Abstract
In decerebrated rabbits, the selective 5-HT1B/1D receptor antagonist GR 127,935 had no significant effects on reflexes evoked in medial gastrocnemius motoneurones by electrical stimulation of the sural nerve, or on arterial blood pressure or heart rate when given by the intrathecal (up to 543 nmol cumulative) or intravenous (up to 1.8 μmol cumulative) routes.
In decerebrated, spinalized rabbits, intrathecal GR 127,935 in doses of up to 543 nmol, had no effect on the sural-gastrocnemius reflex. Furthermore, this drug failed to alter enhancement of the sural-gastrocnemius reflex induced by 8-hydroxy-2-(di-n-propyl)aminotetralin (8-OH-DPAT), given at 300 nmol kg−1 i.v.
In decerebrated, spinalized rabbits, the selective 5-HT1B/1D receptor agonists L-694,247 (cumulative doses of 2–243 nmol kg−1 i.v.) and L-741,604 (cumulative doses of 3–307 nmol kg−1 i.v.), each caused the sural-gastrocnemius reflex to increase to 140% of pre-drug levels, and arterial blood pressure to rise by about 10 mmHg. Subsequent administration of GR 127,935 at 0.9–1.8 μmol kg−1 reversed the pressor effect of the agonists but not the increase in reflexes. The 5-HT1A receptor antagonist WAY-100,635 (185 nmol kg−1 i.v.) also failed to reverse the increase in reflexes, but the 5-HT1B/1D/5-HT2/5-HT7 ligand ritanserin (1.6 μmol kg−1 i.v.) restored reflexes to pre-drug control values after L-741,604 (it was not tested against L-694,247).
These data indicate that 5-HT1B/1D receptors do not significantly modulate transmission in the sural-gastrocnemius reflex pathway, and that the enhancement of reflexes by 8-OH-DPAT and L-741,604 is probably mediated by 5-HT7 receptors.
Keywords: Nociception, serotonin, spinal cord, withdrawal reflex, 5-HT1B receptor, 5-HT1D receptor, 5-HT7 receptor, rabbit
Introduction
The agonist 8-hydroxy-2-(di-n-propylamino)tetralin (8-OH-DPAT) has long been considered to be selective for the 5-HT1A receptor (Hjorth et al., 1987), but some recent studies have suggested that it has significant activity at other 5-HT receptors. For instance, in the decerebrated, spinalized rabbit, 8-OH-DPAT enhanced reflexes evoked in medial gastrocnemius (MG) motoneurones evoked by electrical stimulation of the cutaneous sural nerve (Clarke et al., 1997). The effects of low doses of 8-OH-DPAT were completely reversed by the selective 5-HT1A receptor antagonist WAY-100,635 (Ogilvie & Clarke, 1998), but responses to higher doses of the agonist (up to 300 nmol kg−1 i.v.) were blocked only by coadministration of WAY-100,635 with the 5-HT1B/1D/5-HT2/5-HT7 receptor ligand ritanserin (Clarke et al., 1997). These findings suggest that 5-HT1A- and non-5-HT1A-receptors were involved in the enhancement of reflexes. As 8-OH-DPAT has little or no affinity for 5-HT2 receptors (Van Wijngaarden et al., 1990), it may be concluded that high doses of 8-OH-DPAT activated 5-HT1B/1D and/or 5-HT7 receptors in addition to 5-HT1A-receptors to produce this effect.
To differentiate between these possibilities, we have now investigated the possible functions of 5-HT1B/1D receptors in rabbit spinal cord. As 8-OH-DPAT has been shown to exert some effects through 5-HT1B/1D receptors in the rabbit (Dando et al., 1998), we started from the position that these receptors were likely to be important modulators of spinal function in this species. We first probed for tonic activation of spinal 5-HT1B/1D receptors with the selective antagonist GR 127,935 (Skingle et al., 1996). The same antagonist was then used to test for the involvement of 5-HT1B/1D receptors in mediating the effects of 8-OH-DPAT. Finally, the potent 5-HT1B/1D receptor agonists L-694,247 (Beer et al., 1993) and L-741,604 (Sternfeld et al., 1996) were used to investigate directly the effects of activation of these receptors. Some of the present data have been reported in abstracts (Wigglesworth et al., 1997; Clarke et al., 1999).
Methods
Experiments were performed on 36 rabbits, of either sex and weighing between 1.7 and 3.0 kg. Anaesthesia was induced by an initial intravenous injection of methohexitone sodium (Brietal, Eli Lilly) at a dose of 5–10 mg kg−1 that was supplemented to effect. The mean total dose given was 13 mg kg−1. The trachea was then cannulated and anaesthesia maintained using halothane (2–4%) in a mixture of oxygen and nitrous oxide in a ratio of 30 : 70. The left carotid artery was then cannulated to allow measurement of arterial blood pressure. Two cannulae were inserted into the left jugular vein for administration of drugs. A laminectomy was then performed at L1–L2. A fine polythene cannula (Portex, o.d. 0.61 mm) was inserted under the dura at this point and threaded down so that its tip lay close to segments L7 and S1. Where spinalization was required, a complete transection of the spinal cord was performed at segment L1 by suction after the cord had been anaesthetized with 100 μl 2% lignocaine solution (Lignavet, C-Vet). The rabbits were then decerebrated by suction to the pre-collicular level. Anaesthesia was discontinued and the animals paralysed with pancuronium bromide (David Bull laboratories, 0.4 mg kg−1 initially and then infused at 1 mg h−1). Ventilation was continued artificially with room air supplemented with oxygen. The left leg was clamped by the femur and tibia so that the hip and knee joints were placed at angles of 100 and 90° respectively. the biceps femoris muscle was exposed and bisected, revealing the popliteal fossa. The pool so formed was filled with warmed (38°C) paraffin oil to prevent dessication of tissues.
The sural nerve was dissected free of any connective tissue, cut and placed over two pairs of stimulating electrodes. A centrally-positioned electrode was used to record afferent volleys from the sural nerve. The nerve to MG was cut, desheathed and placed over a pair of platinum recording electrodes, between which the nerve was crushed to obtain a monophasic signal. At least 1 h was allowed to pass between the withdrawal of anaesthesia and recording of reflexes. Reflex responses were evoked by stimulating the sural nerve with 0.1 ms pulses at a strength sufficient to excite all myelinated axons (15–30×threshold). Stimuli were applied as a series of eight shocks, given at a frequency of 1 Hz. Responses were recorded from the MG muscle nerve, averaged, and integrated by computer.
