Abstract
The effects of the selective β2 adrenoceptor agonists salbutamol, terbutaline and salmeterol and the non-selective β adrenoceptor agonist isoprenaline on [3H]-cyclic AMP formation and cyclic AMP response element (CRE) driven luciferase expression, assessed using the construct p6CRE/luc, were studied in primary cultures of human airway smooth muscle (HASM) cells.
Optimal transfection conditions for transient expression of pGL3 Control were 4 μg DNA/well71 in a 6 well plate and 1.8 μl Transfectam/μg DNA. Expression was maximal at 48–72 h.
Salbutamol (maximum response 19%, EC50 0.6 μM), terbutaline (maximum response 38%, EC50 2.3 μM) and salmeterol (maximum response 18%, EC50 0.0012 μM) were all partial agonists for cyclic AMP formation compared with isoprenaline (EC50 0.08 μM). However, all of the β2 adrenoceptor agonists produced increases in CRE-driven luciferase activity, in cultured HASM transfected with the vector p6CRE/luc, which were equivalent or greater (salmeterol) than those seen with isoprenaline.
Both salbutamol and salmeterol were more potent at increasing luciferase expression than in elevating cyclic AMP levels in these cells. The potency ratios (EC50 (cyclic AMP)/EC50 (LUC)) for the agents studied were isoprenaline: 0.2 fold, terbutaline: 3 fold, salbutamol: 24 fold, salmeterol: 38 fold.
These data suggest that important quantitative differences exist in the ability of β2 adrenoceptor agonists to increase whole cell cyclic AMP levels in airway smooth muscle and to drive gene expression via a CRE-driven mechanism.
Keywords: Airway smooth muscle, cyclic AMP, cyclic AMP response element, isoprenaline, salmeterol, terbutaline, salbutamol
Introduction
β2 adrenoceptor agonists are airway smooth muscle relaxant agents and form the main stay bronchodilator drugs used in the treatment of asthma. The majority of the effects of these agents are due to changes in intracellular cyclic AMP. Elevation of cyclic AMP levels in airway myocytes produces membrane hyperpolarization, activation of calcium activated K+ channels, inhibition of histamine-induced inositol phospholipid hydrolysis, alteration of sensitivity of the contractile apparatus to calcium and sequestration of calcium within the cell (reviewed in Torphy & Hall, 1994). Relaxation of airway smooth muscle is likely to be due to a combination of the above and other effects.
In addition to inducing airway smooth muscle relaxation, elevation in intracellular cyclic AMP can potentially alter gene expression in airway smooth muscle and other cells. Cyclic AMP modulates gene expression by protein kinase A mediated activation of cyclic AMP response element binding protein (CREB) via a specific phosphorylation on serine 133 (Gonzalez & Montminy, 1989). This enables CREB to activate gene transcription through cyclic AMP response elements (CREs). Many genes contain within their regulatory regions CREs which generally increase the rate of transcription of the downstream gene. For example, the β2 adrenoceptor gene contains a CRE approximately 270 base pairs upstream from the start codon for the gene. β2 adrenoceptor agonists can therefore potentially induce gene expression of the β2 adrenoceptor gene, or other genes containing CREs within their regulatory regions. However in addition to CREs most genes contain a large number of other regulatory elements (e.g. for the human β2 adrenoceptor gene AP2, glucocorticoid response element (GRE), Sp1, NF-IL6 and other recognition sites (Scott et al., 1999b)) and hence studying the direct effect of agents upon gene expression can provide data which are difficult to interpret. For example, stimulation of mammalian cell lines constitutively expressing the β2 adrenoceptor with agents able to elevate cyclic AMP levels has variably been shown to increase (Collins et al., 1989; 1990; Jones et al., 1998) and, at differing time points, decrease (Collins et al., 1989; Hosoda et al., 1995; McGraw et al., 1997) the rate of transcription of the β2 adrenoceptor gene itself or reporter constructs regulated by the β2 adrenoceptor promoter.
In order to define the effects of β2 adrenoceptor agonists on both cyclic AMP levels and on gene expression within human airway smooth muscle (HASM) cells we have utilized a transiently expressed CRE driven luciferase construct (p6CRE/luc) which has the advantage that reporter gene expression is essentially only induced by changes in intracellular cyclic AMP. In this paper we report the effects of a range of β2 adrenoceptor agonists on intracellular cyclic AMP levels in primary cultures of HASM cells and also the effects of these agents on CRE driven luciferase expression in cells transiently transfected with p6CRE/luc. These studies demonstrate that agents which act as partial agonists in terms of cyclic AMP production (e.g. salbutamol and salmeterol) are able to maximally drive gene expression, implying that the relationship between the ability of agents to elevate cyclic AMP levels and to drive CRE driven gene expression is quantitatively different. Preliminary accounts of this work have been communicated to the British Pharmacological Society (Scott et al., 1997; 1999a).
Methods
Primary culture of HASM cells
Primary cultures of HASM cells were prepared from explants of trachealis muscle obtained from individuals without respiratory disease, within 12 h of death, as previously described (Daykin et al., 1993). We and others have extensively characterized the phenotype of these cells which retain many properties of airway smooth muscle ex vivo (Hall & Kotlikoff, 1995). Tissue was taken from the trachea immediately above the level of the carina. A strip of trachealis about 2×1 cm was dissected clear of surrounding tissue and transported to the laboratory in DMEM containing penicillin G (200 U ml−1), streptomycin (200 μg−1) and amphotericin B (0.5 μg−1). The tissue was washed several times in 10 mls of DMEM containing antibiotics and antifungal agents at double the above concentrations. Overlying mucosa was dissected free from the airway smooth muscle under sterile conditions. Small (0.2×0.2 cm) explants of airway muscle were then excised and about 15 explants placed in each 60 mm Petri dish. After allowing explants to adhere, DMEM containing antibiotics, amphotericin B, 10% foetal calf serum (FCS) and glutamine (2 mM) were added to just cover explants. The medium was changed twice each day for the first 3 days to reduce the incidence of fungal infection. Smooth muscle cell growth usually occurred about 7–10 days after placing explants in culture. When growth commenced, cultures were supplemented with fresh DMEM containing 10% FCS and 2 mM glutamine about every 3 days. When cells were approaching confluence in some parts of the vessel, explants were removed and 24 h later cells were harvested by trypsinization. Cells from an individual dish or flask were then plated in one 75 cm2 flask and grown to confluence. When confluent, each flask was split into four new flasks. Antibiotics and amphotericin were not added to the medium used for all subsequent passages after this stage (passage 2). Cells for experiments were seeded in 6 or 24 well plates unless otherwise stated. All primary cell cultures from each donor were examined using anti-smooth muscle alpha actin antibody (1 : 100 dilution) (Sigma) to confirm the presence of smooth muscle type cells using standard immunocytochemical techniques. Primary cell cultures used for the experiments described in this paper showed >95% of cells staining for smooth muscle actin. Cells from preparations from four individuals were used.
