Abstract
The shear stress of flowing blood profoundly influences the release of endothelium-dependent vasodilative and constrictive factors. Conversely, the influence of these mediators such as nitric oxide (NO) or endothelin-1 (ET-1) on blood rheology remains elusive. In the present study the influence of nitrovasodilators and ET-1 on red blood cell (RBC) shape and whole blood viscosity were investigated.
Incubation of whole blood with sodium-nitroprusside (SNP, 10−5–10−2 M), glyceryl trinitrate (GTN, 0.0001–0.1 mg mL−1), S-nitroso-N-acetylpenicillamine (SNAP, 10−6–10−3 M), and the active metabolite of molsidomine (SIN-1, 10−6–10−3 M), but not molsidomine (10−6–10−3 M), resulted in significantly increased amounts of methaemoglobin, indicating a relevant interaction with RBCs. Treatment with SNP at 10−2 M induced a marked echinocytosis (morphological index: 2.23±0.98 vs −0.17±0.10; P<0.001) and increased blood viscosity (haematocrit 45%) at a high shear rate of 94.5 s−1 (6.46±0.60 vs 5.07±0.35 mPa·s; P<0.01) and a low shear rate of 0.1 s−1 (88.6±36.8 vs 42.1±11.7 mPa·s; P<0.01). Echinocytosis was probably due to cyanide accumulation. SIN-1 at 10−3 M slightly decreased high shear viscosity (4.88±0.28 vs 4.95±0.30 mPa·s; P<0.05). SNAP at 10−3 M slightly increased both high (5.14±0.23 vs 5.05±0.24 mPa·s; P<0.01) and low shear (53.9±7.2 vs 51.2±5.9 mPa·s; P<0.05) viscosity. Molsidomine and GTN failed to influence whole blood viscosity. ET-1 (10−9–10−6 M) had no effect on RBC shape and viscosity.
We conclude that the most important modulators of vascular tone, NO and ET-1, do not affect RBC shape and blood viscosity, which is important from both a physiological and a pharmacological point of view.
Keywords: Erythrocyte, methaemoglobin, nitric oxide, nitrovasodilator, endothelin, rheology, viscosity
Introduction
The vascular endothelium plays a central role in vasomotor control by secreting both relaxing and constrictive mediators (Vanhoutte & Mombouli, 1996). In recent years, these mediators such as nitric oxide (NO) and endothelin-1 (ET-1) have gained much interest.
In human endothelial cells, the NO radical is endogenously synthesized from L-arginine by a constitutively expressed isoform of NO synthase (cNOS) and mediates vasodilatation via activation of soluble guanylate-cyclase in underlying vascular smooth muscle cells (Marletta, 1994; Nathan & Xie, 1994). Basal NO release is modulated by various physiologic stimuli such as hormones, coagulation factors and shear stress (Luscher, 1991). Derangements in vascular NO production are thought to be involved as both cause and consequence of vascular diseases (Cooke & Dzau, 1997; McHugh & Cheek, 1998). Conversely, inhalation of NO as well as drugs which affect the L-arginine-NO-cGMP pathway, the so called nitrovasodilators, are therapeutically used in a broad range of cardiac, respiratory and gastrointestinal disorders (Abrams, 1996; Roberts et al., 1997; Moreau & Lebrec, 1990).
The endothelial secretion of the vasoconstrictive 21 amino acid peptide ET-1 is regulated by stimuli such as growth factors, hypoxia and shear stress (Rosendorff, 1997). However the effect of the latter is controversial, with investigators observing both increases and decreases, probably due to differences in study designs (Ziegler et al., 1998; Malek & Izumo, 1995).
The effects of viscosity and shear stress on NO synthesis are well investigated, however there is only scant knowledge about the effect of NO and related molecules on blood rheology. This is relevant since NO is readily taken up by red blood cells (RBCs) and inactivated by haemoglobin via conversion to methaemoglobin and nitrate (Wennmalm et al., 1992), which may alter the functional properties of these cells. L-arginine has been reported to decrease blood viscosity in vivo in healthy subjects (Giugliano et al., 1997) and in patients with diabetes (Giugliano et al., 1998). NO itself has been demonstrated in some studies to induce an RBC shape transformation and to influence RBC deformability (Caramelo et al., 1994; Korbut & Gryglewski, 1993; Maeda et al., 1987), whereas in an other study no changes in rheologic parameters were noted (Hogman et al., 1994). The influence of nitrovasodilators and ET-1 on blood rheology remains elusive, with only one study investigating the effects of nitrovasodilators on RBC deformability (Korbut & Gryglewski, 1993) and viscosity (Giugliano et al., 1995), and one research group reporting improved filterability of RBC upon treatment with ET-1 (Oonishi et al., 1997; 1998).
Therefore, the present study was designed to evaluate the effects of various nitrovasodilators and ET-1. Blood viscosity was measured at high shear rate, which is determined primarily by RBC deformability, and at low shear rate, which is primarily determined by RBC aggregation (Chien, 1975). Methaemoglobin formation was measured as indirect evidence for the presence of NO or related molecules (Archer, 1993).