The ECG was recorded from subcutaneous electrodes placed either side of the chest and was used to trigger an instantaneous rate meter (Neurolog NL 253) for a record of heart rate. The blood pressure signal was passed through a Hewlett Packard 8856 blood pressure analyzer to obtain mean arterial pressure. Heart rate and blood pressure were sampled simultaneously with nerve recordings.
Drug administration protocols
Three sets of experiments were performed.
Are 5-HT1B/1D receptors tonically active in decerebrated rabbits?
In order to establish whether or not 5-HT1B/1D receptors are tonically occupied in decerebrated rabbits, 12 non-spinalized animals were given a range of doses of GR 127,935 by the intrathecal (i.th.) or i.v. routes. This protocol was used to determine if an effect obtained from i.th. administration was likely to be due to a spinal site of action or leakage of the drug into the circulation. The antagonist was given intrathecally to six animals in incrementing doses of 2, 4, 13, 36, 130 and 360 nmol to give a final cumulative dose of 543 nmol. Injections, none of which exceeded 100 μl in volume, were flushed in with 30 μl Ringer's solution and were separated by intervals of 24 min. Six further animals received GR 127,935 intravenously, in doses of 6, 13, 36, 130, 360, and 1300 nmol per rabbit, giving a cumulative total of 1.8 μmol.
Are 5-HT1B/1D receptors involved in the facilitation of reflexes by 8-OH-DPAT?
These and all subsequent experiments were performed on decerebrated rabbits spinalized at the thoracolumbar junction. Two separate protocols were used in this part of the study.
The effects of GR 127,935 given before 8-OH-DPAT
GR 127,935 was given to six rabbits intrathecally to maximize access to the spinal cord, in doses of 180 and then 360 nmol, providing a total cumulative dose of 540 nmol. 8-OH-DPAT was then given at 300 nmol kg−1 i.v., followed by WAY-100,635 at 185 nmol kg−1 i.v. and, in one animal, ritanserin at 1.6 μmol kg−1 i.v. All drug doses were separated by intervals of 12 min.
The effects of GR 127,935 given after 8-OH-DPAT
Six rabbits received 8-OH-DPAT at 300 nmol kg−1 i.v., followed by GR 127,935 at 540 nmol kg−1 i.th., WAY-100,635 at 185 nmol kg−1 i.v. and then ritanserin at 1.6 μmol kg−1 i.v. All drug doses were separated by intervals of 12 min.
The results of activation of 5-HT1B/1D receptors
Twelve decerebrated and spinalized rabbits received increasing i.v. doses of either L-694,247 of 2, 5, 17, 49 and 170 nmol kg−1, giving a total cumulative dose of 243 nmol kg−1 (100 μg kg−1), followed by GR 127,935 in a dose of 1.8 μmol kg−1 i.v. (n=6), or L-741,604 in doses of 3, 6, 22, 59 and 220 nmol kg−1 giving a total cumulative dose of 307 nmol kg−1, followed by GR 127,935 at 0.9 μmol kg−1 i.v. (n=6). The doses of GR 127,935 were calculated on the published affinities of the drugs at 5-HT1B/1D receptors (Beer et al., 1993; Skingle et al., 1996; Sternfeld et al., 1996). After GR 127,935, all animals were given WAY-100,635 (185 nmol kg−1 i.v.), and the animals receiving L-741,604 were also given a final injection of ritanserin at 1.6 μmol kg−1 i.v. Doses of 5-HT1B/1D agonists were separated by intervals of 24 min, all subsequent injections were given 12 min apart.
Drugs
GR 127,935 (2′-methyl-4′-(5-methyl-(1,2,4) oxadiazol-3-yl)-biphenyl-4-carboxylic acid (4-methoxy-3-(4-methyl-piperazin-1-yl-phenyl)-amide.HCl), a gift of Glaxo Wellcome research, was dissolved in dimethyl sulphoxide (DMSO) and subsequently diluted to a strength of 3.6 mM in Ringer's solution (final DMSO concentration 0.5%). Further 10 fold dilutions were made up in Ringer's solution. L-694,247 (2-(5-(3-(4-methylsulphonylamino) benzyl - 1,2,4 - oxadiazol -5 -yl) - 1H-indole-3-yl)ethylamine) and L-741,604 (N,N-dimethyl-2-[5-(1,2,4-triazol-4-yl)-1H-indol-3-yl]ethylamine) were gifts of Dr R. Hargreaves, Merck, Sharp and Dohme Neuroscience Research. L-694,247 was dissolved in DMSO and diluted to a strength of 4.9 mM in Ringer's solution (final concentration of DMSO 1%): subsequent 10 fold dilutions were made up in Ringer's. L-741,604 was dissolved in Ringer's solution to a concentration of 3 mM and serial 10 fold dilutions were made in the same solvent. WAY-100,635 (N-2,N-(2-pyridinyl)cyclohexanecarboxamide.3HCl), a gift of Wyeth Research, and 8-OH-DPAT (Tocris Cookson) were each dissolved in Ringer's solution to concentrations of 3.7 and 3 mM respectively. Ritanserin (Research Biochemicals Inc.) was dissolved in 20 μl DMSO and suspended at 1.6 mM in 5% d-glucose solution. The composition of the Ringer's solution used in this laboratory is (mM): NaCl 150; NaHCO3 5; KCl 4; CaCl2 2 and MgCl2 50 μM.
Statistical analysis
Reflexes are expressed as a percentage of the mean of the twelve values recorded immediately before the first drug was given. Data points are described as medians and inter-quartile ranges (IQRs) derived from pooled data from each group of experiments. Reflex data did not fit a normal distribution and were therefore analysed using non parametric tests, namely the Friedman's ANOVA on ranks, the Wilcoxon signed ranks and matched pair tests for paired data and Mann-Whitney U-test for unpaired data. Cardiovascular data were suitable for parametric analysis and are therefore expressed as means ±s.e.mean; one-way ANOVA and paired and unpaired t-tests were used for statistical analysis of these variables. All P values are two-tailed. Tests were performed using the Instat3 program from GraphPad Software.