Measurement of cell cyclic AMP content
Cyclic AMP responses to agonists were measured in cultured HASM cells using previously described methods (Hall et al., 1992). In brief, cells were seeded into 24 well plates and when confluent were incubated with [3H]-adenine (2 μCi well−1 in 1 ml of DMEM) for 3 h at 37°C in an incubator constantly gassed with air/5% CO2. After this period cells were washed twice with 1 ml of Hanks/HEPES buffer, allowed to rewarm to 37°C, and then agonists were added for the times specified in the text. At the end of incubations, reactions were terminated by the addition of 50 μl of concentrated HCl and samples stored frozen for at least 2 h. Cyclic AMP in samples was then determined by column chromatography. Variations in column recovery were allowed for by spiking samples with a known amount of [14C]-cyclic AMP, and variations in cell number were controlled for by counting an aliquot of the initial sample for [3H]-adenine.
Construction of CRE/luciferase vector
All DNA manipulations were performed by standard methods unless otherwise described (Sambrook et al., 1989). The synthetic 123 base oligonucleotide 5′-CCAGAAGCCTACGTAGGCGTCGACCTCCTTGGC TGACGTCAGTAGAGAGA TC CCA TTGA CGT CATACT GAGA CGTAGAT CTCCATTGACGTCAAGGAGACTCGAGGCTCCATCGCAGTGATCG-3′ containing three copies of the concensus CRE; shown in bold (Montminy et al., 1990; Foulkes & Sassone-Corsi, 1996) and SalI and XhoI restriction sites (underlined), was used as the template in a PCR reaction using flanking primers 5′-CCAGAAGCCTACGTAGGCGTC-3′ and 5′-CGATCAC-TGCGATGGAGCCTC-3′ to amplify a 123 bp fragment containing three copies of the consensus CRE. The PCR product was digested with SalI and XhoI and inserted between the SalI and XhoI sites of pBluescript-KS (Stratagene) to generate pBS/3CRE. Following sequencing the 3CRE element was excised by digestion with SalI and XhoI and cloned into the unique SalI site of the vector pTK/luc, a gift from Dr D.M. Wallace, Glaxo Wellcome Research and Development, containing the firefly luciferase gene downstream of the minimal Herpes Simplex Virus Thymidine Kinase promoter (−110 to +101), to generate p3CRE/luc. This vector was subsequently digested with SalI and a second copy of the 3CRE element was inserted to generate p6CRE/luc. The subcloning was iteratively repeated to result in the generation of the vectors p3CRE/luc, p6CRE/luc, p12CRE/luc and p24CRE/luc. The vector pCMV-luc and pCMV-sPAP were also a gift from Dr D. Wallace and contain the firefly luciferase or secreted placental alkaline phosphatase (sPAP) genes under the transcriptional control of the cytomegalovirus immediate early promoter sequence.
Characterization of luciferase reporter vectors: transfection of CHO cells
Chinese Hamster Ovary (CHO) cells were plated into 6 well multidishes at a density of 300,000 cells per well, in DMEM-F12 (50 : 50), 10% foetal calf serum, 2 mM Glutamine (all reagents from Life Technologies), and incubated overnight at 37°C with 5% CO2 and 95% humidity to reach 50–80% confluency. The following day transfections were performed using the Lipofectamine reagent (Life Technologies). The test vectors pTK/luc, p3CRE/luc, p6CRE/luc, p12CRE/luc and p24CRE/luc were transfected together with the vector pCMV-sPAP to control for transfection efficiency. Briefly, two solutions were prepared. Solution A: for each transfection 20 ng of luciferase test vector together with 200 ng pCMV-sPAP was diluted into 100 μl Optimem serum-free media (Life Technologies). Solution B: for each transfection 10 μl lipofectamine was diluted into 100 μl Optimem. The two solutions were combined and incubated at room temperature for 15 min to allow the formation of DNA-liposome complexes. Following this, 0.8 ml of Optimem was added to the tube containing the DNA complex and this was then layered onto the cells which had been previously rinsed in Optimen. The cells were then incubated for 5 h after which time the transfection solution was removed and replaced with normal growth media. The cells were left for 18 h at 37°C prior to assay.
In order to assay CRE promoter activity, the media was removed from the cells and replaced with fresh media alone or media containing 20 μM forskolin. Cells were left 4 h at 37°C prior to assay for accumulation of luciferase and sPAP.
All media was removed from the cells and heat inactivated by incubation at 65°C for 30 min to inactivate endogenous alkaline phosphatases. sPAP assays were performed as follows; 12.5 μl of media was mixed with 250 μl of reaction mix (5 mM p-nitrophenolphosphate (PNPP) in DEA buffer: 1 M diethanolamine, 0.28 M NaCl, 0.5 mM MgCl2, pH to 9.85; all reagents from Sigma) and left in the dark until a yellow colour developed (time varied according to levels of sPAP). The absorbance of the yellow solution was measured at 405 nm. The amount of sPAP (units) was calculated per sample using the following equation:
 |
Due to the small values of sPAP measured all values are quoted in mUnits.
Transfection of cultured HASM cells
HASM cells grown in 6 or 24 well plates were transfected at 70–80% confluency, which was found in initial experiments (data not shown) to give the highest rates of transfection. Immediately prior to transfection, the medium was aspirated from the cells and replaced with 5 ml of fresh medium (containing 10% FCS). DNA and Tranfectam reagent (dioctadecylamidoglycylspermine) (Promega, U.K.) was briefly vortexed and then incubated at room temperature for 10 min to facilitate the formation of DNA-liposome complexes before being added to the cells. Initial experiments were performed to optimise transfection conditions for cultured HASM cells: we used 4 μg or 1 μg DNA for wells of 6 and 24 well plates respectively and 1.8 μl of 2 mM Transfectam/μg DNA (see Results). DNA was left in contact with cells for the whole transfection period (48 h) which was found to be the optimal time of expression.
