Abstract
The vasoconstrictor responses of isolated intrapulmonary arteries (IPA) to P2-receptor agonists was investigated during adaptation to extrauterine life in the normal piglet and the effect of pulmonary hypertension was studied following exposure of newborn animals to chronic hypobaric hypoxia (51 kPa) for 3 days.
At resting tone, α,β-methyleneATP (α,β-meATP) (P2X-receptor agonist) contracted intrapulmonary arteries from adult, but not immature pigs, and repeated application desensitized the response.
Adenosine 5′-triphosphate (ATP) induced endothelium-independent relaxation at low concentrations at all ages, a variable contractile response to high concentrations developed by 3 days, becoming larger and consistent by 14 days of age.
Uridine 5′-triphosphate (UTP) evoked a contractile response in normal intrapulmonary arteries from foetal to adult life, the magnitude of the response increasing with age. Endothelial removal and pre-incubation with Nω-nitro-L-arginine methyl ester (L-NAME) (100 μM) increased the contractile response of adult vessels.
Pre-incubation with α,β-meATP (100 μM), increased the contractile response to UTP in both newborn and adult vessels. ATP-induced relaxations were reduced in newborn vessels but there was no effect on the responses of adult vessels.
Responses to UTP, ATP and α,β-meATP of intrapulmonary arteries from newborn piglets exposed to chronic hypobaric hypoxia for 3 days were normal.
In summary, UTP elicited marked vasoconstriction of porcine IPA at all ages. UTP and ATP responses were consistent with activation of the P2Y4-receptor recently identified in vascular smooth muscle by others. α,β-meATP induced a small vasoconstriction in the adult probably via the P2X1-receptor. Responses remained normal in neonatal pulmonary hypertension.
Keywords: Pyrimidine, purine, pulmonary artery, neonatal hypertension, vasoconstriction
Introduction
The pulmonary vascular resistance is high in utero and remains elevated in persistent pulmonary hypertension of the newborn (PPHN), a clinical syndrome most commonly associated with hypoxic lung disease (Haworth, 1979; 1993). Whether or not nucleotides play a role in maintaining a high resistance in either of these circumstances is not known.
ATP can produce either relaxation or contraction depending upon the P2-receptor subtype(s) present in the tissue and the tone of the vessel investigated (Burnstock, 1997), suggesting that the pulmonary arterial response to ATP might change with age in normal pulmonary arteries as the vascular resistance falls after birth due to maturation of vascular reactivity and structure (Allen & Haworth, 1998; Hall & Haworth, 1987; Haworth et al., 1987; Levy et al., 1995; Liu et al., 1992). At low vascular tone, vasoconstriction to ATP is the dominant response, mediated by P2X-receptor(s), partly via the P2X1 subtype on smooth muscle and partly by P2X subtypes which have not yet been clearly identified (Benham & Tsien, 1987; Neely et al., 1991; 1996). Currently there are seven recognized P2X-receptors, including the non-selective ion pore P2X7 (formerly P2Z) (Burnstock, 1997). Transcripts of receptor mRNA for the P2X1,2 and 4-receptor sub-units have been co-localized by in situ hybridization in adult rat aortic and pulmonary arterial smooth muscle (Nori et al., 1998). The metabolically stable ATP analogue α,β-meATP evoked a contractile response in the isolated guinea-pig taenia coli and then desensitized P2X-receptors, without significant vasodilator activity (Kasakov & Burnstock, 1983; Kennedy & Leff, 1995). ATP induces a contraction in isolated adult human and rat pulmonary artery and α,β-meATP can block the response suggesting that these agonists act via a common receptor (Liu et al., 1989a,1989b). However, in the isolated perfused adult rat lung, vasoconstriction to ATP was only partially inhibited by α,β-meATP (Rubino & Burnstock, 1996). Pyrimidines (uridine nucleotides) are also known to evoke α,β-meATP-insensitive vasoconstriction of some systemic and pulmonary vessels (Juul et al., 1993; Matsumoto et al., 1997; Ralevic & Burnstock, 1991; 1998; Saiag et al., 1987; 1990); von Kugelgen & Starke, 1990). They can be released from the vascular endothelium of rabbit thoracic aorta, and from platelets, suggesting physiological sources (Goetz et al., 1971; Saiag et al., 1995). Pyrimidine-induced vasoconstrictions reported for the adult rat small pulmonary artery and adult rabbit coronary artery were thought to be mediated by members of the G-protein coupled, P2Y-receptor family, which can activate phospholipase C through Gq11 and subsequently stimulate a rise of intracellular calcium (Burnstock 1997; Nicholas et al., 1996; Hartley et al., 1998; Matsumoto et al., 1997). Recently, adult rat aorta smooth muscle has been shown to express P2Y2,4, and 6, subtypes, which are known to be activated by pyrimidines (Harper et al., 1998).