Methods
Drugs
Pilot experiments with direct NO-bubbling (medical NO gas, 300 parts per million, AGA Gas AB, Sundbyberg, Sweden) of blood samples had to be abandoned because blood viscosity measurement with a Couette viscometer (see below) is not feasible in the presence of even the smallest amounts of gas microbubbles.
Sodium-nitroprusside (SNP) was obtained from Merck AG (Dietikon, Switzerland). Solutions of SNP were kept in the dark to prevent spontaneous photolysis (Harrison & Bates, 1993). N-[ethoxycarbonyl]-3-[4-morpholinyl] sydnone imine (molsidomine, SIN-10) and 3-morpholinosydnonimine (SIN-1) were from Sigma (Buchs, Switzerland). S-nitroso-N-acetylpenicillamine (SNAP) was purchased from Sigma. A stock solution of 5 10−2 M was prepared in methanol. For use, the stock solution was diluted in methanol and 5% glucose and samples were prepared each containing equal amounts of methanol. Similarly, the same quantity of methanol was added as vehicle to control experiments. Endothelin-1 (ET-1) was also from Sigma. Stock solutions of 10−4 M were prepared in distilled water and stored in tubes coated with silicone at −20°C. Working solutions were prepared in tubes coated with silicone and were handled on ice. A commercial glyceryl trinitrate (GTN) solution, containing 1 mg mL−1 (corresponding to 4.4 10−3 M) GTN in 5% glucose was from Schwarz Pharma AG (Perlinganit®, Liestal, Switzerland).
Preparation of blood samples
Blood was drawn from healthy adult volunteers into K3-EDTA containing tubes (S-Monovette®, Sarstedt AG, Sevelen, Switzerland). A second set of experiments was done using blood anticoagulated with lithium-heparin (Vacutainer Systems®, Becton Dickinson, Meylan Cedex, France). The haematocrit was determined using an electronic particle counter (Coulter JS, IL Instrumentation Laboratory AG, Schlieren, Switzerland). After centrifugation at 1000×g for 5 min, a calculated volume of plasma was removed to obtain a haematocrit of 50%. Blood from one donor was used for two control experiments (addition of vehicle only) and four experiments with addition of one drug at four different concentrations. Drugs or corresponding vehicles were added to yield a final haematocrit of 45%. After incubation for 60 min in a water bath at 37°C (to allow equilibration of simulated body temperature, decay of nitrovasodilators and possible enzymatic and non-enzymatic reactions), blood viscosity and methaemoglobin were determined and RBCs fixed in phosphate-buffered saline (NaCl 122 mM, NaK2PO4 30 mM, glucose 2 g L−1, pH 7.4, mOsm kg−1 300) containing 1% glutaraldehyde for morphological analysis.
Blood viscosity
Blood viscosity at haematocrit of 45% was measured in a Couette viscometer (Contraves LS 30, Mettler-Toledo, Greifensee, Switzerland) at 37°C at two different shear rates of 94.5 and 0.1 s−1, respectively.
Methaemoglobin
Methaemoglobin was determined by multiwavelength photometrical analysis using an ABL 625 System (Radiometer A/S, Copenhagen NV, Denmark).
RBC morphology
RBC morphology was assessed on fixed cells by light microscopy according to the slightly modified nomenclature of Bessis (Bessis, 1972): a discocyte obtained a score of zero; echinocytic transformation was classified as: echinocyte I (score: +1), irregularly contoured discocyte with up to five protrusions; echinocyte II (score: +2), flat cell with multiple spicules; echinocyte III (score: +3), ovoid or spherical erythrocyte with multiple spicules; spheroechinocyte (score: +4), a sphere with multiple short and thin spicules. Stomatocytic transformation was classified as: stomatocyte I (score: −1), cup-shaped erythrocyte (convex-concave erythrocyte); stomatocyte II (score: −2), RBC with a pronounced, often longitudinal concavity; stomatocyte III (score: −3), RBC with more than one concavity; and spherostomatocyte (score: −4), spherical RBC with an irregular area of pits.
For each specimen, 200 RBCs were classified and the morphological index calculated as Σ scores/200 (Ferrell & Huestis, 1984).
Electron microscopy
Fixation was carried out at 20°C. RBCs were primarily fixed in 1% glutaraldehyde in phosphate buffer, pH 7.4, for at least 24 h. The blood was then settled onto Millipore filters and rinsed twice for 5 min each in 0.1 M PIPES pH 7.4, before postfixation in 0.5% osmium tetroxide in 0.1 M PIPES pH 6.8 for 60 min. Following this, the blood was rinsed three times for 5 min each in double distilled water and stained with 2% aqueous uranyl acetate for 60 min. Samples were taken through an ethanol series – 50, 70, 96 and 100% for 5 min respectively. This was followed by 1 : 3, 1 : 1 and 3 : 1 fluorisol:ethanol mixtures for 5 min each (fluorisol -1,1,2-trichloro-1,2,2-trifluro ethane). Finally the samples were placed in 100% fluorisol for 5 min before critical point drying with a Polaron E3000 critical point drier (TAAB, England). Samples were then mounted onto stubs and coated at a low sputtering rate of 0.1 nm s−1 with 8 nm of gold/palladium 80/20 (as measured with a quartz thin film monitor) in a Baltec MED 020 unit (Balzers, Liechtenstein). Scanning electron microscopy examination of the specimens was performed with a Hitachi (Tokyo, Japan) S-4100 field emission SEM fitted with a Autrata yttrium aluminium garnet (YAG) backscattered electron (BSE) detector (Prophysics, Uster, Switzerland) and a Quartz PCI image acquisition system (Quartz Imaging, Vancouver, Canada). The microscope was operated in backscattered electron detection mode at high emission currents (Richards & Gwynn, 1995), which enables low-energy imaging of the sample to produce topographical information.