Measurement of luciferase activity
Luciferase activity was measured in lysates of cultured HASM cells using a commercially available kit (Promega, U.K.) as described in the product information but with minor modifications. Medium was aspirated from wells and cells washed twice with 1 ml (6 well) or 0.5 ml (24 well) of phosphate buffered saline solution. Three hundred (6 well) or 50 μl (24 well) of lysis buffer was then added to each well and the cells incubated for 10–15 min at room temperature. Any cell debris together with the lysis buffer was then removed from individual wells of each 6 or 24 well plate. Lysates were spun at 13,000 r.p.m. for 30 s to pellet large cell debris. Twenty μl of the supernatant was then assayed for luciferase activity in a Turner Luminometer (model 20e): measurement was made for 30 s after an initial delay of 10 s following substrate addition. Protein concentrations in the cell lysate supernatant from 6 well plates were determined by the method of Bradford (Bradford, 1976). All luciferase activities were then corrected for protein content to normalize for variation in cell number and lysis efficiency between experiments. Protein concentrations were not measured in experiments performed in 24 well plates following initial experiments which demonstrated that the variability in protein content between wells in 24 well plates was less than 10%.
Because of the higher levels of luciferase activity achieved following transfection of CHO cells, luciferase activity was measured using a modified version of the methods described above for HASM cells. Briefly, following washing with phosphate buffered saline (PBS). 250 μl of cell culture lysis reagent (diluted 1 : 5) was placed into each well. This was incubated at room temperature for 15 min after which the cell lysate was removed and placed into a clean tube at 4°C to slow down the decay of the luciferase enzyme. The lysate was assayed by adding 10 μl of sample into an individual well in a white 96 well plate. A standard curve of luciferase (Sigma) was also placed onto each plate to allow calculation of the amount of luciferase present in each sample. Luciferase assay reagent was injected directly into each well (50 μl per well) using the injection mode of the Dynatech ML3000 luminometer and the light emission measured within 2 s of mixing the extract with the assay reagent.
In order to control for variations in transfection efficiency, all values for luciferase activity in the CHO cells were expressed as fg luciferase/mUnits sPAP.
Materials
FCS was obtained from Advanced Protein Products (U.K.). 2,8-[3H]-adenine (26 μCi mmol−1) and 8-[14C]-cyclic AMP (42.4 μCi mmol−1) was purchased from New England Nuclear (Stevenage, U.K.). The firefly luciferase vector pGL3 Control (used as a control), Transfectam Reagent and luciferase assay kits were obtained from Promega U.K. All other chemicals were obtained from the Sigma Chemical Co. (Poole, U.K.) unless stated otherwise in the text. The antibodies used for immunocytochemistry were anti smooth muscle actin (Sigma A2547) and mouse IgG whole molecule (host goat) (Sigma F0257). Plasticware was obtained from Costar (U.K.) Ltd. (High Wycombe, U.K.).
Data analysis and statistics
Statistical analysis of data was performed using paired or unpaired t-tests, analysis of variance and Dunnett's test as appropriate. All values in the text represent mean±s.e.mean of n separate experiments. EC50 values were obtained in individual experiments: the values quoted are mean values obtained from n individual experiments.
Results
Optimizing transfection conditions for primary cultures of HASM cells
An initial series of experiments was performed to optimize transfection conditions for cultured HASM cells. Using the control pGL3 vector (containing the firefly luciferase cDNA driven by SV40 early promoter and enhancer elements) we found consistent levels of expression around 100–1000 fold less than those seen in COS or CHO cells when transfections were performed by cationic lipid mediated transfer using Transfectam reagent (Promega): in general, cells at later passage number tended to show somewhat lower levels of expression than early passage cells. In view of this, comparative experiments were always performed with cells of passage number less than 12. The optimal DNA amount was determined as 4 μg well−1 and the optimum Transfectam : DNA ratio as 1.8 μl : 1 μg when experiments were performed in a 6 well plate. Time course experiments demonstrated that expression was observed from 24–168 h with highest levels generally being between 48–72 h. The optimal DNA amount when experiments were performed in a 24 well plate was 1 μg well−1. Hence, for all further experiments, 4 μg DNA (6 well) or 1 μg (24 well) was used with the optimal ratio of Transfectam : DNA, with cells being harvested at 48 h.
Characterization of CRE/luciferase vector (pCRE/luc)
Because the number and position of transcription factor binding sites such as the CRE element has a dramatic effect on promoter regulation we constructed reporter vectors containing 3, 6, 12 or 24 copies of the consensus cyclic AMP response element upstream of the minimal Herpes Simplex Virus Thymidine Kinase promoter and used these cyclic AMP responsive promoters to regulate the expression of the firefly luciferase reporter gene. The construction of the vector p6CRE/luc containing six copies of the consensus CRE is described in the Methods section. The inducibility of these vectors in response to treatment with the synthetic diterpene forskolin, which interacts directly with members of the adenylyl cyclase family to cause the synthesis of cyclic AMP, was assayed following transient transfection of each of these vectors into CHO cells (Figure 1). A basal level of luciferase activity was obtained with the vector pTK/luc which lacks any cyclic AMP regulatable element. This promoter was not stimulated with forskolin. The basal level of luciferase expression of the p3CRE/luc and p6CRE/luc vectors was not significantly enhanced over that obtained with pTK/luc indicating that this activity is due to the weak transcriptional activity of the Herpes Simplex Virus Thymidine Kinase minimal promoter. Upon treatment with forskolin approximately 3 fold and 6 fold increases in luciferase activity were observed with the vectors p3CRE/luc and p6CRE/luc. Surprisingly, the 12CRE and 24CRE containing promoters had a depressed basal activity and little inducibility presumably as a result of vector instability as a result of the repetitive nature of these promoters or to a squelching effect. For this reason the vector p6CRE/luc was chosen for all subsequent studies.
Figure 1.