Persistent pulmonary hypertension of the newborn is a potentially fatal condition, which results in abnormal remodelling of the intrapulmonary arteries during the first few days of life (Allen & Haworth, 1986; Haworth, 1979; 1993). The marked hypertrophy of the IPA smooth muscle cells has been taken to indicate an increase in contractile response to different stimuli, including hypoxia. The role of purines and pyrimidines in this condition is unknown. In an experimental study using the perfused adult rat lung, the vasoconstrictor response to acute alveolar hypoxia could not be attributed to up-regulation of α,β-methylene-sensitive P2X-receptors (McCormack et al., 1989).
In the present study, the response of normal, isolated porcine IPA to cumulative doses of ATP, α,β-meATP and UTP was investigated from foetal to adult life. We also studied P2X-receptor desensitization by α,β-meATP on the response to ATP and UTP. The effect of neonatal pulmonary hypertension was determined in the IPA of newborn piglets which had been exposed to chronic hypobaric hypoxia for 3 days (Tulloh et al., 1997).
Materials
Preparation of tissue
Piglets were produced by pregnant Large White sows at term. Animals were killed by an intraperitoneal injection of sodium pentobarbitone (Expiral 100 mg kg−1) when 5 min – 3 h old and at 3 and 14 days of age. In addition, heart-lung blocks from foetal (5 days pre-term), 3, 14 day and adult animals (9-months-old) were delivered from a recognized tissue supplier in cold physiological salt solution. Normal newborn piglets were exposed to chronic hypobaric hypoxia (51 kPa) for a period of 3 days in a hypobaric chamber. Piglets exposed to hypoxia were regularly provided with fresh water and piglet feed (Sow Milk Equivalent, SCA Nutrition Ltd., U.K.) ad libitum, in addition to tube feeding as required. The chamber was illuminated and maintained at a temperature of 25°C (Tulloh et al., 1997). The treatment of all animals followed the guidelines set down in the British Home Office regulations and in the ‘Principles of Laboratory Animal Care' (National Institutes of Health, publication number 80/23, revised 1978).
Organ bath experiments
The main intrapulmonary conduit artery (IPA) was dissected from the middle third region of a lower lobe, placed in calcium containing physiological salt solution (PSS: NaCl 119; KCl 4.7; NaHCO3 25; MgSO4 1.2; KHPO4 1.2, CaCl2 2.5, glucose 11 mM) and cleaned of lung parenchyma and connective tissue. The vessel was cut into rings 2–4 mm long, external diameter range of 1.5 mm (foetal) to 3.5 mm (adult). The endothelium was removed from selected rings by mechanical rubbing with a metal tool. Two horizontal tungsten wires (120 μm diameter) were inserted through the vessel lumen and then mounted for isometric force recording in 5 ml organ baths. Isometric force data was recorded in a digital format using Chart software on a MacLab computer system. The rings were allowed to stabilize for at least 40 min, during which time the PSS was replaced once and the tension gradually increased to 1000 mg, in vessels from all animals. Contraction to 30 mM potassium chloride (KCl) established the presence of smooth muscle function in all preparations. In order to verity that the endothelium was intact or had been removed effectively, in animals aged 3 days or more acetylcholine (ACh) (1–10 μM) was added in the presence of a stable contraction to PGF2α (10 or 30 μM), to detect endothelium-dependent relaxation (Liu et al., 1992). Conduit porcine IPA from younger or pulmonary hypertensive animals do not relax in response to ACh (Tulloh et al., 1997). A contractile response to a second bolus of KCl (30 mM) was determined, against which responses could be evaluated (in combination with the tension remaining after 100 μM papaverine). The KCl was then washed out, allowing the baseline to stabilize again. A cumulative-concentration response curve to either α,β-meATP (0.01–100 μM), ATP (0.001–30 mM) or UTP (0.001–30 mM) was then carried out in rings from normal animals, from foetal to adult life, and in rings from neonatal hypoxic animals.
In order to investigate the role of the endothelium in the response to UTP, IPAs with endothelium from adult pigs were pre-incubated with either L-NAME (100 μM) or indomethacin (10 μM), both inhibitors being added before carrying out a cumulative-concentration response study to UTP. The effect of P2X-receptor desensitization upon the responses was investigated in normal newborn and adult porcine IPAs. Twenty minutes after carrying out a cumulative dose-response study with α,β-meATP, a 100 μM bolus of α,β-meATP was added to confirm a P2X-receptor block. Then in the continued presence of α,β-meATP, a cumulative dose response study to either ATP or UTP was performed on the same vessel. Finally, in each experiment, a bolus of papaverine (100 μM) was given in order to remove all remaining tone. The change in tension between the peak of the contractile response to 30 mM KCl and the relaxation to 100 μM papaverine was taken as the measure of greatest contractile activity, as 100% against which the response to P2-agonists was assessed.
Drugs
The drugs were purchased from Sigma and dissolved in distilled water unless stated otherwise: acetylcholine (hydrochloride salt), adenosine 5′-triphosphate (di-sodium salt), indomethacin, L-NAME, papaverine (hydrochloride salt), prostaglandin F2α (Sigma and Cayman Chemical Company (made up in absolute ethanol), uridine 5′-triphosphate (sodium salt), α,β-methylene adenosine 5′-triphosphate (from RBI).
