T. Dysfunction of cholinergic and dopaminergic neuronal systems in β-amyloid protein-infused rats. J. Neurochem. 1996;66:1113–1117. doi: 10.1046/j.1471-4159.1996.66031113.x. [DOI] [PubMed] [Google Scholar]
- IWATSUBO T., ODAKA A., SUZUKI N., MIZUSAWA H., NUKINA N., IHARA Y. Visualization of Aβ42(43) and Aβ40 in senile plaques with end-specific Aβ monoclonals: evidence that an initially deposited species is Aβ42(43) Neuron. 1994;13:45–53. doi: 10.1016/0896-6273(94)90458-8. [DOI] [PubMed] [Google Scholar]
- JAFFE A.B., TORAN-ALLERAND C.D., GREENGARD P., GANDY S.E. Estrogen regulates metabolism of Alzheimer amyloid β precursor protein. J. Biol. Chem. 1994;269:13065–13068. [PubMed] [Google Scholar]
- JARRETT J.T., LANSBURY P.T., JR Seeding ‘one-dimensional crystallization' of amyloid: a pathogenic mechanism in Alzheimer's disease and scrapie. Cell. 1993;73:1055–1058. doi: 10.1016/0092-8674(93)90635-4. [DOI] [PubMed] [Google Scholar]
- JOHNSON-WOOD K., LEE M., MOTTER R., HU K., GORDON G., BARBOUR R., KHAN K., GORDON M., TAN H., GAMES D., LIEBERBURG I., SCHENK D., SEUBERT P., MCCONLOGUE L. Amyloid precursor protein processing and Aβ42 deposition in a transgenic mouse model of Alzheimer disease. Proc. Natl. Acad. Sci. U.S.A. 1997;94:1550–1555. doi: 10.1073/pnas.94.4.1550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- JORM A.F., KORTEN A.E., HENDERSONM A.S. The prevalence of dementia: a quantitative integration of the literature. Acta Psychiatr. Scand. 1987;76:465–479. doi: 10.1111/j.1600-0447.1987.tb02906.x. [DOI] [PubMed] [Google Scholar]
- KAR S., ISSA A.M., SETO D., AULD D.S., COLLIER B., QUIRION R. Amyloid β-peptide inhibits high-affinity choline uptake and acetylcholine release in rat hippocampal slices. J. Neurochem. 1998;70:2179–2187. doi: 10.1046/j.1471-4159.1998.70052179.x. [DOI] [PubMed] [Google Scholar]
- KOH J.-Y., YANG L.L., COTMAN C.W. β-amyloid protein increases the vulnerability of cultured cortical neurons to excitotoxic damage. Brain Res. 1990;533:315–320. doi: 10.1016/0006-8993(90)91355-k. [DOI] [PubMed] [Google Scholar]
- MATTSON M.P., CHENG B., DAVIS D., BRYANT K., LIEBERBURG I., RYDEL R.E. β-amyloid peptides destabilize calcium homeostasis and render human cortical neurons vulnerable to excitotoxicity. J. Neurosci. 1992;12:376–389. doi: 10.1523/JNEUROSCI.12-02-00376.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MAURICE T., HIRAMATSU M., ITOH J., KAMEYAMA T., NABESHIMA T. Behavioural evidence for a modulating role of σ ligands in memory processes. I. Attenuation of dizocilpine (MK-801)-induced amnesia. Brain Res. 1994;647:44–56. doi: 10.1016/0006-8993(94)91397-8. [DOI] [PubMed] [Google Scholar]
- MORRIS R.G.M. Developments of water-maze procedure for studying spatial learning in the rat. J. Neurosci. Methods. 1984;11:47–60. doi: 10.1016/0165-0270(84)90007-4. [DOI] [PubMed] [Google Scholar]
- MORRIS R.G.M., SCHENK F., TWEEDIE F., JARRARD L.E. Ibotenate lesions of hippocampus and/or subiculum: dissociating components of allocentric spatial learning. Eur. J. Neurosci. 1990;2:1016–1028. doi: 10.1111/j.1460-9568.1990.tb00014.x. [DOI] [PubMed] [Google Scholar]