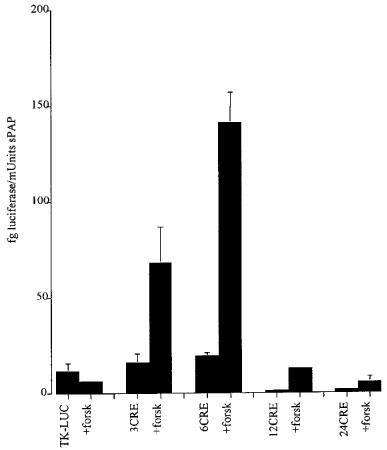
The effect of forskolin (20 μM) (forsk) on luciferase activity in CHO cells transiently transfected with the vectors pTK/luc, p3CRE/luc, p6CRE/luc, p12CRE/luc and p24CRE/luc (see Methods for details). The vectors were cotransfected with the control vector pCMV/sPAP. Data are expressed as fg luciferase/mUnits sPAP. Each bar represents the mean±s.e.mean and all transfections were performed in triplicate. Where error bars are not shown they lie within the bar.
Time course of effect of isoprenaline on luciferase activity in HASM cells
The time course of isoprenaline induced increase in luciferase activity is shown in Figure 2: essentially linear increases in luciferase activity were observed over the 24 h time course of these experiments. The time course of the responses to the other β-agonists studied did not differ significantly from the time course of the response to isoprenaline. Thus, for all further experiments performed in cultured HASM cells using the reporter gene system, β-agonists were added for 24 h.
Figure 2.
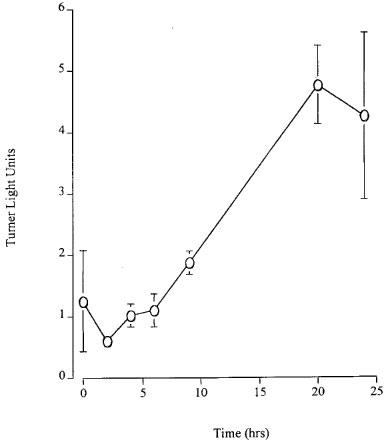
Effect of different exposure times to isoprenaline (1 μM) on increases in luciferase activity in primary cultures of HASM cells transiently transfected with p6CRE/luc. Each data point represents the mean±s.e.mean of data obtained in n individual experiments (n=3) performed in triplicate.
Regulation of reporter gene expression by cyclic AMP in HASM cells
The maximum induction observed (seen in cells stimulated with 10 μM isoprenaline for 24 h) was 5.54±0.70 fold compared with cells transfected with p6CRE/luc in the absence of agonist (P< 0.0001, n=39). Isoprenaline had no effect upon luciferase activity in cells transfected with the pGL3 Control vector (data not shown), implying that transfection efficiency was not altered by incubation with isoprenaline. In order to confirm that the increase in luciferase activity induced by isoprenaline was mediated through elevation of cyclic AMP via stimulation of β2 adrenoceptors, we preincubated cells with the β2 adrenoceptor selective antagonist ICI 118551 (Bilski et al., 1983) before stimulation with isoprenaline (Figure 3). ICI 118551 had no significant effect upon expression of the p6CRE/luc construct in the absence of agonist, but inhibited isoprenaline induced luciferase gene expression at a concentration (50 nM) selective for β2 adrenoceptors (Hall et al., 1992).
Figure 3.

The effect of ICI 118 551 (50 nM) (ICI) preincubation on luciferase activity in primary cultures of HASM cells treated with or without isoprenaline (10 μM) (iso) and transiently transfected with p6CRE/luc or pGL3 control. Each bar represents the mean±s.e.mean of data obtained in individual experiments (n=4). Data were analysed by paired students t-tests. *Significantly different from p6CRE/luc alone, P<0.05 and †significantly different from p6CRE/luc+iso, P<0.05. p6CRE/luc+ICI 118 551 was not significantly different from p6CRE/luc alone.
Time course of cyclic AMP responses to β adrenoceptor agonists
Figure 4a–d shows the time course of cyclic AMP responses to salmeterol, salbutamol, terbutaline and isoprenaline. The response to all drugs rose over the initial 4–8 h then tailed off at later time points for all agents except salmeterol: however, the levels of cyclic AMP were still higher than baseline at 6 h for all the agents studied. The magnitude of the maximum response to isoprenaline was as expected greater than the response to the other agonists studied (P<0.01), and, although the cyclic AMP response to isoprenaline returned to basal levels earlier than with the other agonists, the area under the curve was still greater than with salbutamol, salmeterol or terbutaline.
Figure 4.
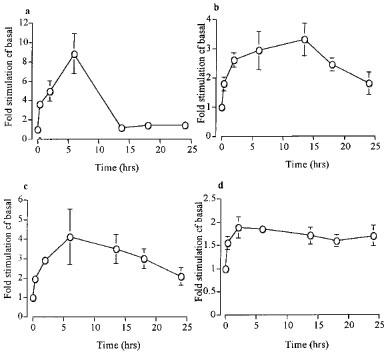
The effect of different exposure times to (a) isoprenaline (1 μM) (b) terbutaline (1 μM) (c) salbutamol (1 μM) and (d) salmeterol (1 μM) on [3H]-cyclic AMP formation in primary cultures of HASM cells. Each data point represents the mean±s.e.mean of data obtained in individual experiments (n=4). Data are expressed as fold stimulation c.f. basal (unstimulated) levels. Where error bars are not shown they lie within the data point.
Effects of β2 adrenoceptor agonists on cyclic AMP levels in HASM cells
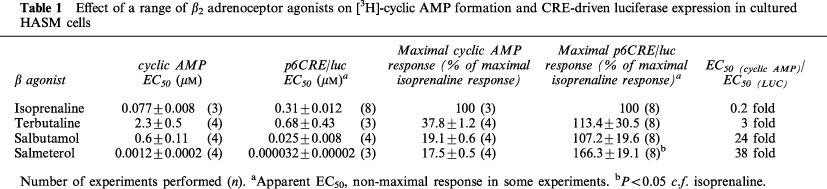
The effect of acute incubation (20 min) with a range of concentrations of the non-selective β adrenoceptor agonist isoprenaline, the short acting β2 selective agonists salbutamol and terbutaline and the long acting β2 selective agonist salmeterol on [3H]-cyclic AMP formation is shown in Figure 5a–d. All agents produced concentration related cyclic AMP responses: the EC50 values are shown in Table 1. Compared with isoprenaline the agents salmeterol, salbutamol and terbutaline all produced markedly smaller maximal responses (Table 1), with salmeterol producing the smallest response (17.5±0.5%, n=4) compared to the maximum response observed with isoprenaline (Figure 5d).
Figure 5.