Statistical analysis
Excel (version 7a) and SPSS (version 6.1.3) PC were used to do the following data analysis. The effect of age on the contractile response to 30 mM potassium chloride (including the basal tone revealed by giving 100 μM papaverine) was assessed using a one-way analysis of variance (ANOVA) with post-hoc Bonferroni testing. One-way ANOVA was used for each P2-receptor agonist to determine the concentration at which the effect of age was greatest. Bonferroni post-hoc testing was used to identify between which age groups the responses seen at this concentration were significantly different. A two-sample Student t-test was used to test the following: the influence of the endothelium on responses; the effect of L-NAME and indomethacin on the response to UTP in the adult; the effect of P2X-receptor desensitization by pre-incubation with α,β-meATP on the response to ATP and UTP. The effect of exposure to chronic hypobaric hypoxia from birth for 3 days was compared to normal age-matched data using a two-sample Student t-test.
Results
Responses of intrapulmonary arteries from normal pigs
A contractile response to a bolus addition of potassium chloride (KCl) was present at all ages and the increase in tension was similar in IPA from foetal life until 14 days of age (Figure 1), but was significantly greater in normal adult vessels (P<0.05). Removing the endothelium did not change the response at any age.
Figure 1.
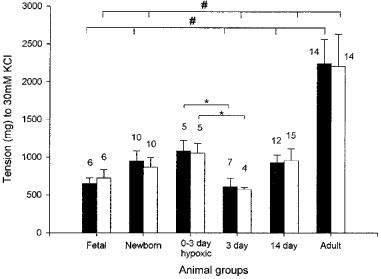
The response of isolated porcine intrapulmonary arteries to 30 mM potassium chloride, at different ages, in normal animals and in those exposed to hypoxia, with and without endothelium. Each column shows the mean±s.e.mean. The number of animals used is indicated above each column. Solid columns indicate arteries with endothelium and empty columns those without. #P<0.05 ANOVA, *P<0.05 Student t-test.
In IPA from normal foetal, newborn and 3 day-old piglets, cumulative addition of ATP induced a concentration-dependent relaxation which was independent of the endothelium (Figure 2a). In addition, small transient contractions were evoked at similar high concentrations in the 3 day-old, but not in younger animals. By contrast, in normal 2 week-old and adult pigs ATP-induced only a small relaxation, with a greater contractile response at high concentrations. Thus, there was a significant reversal from a pre-dominantly relaxant response to a contractile one as age increased (P<0.05).
Figure 2.
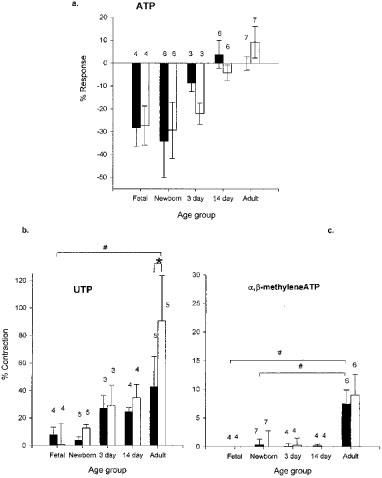
Histograms showing the mean response to (a) ATP, 20 mM; (b) UTP, 20 mM; (c) α,β-meATP, 10 μM, at different ages. The data shown for each agonist gave the most significant (ANOVA) change in response with age and was derived from cumulative concentration-response curves. Columns show mean±s.e.mean. Solid columns indicate arteries with endothelium and empty columns those without. The number of animals used is indicated above each column. #P<0.05 ANOVA, *P<0.05 Student t-test.
In IPA from piglets of all ages, cumulative addition of UTP induced a contractile response in vessels with and without endothelium (Figure 2b). The response in rings without endothelium increased significantly with age and was significantly greater in the adult than in foetal animals (P<0.05). In adult IPA the contractile response was significantly greater in rings without endothelium (P<0.05). Representative traces of ATP- and UTP-cumulative concentration response curves in newborn and adult IPA are presented (Figure 3a,b,c,d).
Figure 3.
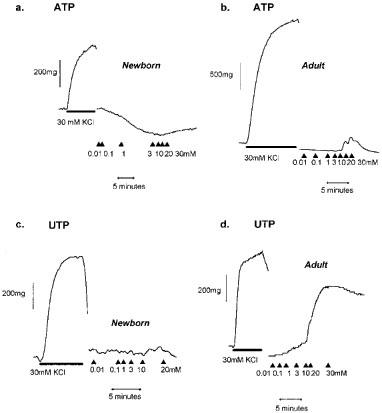
Representative traces for the responses of isolated porcine intrapulmonary arteries, with endothelium, to a bolus of 30 mM KCl and cumulative doses of either ATP or UTP at resting tension. Responses are shown for ATP (a, b) and UTP (c, d) in newborn and adult vessels respectively.
Cumulative-addition of α,β-meATP only induced a significant response in adult IPA, when a small transient contraction was evoked at 10 μM (Figure 2c). Removing the endothelium had no significant effect on the response at any age. In the normal adult porcine IPA the rank order of vasoconstrictor activity was UTP>ATP>α,β-meATP.