Statistical analysis
Results are presented as means±s.d. One-way analysis of variance (ANOVA) or, where appropriate, ANOVA for repeated measures with Tukey-Kramer's Multiple Comparisons Test were performed using InStat version 3.00 (GraphPad, San Diego, CA, U.S.A). P<0.05 was considered significant.
Results
Sodium-nitroprusside (SNP)
As shown in Figure 1A, incubation of EDTA-anticoagulated whole blood with SNP for 1 h resulted in a dose-dependent methaemoglobin formation. However, high doses (10−2 M) were required for a significant effect. Whereas low concentrations of SNP failed to alter RBC shape, a marked and dose-dependent induction of echinocytosis was observed at higher doses, which is illustrated in Figure 2. The morphological index, therefore, showed a dose-dependent increase (Figure 1B). Concomitantly, this echinocytic transformation was accompanied by a significant increase of whole blood viscosity at high (Figure 1C) and low (Figure 1D) shear rate. Further experiments revealed that the effect of SNP on RBC shape was instantaneous, and reversible after washing cells three times with physiologic saline (data not shown).
Figure 1.
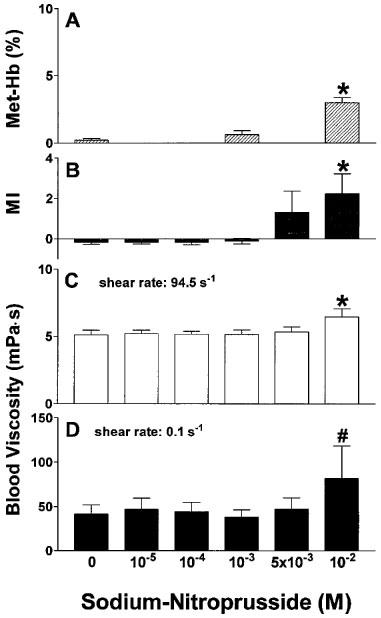
Effects of sodium-nitroprusside on methaemoglobin concentration (A), RBC morphological index (B) and whole blood viscosity at high (C) and low (D) shear rate after incubation for 60 min at 37°C. n=4–9. *P<0.001 compared to control; #P<0.01 compared to control.
Figure 2.
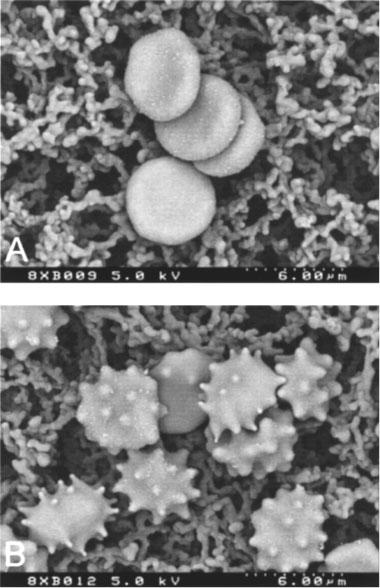
Scanning electron micrographs of control RBCs (A) and RBCs incubated with 10−2 M sodium-nitroprusside (B).
Molsidomine and SIN-1 in EDTA-anticoagulated blood
As shown in Figure 3A, RBC shape remained unaffected upon treatment with molsidomine in concentrations of 10−6–10−3 M. Similarly, molsidomine failed to influence the viscosity of whole blood anticoagulated with EDTA at high and low shear rates (Figure 3B,C), and methaemoglobin levels did not increase (data not shown). In contrast, SIN-1 dose-dependently increased methaemoglobin levels (Figure 4A) with a threshold concentration of 10−4 M for significance. Although RBC shape was not affected by SIN-1 at any concentration examined (morphological index, Figure 4B), SIN-1 at 10−3 M induced a slight but statistically significant decrease of high shear viscosity (Figure 4C) when compared to corresponding controls, but had no effect on low shear viscosity (Figure 4D).
Figure 3.
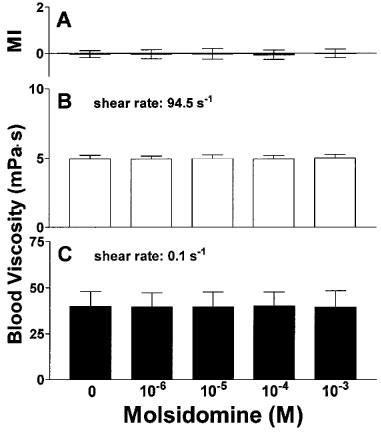
Influence of molsidomine on RBC morphological index (A) and whole blood viscosity at high (B) and low (C) shear rate. n=7.
