Abstract
We investigated the relationship between angiotensin II formation and the development of atherosclerotic lesions in the aorta of monkeys (Macaca fascicularis) fed a high-cholesterol (4% cholesterol and 6% corn oil) diet for 6 months, and studied the effects of an angiotensin converting enzyme (ACE) inhibitor, trandolapril (10 mg kg−1 per day, p.o.), and an angiotensin II type 1 receptor antagonist, 2-butyl-4-(methylthio)-1-[[2′[[[(propylamino)carbonyl]amino]sulfonyl](1,1′-biphenyl)-4-yl]methyl]-1H-imidazole-5-carboxylate (HR 720; 20 mg kg−1 per day, p.o.).
The level of low-density lipoprotein was significantly increased by the cholesterol diet, whereas that of high-density lipoprotein was significantly decreased. The relative areas of the atherosclerotic lesions in the thoracic aorta in the normal and cholesterol-diet groups were 1.3±0.3 and 64±10%, respectively.
Plasma renin and ACE activities showed no differences between the normal and cholesterol-diet groups. ACE activity and the concentration of angiotensin II were significantly increased in the aorta of the cholesterol-fed monkeys.
Trandolapril and HR 720 decreased significantly the area of the atherosclerotic lesions in the thoracic aorta of cholesterol-fed monkeys, but not the mean blood pressure and the levels of low-density and high-density lipoproteins.
In plasma and aorta, trandolapril, but not HR 720, decreased significantly the ACE activities in the cholesterol-fed monkeys, while both of these drugs decreased significantly the angiotensin II levels.
In conclusion, blockade of angiotensin II function in vascular tissues by trandolapril or HR 720 may play an important role in preventing the development of atherosclerotic lesions.
Keywords: Angiotensin II, inhibitor, antagonist, atherosclerosis, monkey
Introduction
It is well known that hypertension is a risk factor for atherosclerosis, but a reduction in blood pressure does not necessarily result in blocking lesion development in animal models of atherosclerosis (Spence et al., 1977; Blumlein et al., 1984), suggesting that additional mechanisms contribute to it. Angiotensin II has been shown to be a potent vasoconstrictor, and it also promotes the growth of vascular smooth muscle cells via the activation of several growth factors such as platelet-derived growth factor and fibroblast growth factor (Itoh et al., 1993). Angiotensin II is generated from angiotensin I by angiotensin converting enzyme (ACE) in plasma, whereas biochemical and molecular biological techniques have demonstrated that the major components of the system are also detectable in cardiovascular tissues (Lindpaintner et al., 1989). In immunohistochemical studies, ACE is found in endothelium, as is well known (Bruneval et al., 1986; Laliberte et al., 1987).
Mitani et al. (1996) demonstrated that ACE activity in atherosclerotic lesions in hyperlipidemic rabbits was increased significantly compared with that in the normal rabbit aorta. Previous reports showed that an ACE inhibitor, captopril, reduced the progression of atherosclerosis in Watanabe heritable hyperlipidemic (WHHL) rabbits (Chobanian et al., 1990) and in high-cholesterol-fed monkeys (Aberg & Ferrer, 1990). Captopril is a sulphydryl ACE inhibitor, and the atherosclerotic activity was attributed to its antioxidant effects or to the reduction of blood pressure. However, some reports have indicated that non-sulphydryl ACE inhibitors also decreased the development of atherosclerosis in rabbit and monkey (Chobanian et al., 1992; Schuh et al., 1993; Song et al., 1998), suggesting that the anti-atherosclerotic effect of ACE inhibitors is not dependent on the presence of a sulphydryl group. On the other hand, ACE is also called kininase II (Yang et al., 1971), which degrades bradykinin to inactive fragments, and it is thought that the inhibitory effects of ACE inhibitor on atherosclerosis may be due to the enhancement of nitric oxide (NO) and prostacyclin via the accumulation of bradykinin (Finta et al., 1993; Riezebos et al., 1994a,1994b). However, the decrease of angiotensin II or the increase of bradykinin by an ACE inhibitor has not yet been demonstrated in vascular tissues.
To clarify the relationship between atherosclerosis and angiotensin II formation in vascular tissue, we investigated the angiotensin II system including the levels of renin, ACE and angiotensin II, and studied the effects of an ACE inhibitor, trandolapril (Brown et al., 1988), and an angiotensin II type 1 (AT1) receptor antagonist, HR 720 (Jin et al., 1997).
Methods
Animals
Twenty-three monkeys (Macaca fascicularis) of either sex were purchased from Keari Co. (Osaka, Japan). The body weight of these monkeys ranged between 3.5 and 5.1 kg. All monkeys were housed at room temperature (23–26°C) with a 12-h light–dark cycle and had free access to food and water. To measure lipid levels and ACE activity, blood samples were taken from the saphenous vein. At the end of the 6-month treatment period, the monkeys were anaesthetized with ketamine hydrochloride (10 mg kg−1, i.m.) and sacrificed by bleeding from the carotid artery. The experimental procedures for animals were in accordance with the Guide for the Care and Use of Laboratory Animals (Animal Research Laboratory, Osaka Medical College).