Effect of nitric oxide synthase and prostaglandin synthase inhibitors in adult porcine IPAs
The resting tone did not change significantly following incubation with either 100 mM L-NAME (increased by 22±12%) or 10 μM indomethacin (decreased by 15±6%) or with a combination of the two (increased by 4±7%). The effect of the synthase inhibitors was assessed from the newly established baseline. The significant increase in UTP-induced contraction following removal of the endothelium from the normal adult pig IPA was mimicked by pre-incubation with L-NAME (100 μM) (Figure 4a). Pre-incubation with indomethacin (10 μM) had no effect (Figure 4b), while a combination of the two inhibitors reduced the effect of L-NAME given alone (Figure 4c).
Figure 4.
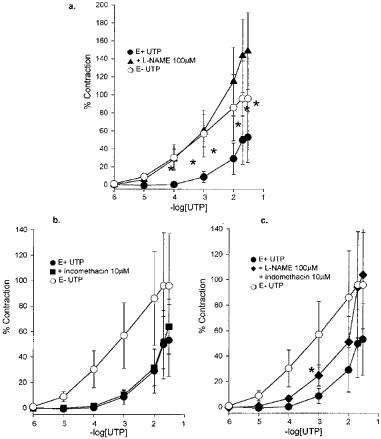
Porcine intrapulmonary arteries from four normal adults with and without endothelium exposed to UTP, and in vessels with endothelium following pre-incubation with (a) L-NAME (100 μM), (b) indomethacin (10 μM) or (c) L-NAME (100 μM) and indomethacin (10 μM), showing the mean response±s.e.mean. *P<0.05 between the treated vessels and the response of vessels with endothelium, Student t-test.
P2X-receptor desensitization experiments
In IPA from normal adult animals a 20 min pre-incubation with 100 μM α,β-meATP, followed by a repeated dose of 100 μM α,β-meATP desensitized the contractile response in vessels with or without endothelium (Figure 5a). In adult vessels, the response to ATP was unchanged by pre-incubation with α,β-meATP (Figure 5b). UTP-induced contractions displayed a significantly augmented potency and magnitude in IPA with and without endothelium (Figure 5c).
Figure 5.
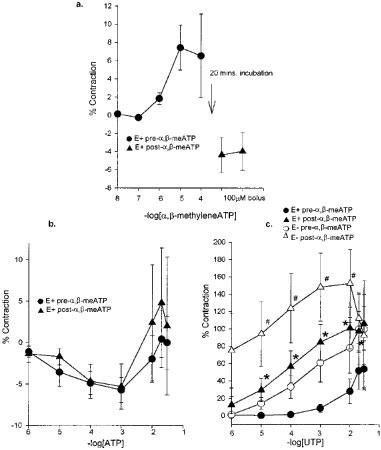
The effect of P2X-receptor desensitization by pre-incubation with (100 μM) α,β-meATP in isolated intrapulmonary arteries with endothelium from normal adult pigs. Effect on the response to (a) α,β-meATP (n=6); (b) ATP (n=6); (c) UTP (n=5), showing the mean response±s.e.mean. Empty symbols denote vessels without endothelium. ‘n' indicates the number of animals used and applies to subsequent legends. * With and # without endothelium, P<0.05 between treated and untreated vessels.
In normal newborn piglets, the same protocol prevented the normal ATP-induced relaxation response (P<0.05 Figure 6a). After pre-incubation with α,β-meATP, UTP appeared to induce an increased contractile response but this was only statistically significant in vessels in which the endothelium had been removed (Figure 6b).
Figure 6.
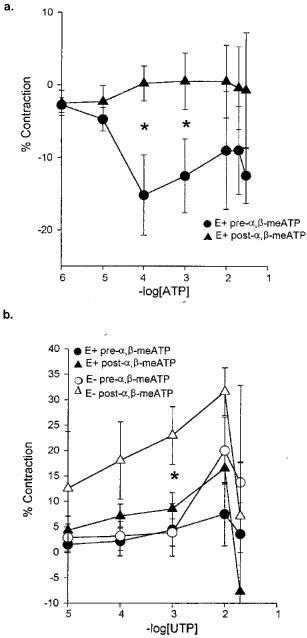
The effect of P2X-receptor desensitization by pre-incubation with (100 μM) α,β-meATP in isolated intrapulmonary arteries with endothelium from normal newborn pigs. Effect on the response to (a) ATP (n=3); (b) UTP (n=3) and (c) UTP (n=3) without endothelium, showing the mean response±s.e.mean. *P<0.05.
Responses of intrapulmonary arteries from piglets with neonatal pulmonary hypertension
The response to a bolus addition of KCl in IPA from animals exposed to chronic hypobaric hypoxia from birth for a period of 3 days was similar to the normal response at birth and greater than that in the normal at 3 days of age (Figure 1) (P<0.05). Removal of the endothelium did not alter this observation.
The response to ATP and α,β-meATP in IPAs from the hypoxic piglets was similar to that in normal age-matched healthy animals (Figure 7a,b). The contractile response to UTP was more similar to the normal response at birth than the normal at 3 days of age with (Figure 7c), or without (data not shown) endothelium.
Figure 7.