Figure 4.
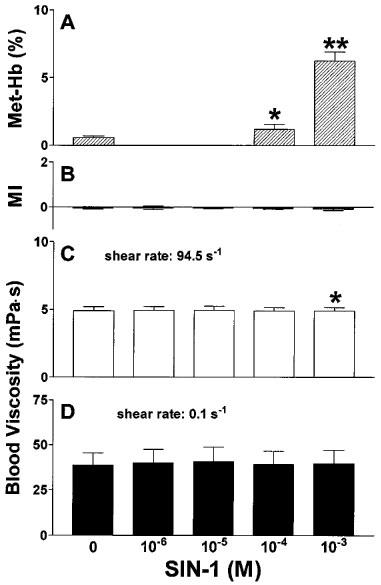
Methaemoglobin formation in whole blood after incubation with the indicated concentrations of SIN-1 for 60 min at 37°C (A), influence of SIN-1 on the morphological index (B) and whole blood viscosity at high (C) and low (D) shear rate. n=6–8. *P<0.05 compared to control. **P<0.001 compared to control and 10−4 M.
Glyceryl trinitrate (GTN)
GTN in EDTA-anticoagulated blood dose-dependently increased methaemoglobin levels with a threshold concentration of 0.1 mg mL−1 for a significant effect (Figure 5A), but failed to influence RBC shape (Figure 5B) and whole blood viscosity (Figure 5C,D) at any concentration examined.
Figure 5.
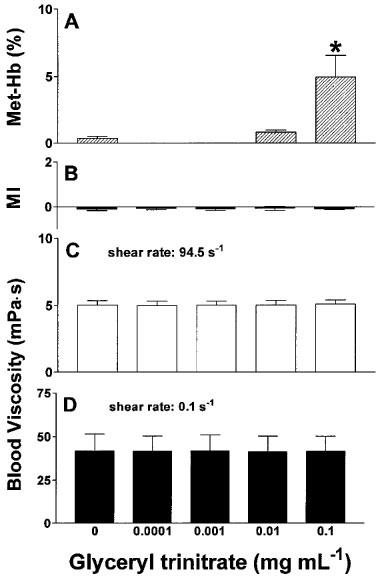
Effects of glyceryl trinitrate on methaemoglobin concentration (A), RBC morphological index (B) and whole blood viscosity at high (C) and low (D) shear rate after incubation for 60 min at 37°C. n=7. *P<0.001 compared to control and 0.01 mg mL−1.
Endothelin-1 (ET-1)
Treatment of EDTA-anticoagulated blood with ET-1 in concentrations up to 10−6 M did not influence RBC shape (Figure 6A) and left whole blood viscosity at high and low shear rates unaffected (Figure 6B,C).
Figure 6.
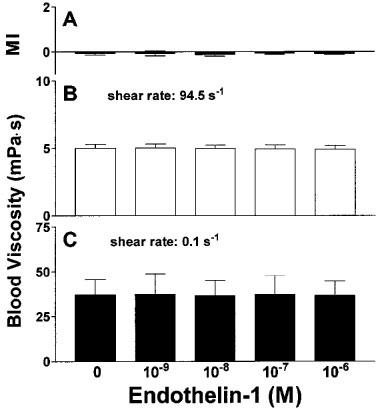
Influence of ET-1 on RBC morphological index (A) and whole blood viscosity at high (B) and low (C) shear rate. n=4–5.
Experiments using blood anticoagulated with heparin
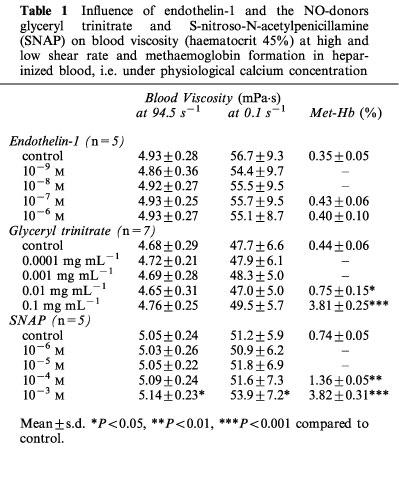
Since the influence of ET-1 on RBC filterability depends on the extracellular calcium concentration (Oonishi et al., 1997; 1998), we performed additional experiments using lithium-heparin instead of the Ca2+-chelating agent EDTA as an anticoagulant. As shown in Table 1, ET-1 in concentrations up to 10−6 M in the presence of physiological Ca2+-concentration did not affect whole blood viscosity at high or low shear rate. As a negative control for experiments with NO donors, methaemoglobin levels were also measured and were found to remain stable as expected. In contrast to ET-1, GTN dose-dependently increased methaemoglobin levels with a concentration of 0.01 mg mL−1 for a significant effect, but also failed to change whole blood viscosity under physiological Ca2+-concentrations (Table 1). In these experiments with heparinized blood the spontaneous NO donor S-nitroso-N-acetylpenicillamine (SNAP) was also tested. As shown in Table 1, SNAP slightly increased both high and low shear viscosity. However, a high concentration of 10−3 M was required for a statistically significant effect. In contrast, a marked and dose-dependent methaemoglobin formation could be observed. The morphological index remained unchanged, i.e., incubation of heparin-anticoagulated blood with SNAP did not influence RBC shape (−0.01±0.04 for control vs −0.04±0.02 at 10−3 M; n=5; P=0.16).