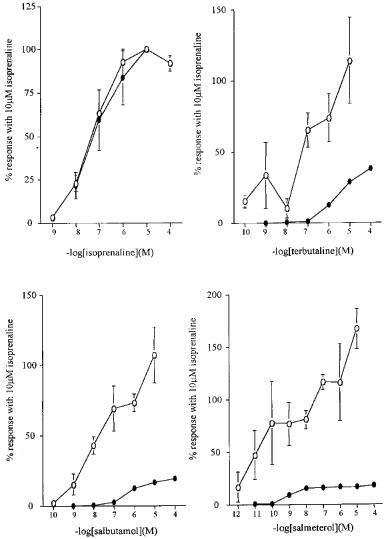
The effects of different concentrations of (a) isoprenaline, (b) terbutaline, (c) salbutamol and (d) salmeterol on [3H]-cyclic AMP formation (filled circles) and CRE-driven luciferase activity (open circles) in primary cultures of HASM cells (n=3–8). Each data point represents the mean±s.e.mean of data obtained in n individual experiments. Where error bars are not shown they lie within the data point. All data are shown as a percentage of the maximum [3H]-cyclic AMP (filled circles) or luciferase (open circles) response observed with 10 μM isoprenaline.
Table 1.
Effect of a range of β2 adrenoceptor agonists on [3H]-cyclic AMP formation and CRE-driven luciferase expression in cultured HASM cells
Effect of a range of β2 adrenoceptor agonists on CRE driven gene expression
The concentration-effect curves for luciferase activity following 24 h exposure to agonists is shown in Figure 5. Interestingly, salbutamol and terbutaline, despite producing much smaller cyclic AMP responses in these cells compared to isoprenaline (see Figure 5), produced similar maximal increases in CRE-driven luciferase activity in HASM cells. Salmeterol, which only produced a cyclic AMP response which was 17.5±0.5%, n=4, of the maximal response with isoprenaline (Figure 5d), produced a significantly greater luciferase response than the full agonist isoprenaline (P<0.05, n=4). This effect was not due to different levels of transfection achieved in the presence of these drugs because the level of luciferase activity in cell lysates from HASM cells transfected with pGL3 and exposed to the range of agonists was not different from cells not exposed to agonist (data not shown).
Discussion
In this paper we have shown that primary cultures of HASM cells can be reliably transfected with reporter constructs using cationic lipid mediated transfer of DNA, and that levels of gene expression can be achieved suitable for the study of regulatory elements of interest in airway cells. Overall, the absolute levels of expression achieved were generally 100–1000 fold less than the levels we have observed in transformed cell lines such as COS or CHO cells, in accordance with findings in other nontransformed cell culture systems (Pickering et al., 1994). Although effective gene transfer could be achieved through a range of different transfection techniques, in our experience only cationic lipid mediated transfer provided reliable levels of transfection on a day to day basis using these vectors. The amounts of DNA required for successful transfection and the ratio of lipid : DNA which was optimal were similar to those previously described in other primary cell lines (Loeffler et al., 1990; Pickering et al., 1994; Staedel et al., 1994; Fortunati et al., 1996).
The main aim of this study was to determine if pharmacological manipulation of cyclic AMP levels using a range of β adrenoceptor agonists in cultured HASM cells could result in changes in gene expression of a model system using a reporter gene driven by CREs. Many potential target genes in airway cells contain CREs, and it is likely that at least some of the longer term effects of elevation of intracellular cyclic AMP levels upon receptor expression (Hall et al., 1993) or proliferation (Panettieri et al., 1993; Noveral & Grunstein, 1994; Tomlinson et al., 1995) are mediated by direct effects of cyclic AMP/protein kinase A stimulating CREB binding to CREs in target genes. However, elevation of cyclic AMP levels can induce many other effects upon these cells including phosphorylation of a range of other intracellular targets by protein kinase A and hence it is difficult to be certain that effects on the expression of genes whose noncoding regions contain multiple regulatory elements are direct rather than indirect: indeed, it is probable that the net effect of cyclic AMP upon gene expression of these target genes is due to a combination of direct effects upon CREs and indirect effects via other regulatory mechanisms. One example of this kind of transcriptional cross talk is shown by the observation that CREB and the glucocorticoid receptor may be able to interact with each other via protein-protein interaction to prevent both glucocorticoid induced gene expression in rat lung (Peters et al., 1995) and cyclic AMP-mediated gene expression of the human glycoprotein hormone α subunit (Schule & Evans, 1990) by reducing the levels of glucocorticoid receptor and CREB available for binding to GREs and CREs respectively. In order to define mechanisms underlying cyclic AMP regulation of CRE activation more precisely, we utilized a simplified approach to the study of cyclic AMP induced changes in gene expression. We transfected cells with a construct containing multiple CREs, but no other known regulatory elements, coupled to the gene for firefly luciferase driven by a minimal Herpes Simplex Virus Thymidine Kinase promoter and then studied the effect of manipulation of cyclic AMP levels upon luciferase expression in these cells.
A number of workers have described the development of reporter gene systems for the study of G protein coupled receptor mediated cyclic AMP signalling events. These reporters consist of multiple copies of the consensus CRE, TGACGTCA (Montminy et al, 1990; Foulkes & Sassone-Corsi, 1996) placed upstream of a minimal promoter sequence and are designed to promote cyclic AMP driven expression of reporter genes such as β-galactosidase (Chen et al., 1995), secreted placental alkaline phosphatase (sPAP) (McDonnell et al., 1998) or firefly luciferase (Stratowa et al., 1995). Such systems have been used to detect signalling of the adenosine A1 and A2A receptors (Castanon & Spevak, 1994; Stratowa et al., 1995), the β2 adrenoceptor (McDonnell et al., 1998), the dopamine D1 and D5 receptors (Himmler et al., 1993) and the serotonin 5-HT1B and Calcitonin (C1a) receptors (George et al., 1997) expressed endogenously and in recombinant cell lines. However, to date, there have been no reports of the use of such constructs in primary cell culture systems.
In our experiments, long term exposure to elevated cyclic AMP levels, achieved by stimulation of the β2 adrenoceptor present upon these cells, resulted in a time dependent increase in the level of luciferase expression. In parallel experiments we followed the time course of cyclic AMP levels in the cells: following stimulation by isoprenaline cyclic AMP levels reached a peak at around 4–8 h before slowly declining. We have previously shown that long term (>6 h) exposure of cells to agonist results in β2 adrenoceptor downregulation, with a reduction in cell surface receptor number (Hall et al., 1993; Green et al., 1995), which is the probable explanation for this decline in cyclic AMP with time. The time course of isoprenaline induced gene expression shows a continued rise over the 24 h period. However, the absolute levels of luciferase present in the cell will depend upon the balance between gene expression, translation and breakdown of luciferase and hence changes in luciferase levels would be expected to lag behind changes in cyclic AMP levels, as was observed. The EC50 values for the effects of the β-agonists studied on cyclic AMP are as one might expect generally higher than effects on relaxation of airway smooth muscle by a factor of approximately 10 fold.