Experimental protocol
Six monkeys (four males, two females) were fed a normal diet for 6 months, and these monkeys were called the normal-diet group. The remaining 16 monkeys fed a high-cholesterol diet for 6 months were grouped as follows: the high-cholesterol-diet group (four males, two females), the group administrated orally with trandolapril (10 mg kg−1 per day, three males, two females) and the group administrated orally with HR 720 (20 mg kg−1 per day, three males, two females). The normal diet and the high cholesterol diet, which contains 4% cholesterol and 6% corn oil, were purchased from Oriental Yeast Co. (Osaka, Japan).
Under anaesthesia with ketamine hydrochloride (10 mg kg−1, i.m.), the mean blood pressure and heart rate were monitored directly via a Surflo catheter (Termo, Tokyo, Japan) with a transducer (MP-4, Nihon Kohden, Tokyo, Japan) at 3 and 6 months. Body weight was measured at 3 and 6 months. The plasma and serum were separated by centrifugation at 3000 r.p.m. for 15 min at 4°C.
Cholesterol and lipoproteins
The level of total cholesterol was measured with the use of cholesterol oxidase (Richmond, 1976). The level of low-density lipoprotein (LDL) was determined with the use of heparin and calcium (Noma et al., 1978) and that of high density lipoprotein (HDL)-cholesterol was determined by precipitation with sodium phosphotungstate and magnesium chloride (Seigler & Wu, 1981).
ACE and renin activity
Tissue extracts for measurement of ACE activity were prepared as described previously (Miyazaki et al., 1984). Tissues were minced and homogenized in 5 volumes (w v−1) of 20 mM Tris-HCl buffer, pH 8.3, containing (mM): Mg(CH3COO)2 5, KCl 30, sucrose 250 and 0.5% NP-40. The homogenate was centrifuged at 20,000×g for 30 min at 4°C. The supernatant was used for the measurement of ACE activity and protein concentration.
The ACE activity in plasma or tissue extract was measured using a synthetic substrate, hippuryl-His-Leu (HHL), specifically designed for ACE (Peptide Institute, Inc., Osaka, Japan). Fifty microliters of tissue extract or plasma were incubated for 30 min at 37°C with 5 mM HHL in 250 μl of 10 mM phosphate buffer, pH 8.3, containing 0.6 M NaCl. The reaction was terminated by addition of 750 μl of 3% metaphosphoric acid, and then the mixture was centrifuged at 20,000×g for 5 min at 4°C. The supernatant was analysed using a reversed phase column (RP-18, 4 mm i.d. ×250 mm, IRICA Instrument, Kyoto, Japan).
The plasma renin activity was measured by radioimuno-assay of [125I]-angiotensin I using a SRL kit (TFB, Tokyo, Japan) at 3 and 6 months.
Angiotensin II and protein concentrations
The angiotensin II concentration in vascular tissues was measured using the procedure of Kim et al. (1992). The tissues were immediately heated to 100°C in 10 volumes (w v−1) of boiled distilled water for 10 min and then cooled in ice water. They were subsequently homogenized using a glass homogenizer. The homogenate was centrifuged at 20,000×g for 30 min at 4°C and the supernatant was applied to a Sep-pak C18 cartridge (Millipore Waters, Bedford, MA, U.S.A.) which was washed with methanol and then equilibrated with 0.1% trifluoroacetic acid. The cartridge was washed with methanol/water/trifluoroacetic acid (10 : 89.9 : 0.1 v v v−1) and eluted with methanol/water/trifluoroacetic acid (80 : 19.9 : 0.1 v v v−1). The eluted medium was dried and dissolved in 10 mM phosphoric acid (pH 3.4) and applied to an ODS-80Tm column (4.6×250 mm I.D., Tosoh, Yamaguchi, Japan). The column was eluted with a linear gradient (30–75%) of methanol in 10 mM phosphoric acid (pH 3.4) at a flow rate of 1.0 ml min−1. Each fraction was subjected to specific radioimmunoassay of angiotensin II.
The protein concentration of the extract was measured by bicinchoninic acid protein assay reagent (Pierce Chemical, Rockford, IL, U.S.A.) using bovine serum albumin as a standard.
Pathological study
The areas of the atherosclerotic lesions of the thoracic aortas were measured as described previously (Catalano & Lillie, 1975). The thoracic aorta was fixed with neutral buffered formalin. The fixed tissue was stained with oil red O for visualization of the presence of lipid deposits. The atheromatous area was calculated as the ratio of the oil-red stained area to all of the intima area with an image analyzer (VM-30, Olympus Co. Ltd., Tokyo, Japan).