Table 1.
Influence of endothelin-1 and the NO-donors glyceryl trinitrate and S-nitroso-N-acetylpenicillamine (SNAP) on blood viscosity (haematocrit 45%) at high and low shear rate and methaemoglobin formation in heparinized blood, i.e. under physiological calcium concentration
Discussion
The endogenous L-arginine/NO pathway can be replaced pharmacologically by the administration of nitrovasodilators. The way these compounds are metabolized to the vasoactive mediator differs significantly depending on the specific chemistry of the drug (Harrison & Bates, 1993). SNP undergoes a non-enzymatic one-electron reduction resulting in the release of NO and cyanide. The spontaneous degradation in the presence of light does not seem to be of physiological importance (Harrison & Bates, 1993). The pharmacologically inactive prodrug molsidomine is deacetylated in the liver resulting in the vasoactive metabolite SIN-1 (Meinertz et al., 1986), which spontaneously liberates NO (Feelisch, et al., 1989). In the vasculature in vivo, GTN undergoes enzymatic biotransformation mainly through glutathione S-transferases and a NADPH-cytochrome P450 reductase to release the active compound NO (Bennett et al., 1994). In whole blood in vitro, RBCs (Jozwik et al., 1997) as well as platelets (Weber et al., 1996) are involved in GTN metabolism. Furthermore, a haemoglobin mediated biotransformation of GTN has been described (Bennett et al., 1986). In contrast to the other nitrovasodilators of this study, the nitrosothiol derivative SNAP is not used therapeutically. It is thought to spontaneously liberate NO, although denitrosation and subsequent release of NO may be catalyzed by specific membrane-bound enzymes (Kowaluk & Fung, 1990).
In accordance with these pharmacological properties of the drugs used, incubation of whole blood with SNP, SIN-1, GTN and SNAP, but not molsidomine, resulted in significantly increased amounts of methaemoglobin indicating relevant generation of NO or related molecules in vitro and penetration of the molecules into the RBC. An avid binding by RBCs of NO released from endothelial cells into the vessel lumen also occurs in vivo (Rimar & Gillis, 1993). The smaller amount of methaemoglobin measured with SNP than with GTN or SIN-1 is due to the fact that incubation of whole blood with SNP also produces cyanomethaemoglobin, which interferes with multiwavelength photometric analysis of haemoglobin (Zijlstra & Buursma, 1993).
Of those nitrovasodilators tested, only SNP affected the RBC shape. A reversible echinocytic shape transformation was observed. This resulted in an increased blood viscosity at high and low shear rates, which is due to an increased intercellular interaction and mechanical hindrance of spiculated cells (Reinhart & Chien, 1986). These changes were seen at concentrations well above those measured during therapeutic application of SNP in vivo, which are in the order of 10−6 M (Vesey et al., 1990), It remains to be seen, therefore, if these changes can occur in vivo, which could impair microcirculatory blood flow. An echinocytic shape transformation indicates an expansion of the outer hemileaflet of the lipid bilayer relative to the inner hemileaflet, most probably by a preferential intercalation of a drug into the outer hemileaflet (Sheetz & Singer, 1976). Since this effect was not seen with other NO-donors, it must be assumed that not NO, but some degradation product of SNP is responsible, probably cyanide. It has been previously shown that treatment of rat erythrocytes with methyl isocyanate indeed induces marked echinocytosis (Agrawal et al., 1990).
In contrast to the marked effect of SNP, the compounds SIN-1 and SNAP at highest concentrations only marginally changed blood viscosity. Whereas SIN-1 slightly decreased whole blood viscosity at a high shear rate (−1.3% as compared to control; Figure 4), SNAP slightly increased both high shear (+1.8%) and low shear (+5.3%) viscosity (Table 1). These small changes at very high concentrations are probably of negligible clinical relevance.
The drug concentrations tested in this study range from pharmacological to highly suprapharmacological concentrations. Pharmacological are SIN-1 concentrations of approximately 6 10−8 M after a small oral dose of 4 mg molsidomine (Rosenkranz et al., 1996) or plasma GTN levels of 1.6 to 5.2 ng mL−1 (Noonan & Benet, 1985) during a GTN infusion rate of 2.3 mg h−1 (up to 10 mg h−1 are clinically used). These concentrations are similar to the lowest concentrations in our study.