Results
Studies on tonic activity at 5-HT1B/1D receptors in non-spinalized preparations
In non-spinalized rabbits, administration of the 5-HT1D antagonist GR 127,935, given by either the intravenous or intrathecal routes (n=6 for both) had no consistent or significant effects on reflex responses (Friedman's ANOVA, P=0.127 and 0.0906 respectively, Figure 1), although there was a tendency for responses to increase when the drug was given intrathecally. GR 127,935 had no consistent or significant effects on mean arterial blood pressure (control values 91±5 and 81±6 mmHg for the i.th. and i.v. treated groups respectively) or heart rate (control values 286±3 and 293±28 beats min−1 for the i.th. and i.v. treated groups respectively) when given by either route.
Figure 1.
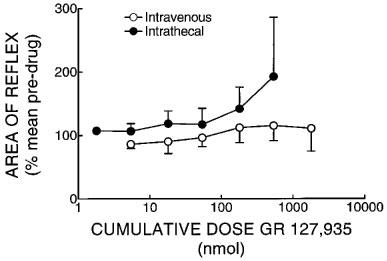
The effects on the sural-MG reflex of GR 127,935 given by the intrathecal and intravenous routes to decerebrated, non-spinalized rabbits. None of the changes observed was statistically significant (see text). Each point is the median from six experiments and the vertical lines indicate the third or first quartiles.
Effects of 5-HT1B/1D receptor blockade on enhancement of reflexes by 8-OH-DPAT
GR 127,935 given first
In spinalized rabbits, administration of GR 127,935 at 180 and then 360 nmol kg−1 i.th. produced no significant changes in the sural-MG reflex. In the presence of this antagonist, 8-OH-DPAT (300 nmol kg−1 i.v.) caused significant facilitation of the MG responses to a median of 313% (IQR 176–404%) of pre-drug controls (Wilcoxon test, P=0.03, n=6, Figure 2). This increase was not significantly different from that observed following the same dose of 8-OH-DPAT given alone (see next section, Mann-Whitney test, P=0.4). Subsequent administration of WAY-100,635 (185 nmol kg−1 i.v.) resulted in a significant decrease in reflex responses compared to post-8-OH-DPAT levels (Wilcoxon test, P=0.03, Figure 2), but reflexes remained significantly greater than pre-8-OH-DPAT values (Wilcoxon test, P=0.03). In one experiment, ritanserin (1.6 μmol kg−1 i.v.) was given after WAY-100,635. This resulted in a decrease of the response to a level that was not different from the pre-drug control.
Figure 2.
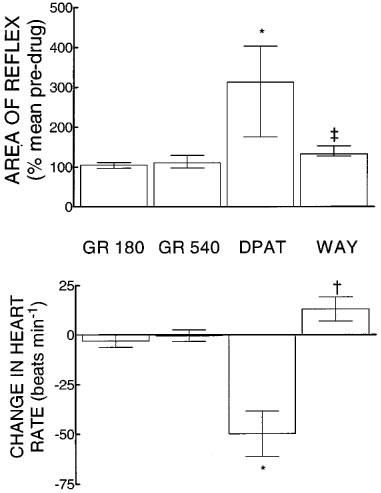
Upper panel. Changes in the sural-MG reflex of spinalized rabbits induced by GR 127,935 (GR180 and GR 540, 180 and then 540 nmol kg−1 i.th. cumulative), followed by 8-OH-DPAT (DPAT, 300 nmol kg−1 i.v.) and then WAY-100,635 (WAY, 185 nmol kg−1 i.v.). The height of each column is the median value from six experiments and the vertical lines indicate inter-quartile ranges. Lower panel: Changes in heart rate induced by the drug regimen described above. The height of each column represents the mean value from six experiments and the vertical lines indicate s.e.mean. Both panels: * indicates a significant difference from pre-drug controls; ‡ indicates significantly less than 8-OH-DPAT level but significantly greater than pre-drug levels; † indicates significantly less than 8-OH-DPAT level and not significantly different from control.
GR 127,935 had no significant effect on heart rate (Figure 2). 8-OH-DPAT caused a powerful bradycardia, with a mean decrease in heart rate of 50±11 beats min−1 from a control level of 269±18 beats min−1 (paired t-test, P=0.003), that was fully reversed by WAY-100,635 (Figure 2). In keeping with previous studies (Clarke et al., 1997), none of the drugs caused any significant or consistent changes in blood pressure (control value 63±4 mmHg).
8-OH-DPAT given first
8-OH-DPAT at 300 nmol kg−1 i.v. produced significant enhancement of the reflex to a median of 168% (IQR 150–234%) of pre-drug levels (Wilcoxon, P=0.03, n=6, Figure 3). Subsequent administration of GR 127,935 at 540 nmol kg−1 i.th. had no further significant effect on the response (median 253% of pre-drug values, IQR 179–414%) leaving the reflex significantly greater than the pre-8-OH-DPAT level (Wilcoxon test, P=0.03, Figure 3). After WAY-100,635 (185 nmol kg−1 i.v.) the reflex was a median of 157% (IQR 147–165%) of pre-drug values, still significantly greater than controls (Wilcoxon test, P=0.03), although there was a tendency for the reflex to decrease following the 5-HT1A receptor antagonist (Figure 3). Ritanserin, at 1.6 μmol kg−1, produced a significant decrease in the reflex compared to post-WAY-100,635 levels (Wilcoxon test, P=0.03) to a median of 126% (IQR 115–150%) of pre-8-OH-DPAT levels. This was not significantly different from pre-drug reflexes (Wilcoxon test, P=0.08).
Figure 3.
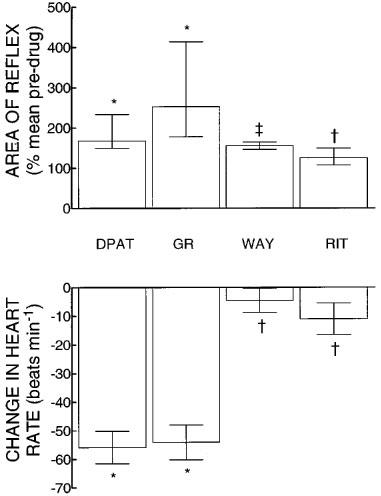
Upper panel. Changes in the sural-MG reflex of spinalized rabbits induced by 8-OH-DPAT (DPAT, 300 nmol kg−1 i.v.) followed by GR 127,935 (GR, 540 nmol kg−1 i.th.); WAY-100,635 (WAY, 185 nmol kg−1 i.v.) and then ritanserin (RIT, 1.6 μmol kg−1 i.v.). The height of each column is the median value from six experiments and the vertical lines indicate inter quartile ranges. Lower panel: Changes in heart rate induced by the drug regimen described above. The height of each column represents the mean value from six experiments and the vertical lines indicate s.e.mean. Both panels: * indicates a significant difference from pre-drug controls; ‡ indicates significantly less than 8-OH-DPAT level but significantly greater than pre-drug levels; † indicates significantly less than 8-OH-DPAT level and not significantly different from control.