Statistical analysis
All values were expressed as means±s.e.mean. Data were analysed by a multiple comparison test (Dunnet's method) and differences were considered to be significant at P<0.05.
Drugs
An ACE inhibitor, trandolapril (Brown et al., 1988), and an angiotensin II type 1 receptor antagonist, HR 720 (Jin et al., 1997), were provided by Nippon Rousell (Tokyo, Japan). Angiotensin I and angiotensin II were purchased from Peptide Institute (Minoh, Japan).
Results
Animal model
All monkeys appeared to be healthy, and no differences in the body weight, mean blood pressure and heart rate were found among the animals of the normal-diet group and the those of the high-cholesterol-diet group throughout the experimental period (Table 1). The areas of the atherosclerotic lesions in the thoracic aortas in the normal-diet and high-cholesterol-diet groups were 1.3±0.3 and 64±10%, respectively (Figures 1 and 2). After a period of 1–6 months in the monkeys fed a normal-diet and a high-cholesterol diet, the levels of total cholesterol and LDL in the high-cholesterol-diet group were significantly increased compared with these levels in the normal-diet group, whereas the level of HDL-cholesterol in the high-cholesterol-diet group was significantly decreased (Figure 3). Plasma renin activity in the high-cholesterol-diet group was not changed compared with that in the normal-diet group (Figure 4). Plasma ACE activity was not affected by a high-cholesterol diet, while the aorta ACE activity in the high-cholesterol-diet group was significantly increased compared to that in the normal-diet group (Figure 5). The levels of angiotensin II in the thoracic aorta in monkeys fed a normal diet or a high-cholesterol diet were 24±3 or 33±3 pg g−1 tissue, respectively, and this difference was statistically significant (Figure 6).
Table 1.
Time course of body weight, mean arterial pressure and heart rate in monkeys fed a normal diet, a high-cholesterol diet, or a high-cholesterol diet plus treatment with trandolapril or HR 720
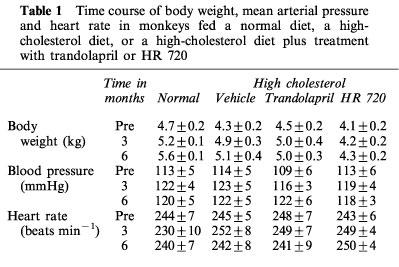
Figure 1.
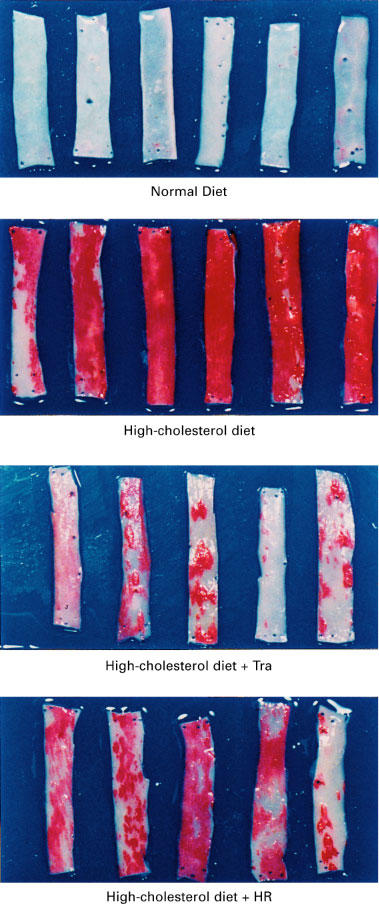
Segments of the aorta stained with oil red O from monkeys fed a normal diet, a high-cholesterol diet, or a high-cholesterol diet plus treatment with trandolapril (Tra) or HR 720 (HR).
Figure 2.
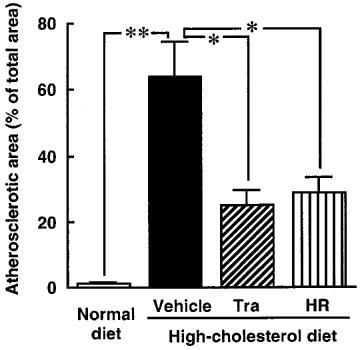
The calculated atherosclerotic lesion in monkeys fed a normal diet, a high-cholesterol diet, or a high-cholesterol diet plus treatment with trandolapril (Tra) or HR 720 (HR). *P<0.05 and **P<0.01 vs the high-cholesterol-diet group.
Figure 3.