Only a few studies have investigated the effects of NO on RBC shape and rheology with controversial results. While Maeda et al. (1987) found an echinocytic transformation after NO-exposure, echinocytosis has also been observed upon removal of NO (Caramelo et al., 1994). Hogman et al. (1994) reported on an unchanged blood rheology after inhaled NO, while others found an enhanced or reduced RBC deformability depending on the NO level (Korbut & Gryglewski, 1993). Transdermally applied GTN increased blood viscosity in diabetic patients but decreased it in controls (Giugliano et al., 1995). In the light of these contradictory data our observation that NO has no influence on blood viscosity is probably reasonable.
Recently, the presence of ET-1 receptors on human RBCs has been demonstrated (Rivera & Brugnara, 1998; Oonishi et al., 1998). ET-1 induced an improvement of RBC filterability under mechanical stress through activation of protein kinase C (Oonishi et al., 1997; 1998). RBC filtration is determined by other factors than blood viscosity is, primarily the surface area/volume ratio of RBCs (Reinhart & Chien, 1986) and is, therefore, not comparable with blood viscosity measurements. Our data indicate that whole blood viscosity, probably the rheological parameter most relevant for blood flow in vivo, is not affected by ET-1, neither in the presence nor absence of extracellular calcium.
We conclude that the most important modulators of vascular tone and hence regulators of blood flow, namely the vasodilator NO and the vasoconstrictor ET-1, do not affect the RBC shape and blood viscosity to a significant degree in vitro. Of the nitrovasodilators tested, only SNP causes echinocytes and elevates blood viscosity at high concentrations. These effects may be mediated by the release of cyanide rather than NO.
Acknowledgments
We are indebted to the numerous voluntary blood donors as well as to the staff of the haematological laboratory at the Kantonsspital Chur for haematocrit determinations. We also thank Dr R.G. Richards, AO ASIF Research Institute, Davos, Switzerland, for consultations regarding the scanning electron microscopy and John Contesse for technical support. This work was supported by a grant from the Bonizzi-Theler Foundation.
Abbreviations
- cNOS
constitutive nitric oxide synthase
- ET-1
endothelin-1
- GTN
glyceryl trinitrate
- NO
nitric oxide
- SIN-1
3-morpholinosydnonimine
- SNAP
S-nitroso-N-acetylpenicillamine
- SNP
sodium-nitroprusside
References
- ABRAMS J. Beneficial actions of nitrates in cardiovascular disease. Am. J. Cardiol. 1996;77:31C–37C. doi: 10.1016/s0002-9149(96)00186-5. [DOI] [PubMed] [Google Scholar]
- AGRAWAL D., GUPTA G.S., SHUKLA J.S., DUTTA K.K., RAY P.K. Effect of methyl isocyanate (MIC) on rat erythrocytes. Arch. Toxicol. 1990;64:332–335. doi: 10.1007/BF01972995. [DOI] [PubMed] [Google Scholar]
- ARCHER S. Measurement of nitric oxide in biological models. FASEB J. 1993;7:349–360. doi: 10.1096/fasebj.7.2.8440411. [DOI] [PubMed] [Google Scholar]
- BENNETT B.M., KOBUS S.M., BRIEN J.F., NAKATSU K., MARKS G.S. Requirement for reduced, unliganded hemoprotein for the hemoglobin- and myoglobin-mediated biotransformation of glyceryl trinitrate. J. Pharmacol. Exp. Ther. 1986;237:629–635. [PubMed] [Google Scholar]
- BENNETT B.M., MCDONALD B.J., NIGAM R., SIMON W.C. Biotransformation of organic nitrates and vascular smooth muscle cell function. Trends Pharmacol. Sci. 1994;15:245–249. doi: 10.1016/0165-6147(94)90319-0. [DOI] [PubMed] [Google Scholar]
- BESSIS M. Red cell shapes: an illustrated classification and its rationales. Nouv. Rev. Fr. Hematol. 1972;12:721–746. [PubMed] [Google Scholar]
- CARAMELO C., RIESCO A., OUTEIRINO J., MILLAS I., BLUM G., MONZU B., CASADO V., SANCHEZ L., MOSQUERA J.R., CASADO S., LOPEZ FARRE A. Effects of nitric oxide on red blood cells: changes in erythrocyte resistance to hypotonic hemolysis and potassium efflux by experimental maneuvers that decrease nitric oxide. Biochem. Biophys. Res. Commun. 1994;199:447–454. doi: 10.1006/bbrc.1994.1249. [DOI] [PubMed] [Google Scholar]