Because there is a lag between cyclic AMP induced activation of luciferase gene expression and the production of functional luciferase, it is difficult to be certain in time course experiments what the minimum exposure to elevated cyclic AMP levels is that would result in altered gene expression. Obviously, expression of individual genes will in reality be subject to multiple, probably conflicting effects, and our model is an oversimplification in that respect. Nonetheless, we would predict that exposure to both short and long acting β2 adrenoceptor agonists in the airways would be able to induce CRE driven changes in gene expression.
When we studied the effects of a range of β2 adrenoceptor agonists on cyclic AMP and CRE driven luciferase responses, interesting differences were observed. In short term experiments, the changes in cyclic AMP induced by salbutamol, terbutaline and salmeterol were all much smaller than the increase in cyclic AMP levels observed with isoprenaline, in keeping with previous observations that these drugs act as partial agonists at the airway smooth muscle β2 adrenoceptor (Dougall et al., 1991). In previous work we have shown by both the use of selective antagonists (Hall et al., 1992) and in binding studies (Green et al., 1995) that these cells express a single population of β adrenoceptors of the β2 subtype, therefore the effects of isoprenaline are not due to stimulation of another receptor population. It is also interesting to note that salmeterol demonstrated an equivalent rapid increase in cyclic AMP accumulation in cultured HASM cells compared to the other agonists used in this study, which is distinct from its slow onset of action in airway smooth muscle (Naline et al., 1994; Anderson et al., 1996). This finding is in agreement with similar studies performed in rat B50 neuroblastoma cells (McCrea & Hill, 1993), bovine tracheal smooth muscle (Ellis et al., 1995), CHO-K1 cells transfected with the human β2 adrenoceptor (McDonnell et al., 1998), and for activation of adenylyl cyclase activity in mouse L-cells transfected with the hamster β2 adrenoceptor (Clark et al., 1996) and the human epithelial cell line BEAS-2B (January et al., 1998). This is presumably related to cell monolayers being used instead of tissue which significantly reduces the diffusion barrier to the drug. Interestingly, when we examined the effects of these agents on CRE driven gene expression, salbutamol and terbutaline induced similar increases in luciferase activity compared to isoprenaline itself, suggesting that maximal gene expression effects may be achieved with much smaller increases in whole cell cyclic AMP levels. Salmeterol, which elicited the smallest maximal cyclic AMP response, demonstrated greater effects on gene expression, even at low agonist concentrations which had little effect on cyclic AMP responses, compared to the other agonists. This disparity is unlikely to be explained by the difference in time points used in the studies, because when we looked at the area under the curve for the cyclic AMP time courses for the different agonists, isoprenaline still produced a markedly greater integrated cyclic AMP response.
The explanation for these differences is unclear, but the data suggest a marked gain in efficiency in the signalling cascade within the cell for the partial agonists, especially salmeterol, between the initial agonist-receptor interaction at the cell surface and the activation of gene transcription in the nucleus. This indicates that stimulation of gene transcription is a much more sensitive response than that of cyclic AMP accumulation for the partial agonists. More evidence in favour of this, is that the EC50 values for salmeterol and salbutamol obtained from the luciferase gene expression were an order of magnitude lower than those obtained from the cyclic AMP responses (salmeterol; EC50 (cyclic AMP)/EC50 (LUC)=38 fold, salbutamol; EC50 (cyclic AMP)/EC50 (LUC)=24 fold). Another potential explanation for the differences between the cyclic AMP and gene expression data is that changes in subcellular pools of cyclic AMP, which are not detectable when measuring changes in whole cell cyclic AMP responses, may be important in driving gene expression through CRE dependent mechanisms. However, whatever the explanation for the differences, if translated into airways in vivo, it is possible that salmeterol may have greater effects on CRE-driven gene expression than other β2-agonists currently in clinical use. Elevation of cell cyclic AMP content has been shown to have a range of important effects likely to be due in part to altered gene expression, including inhibition of proliferative signalling in airway myofibroblasts (Tomlinson et al., 1995).
Interestingly, similar results have been obtained in CHO cells expressing the β2 adrenoceptor at similar levels (50 fmol mg protein−1) to those seen in cultured HASM, suggesting that this is a general property of salmeterol at the β2 adrenoceptor rather than a cell specific effect (McDonnell et al., 1998). In this CHO cell system, salmeterol produced a maximal cyclic AMP response that was only 22% of the response obtained with isoprenaline but induced a similar increase in CRE-driven gene expression as that seen with isoprenaline, again suggesting a marked gain in efficiency within the intracellular signal cascade.
In summary therefore, we have shown that manipulation of cyclic AMP levels in cultured HASM cells produces changes in gene expression of a reporter construct under the control of cyclic AMP response elements. This model will provide a useful system for studying control of gene expression in airway cells, and demonstrates that pharmacological agents used in the treatment of airway disease are likely to induce changes in gene expression of airway genes containing CREs. Potentially important quantitative differences were observed in the ability of β2 adrenoceptor agonists to stimulate CRE driven gene expression compared with effects on cell cyclic AMP content. In addition, this approach is a potentially useful one to use to study possible interactions between transcription factors which may alter the way in which regulation of gene expression occurs.
Acknowledgments
This work was funded in part by the National Asthma Campaign.