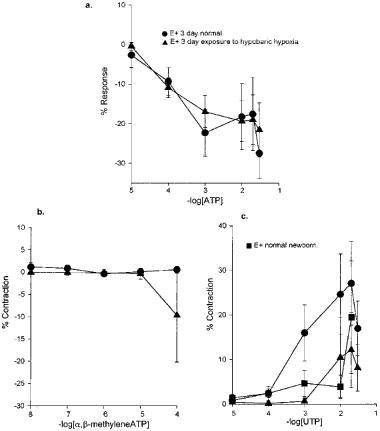
The effect of chronic pulmonary hypertension induced by hypoxia from birth for a period of 3 days on the response of isolated intrapulmonary arteries, with endothelium, to (a) ATP (n=4); (b) α,β-methyleneATP (n=3); (c) UTP (n=3), showing the mean response±s.e.mean, normal newborn, normal 3 day-old both with endothelium. *P<0.05.
Discussion
The present study has demonstrated that under resting conditions the contractile response of the porcine conduit intrapulmonary artery to P2-receptor agonists increased significantly between foetal and adult life. The contractile response was low in the perinatal animals suggesting that the agonists tested may not have physiological importance in the presence of the high pulmonary tone present at this age. A contractile response to UTP was evident in the normal foetal and newborn preparations, much earlier than ATP-induced contraction. UTP also had a greater contractile effect than either ATP or α,β-meATP at all ages. The explanation for the increase in contractile response to the different nucleotides with age is unclear. The response to purines is generally tone-dependent, vasoconstriction being favoured at low tone, such as is found in IPAs of mature normal animals. But the response to other contractile agonists also increases with age (Levy et al., 1995) and could be related to a progressive increase in smooth muscle cell contractile myofilament volume density (Hall & Haworth, 1987).
α,β-meATP only constricted adult porcine IPA and the responses were transient, and tachyphylactic, as described in the isolated IPAs of man and the adult rat (Liu et al., 1989a, 1989b). Following reports of P2X1,2,4-receptor mRNA located on adult rat IPA smooth muscle it may be suggested that α,β-meATP acts at the P2X1-receptor subtype in the present study (Nori et al., 1998). It was interesting to note the absence of any contractile response in the IPAs of younger piglets, because the rat tail artery shows a greater P2X-receptor mediated vasoconstriction in the young rat than in the adult (Bao et al., 1989). In the adult porcine IPA, α,β-meATP evoked a smaller contractile response at lower concentrations than either ATP or UTP. a,β-meATP has been shown to desensitize certain subtypes of P2X-contractile receptors (P2X1 and 3) (Burnstock, 1997). In the present study, α,β-meATP reduced the relaxation response to low concentrations of ATP in the newborn pig IPA, and actually increased the contractile response to UTP. In the adult porcine IPA, the ATP- induced contractile responses were not blocked and the UTP-induced responses were increased by pre-incubation with α,β-meATP. Other workers have also found the contractile response to ATP was not inhibited following P2X-receptor desensitization by α,β-meATP in systemic vessels (Juul et al., 1993; Maguire et al., 1996; O'Connor et al., 1990) and potentiation of UTP-induced vasoconstriction has been reported in the isolated adult rat pelvic artery (Maguire et al., 1996). In addition, α,β-meATP reduced ATP induced vasoconstriction of perfused adult rat lungs but it did not inhibit the UTP response (Rubino & Burnstock, 1996). α,β-meATP has been shown to be an inhibitor of ectoATPase activity on bovine aortic endothelial cells (Chen & Lin, 1997). EctoATPase inhibition may increase the levels of ATP and UTP and therefore increase the contractile response at low tone (Zimmerman, 1996).
UTP evoked a marked vasoconstriction of IPA at all ages in the present study. The role of UTP in the cardiovascular system, normal or abnormal, is uncertain. High concentrations are present in platelets and UTP can be released from the endothelium under conditions of increased shear stress and hypoxia (Saiag et al., 1995), suggesting the involvement of UTP in high flow and hypoxia-induced vasoconstriction in vivo. Ours is the first study to demonstrate a vasoconstrictor action for UTP which was greater than that of purines, as it was throughout life in porcine IPA. The observation that UTP induces a greater contractile response in the adult porcine IPA without endothelium would suggest UTP-stimulated release of an EDRF (Purkiss et al., 1994). This was confirmed in the present study with L-NAME. A pyrimidine-preferring P2Y receptor (G-protein coupled receptor superfamily) is thought to mediate the contractile response of the adult rabbit coronary artery induced by UDP and UTP (Matsumoto et al., 1997). In the present study UTP evoked an α,β-meATP-insensitive vasoconstriction, suggesting action at a P2Y2-receptor, recently identified in adult rat small pulmonary artery (Hartley et al., 1998). However, the observation that ATP seemed to act as a partial agonist also evoking an α,β-meATP-insensitive vasoconstriction would lead us to conclude that the responses to both UTP and ATP were mediated by a P2Y4-receptor. This conclusion is based on receptor expression studies by Nicholas et al. (1996), demonstrating that UTP was more potent than ATP at the P2Y4-receptor, while both nucleotides were equipotent at the P2Y2 and that the P2Y6 was only potently activated by UDP.