Once again, none of the drugs, alone or in combination, caused any significant changes in arterial blood pressure (control value 63±4 mmHg). 8-OH-DPAT induced a large fall in heart rate by a mean of 56±6 beats min−1 from a pre-drug level of 304±5 beats min−1 (P=0.001, paired t-test), that was unaffected by subsequent administration of GR 127,935 but was completely reversed by WAY-100,635 (P=0.003 compared to post-8-OH-DPAT heart rate, paired t-test, Figure 3).
Effects of the 5-HT1B/1D agonists L-694,247 and L-741,604 in spinalized preparations
When given i.v. to spinalized rabbits, the 5-HT1B/1D agonists L-694,247 and L-741,604 each caused dose-dependent, significant facilitation of the sural-MG reflex response (Friedman's ANOVA, P=0.0002 and n=6 for both, Figure 4). This increase became significant at the third dose (23 and 31 nmol kg−1 cumulative for L-694,247 and L-741,604, respectively) of each agonist (Wilcoxon, P=0.03). After the final dose of L-694,247, reflexes were a median of 146% (IQR 125–166%) of pre-drug control values. The corresponding value after L-741,604 was 143% (IQR 124–153%) of controls. Subsequent administration of GR 127,935 (0.9–1.8 μmol kg−1) and then WAY-100,635 (185 nmol kg−1) had no effect after either agonist (Figure 4), but when ritanserin (1.6 μmol kg−1 i.v.) was given after L-741,604, reflexes returned to pre-agonist control levels (Figure 4).
Figure 4.
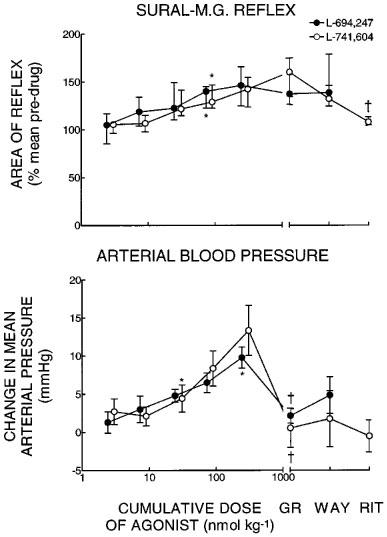
Dose-effect curves for L-694,247 and L-741,604 given i.v. to spinalized rabbits, showing changes induced by subsequent doses of GR 127,935 (GR, 1.8 μmol kg−1 i.v. for L-694,247, 0.9 μmol kg−1 for L-741,604); WAY-100,635 (WAY 185 nmol kg−1 i.v.) and ritanserin (RIT, 1.6 μmol kg−1 i.v.). Upper panel: effects on the sural-MG reflex, each point is the median from six experiments and the vertical lines indicate inter-quartile ranges. Lower panel: as above for arterial blood pressure, each point is the mean from six experiments and the vertical lines indicate s.e.mean. *Indicates lowest dose of L-694,247/L-741,604 inducing a significant change from pre-drug control; † indicates significantly different from pre-GR 127,935 values and not significantly different from control.
Both 5-HT1B/1D agonists also induced significant increases in blood pressure (Figure 4). The mean increase in arterial pressure after L-694,247 was 10±1 mmHg over a pre-drug average of 65±5 mmHg (ANOVA, P=0.02) and this effect became significant with the highest dose of the drug (paired t-test, P=0.03). After L-741,604, blood pressure rose by a mean of 13±3 mmHg from a pre-drug level of 71±5 mmHg (ANOVA, P=0.002), and the lowest effective dose was 31 nmol kg−1. In both cases these effects were reversed by GR 127,935 (Figure 4, paired t-tests against the final dose of agonist, P=0.03 and 0.003 for L-694,247 and L-741,604 respectively). Neither WAY-100,635, nor ritanserin induced any further significant changes in blood pressure (Figure 4, paired t-tests, P>0.05). None of the drugs caused any significant changes in heart rate compared with control.
Discussion
5-HT1B/1D receptors: involvement in modulating spinal reflexes and in cardiovascular control
The original purpose of the present study was to investigate the extent to which the non-5-HT1A-receptor-mediated effects of 8-OH-DPAT could be attributed to an action at 5-HT1B/1D receptors. Rabbits are known to possess 5-HT1B and 5-HT1D receptors with a similar pharmacology to those found in human tissues (Limberger et al., 1991; Hoyer et al., 1992; Harwood et al., 1995; Wurch et al., 1997), and others have shown that 8-OH-DPAT can induce effects at 5-HT1B/1D receptors in this species (Dando et al., 1998). However, the present results indicate that these receptors have little or no role to play in modulating transmission between sural nerve afferents and MG motoneurones, and are certainly not responsible for the reflex enhancing actions of 8-OH-DPAT. In non-spinalized animals, the selective 5-HT1B/1D antagonist GR 127,935 induced a small increase in spinal reflexes when given by the i.th. route, but this effect was not statistically significant. The drug failed to produce any changes at all in cardiovascular variables. In view of the high affinity of the antagonist for 5-HT1B/1D receptors, and by comparison with the effects of antagonists for other receptors with similar affinities at their preferred binding sites (Ogilvie et al., 1999), it is highly unlikely that insufficient drug was given to block 5-HT1B/1D receptors. It appears that there is no tonic activation of 5-HT1B/1D receptors in rabbit spinal cord or cardiovascular system under the conditions of these experiments.
In spinalized preparations, the potent 5-HT1B/1D agonists L-694,247 and L-741,604 both increased sural-MG reflexes and arterial blood pressure. The two agonists were indistinguishable in their effects: it appears that the very high binding affinity of L-694,247 is offset by the enhanced lipophilicity of the L-741,604 molecule (Beer et al., 1993; Sternfeld et al., 1996). The increase in blood pressure induced by both drugs was fully reversed by GR 127,935. This result indicates that:- (i) the pressor effect of the agonists was indeed mediated through 5-HT1B and/or 5-HT1D receptors; (ii) sufficient doses of the agonists had been given to activate these receptors; and (iii) enough antagonist had been given to overcome either agonist. The vaso- and venoconstrictor actions of 5-HT1B/1D agonists in rabbit are well known (e.g. Deckert et al., 1994; Choppin & O'Connor, 1994; 1995; Valentin et al., 1996; De Vries et al., 1997; Ellwood & Curtis, 1997), and no doubt explain the blood pressure changes caused by L-694,247 and L-741,604.