- NABESHIMA T., ITOH A.Toxicity of β-amyloid protein: neurochemical histological and behavioral changes Alzheimer's Disease: Biology, Diagnosis and Therapeutics 1997John Wiley & Sons Ltd., England; 623–630.In Iqbal, K., Winblad, B., Nishimura, T., Takeda, M. & Wisniewski, H.M. (eds) [Google Scholar]
- NITTA A., FUKUTA T., HASEGAWA T., NABESHIMA T. Continuous infusion of β-amyloid protein into cerebral ventricle induces learning impairment and neuronal and morphological degeneration. Jpn. J. Pharmacol. 1997;73:51–57. doi: 10.1254/jjp.73.51. [DOI] [PubMed] [Google Scholar]
- NITTA A., ITOH A., HASEGAWA T., NABESHIMA T. β-Amyloid protein-induced Alzheimer's disease animal model. Neurosci. Lett. 1994;170:63–66. doi: 10.1016/0304-3940(94)90239-9. [DOI] [PubMed] [Google Scholar]
- OHKURA T., ISSE K., AKAZAWA K., HAMAMOTO M., YAOI Y., HAGINO N. Evaluation of estrogen treatment in female patients with dementia of the Alzheimer type. Endocri. J. 1994;41:361–371. doi: 10.1507/endocrj.41.361. [DOI] [PubMed] [Google Scholar]
- PAXINOS G., WATSON C. The Rat Brain in the Stereotaxic Coordinates. Academic Press, New York; 1986. [Google Scholar]
- SELDEN N.R.W., COLE B.J., EVERITT B.J., ROBBINS T.W. Damage to ceruleo-cortical noradrenergic projections impairs locally cued but enhances spatially cued water maze acquisition. Behav. Brain Res. 1990;39:29–51. doi: 10.1016/0166-4328(90)90119-y. [DOI] [PubMed] [Google Scholar]
- SELKOE D.J. Amyloid b-protein and the genetics of Alzheimer's disease. J. Biol. Chem. 1996;271:18295–18298. doi: 10.1074/jbc.271.31.18295. [DOI] [PubMed] [Google Scholar]
- SHIMIZU H., BRAY G.A. Effects of castration, estrogen replacement and estrus cycle on monoamine metabolism in the nucleus accumbens, measured by microdialysis. Brain Res. 1993;621:200–206. doi: 10.1016/0006-8993(93)90107-x. [DOI] [PubMed] [Google Scholar]
- SINGH M., MEYER E.M., MILLARD W.J., SIMPKINS J.W. Ovarian steroid deprivation results in a reversible learning impairment and compromised cholinergic function in female Sprague-Dawley rats. Brain Res. 1994;644:305–312. doi: 10.1016/0006-8993(94)91694-2. [DOI] [PubMed] [Google Scholar]
- SINGH M., MEYER E.M., SIMPKINS J.W. The effect of ovariectomy and estradiol replacement on brain-derived neurotrophic factor messenger ribonucleic acid expression in cortical and hippocampal brain regions of female Sprague-Dawley rats. Endocrinology. 1995;136:2320–2324. doi: 10.1210/endo.136.5.7720680. [DOI] [PubMed] [Google Scholar]
- STURCHLER-PIERRAT C., ABRAMOWSKI D., DUKE M., WIEDERHOLD K.-H., MISTL C., ROTHACHER S., LEDERMANN B., BURKI K., FREY P., PAGANETTI P.A., WARIDEL C., CALHOUN M., JUCKER M., PROBST A., STAUFENBIEL M., SOMMER B. Two amyloid precursor protein transgenic mouse models with Alzheimer disease-like pathology. Proc. Natl. Acad. Sci. U.S.A. 1997;94:13287–13292. doi: 10.1073/pnas.94.24.13287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TANAKA T., YAMADA K., SENZAKI K., NARIMATSU H., NISHIMURA K., KAMEYAMA T., NABESHIMA T. NC-1900, an active fragment analog of arginine vasopressin, improves learning and memory deficits induced by β-amyloid protein in rats. Eur. J. Pharmacol. 1998;349:15–22. doi: 10.1016/s0014-2999(98)00344-6. [DOI] [PubMed] [Google Scholar]