Abbreviations
- CHO
Chinese Hamster Ovary
- CRE
cyclic AMP response element
- CREB
cyclic AMP response element binding protein
- HASM
human airway smooth muscle
- IBMX
isobutyl-1-methyl-xanthine
- sPAP
secreted placental alkaline phosphatase
References
- ANDERSON G.P., LOTVALL J., LINDEN A. Relaxation kinetics of formoterol and salmeterol in the guinea pig trachea. Lung. 1996;174:159–170. doi: 10.1007/BF00173308. [DOI] [PubMed] [Google Scholar]
- BILSKI A.J., HALLIDAY S.E., FITZGERALD J.D., WALE J.L. The pharmacology of a β2 selective adrenoceptor antagonist (ICI 118551) J. Cardiovasc. Res. 1983;5:430–437. doi: 10.1097/00005344-198305000-00013. [DOI] [PubMed] [Google Scholar]
- BRADFORD M. A rapid and Sensitive Method for the Quantitation of Microgram Quantities of Protein Utilizing the Principal of Protein-Dye Binding. Anal. Biochem. 1976;72:248–254. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
- CASTANON M.J., SPEVAK W. Functional coupling of human adenosine receptors to a ligand-dependent reporter gene system. Biochem. Biophys. Res. Commun. 1994;198:626–631. doi: 10.1006/bbrc.1994.1091. [DOI] [PubMed] [Google Scholar]
- CHEN W., SHIELDS T.S., STORK P.J.S., CONE R.D. A colorimetric assay for measuring activation of Gs- and Gq- coupled signalling pathways. Analytical Biochem. 1995;226:349–354. doi: 10.1006/abio.1995.1235. [DOI] [PubMed] [Google Scholar]
- CLARK R.B., ALLAL C., FRIEDMAN J., JOHNSON M., BARBER R. Stable activation and desensitization of β2-adrenergic receptor stimulation of adenylyl cyclase by salmeterol: evidence for quasi-irreversible binding to an exosite. Mol. Pharmacol. 1996;49:182–189. [PubMed] [Google Scholar]
- COLLINS S., ALTSCHMIED J., HERBSMAN O., CARON M.G., MELLON P.L., LEFKOWITZ R.J. A cAMP Response Element in the β2-Adrenergic Receptor Gene Confers Transcriptional Regulation by cAMP. J. Biol. Chem. 1990;265:19330–19335. [PubMed] [Google Scholar]
- COLLINS S., BOUVIER M., BOLANOWSKI M.A., CARON M.G., LEFKOWITZ R.J. cAMP stimulates transcription of the β2-adrenergic receptor gene in response to short-term agonist exposure. Proc. Natl. Acad. Sci. U.S.A. 1989;86:4853–4857. doi: 10.1073/pnas.86.13.4853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DAYKIN K., WIDDOP S., HALL I.P. Control of histamine-induced inositol phospholipid hydrolysis in cultured human smooth muscle cells. Eur. J. Pharmacol. (Mol. Pharmacol.) 1993;264:135–140. doi: 10.1016/0922-4106(93)90090-v. [DOI] [PubMed] [Google Scholar]
- DOUGALL I.G., HARPER D., JACKSON D.M., LEFF P. Estimation of the efficacy and affinity of the β2-adrenoceptor agonist salmeterol in guinea-pig trachea. Br. J. Pharmacol. 1991;104:1057–1061. doi: 10.1111/j.1476-5381.1991.tb12549.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ELLIS K.E., MISTRY R., BOYLE J.P., CHALLIS R.A.J. Correlation of cyclic AMP accumulation and relaxant actions of salmeterol and salbutamol in bovine tracheal smooth muscle. Br. J. Pharmacol. 1995;116:2510–2516. doi: 10.1111/j.1476-5381.1995.tb15103.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FORTUNATI E., BOUT A., ZANTA M.A., VALERIO D., SCARPA M. In vitro & in vivo gene transfer to pulmonary cells mediated by cationic liposomes. Biochim. Biophys. Acta. 1996;1306:55–62. doi: 10.1016/0167-4781(95)00217-0. [DOI] [PubMed] [Google Scholar]
- FOULKES N.S., SASSONE-CORSI P. Transcription factors coupled to the cAMP-signalling pathway. Biochim. Biophys. Acta. 1996;1288:F101–F121. doi: 10.1016/s0304-419x(96)00025-x. [DOI] [PubMed] [Google Scholar]
- GEORGE S.E., BUNGAY P.J., NAYLOR L.H. Functional Coupling of Endogenous Serotonin (5-HT1B) and Calcitonin (C1a) Receptors in CHO Cells to a Cyclic AMP-Responsive Luciferase Reporter Gene. J. Neurochem. 1997;69:1278–1285. doi: 10.1046/j.1471-4159.1997.69031278.x. [DOI] [PubMed] [Google Scholar]
- GONZALEZ G.A., MONTMINY M.R. Cyclic AMP stimulates somatostatin gene-transcription by phosphorylation of CREB at serine-133. Cell. 1989;59:675–680. doi: 10.1016/0092-8674(89)90013-5. [DOI] [PubMed] [Google Scholar]
- GREEN S.A., TURKI P., HALL I.P., LIGGETT S.B. Influence of β2 adrenergic receptor genotypes on signal transduction in HASM cells. Am. J. Respir. Cell Mol. Biol. 1995;13:25–33. doi: 10.1165/ajrcmb.13.1.7598936. [DOI] [PubMed] [Google Scholar]
- HALL I.P., DAYKIN K., WIDDOP S. β2 adrenoceptor desensitisation in cultured HASM. Clin. Sci. 1993;84:151–157. doi: 10.1042/cs0840151. [DOI] [PubMed] [Google Scholar]
- HALL I.P., KOTLIKOFF M. Use of cultured airway myocytes for study of airway smooth muscle. Am. J. Physiol. 1995;268:L1–L11. doi: 10.1152/ajplung.1995.268.1.L1. [DOI] [PubMed] [Google Scholar]
- HALL I.P., WIDDOP S., TOWNSEND P., DAYKIN K. Control of cyclic AMP levels in primary cultures of human tracheal smooth muscle cells. Br. J. Pharmacol. 1992;107:422–428. doi: 10.1111/j.1476-5381.1992.tb12762.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HIMMLER A., STRATOWA C., CZERNILOFSKY A.P. Functional testing of Dopamine-D1 and Dopamine-D5 Receptors expressed in stable cyclic-AMP responsive luciferase reporter cell-lines. J. Recept. Res. 1993;13:79–94. doi: 10.3109/10799899309073647. [DOI] [PubMed] [Google Scholar]
- HOSODA K., FITZGERALD L.R., VAIDYA V.A., FEUSSNER G.K., FISHMAN P.H., DUMAN R.S. Regulation of β2-Adrenergic Receptor mRNA and Gene Transcription in Rat C6 Glioma Cells: Effects of Agonist, Forskolin and Protein Synthesis Inhibition. Mol. Pharmacol. 1995;48:206–211. [PubMed] [Google Scholar]