Potentiation of reflexes induced by the 5-HT1B/1D agonists was not reversed by GR 127,935. Although both L-694,247 and L-741,604 have moderate affinity for 5-HT1A receptors (Sternfield et al., 1996), the reflex enhancing action of the drugs could not have been mediated through these sites as it was not reversed by the selective 5-HT1A antagonist, WAY-100,635. Unlike other 5-HT1B/1D agonists, L-694,247 has no agonist activity at 5-HT1F receptors (Wainscott et al., 1998), so that these sites are not candidates for increasing reflex responses. Ritanserin, the 5-HT2/1B/1D/7 ligand, reversed the effect of L-741,604. As neither this agonist, nor L-694,247, has significant affinity for 5-HT2 receptors (Sternfeld et al., 1996), one might conclude that the potentiation of reflexes was due to an action at 5-HT7 receptors (although it is not possible to exclude the involvement of a novel 5-HT receptor). No data on the activity of these compounds at 5-HT7 receptors have been published, but this conclusion is consistent with our observations on 8-OH-DPAT (see below). Furthermore, the archetypal 5-HT1B/1D receptor ligand sumatriptan (from which L-694,247 and L-741,604 were derived) is known to be a ligand at 5-HT7 receptors (Bard et al., 1993; Ruat et al., 1993). A common clinical side-effect of the ‘triptan' class of 5-HT receptor agonists is tightness of the chest (Mathew, 1997). Assuming that activity at 5-HT7 receptors is a general feature of these drugs, it is possible that this sensation could result from a 5-HT7 receptor-induced excitation of thoracic motoneurones and that the problem could be overcome by the synthesis of more selective 5-HT1B/1D agonists.
The apparent non-involvement of 5-HT1B/1D receptors in modulating reflexes in the present experiments is consistent with an earlier report showing that a 5-HT1B/1D receptor agonist had no effect on tail flick reflex latency in the rat (Mjellem et al., 1992) and the recent observation that naratriptan does not depress the responses of nociceptive dorsal horn neurones in the same species (Cumberbatch et al., 1998b). However, Gjerstad et al. (1997) found that the 5-HT1B agonist CP 93,129 selectively depressed the responses to Aδ fibre stimulation in rat dorsal horn cells, and other agonists for these receptors have been shown to inhibit the tail flick reflex of the mouse (Eide et al., 1990; Alhaider & Wilcox, 1993), and the clasp-knife reflex in the cat (Miller et al., 1995). Clearly more experiments are required to establish what role(s), if any, 5-HT1B/1D receptors might have in the spinal cord. Autoradiographic studies suggest that they are present in the grey matter (Marlier et al., 1991; Thor et al., 1993), and it is possible that the design of the present experiments led us to miss aspects of their function. Several observations gave tantalizing hints of an inhibitory action: GR 127,935 tended to increase reflexes after 8-OH-DPAT; after L-741,604; and in non-spinalized rabbits, and 8-OH-DPAT induced a larger median change in reflexes after GR 127,935 than when given alone. These ‘effects' were all close to achieving statistical significance. The absence of any cardiovascular changes when GR 127,935 was given alone suggests that this drug was not acting as a partial agonist in the present study (Watson et al., 1996). Some 5-HT1B/1D receptors are located on the terminals of descending fibres (Matsumoto et al., 1990; 1992), so that the agonists might show greater activity when the spinal cord is intact. Also, we must consider that fact that none of the drugs used can distinguish between the 5-HT1B (i.e. 5-HT1Dβ) and 5-HT1D (i.e. 5-HT1Dα) receptor types. It is not impossible that the two receptors could exert opposite and equal effects on the sural-MG reflex. Although this does not seem very likely, the development of drugs that select between the types (e.g. Price et al., 1997; Selkirk et al., 1998) will allow the possibility to be tested in the future. In the human trigeminovascular system it has been shown that 5-HT1B receptors are localized on intracranial blood vessels whereas 5-HT1D sites are predominantly found on trigeminal afferent terminals, from whence they can regulate the release of neurotransmitters (Longmore et al., 1997).
Although the functions of 5-HT1B/1D receptors in the spinal cord are not yet established, there is no doubting the importance of these sites in the trigeminal system. Sumatriptan and related 5-HT1B/1D receptor agonists inhibit the responses of trigeminal nucleus caudalis neurones to stimulation of dural nociceptors in cat and rat (Goadsby & Hoskin, 1996; Hoskin et al., 1996; Cumberbatch et al., 1997; 1998a,1998b; Hoskin & Goadsby, 1998), an action that probably contributes to the anti-migraine actions of this class of drugs. These observations raise the intriguing possibility that the spinal and trigeminal nociceptive systems are differentially sensitive to 5-HT1B/1D receptor agonists (Cumberbatch et al., 1998b). The actions of these drugs on trigeminally-organized motor reflexes, which would make for an appropriate comparison with the results of the present study, have not been reported.
The receptors mediating the reflex enhancing actions of 8-OH-DPAT
In a previous study it was shown that the reflex enhancing actions of 8-OH-DPAT were mediated by 5-HT1A receptors and another, ritanserin-sensitive, site likely to be a 5-HT1B/1D or a 5-HT7 receptor (Clarke et al., 1997). The present experiments have shown that none of the effects of 8-OH-DPAT were significantly altered by GR 127,935, whether it was given before or after the agonist. If anything, the effects of 8-OH-DPAT on reflexes were enhanced by treatment with GR 127,935 (see above). It is evident that the facilitatory effects of 8-OH-DPAT are not dependent on an action at 5-HT1B/1D receptors. Process of elimination leads us to believe that the non-5-HT1A receptors activated by this drug are of the 5-HT7 type (Lovenberg et al., 1993; Ruat et al., 1993; Bard et al., 1993). 5-HT7 receptors are thought to be present in spinal cord (Gustafson et al., 1996) and mRNA coding for the receptor has been observed in dorsal root ganglion neurones of rat and human (Pierce et al., 1996; 1997). Nonetheless, it is necessary to test the involvement of these receptors in the present system with a 5-HT7 selective antagonist. One such agent has been described recently (Forbes et al., 1998), but it is not yet available for general use.