- CHIEN S.Biophysical behaviour or red cells in suspension The Red Blood Cell 1975Academic Press, New York; 1031–1133.In: MacN. Surgenor, D ed [Google Scholar]
- COOKE J.P., DZAU V.J. Nitric oxide synthase: role in the genesis of vascular disease. Annu. Rev. Med. 1997;48:489–509. doi: 10.1146/annurev.med.48.1.489. [DOI] [PubMed] [Google Scholar]
- FEELISCH M., OSTROWSKI J., NOACK E. On the mechanism of NO release from sydnonimines. J. Cardiovasc. Pharmacol. 1989;14 Suppl 11:S13–S22. [PubMed] [Google Scholar]
- FERRELL J.E., HUESTIS W.H. Phosphoinositide metabolism and the morphology of human erythrocyte. J. Cell Biol. 1984;98:1992–1998. doi: 10.1083/jcb.98.6.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GIUGLIANO D., MARFELLA R., ACAMPORA R., GIUNTA R., COPPOLA L., D'ONOFRIO F. Effects of perindopril and carvedilol on endothelium-dependent vascular functions in patients with diabetes and hypertension. Diabetes Care. 1998;21:631–636. doi: 10.2337/diacare.21.4.631. [DOI] [PubMed] [Google Scholar]
- GIUGLIANO D., MARFELLA R., VERRAZZO G., ACAMPORA R., COPPOLA L., COZZOLINO D., D'ONOFRIO F. The vascular effects of L-Arginine in humans. The role of endogenous insulin. J. Clin. Invest. 1997;99:433–438. doi: 10.1172/JCI119177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GIUGLIANO D., MARFELLA R., VERRAZZO G., ACAMPORA R., DONZELLA C., QUATRARO A., COPPOLA L., D'ONOFRIO F. Abnormal rheologic effects of glyceryl trinitrate in patients with non-insulin-dependent diabetes mellitus and reversal by antioxidants. Ann. Intern Med. 1995;123:338–343. doi: 10.7326/0003-4819-123-5-199509010-00003. [DOI] [PubMed] [Google Scholar]
- HARRISON D.G., BATES J.N. The nitrovasodilators. New ideas about old drugs. Circulation. 1993;87:1461–1467. doi: 10.1161/01.cir.87.5.1461. [DOI] [PubMed] [Google Scholar]
- HOGMAN M., FROSTELL C., ARNBERG H., SANDHAGEN B., HEDENSTIERNA G. Prolonged bleeding time during nitric oxide inhalation in the rabbit. Acta Physiol. Scand. 1994;151:125–129. doi: 10.1111/j.1748-1716.1994.tb09728.x. [DOI] [PubMed] [Google Scholar]
- JOZWIK M., JOZWIK M., JOZWIK M., SZCZYPKA M., GAJEWSKA J., LASKOWSKA-KLITA T. Antioxidant defence of red blood cells and plasma in stored human blood. Clin. Chim. Acta. 1997;267:129–142. doi: 10.1016/s0009-8981(97)00148-4. [DOI] [PubMed] [Google Scholar]
- KORBUT R., GRYGLEWSKI R.J. Nitric oxide from polymorphonuclear leukocytes modulates red blood cell deformability in vitro. Eur. J. Pharmacol. 1993;234:17–22. doi: 10.1016/0014-2999(93)90700-r. [DOI] [PubMed] [Google Scholar]
- KOWALUK E.A., FUNG H.L. Spontaneous liberation of nitric oxide cannot account for in vitro vascular relaxation by S-nitrosothiols. J. Pharmacol. Exp. Ther. 1990;255:1256–1264. [PubMed] [Google Scholar]
- LUSCHER T.F. Endothelium-derived nitric oxide: the endogenous nitrovasodilator in the human cardiovascular system. Eur. Heart J. 1991;12 Suppl E:2–11. doi: 10.1093/eurheartj/12.suppl_e.2. [DOI] [PubMed] [Google Scholar]
- MAEDA N., IMAIZUMI K., KON K., SHIGA T. A kinetic study on functional impairment of nitric oxide-exposed rat erythrocytes. Environ. Health Perspect. 1987;73:171–177. doi: 10.1289/ehp.8773171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MALEK A.M., IZUMO S. Control of endothelial cell gene expression by flow. J. Biomech. 1995;28:1515–1528. doi: 10.1016/0021-9290(95)00099-2. [DOI] [PubMed] [Google Scholar]
- MARLETTA M.A. Nitric oxide synthase: aspects concerning structure and catalysis. Cell. 1994;78:927–930. doi: 10.1016/0092-8674(94)90268-2. [DOI] [PubMed] [Google Scholar]
- MCHUGH J., CHEEK D.J. Nitric oxide and regulation of vascular tone: pharmacological and physiological considerations. Am. J. Crit. Care. 1998;7:131–140. [PubMed] [Google Scholar]
- MEINERTZ T., BRANDSTAETTER A., TRENK D., JAEHNCHEN E., OSTROWSKI J.Relationship between pharmacokinetics and pharmacodynamics of molsidomine and its metabolites in man Ischemic Heart Disease and Heart Failure 1986Urban & Schwarzenberg; 24–27.In: Bing, R.J. & Strauch, M. (eds) [DOI] [PubMed] [Google Scholar]
- MOREAU R., LEBREC D. Nitrovasodilators and portal hypertension. J. Hepatol. 1990;10:263–267. doi: 10.1016/0168-8278(90)90129-f. [DOI] [PubMed] [Google Scholar]