Chronic hypoxia produces vascular remodelling of the intrapulmonary arteries in the newborn piglet and other species including man, which involves an increase in smooth muscle (Allen & Haworth, 1986; Haworth, 1993). However, in both present and previous studies on chronically hypoxic piglets, the increase in smooth muscle was not associated with an increase in the contractile response to potassium chloride (Tulloh et al., 1997). Hypoxic exposure prevented the normal postnatal reduction in response from taking place. The responses evoked by P2-receptor agonists were also similar to those of vessels from normal piglets at birth, prior to entering the hypobaric chamber. Reports from our own group and others make it clear that the vasoconstrictor response to other agonists is not always enhanced following exposure to chronic hypoxia (Tulloh et al., 1997; Porcelli & Bergman, 1983; Karamsetty et al., 1995; Maclean et al., 1996).
Chronic hypobaric hypoxia may have remodelled the IPA in such a way as to produce a ‘pre-contracted, high tone vessel' which would favour a relaxation response, judging by the tone-dependent nature of the responses evoked by P2-agonists. An alternative explanation would be that the smooth muscle sensitivity to ATP induced relaxation was up-regulated to balance the vasoconstricting effect of hypoxia in vivo. An initial up-regulation of IPA vasodilatation to EDRF and activation of K+-ATP channels has been shown in response to acute hypoxia, prior to a secondary loss of vasodilatation (Wadsworth, 1994).
The present study suggests that the P2X1-receptor mediates the α,β-meATP vasoconstriction response in the adult. However, UTP was found to be a more potent vasoconstrictor than ATP at all ages, suggesting the α,β-meATP-insensitive contractile responses were mediated by the P2Y4-receptor subtype. Neonatal pulmonary hypertension induced by chronic hypoxia did not affect these conclusions.
Acknowledgments
We thank Dr P.J. Boels for his pharmacological and statistical advice. This work was funded by the British Heart Foundation.
Abbreviations
- Ach
Acetylcholine
- ANOVA
analysis of variance
- ATP
adenosine 5′-triphosphate
- IPA
intrapulmonary artery
- KCl
potassium chloride
- L-NAME
Nw-nitro-L-arginine methyl ester
- α,β-meATP
α,β-methylene adenosine 5′-triphosphate
- PAP
pulmonary arterial pressure
- PGF2α
prostaglandin F2α
- PPHN
persistent pulmonary hypertension of the newborn
- PSS
physiological salt solution
- UTP
uridine 5′-triphosphate
References
- ALLEN K., HAWORTH S.G. Human postnatal pulmonary arterial remodelling: ultrastructural studies of smooth muscle cell and connective tissue maturation. Lab. Invest. 1988;59:702–709. [PubMed] [Google Scholar]
- ALLEN K.M., HAWORTH S.G. Impaired adaptation of pulmonary circulation to extrauterine life in newborn pigs exposed to hypoxia: an ultrastructural study. J. Pathol. 1986;150:205–212. doi: 10.1002/path.1711500309. [DOI] [PubMed] [Google Scholar]
- BAO J.X., ERIKSSON I.E., STJARNE L. Age-related variations in the relative importance of noradrenaline and ATP as mediators of the contractile response of rat tail artery to sympthetic nerve stimulation. Acta Physiol. Scand. 1989;136:287–288. doi: 10.1111/j.1748-1716.1989.tb08663.x. [DOI] [PubMed] [Google Scholar]
- BENHAM C.D., TSIEN R.W. A novel receptor-operated Ca2+ permeable channel activated by ATP in smooth muscle. Nature. 1987;328:275–278. doi: 10.1038/328275a0. [DOI] [PubMed] [Google Scholar]
- BURNSTOCK G. The past, present and future of purine nucleotides as signalling molecules. Neuropharmacology. 1997;36:1127–1139. doi: 10.1016/s0028-3908(97)00125-1. [DOI] [PubMed] [Google Scholar]
- CHEN B.C., LIN W. Inhibition of ecto-ATPase by the P2-purinoceptor agonists, ATPγS, α,β-meATP, and AMP-PNP, in endothelial cells. Biochem. Physical Res. Commun. 1997;233:442–446. doi: 10.1006/bbrc.1997.6478. [DOI] [PubMed] [Google Scholar]
- GOETZ U., DA-PRADA M., PLETSCHER A. Adenine-, guanine- and uridine-5′-phosphonucleotides in blood platelets and storage organelles of various species. J. Pharmacol. Exp. Ther. 1971;178:210–215. [PubMed] [Google Scholar]