Acknowledgments
Some of this work was supported by the Wellcome Trust. We are very grateful to Merck, Sharp and Dohme Neuroscience Research and Glaxo Wellcome Research for the supply of drugs. J. Ogilvie was a University of Nottingham scholar.
Abbreviations
- GR 127,935
2′-methyl-4′-(5-methyl-(1,2,4) oxadiazol-3-yl)-biphenyl-4-carboxylic acid (4-methoxy-3-(4-methyl-piperazin-1-yl-phenyl)-amide.HCl
- IQR
inter-quartile range
- L-694,247
2-(5-(3-(4-methylsulphonylamino)benzyl-1,2,4-oxadiazol-5-yl)-1H-indole-3-yl)ethylamine
- L-741,604
N,N-dimethyl-2-[5-(1,2,4-triazol-4-yl)-1H-indol-3-yl]ethylamine
- MG
medial gastrocnemius
- 8-OH-DPAT
8-hydroxy-2-(di-n-propylamino)tetralin
- WAY-100,635
N-2,N-(2-pyridinyl)cyclohexanecarboxamide.3HCl
References
- ALHAIDER A.A., WILCOX G.L. Differential roles of 5-hydroxytryptamine1A and 5-hydroxytryptamine1B receptor subtypes in modulating spinal nociceptive transmission in mice. J. Pharmacol. Exp. Ther. 1993;265:378–385. [PubMed] [Google Scholar]
- BARD J.A., ZGOMBICK J., ADHAM N., VAYSSE P., BRANCHEK T.A., WEINSHANK R.L. Cloning of a novel human serotonin receptor (5-HT7) positively linked to adenylate cyclase. J. Biol. Chem. 1993;268:23422–23426. [PubMed] [Google Scholar]
- BEER M.S., STANTON J.A., BEVAN Y., HEALD A., REEVE A.J., STREET L.J., MATASSA V.G., HARGREAVES R.J., MIDDLEMISS D.N. L-694,247: A potent 5-HT1D receptor agonist. Br. J. Pharmacol. 1993;110:1196–1200. doi: 10.1111/j.1476-5381.1993.tb13941.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CHOPPIN A., O'CONNOR S.E. 5-hydroxytryptamine1D-like receptors mediate contraction of partially depolarised rabbit renal arteries. J. Pharmacol. Exp. Ther. 1994;270:650–655. [PubMed] [Google Scholar]
- CHOPPIN A., O'CONNOR S.E. Presence of vasoconstrictor 5-HT1-like receptors revealed by precontraction of rabbit isolated mesenteric artery. Br. J. Pharmacol. 1995;114:309–314. doi: 10.1111/j.1476-5381.1995.tb13228.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CLARKE R.W., OGILVIE J., HOUGHTON A.K. Enhancement and depression of spinal reflexes by 8-hydroxy-2-(di-n-propylamino)tetralin in the decerebrated and spinalized rabbit: involvement of 5-HT1A- and non-5-HT1A-receptors. Br. J. Pharmacol. 1997;122:631–638. doi: 10.1038/sj.bjp.0701430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CLARKE R.W., OGILVIE J., KINGSTON T.O.W.5-HT1B/1D receptors do not influence transmission in a withdrawal reflex pathway in the decerebrated and spinalized rabbit J. Physiol. 1999. in press
- CUMBERBATCH M.J., HILL R.G., HARGREAVES R.J. Rizatriptan has central antinociceptive effects against durally evoked responses. Eur. J. Pharmacol. 1997;328:37–40. doi: 10.1016/s0014-2999(97)83024-5. [DOI] [PubMed] [Google Scholar]
- CUMBERBATCH M.J., HILL R.G., HARGREAVES R.J. The effects of 5-HT1A, 5-HT1B and 5-HT1D receptor agonists on trigeminal nociceptive neurotransmission in anaesthetized rats. Eur. J. Pharmacol. 1998a;362:43–46. doi: 10.1016/s0014-2999(98)00764-x. [DOI] [PubMed] [Google Scholar]
- CUMBERBATCH M.J., HILL R.G., HARGREAVES R.J. Differential effects of the 5-HT1B/1D receptor agonist naratriptan on trigeminal versus spinal nociceptive responses. Cephalalgia. 1998b;18:659–663. doi: 10.1046/j.1468-2982.1998.1810659.x. [DOI] [PubMed] [Google Scholar]
- DANDO S.B., SKINNER M.R., JORDAN D., RAMAGE A.G. Modulation of the vagal bradycardia evoked by the stimulation of upper airway receptors by central 5-HT1 receptors in anaesthetized rabbits. Br. J. Pharmacol. 1998;125:409–417. doi: 10.1038/sj.bjp.0702085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DECKERT V., PRUNEAU D., ELGHOZI J.L. Mediation by 5-HT1D receptors of 5-hydroxytryptamine-induced contractions of rabbit middle and posterior cerebral arteries. Br. J. Pharmacol. 1994;112:939–945. doi: 10.1111/j.1476-5381.1994.tb13171.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DE VRIES P., APAYDIN S., VILLALON C.M., HEILIGERS J.P.C., SAXENA P.R. Interactions of GR 127935, a 5-HT1B/D receptor ligand, with functional 5-HT receptors. Naunyn-Schmiedeberg's Arch. Pharmacol. 1997;355:423–430. doi: 10.1007/pl00004964. [DOI] [PubMed] [Google Scholar]
- EIDE P.K., JOLY N.M., HOLE K. The role of spinal cord 5-HT1A and 5-HT1B receptors in the modulation of a spinal nociceptive reflex. Brain Res. 1990;536:195–200. doi: 10.1016/0006-8993(90)90025-7. [DOI] [PubMed] [Google Scholar]
- ELWOOD A.J., CURTIS M.J. Involvement of 5-HT1B/1D and 5-HT2A receptors in 5-HT-induced contraction of endothelium denuded rabbit epicardial coronary arteries. Br. J. Pharmacol. 1997;122:875–884. doi: 10.1038/sj.bjp.0701470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FORBES I.T., DABBS S., DUCKWORTH D.M., JENNINGS A.J., KING F.D., LOVELL P.J., BROWN A.M., COLLIN L., HAGAN J.J., MIDDLEMISS D.N., RILEY G.J., THOMAS D.R., UPTON N. (R)-3,N-dimethyl-N-[1-methyl-3-(4-methyl-piperidin-1-yl)propyl]benzenesulfonamide: The first selective 5-HT7 receptor antagonist. J. Med. Chem. 1998;41:655–657. doi: 10.1021/jm970519e. [DOI] [PubMed] [Google Scholar]