- NATHAN C., XIE Q.W. Nitric oxide synthase: roles, tolls, and controls. Cell. 1994;78:715–718. doi: 10.1016/0092-8674(94)90266-6. [DOI] [PubMed] [Google Scholar]
- NOONAN P.K., BENET L.Z. Incomplete and delayed bioavailability of sublingual nitroglycerin. Am. J. Cardiol. 1985;55:184–187. doi: 10.1016/0002-9149(85)90325-x. [DOI] [PubMed] [Google Scholar]
- OONISHI T., SAKASHITA K., SUEMATSU N., UYESAKA N.Endothelin-1 improves impaired red cell filterability under mechanical stress Blood 19979010 Suppl 17bAbstract 2713 [Google Scholar]
- OONISHI T., SAKASHITA K., SUEMATSU N., UYESAKA N.Endothelin-1 improves the impaired filterability of red blood cells through activation of protein kinase C Blood 19989210 Suppl 16aAbstract 13 [DOI] [PubMed] [Google Scholar]
- REINHART W.H., CHIEN S. Red cell rheology in stomatocyte-echinocyte transformation: roles of cell geometry and cell shape. Blood. 1986;67:1110–1118. [PubMed] [Google Scholar]
- RICHARDS R.G., AP GWYNN I. Backscattered electron imaging of the undersurface of resin-embedded cells by field-emission scanning electron microscopy. J. Microsc. 1995;177:43–52. doi: 10.1111/j.1365-2818.1995.tb03532.x. [DOI] [PubMed] [Google Scholar]
- RIMAR S., GILLIS C.N. Selective pulmonary vasodilation by inhaled nitric oxide is due to hemoglobin inactivation. Circulation. 1993;88:2884–2887. doi: 10.1161/01.cir.88.6.2884. [DOI] [PubMed] [Google Scholar]
- RIVERA A., BRUGNARA C.Normal and sickle erythrocytes express an endothelin-1 receptor, which is functionally coupled to the gardo's channel: a new potential pathway for red blood cell dehydration Blood 19989210 Suppl 113aAbstract 42 [Google Scholar]
- ROBERTS J.D., JR, FINEMAN J.R., MORIN F.C., 3RD, SHAUL P.W., RIMAR S., SCHREIBER M.D., POLIN R.A., ZWASS M.S., ZAYEK M.M., GROSS I., HEYMANN M.A., ZAPOL W.M. Inhaled nitric oxide and persistent pulmonary hypertension of the newborn. The Inhaled Nitric Oxide Study Group. N. Engl. J. Med. 1997;336:605–610. doi: 10.1056/NEJM199702273360902. [DOI] [PubMed] [Google Scholar]
- ROSENDORFF C. Endothelin, vascular hypertrophy, and hypertension. Cardiovasc. Drugs Ther. 1997;10:795–802. doi: 10.1007/BF00053038. [DOI] [PubMed] [Google Scholar]
- ROSENKRANZ B., WINKELMANN B.R., PARNHAM M.J. Clinical pharmacokinetics of molsidomine. Clin. Pharmacokinet. 1996;30:372–384. doi: 10.2165/00003088-199630050-00004. [DOI] [PubMed] [Google Scholar]
- SHEETZ M.P., SINGER S.J. Equilibrium and kinetic effects of drugs on the shape of human erythrocytes. J. Cell Biol. 1976;70:247–251. doi: 10.1083/jcb.70.1.247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VANHOUTTE P.M., MOMBOULI J.-V. Vascular endothelium: vasoactive mediators. Prog. Cadiovasc. Dis. 1996;39:229–238. doi: 10.1016/s0033-0620(96)80003-x. [DOI] [PubMed] [Google Scholar]
- VESEY C.J., SWEENEY B., COLE P.V. Decay of nitroprusside. II: In vivo. Br. J. Anaesth. 1990;64:704–709. doi: 10.1093/bja/64.6.704. [DOI] [PubMed] [Google Scholar]
- WEBER A.A., NEUHAUS T., SEUL C., DUSING R., SCHROR K., SACHINIDIS A., VETTER H. Biotransformation of glyceryl trinitrate by blood platelets as compared to vascular smooth muscle cells. Eur. J. Pharmacol. 1996;309:209–213. doi: 10.1016/0014-2999(96)00338-x. [DOI] [PubMed] [Google Scholar]
- WENNMALM A., BENTHIN G., PETERSSON A.S. Dependence of the metabolism of nitric oxide (NO) in healthy human whole blood on the oxygenation of its red cell haemoglobin. Br. J. Pharmacol. 1992;106:507–508. doi: 10.1111/j.1476-5381.1992.tb14365.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ZIEGLER T., BOUZOURENE K., HARRISON V.J., BRUNNER H.R., HAYOZ D. Influence of oscillatory and unidirectional flow environments on the expression of endothelin and nitric oxide synthase in cultured endothelial cells. Arterioscler. Thromb. Vasc. Biol. 1998;18:686–692. doi: 10.1161/01.atv.18.5.686. [DOI] [PubMed] [Google Scholar]
- ZIJLSTRA W.G., BUURSMA A. Rapid multicomponent analysis of hemoglobin derivatives for controlled antidotal use of methemoglobin-forming agents in cyanide poisoning. Clin. Chem. 1993;39:1685–1689. [PubMed] [Google Scholar]