- HALL S.M., HAWORTH S.G. Conducting pulmonary arteries: structural adaptation to extrauterine life. Cardiovascular Res. 1987;21:208–216. doi: 10.1093/cvr/21.3.208. [DOI] [PubMed] [Google Scholar]
- HARPER S., WEBB T.E., CHARLTON S.J., NG L.L., BOARDER M.R. Evidence that P2 Y4 nucleotide receptors are involved in the regulation of rat aortic smooth muscle cells by UTP and ATP. Br. J. Pharmacol. 1998;124:703–710. doi: 10.1038/sj.bjp.0701895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HARTLEY S.A., KATO K., SALTER K.J., KOZLOWSKI R.Z. Functional evidence for a novel suramin-insensitive pyrimidine receptor in rat small pulmonary arteries. Circ. Res. 1998;83:940–946. doi: 10.1161/01.res.83.9.940. [DOI] [PubMed] [Google Scholar]
- HAWORTH S.G.Pulmonary vascular structure in persistent fetal circulation Paediatric Cardiology. Heart Disease in the Newborn 1979Churchill Livingstone: Edinburgh; 67–78.In: Godman, M.J. & Marquis, R.M. (eds) [Google Scholar]
- HAWORTH S.G., HALL S.M., CHEW M., ALLEN K. Thinning of fetal pulmonary arterial wall and postnatal remodelling: ultrastructural studies on the respiratory unit arteries of the pig. Virchows Arch A. 1987;411:161–171. doi: 10.1007/BF00712740. [DOI] [PubMed] [Google Scholar]
- HAWORTH S.G.Persistent Fetal Circulation: Principles of Diagnosis and Management Fetus and Neonate 1993Cambridge Press: Cambridge; 396–423.In: Hanson, M.A., Spender, J.A.D. & Rodeck, C.H. (ed) [Google Scholar]
- JUUL B., PLESNER L., AALKJAER C. Effects of ATP and related nucleotides on the tone of the isolated rat mesenteric resistance arteries. J. Pharmacol. Exp. Ther. 1993;264:1234–1240. [PubMed] [Google Scholar]
- KARAMSETTY V.S.N.M.R., KANE K.A., WADSWORTH R.M. The effects of chronic hypoxia on the pharmacological responsiveness of the pulmonary artery. Pharmac. Ther. 1995;68:233–246. doi: 10.1016/0163-7258(95)02007-1. [DOI] [PubMed] [Google Scholar]
- KASAKOV L., BURNSTOCK G. The use of the slowly degradable analogue α,β-methylene ATP, to produce desensitisation of the P2-purinoceptor: effect on non-adrenergic non-cholinergic responses of the guinea-pig urinary bladder. Eur. J. Pharmacol. 1983;86:291–294. doi: 10.1016/0014-2999(82)90330-2. [DOI] [PubMed] [Google Scholar]
- KENNEDY C., LEFF P. How should P2-purinoceptors be classified pharmacologically. TIPS. 1995;16:168–173. doi: 10.1016/s0165-6147(00)89010-0. [DOI] [PubMed] [Google Scholar]
- LEVY M., TULLOH R.M., KOMAI H., STUART-SMITH K., HAWORTH S.G. Maturation of the contractile response and its endothelial modulation in newborn porcine intrapulmonary arteries. Pediatr. Res. 1995;38:25–29. doi: 10.1203/00006450-199507000-00005. [DOI] [PubMed] [Google Scholar]
- LIU S.F., HISLOP A.A., HAWORTH S.G., BARNES P.J. Developmental changes in endothelium-dependent pulmonary vasodilatation in pigs. Br. J. Pharmacol. 1992;106:324–330. doi: 10.1111/j.1476-5381.1992.tb14335.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LIU S.F., MCCORMACK D.G., EVANS T.W., BARNES P.J. Characterization and distribution of P2-purinoceptor subtypes in rat pulmonary vessels. J. Pet. 1989a;251:1204–1210. [PubMed] [Google Scholar]
- LIU S.F., MCCORMACK D.G., EVANS T.W., BARNES P.J. Evidence for two P2-purinoceptor subtypes in human small pulmonary arteries. Br. J. Pharmacol. 1989b;98:1014–1020. doi: 10.1111/j.1476-5381.1989.tb14633.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MACLEAN M.R., SWEENEY G., BAIRD M., MCCULLOCH K.M., HOUSLAY M., MORECROFT I. 5-Hydroxytryptamine receptors mediating vasoconstriction in pulmonary arteries from control and pulmonary hypertensive rats. Br. J. Phrmacol. 1996;119:917–930. doi: 10.1111/j.1476-5381.1996.tb15760.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MAGUIRE M.H., DOBRONYI I., HUNG K.S., SATCHELL D.G.Do different receptors mediate ATP- and UTP-elicited contraction of rat pelvic artery Drug Develop Res. 199637165 [Google Scholar]
- MATSUMOTO T., NAKANE T., CHIBA S. UTP induces responses in the isolated and perfused canine epicardial coronary artery via UTP-preferring P2Y receptors. Br. J. Pharmacol. 1997;122:1625–1632. doi: 10.1038/sj.bjp.0701559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MCCORMACK D.G., BARNES P.J., EVANS T.W. Purinoceptors in the pulmonary circulation of the rat and their role in hypoxic vasoconstriction. Br. J. Pharmacol. 1989;98:367–372. doi: 10.1111/j.1476-5381.1989.tb12606.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NEELY C.F., HAILE D.M., CAHILL B.E., KADOWITZ P.J. Adenosine and ATP produce vasoconstriction in the feline pulmonary vascular bed by different mechanisms. J. Pet. 1991;258:753–761. [PubMed] [Google Scholar]