- GJERSTAD J., TJOLSEN A., HOLE K. A dual effect of 5-HT1B receptor stimulation on nociceptive dorsal horn neurones in rats. Eur. J. Pharmacol. 1997;335:127–132. doi: 10.1016/s0014-2999(97)01183-7. [DOI] [PubMed] [Google Scholar]
- GOADSBY P.J., HOSKIN K.L. Inhibition of trigeminal neurons by intravenous administration of the serotonin (5-HT1B/D) receptor agonist zolmitriptan (311C90): Are brain stem sites therapeutic target in migraine. Pain. 1996;67:355–359. doi: 10.1016/0304-3959(96)03118-1. [DOI] [PubMed] [Google Scholar]
- GUSTAFSON E.L., DURKIN M.M., BARD J.A., ZGOMBICK J., BRANCHEK T.A. A receptor autoradiographic and in situ hybridization analysis of the distribution of the 5-ht7 receptor in rat brain. Br. J. Pharmacol. 1996;117:657–666. doi: 10.1111/j.1476-5381.1996.tb15241.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HARWOOD G., LOCKYER M., GILES H., FAIRWEATHER N. Cloning and characterisation of the rabbit 5-HT1Dα and 5-HT1Dβ receptors. FEBS Lett. 1995;377:73–76. doi: 10.1016/0014-5793(95)01308-3. [DOI] [PubMed] [Google Scholar]
- HJORTH S., CARLSSON A., MAGNUSSON T., ARVIDSSON L.-E.In vivo biochemical characteristics of 8-OH-DPAT: Evidence for 5-HT receptor selectivity and agonist action in the rat CNS Brain 5-HT1A receptors: Behavioural and neurochemical pharmacology 1987Horwood: Chichester; 105–94.In: Dourish, C.T., Ahlenius, S. & Hudson, P.H. (eds) [Google Scholar]
- HOSKIN K.L., GOADSBY P.J. Comparison of more and less lipophilic serotonin (5-HT1B/1D) agonists in a model of trigeminovascular nociception in cat. Exp. Neurol. 1998;150:45–51. doi: 10.1006/exnr.1997.6749. [DOI] [PubMed] [Google Scholar]
- HOSKIN K.L., KAUBE H., GOADSBY P.J. Sumatriptan can inhibit trigeminal afferents by an exclusively neural mechanism. Brain. 1996;119:1419–1428. doi: 10.1093/brain/119.5.1419. [DOI] [PubMed] [Google Scholar]
- HOYER D., LERY H., WAEBER C., BRUINVELS A.T., NOZULAK J., PALACIOS J.M. ‘5-HT1R' or 5-HT1D sites? Evidence for 5-HT1D binding sites in rabbit brain. Naunyn-Schmiedeberg's Arch. Pharmacol. 1992;346:249–254. doi: 10.1007/BF00173536. [DOI] [PubMed] [Google Scholar]
- LIMBERGER N., DEICHER R., STARKE K. Species differences in presynaptic serotonin autoreceptors: mainly 5-HT1B but possibly in addition 5-HT1D in the rat, 5-HT1D in the rabbit and guinea-pig brain cortex. Naunyn-Schmiedeberg's Arch. Pharmacol. 1991;343:353–364. doi: 10.1007/BF00179039. [DOI] [PubMed] [Google Scholar]
- LONGMORE J., SHAW D., SMITH D., HOPKINS R., MCALLISTER G., PICKARD J.D., SIRINATHSINGHJI D.J.S., BUTLER A.J., HILL R.G. Differential distribution of 5-HT1D- and 5-HT1B- immunoreactivity within the human trigemino-cerebrovascular system: implications for the discovery of new antimigraine drugs. Cephalalgia. 1997;17:833–842. doi: 10.1046/j.1468-2982.1997.1708833.x. [DOI] [PubMed] [Google Scholar]
- LOVENBERG T.W., BARON B.M., DE LECEA L., MILLER J.D., PROSSER R.A., REA M.A., FOYE P.E., RACKE M., SLONE A.L., SIEGEL B.W., DANIELSON P.E., SUTCLIFFE J.G., ERLANDER M.G. A novel adenylyl cyclase-activating serotonin receptor (5-HT7) implicated in the regulation of mammalian circadian rhythms. Neuron. 1993;11:449–458. doi: 10.1016/0896-6273(93)90149-l. [DOI] [PubMed] [Google Scholar]
- MARLIER L., TEILHAC J.R., CERRUTI C., PRIVAT A. Autoradiographic mapping of 5-HT1, 5-HT1A, 5-HT1B and 5-HT2 receptors in the rat spinal cord. Brain Res. 1991;550:15–23. doi: 10.1016/0006-8993(91)90400-p. [DOI] [PubMed] [Google Scholar]
- MATHEW N.T. Serotonin 1(D) (5-HT1D) agonists and other agents in acute migraine. Neurologic Clinics. 1997;15:61–83. doi: 10.1016/s0733-8619(05)70295-4. [DOI] [PubMed] [Google Scholar]
- MATSUMOTO I., COMBS M., BRANNAN S., JONES D.J. Autoreceptor- and hetero-receptor-mediated regulation of monoamine release in spinal cord synaptosomes. Ann. N.Y. Acad. Sci. 1990;604:609–611. [Google Scholar]
- MATSUMOTO I., COMBS M.R., JONES D.J. Characterization of 5-Hydroxytryptamine1B receptors in rat spinal cord via 125I-iodocyanopindolol binding and inhibition of 3H-5-Hydroxytryptamine release. J. Pharmacol. Exp. Ther. 1992;260:614–626. [PubMed] [Google Scholar]
- MILLER J.F., PAUL K.D., RYMER W.Z., HECKMAN C.J. 5-HT1B/1D agonist CGS-12066B attenuates clasp knife reflex in the cat. J. Neurophysiol. 1995;74:453–456. doi: 10.1152/jn.1995.74.1.453. [DOI] [PubMed] [Google Scholar]
- MJELLEM N., LUND A., EIDE P.K., STORKSON R., TJOLSEN A. The role of 5-HT1A and 5-HT1B receptors in spinal nociceptive transmission and in the modulation of NMDA induced behaviour. Neuroreport. 1992;3:1061–1064. doi: 10.1097/00001756-199212000-00007. [DOI] [PubMed] [Google Scholar]