- JANUARY B., SEIBOLD A., ALLAL C., WHALEY B.S., KNOLL B.J., MOORE R.H., DICKEY B.F., BARBER R., CLARK R.B. Salmeterol-induced desensitization, internalization and phosphorylation of the human β2-adrenoceptor. Br. J. Pharmacol. 1998;123:701–711. doi: 10.1038/sj.bjp.0701658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- JONES S.M., FOREMAN S.K., CORNETT L.E. The paradox of prolonged β2-adrenergic receptor stimulation. Am. J. Respir. Crit. Care Med. 1998;157:A746. [Google Scholar]
- LOEFFLER J.Ph., BARTHEL F., FELTZ P., BEHR J.P., SASSONE-CORSI P., FELTZ A. Lipopolyamine-mediated transfection allows gene expression studies in primary neuronal cells. J. Neurochem. 1990;54:1812–1815. doi: 10.1111/j.1471-4159.1990.tb01240.x. [DOI] [PubMed] [Google Scholar]
- MCCREA K.E., HILL S.J. Salmeterol, a long activating β2 adrenoceptor agonist mediating cyclic AMP accumulation in a neuronal cell line. Br. J.Pharmacol. 1993;110:619–626. doi: 10.1111/j.1476-5381.1993.tb13856.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MCDONNELL J., LATIF M.J., REES E.S., BEVAN N.J., HILL S.J. Influence of receptor number on the stimulation by salmeterol of gene transcription in CHO-K1 cells transfected with the human β2-adrenoceptor. Br. J. Pharmacol. 1998;125:717–726. doi: 10.1038/sj.bjp.0702139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MCGRAW D.W., LARY C., JACOBI S., CORNETT L.E. Functional analysis of β2-adrenergic receptor promoter activity after prolonged β-agonist exposure. J. Allergy Clin. Immunol. 1997;99:S410. [Google Scholar]
- MONTMINY M.R., GONZALEZ G.A., YAMAMOTO K.K. Regulation of cAMP-inducible genes by CREB. Trends Neuro-sci. 1990;13:184–188. doi: 10.1016/0166-2236(90)90045-c. [DOI] [PubMed] [Google Scholar]
- NALINE E., ZHANG Y., QIAN Y., MAIRON N., ANDERSON G.P., GRANDORDY B., ADVENIER C. Relaxant effects and durations of action of formoterol and salmeterol on the isolated human bronchus. Eur. Respir. J. 1994;7:914–920. [PubMed] [Google Scholar]
- NOVERAL J.P., GRUNSTEIN M.M. Adrenergic receptor-mediated regulation of cultured rabbit airway smooth-muscle cell-proliferation. Am. J. Physiol. 1994;267:L291–L299. doi: 10.1152/ajplung.1994.267.3.L291. [DOI] [PubMed] [Google Scholar]
- PANETTIERI R.A., COHEN M.D., BILGEN G. Airway smooth muscle proliferation is inhibited by microinjection of the catalytic subunit of cAMP-dependent protein kinase A. Am. Rev. Respir. Dis. 1993;147:A252. [Google Scholar]
- PETERS M.J., ADCOCK I.M., BROWN CR., BARNES P.J. Beta-adrenoceptor agonists interfere with glucocorticoid receptor DNA-binding in rat lung. Eur. J. Pharmacol. (Mol. Pharm.) 1995;289:275–281. doi: 10.1016/0922-4106(95)90104-3. [DOI] [PubMed] [Google Scholar]
- PICKERING J.G., JEKANOWSKI J., WEIR L., TAKESHITA S., LOSORDO D.W., ISNER J.M. Liposome-mediated gene transfer into vascular smooth muscle cells. Circulation. 1994;89:13–21. doi: 10.1161/01.cir.89.1.13. [DOI] [PubMed] [Google Scholar]
- SAMBROOK J., FRITSCH E.F., MANIATIS T.Molecular Cloning: A Laboratory Manual 1989Cold Spring Harbor Laboratory Press, Cold Spring Harbor, New York; 2nd ed [Google Scholar]
- SCHULE R., EVANS R.M. Cross-coupling of signal-transduction pathways: zinc finger meets leucine zipper. Trends Genet. 1990;7:377–381. doi: 10.1016/0168-9525(91)90259-s. [DOI] [PubMed] [Google Scholar]
- SCOTT M.G.H., HILL P., REES S., BROWN S., LEE M., HALL I.P. Control of gene expression by elevation of cell cyclic AMP content in primary cultures of HASM cells (HASM) transfected with a cyclic AMP-responsive reporter construct. Br. J. Pharmacol. 1997;120:P9. [Google Scholar]
- SCOTT M.G.H., JOBSON T.M., SWAN C., REES E.S., HALL I.P. Effects of a range of beta-2 agonists on intracellular cyclic AMP content and cyclic AMP driven gene expression in primary cultures of human airway smooth muscle cells. Br. J. Pharmacol. 1999a;126:P122. doi: 10.1038/sj.bjp.0702829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SCOTT M.G.H., SWAN C., WHEATLEY A.P., HALL I.P. Identification of novel polymorphisms within the promoter region of the human β2 adrenergic receptor gene. Br. J. Pharmacol. 1999b;126:841–844. doi: 10.1038/sj.bjp.0702385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- STAEDEL C., REMY J.S., HUA Z., BROKER T.R., CHOW L.T., BEHR J.P. High-efficiency transfection of primary human keratinocytes with positively charged lipopolyamine : DNA complexes. J. Invest. Dermatol. 1994;102:768–772. doi: 10.1111/1523-1747.ep12377673. [DOI] [PubMed] [Google Scholar]
- STRATOWA C., HIMMLER A., CZERNILOFSKY A.P. Use of a luciferase reporter system for characterizing G-protein coupled receptors. Curr. Opinion Biotech. 1995;6:574–581. [Google Scholar]
- TOMLINSON P.R., WILSON J.W., STEWART A.G. Salbutamol inhibits the proliferation of HASM cells grown in culture - relationship to elevated cyclic AMP levels. Biochem. Pharmacol. 1995;49:1809–1819. doi: 10.1016/0006-2952(94)00532-q. [DOI] [PubMed] [Google Scholar]
- TORPHY T.J., HALL I.P.Cyclic AMP and the control of airways smooth muscle tone Airway smooth muscle biochemical control of contraction and relaxation 1994Birhauser Verlag, Switzerland; 215–223.ed. Raeburn, Giembycz [Google Scholar]