- TANG M.-X., JACOBS D., STERN Y., MARDER K., SCHOFIELD P., GURLAND B., ANDREWS H., MAYEUX R. Effects of oestrogen during menopause on risk and age at onset of Alzheimer's disease. Lancet. 1996;348:429–432. doi: 10.1016/S0140-6736(96)03356-9. [DOI] [PubMed] [Google Scholar]
- XU H., GOURAS G.K., GREENFIELD J.P., VINCENT B., NASLUND J., MAZZARELLI L., FRIED G., JOVANOVIC J.N., SEEGER M., RELKIN N.R., LIAO F., CHECLER F., BUXBAUM J.D., CHAIT B.T., THINAKARAN G., SISODIA S., WANG R., GREENGARD P., GANDY S. Estrogen reduces neuronal generation of Alzheimer β-amyloid peptides. Nature Med. 1998;4:447–451. doi: 10.1038/nm0498-447. [DOI] [PubMed] [Google Scholar]
- YAMADA K., NITTA A., SAITO T., HU J., NABESHIMA T. Changes in ciliary neurotrophic factor content in the rat brain after continuous intracerebroventricular infusion of β-amyloid (1-40) protein. Neurosci. Lett. 1995;201:155–158. doi: 10.1016/0304-3940(95)12161-7. [DOI] [PubMed] [Google Scholar]
- YAMADA K., NODA Y., HASEGAWA T., KOMORI T., NIKAI T., SUGIHARA H., NABESHIMA T. The role of nitric oxide in dizocilpine-induced impairment of spontaneous alternation behaviour in mice. J. Pharmacol. Exp. Ther. 1996;276:460–466. [PubMed] [Google Scholar]
- YAMADA K., TANAKA T., HAN D., SENZAKI K., KAMEYAMA T., NABESHIMA T. Protective effects of idebenone and α-tocopherol on β-amyloid-(1-42)-induced learning and memory deficits in rats: implication of oxidative stress in β-amyloid-induced neurotoxicity in vivo. Eur. J. Neurosci. 1999b;11:83–90. doi: 10.1046/j.1460-9568.1999.00408.x. [DOI] [PubMed] [Google Scholar]
- YAMADA K., TANAKA T., MAMIYA T., SHIOTANI T., KAMEYAMA T., NABESHIMA T. Improvement of nefiracetam of β-amyloid-(1-42)-induced learning and memory impairments in rats. Br. J. Pharmacol. 1999a;126:235–244. doi: 10.1038/sj.bjp.0702309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- YAMADA K., TANAKA T., SENZAKI K., KAMEYAMA T., NABESHIMA T. Propentofylline improves learning and memory deficits in rats induced by β-amyloid protein-(1-40) Eur. J. Pharmacol. 1998;349:15–22. doi: 10.1016/s0014-2999(98)00166-6. [DOI] [PubMed] [Google Scholar]
- YAMADA K., TANG Y.-P., KANO Y., MIYAZAKI T., MURATA Y., SEO H., NABESHIMA T. Continuous infusion of β-amyloid into the cerebral ventricle of rats promotes expression of BDNF messenger RNA in the hippocampus. Neurosci. Res. 1997;22 Suppl.:S36. [Google Scholar]
- YAMAMURA K., IWATA H., OSADA T., YANO K., YOTSUYANAGI T., NABESHIMA T. Prevention of bone loss by percutaneous estradiol implants in ovariectomized rats. J. Biomed. Mater. Res. 1995;29:1249–1253. doi: 10.1002/jbm.820291012. [DOI] [PubMed] [Google Scholar]
- YANKNER B.A. Mechanisms of neuronal degeneration in Alzheimer's disease. Neuron. 1996;16:921–932. doi: 10.1016/s0896-6273(00)80115-4. [DOI] [PubMed] [Google Scholar]
- YANKNER B.A., DUFFY L.K., KIRSCHNER D.A. Neurotrophic and neurotoxic effects of amyloid β protein: reversal by tachykinin neuropeptides. Science. 1990;250:279–282. doi: 10.1126/science.2218531. [DOI] [PubMed] [Google Scholar]