- NEELY C.F., MATOT I., BATRA V.K., BO X., BURNSTOCK G. P2X purinoceptors in the feline pulmonary vascular bed: distribution and selective in vivo pharmacological probes. Am. J. Physiol. 1996;270:L889–L897. doi: 10.1152/ajplung.1996.270.6.L889. [DOI] [PubMed] [Google Scholar]
- NICHOLAS R.A., LAZAROWSKI E.R., WATT W.C., LI Q., BOYER J., HARDEN T.K. Pharmacological and second messenger signalling selectives of cloned P2Y receptors. J. Auto. Pharmacol. 1996;16:319–323. doi: 10.1111/j.1474-8673.1996.tb00044.x. [DOI] [PubMed] [Google Scholar]
- NORIS S., FUMAGALLI L., BO X., BOGDANOV Y., BURNSTOCK G. Coexpression of mRNAs for P2X1, P2X2 and P2X4 receptors in rat vascular smooth muscle: an in situ hybridisation and RT–PCR study. J. Vasc. Res. 1998;35:179–185. doi: 10.1159/000025582. [DOI] [PubMed] [Google Scholar]
- O'CONNOR S.E., WOOD B.E., LEFF P. Characterization of P2x-receptors in rabbit isolated ear artery. Br. J. Pharmacol. 1990;101:640–644. doi: 10.1111/j.1476-5381.1990.tb14133.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PORCELLI R.J., BERGMAN M.J. Effects of chronic hypoxia on pulmonary vascular responses to biogenic amines. J. Appl. Physiol. 1983;55:534–540. doi: 10.1152/jappl.1983.55.2.534. [DOI] [PubMed] [Google Scholar]
- PURKISS J.R., WILKINSON G.F., BOARDER M.R. Differential regulation of inositol 1,4,5-triphosphate by co-existing P2Y-purinoceptors and nucleotide receptors on bovine aortic endothelial cells. Br. J. Pharmacol. 1994;111:723–728. doi: 10.1111/j.1476-5381.1994.tb14797.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RALEVIC V., BURNSTOCK G. Effects of purines and pyrimidines on the rat mesenteric arterial bed. Br. J. Pharmacol. 1991;69:1583–1590. doi: 10.1161/01.res.69.6.1583. [DOI] [PubMed] [Google Scholar]
- RALEVIC V., BURNSTOCK G. Receptors for purines and pyrimidines. Pharmacol. Rev. 1998;50:413–492. [PubMed] [Google Scholar]
- RUBINO A., BURNSTOCK G. Evidence for a P2-purinoceptor mediating vasoconstriction by UTP, ATP and related nucleotides in the isolated pulmonary vascular bed of the rat. Br. J. Pharmacol. 1996;118:1415–1420. doi: 10.1111/j.1476-5381.1996.tb15554.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SAIAG B., BODIN P., SHACOORI V., CATHERINE M., RAULT B., BURNSTOCK G. Uptake and flow-induced release of uridine nucleotides from isolated vascular endothelial cells. Endothelium. 1995;2:279–285. [Google Scholar]
- SAIAG B., MILON D., ALLAIN H., RAULT B., DRIESSCHE J.V.D. Constriction of the smooth muscle of rat tail and femoral arteries and dog saphenous vein is induced by uridine triphosphate via a ‘pyrimidinoceptors' and by adenosine triphosphate via P2X-purinoceptors. Blood Vessels. 1990;27:352–364. doi: 10.1159/000158829. [DOI] [PubMed] [Google Scholar]
- SAIAG B., MILON D., GUELOU M.C., VAN DEN DRIESSCHE J., RAULT B. Heterogeneity of purinergic P2 receptors at the level of the caudal artery of the rat and the saphenous vein of the dog. C R Seances Soc. Biol. Fil. 1987;181:168–177. [PubMed] [Google Scholar]
- TULLOH R.M.R., HISLOP A.A., BOELS P.J., DEUTSCH J., HAWORTH S.G. Chronic hypoxia inhibits postnatal maturation of porcine intrapulmonary artery relaxation. Am. J. Physiol. 1997;272:H2436–H2445. doi: 10.1152/ajpheart.1997.272.5.H2436. [DOI] [PubMed] [Google Scholar]
- VON KUGELGENI T., STARKE K. Evidence for two separate vasoconstriction-mediating nucleotide receptors, both distinct from the P2X-receptor, in rabbit basilar artery: a receptor for pyrimidine nucleotides and a receptor for purine nucleotides. Arch. Pharmacol. 1990;341:538–546. doi: 10.1007/BF00171734. [DOI] [PubMed] [Google Scholar]
- WADSWORTH R.M. Vasoconstrictor and vasodilator effects of hypoxia. Trends Pharmacol Sci. 1994;15:47–53. doi: 10.1016/0165-6147(94)90109-0. [DOI] [PubMed] [Google Scholar]
- ZIMMERMAN H. Extracellular purine metabolism. Drug Metab. Res. 1996;39:337–352. [Google Scholar]