- OGILVIE J., CLARKE R.W. Effect of RX 821002 at 5-HT1A-receptors in rabbit spinal cord in vivo. Br. J. Pharmacol. 1998;123:1138–1142. doi: 10.1038/sj.bjp.0701729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- OGILVIE J., SIMPSON D.A.A., CLARKE R.W. Tonic adrenergic and serotonergic inhibition of a withdrawal reflex in rabbits subjected to different levels of surgical preparation. Neuroscience. 1999;89:1247–1258. doi: 10.1016/s0306-4522(98)00416-3. [DOI] [PubMed] [Google Scholar]
- PIERCE P.A., XIE G.X., LEVINE J.D., PEROUTKA S.J. 5-hydroxytryptamine receptor subtype messenger RNAs in rat peripheral sensory and sympathetic ganglia: A polymerase chain reaction study. Neuroscience. 1996;70:553–559. doi: 10.1016/0306-4522(95)00329-0. [DOI] [PubMed] [Google Scholar]
- PIERCE P.A., XIE G.X., MEUSER T., PEROUTKA S.J. 5-hydroxytryptamine receptor subtype messenger rnas in human dorsal root ganglia: a polymerase chain reaction study. Neuroscience. 1997;81:813–819. doi: 10.1016/s0306-4522(97)00235-2. [DOI] [PubMed] [Google Scholar]
- PRICE G.W., BURTON M.J., COLLIN L.J., DUCKWORTH M., GASTER L., GOTHERT M., JONES B.J., ROBERTS C., WATSON J.M., MIDDLEMISS D.N. SB-216641 and BRL-15572–compounds to pharmacologically discriminate h5-HT1B and h5-HT1D receptors. Naunyn-Schmiedeberg's Arch. Pharmacol. 1997;356:312–320. doi: 10.1007/pl00005056. [DOI] [PubMed] [Google Scholar]
- RUAT M., TRAIFFORT E., LEURS R., TARDIVEL-LACOMBE J., DIAZ J., ARRANG J.-M., SCHWARTZ J.-C. Molecular cloning, characterization, and localization of a high affinity serotonin receptor (5-HT7) activating cAMP formation. Proc. Natl. Acad. Sci. U.S.A. 1993;90:8547–8551. doi: 10.1073/pnas.90.18.8547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SELKIRK J.V., SCOTT C., HO M., BURTON M.J., WATSON J., GASTER L.M., COLLIN L., JONES B.J., MIDDLEMISS D.N., PRICE G.W. SB-224289–a novel selective (human) 5-HT1B receptor antagonist with negative intrinsic activity. Br. J. Pharmacol. 1998;125:202–208. doi: 10.1038/sj.bjp.0702059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SKINGLE M., BEATTIE D.T., SCOPES D.I.C., STARKEY S.J., CONNOR H.E., FENIUK W., TYERS M.B. GR127935: A potent and selective 5-HT1D receptor antagonist. Behav. Brain Res. 1996;73:157–161. doi: 10.1016/0166-4328(96)00089-7. [DOI] [PubMed] [Google Scholar]
- STERNFELD F., BAKER R., BROUGHTON H.B., GUIBLIN A.R., JELLEY R.A., MATASSA V.G., REEVE A.J., BEER M.S., STANTON J.A., HARGREAVES R.J., SHEPHEARD S.L., LONGMORE J., RAZZAQUE Z., GRAHAM M.I., SOHAL B., STREET L.J. The chemical evolution of N,N-dimethyl-2-[5-(1,2,4-triazol-4-yl)-1H-indol-3-yl]ethylamine (L-741,604) and analogues: Potent and selective agonists for 5-HT1D receptors. Bioorg. Med.Chem. Lett. 1996;6:1825–1830. [Google Scholar]
- THOR K.B., NICKOLAUS S., HELKE C.J. Autoradiographic localization of 5-hydroxytryptamine1A, 5-hydroxytryptamine1B and 5-hydroxytryptamine1C/2 binding sites in the rat spinal cord. Neuroscience. 1993;55:235–252. doi: 10.1016/0306-4522(93)90469-v. [DOI] [PubMed] [Google Scholar]
- VALENTIN J.P., BONNAFOUS R., JOHN G.W. Influence of the endothelium and nitric oxide on the contractile responses evoked by 5-HT1D receptor agonists in the rabbit isolated saphenous vein. Br. J. Pharmacol. 1996;119:35–42. doi: 10.1111/j.1476-5381.1996.tb15674.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VAN WIJNGAARDEN I., TULP M.T.H., SOUDIJN W. The concept of selectivity in 5-HT receptor research. Eur. J. Pharmacol. 1990;188:301–312. doi: 10.1016/0922-4106(90)90190-9. [DOI] [PubMed] [Google Scholar]
- WAINSCOTT D.B., JOHNSON K.W., PHEBUS L.A., SCHAUS J.M., NELSON D.L. Human 5-HT1F receptor-stimulated 35S-GTPγS binding: correlation with inhibition of guinea pig dural plasma protein extravasation. Eur. J. Pharmacol. 1998;352:117–124. doi: 10.1016/s0014-2999(98)00336-7. [DOI] [PubMed] [Google Scholar]
- WATSON J.M., BURTON M.J., PRICE G.W., JONES B.J., MIDDLEMISS D.N. GR127935 acts as a partial agonist at recombinant human 5-HT1Dα and 5-HT1Dβ receptors. Eur. J. Pharmacol. 1996;314:365–372. doi: 10.1016/s0014-2999(96)00579-1. [DOI] [PubMed] [Google Scholar]
- WIGGLESWORTH M., APPLEBY L., OGILVIE J., CLARKE R.W.Enhancement of polysynaptic reflexes in the decerebrated rabbit by 8-hydroxy-2-(di-n-propyl-amino)tetralin (8-OH-DPAT) does not involve 5-HT1D receptors J. Physiol. 1997504P [Google Scholar]
- WURCH T., PALMIER C., COLPAERT F.C., PAUWELS P.J. Recombinant saphenous vein 5-HT1B receptors of the rabbit: Comparative pharmacology with human 5-HT1B receptors. Br. J. Pharmacol. 1997;120:153–159. doi: 10.1038/sj.bjp.0700868. [DOI] [PMC free article] [PubMed] [Google Scholar]