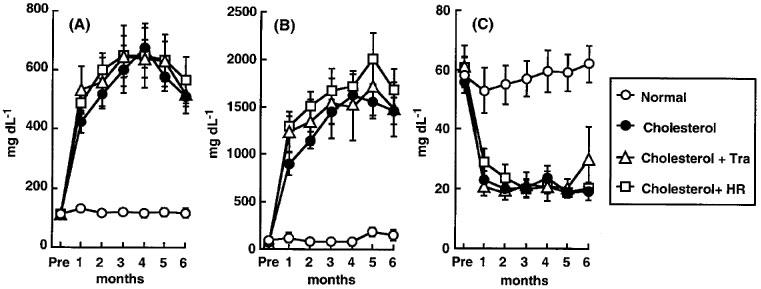
Time course of total cholesterol (A), LDL (B) and HDL-cholesterol (C) in monkeys fed a normal diet, a high-cholesterol diet, or a high-cholesterol diet plus treatment with trandolapril (Tra) or HR 720 (HR).
Figure 4.
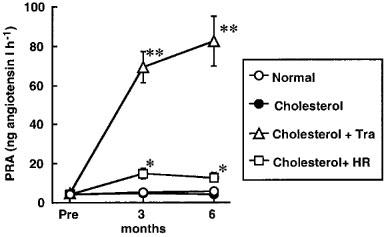
Time course of PRA in monkeys a normal diet, a high-cholesterol diet, or a high-cholesterol diet plus treatment with trandolapril (Tra) or HR 720 (HR). *P<0.05 and **P<0.01 vs the high-cholesterol-diet group.
Figure 5.
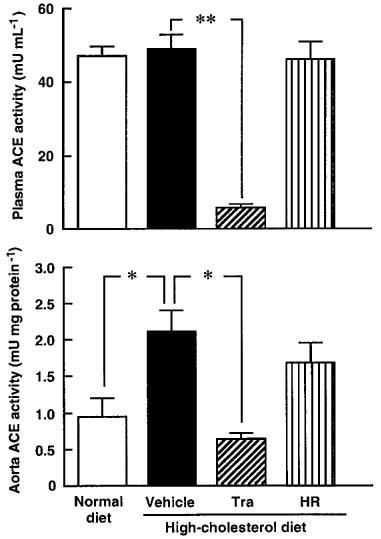
ACE activities in plasma and aorta in monkeys fed a normal diet, a high-cholesterol diet, or a high-cholesterol diet plus treatment with trandolapril (Tra) or HR 720 (HR) after 6 months. *P<0.05 and **P<0.01 vs the high-cholesterol-diet group.
Figure 6.
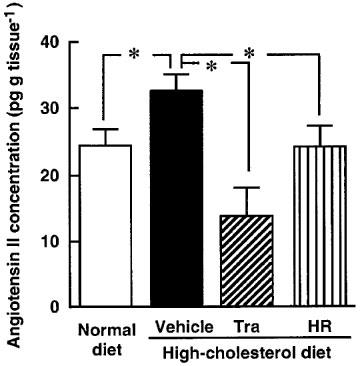
Angiotensin II concentration in aorta in monkeys fed a normal diet, a high-cholesterol diet, or a high-cholesterol diet plus treatment with trandolapril (Tra) or HR 720 (HR) after 6 months. *P<0.05 vs the high-cholesterol-diet group.
Effects of ACE inhibitor and angiotensin II antagonist
In the high-cholesterol-diet groups treated with trandolapril (10 mg kg−1 day−1) and HR 720 (20 mg kg−1 day−1), the mean blood pressure and heart rate were not affected by the drugs (Table 1). The atherosclerotic lesions in the groups treated with trandolapril and HR 720 were significantly reduced to 39 and 45%, respectively, compared with those in the drug-free high-cholesterol-diet group (Figures 1 and 2). The doses of trandolapril and HR 720 did not influence the concentrations of total cholesterol, LDL and HDL-cholesterol in the high-cholesterol-diet group (Figure 3). The renin activities were significantly increased 20.6 and 3.2 fold, respectively, by treatment with trandolapril and HR 720 compared with that in the drug-free high-cholesterol-diet group (Figure 4). The ACE activities in the plasma and aorta were significantly reduced by trandolapril, but not by HR 720 (Figure 5). The levels of angiotensin II concentration in the animals of the high-cholesterol-diet group were significantly reduced to 42.5 and 74.4% by trandolapril and HR 720, respectively (Figure 6).
Discussion
In the present study, there were no differences in the blood pressure and heart rate of the monkeys fed a normal-diet or a high-cholesterol-diet. Although hypertension is an important risk factor in the development of atherosclerosis (Middeke & Holzgreve, 1988; Sasaki et al., 1994), the findings of the present study are not related to hypertension. The plasma levels of total cholesterol and LDL in the high-cholesterol-diet monkeys were significantly increased compared with those in the normal-diet monkeys, whereas the levels of HDL-cholesterol were significantly reduced. It is well known that LDL levels are increased and that the HDL levels are reduced in patients with atherosclerosis (Miller, 1982). The role of LDL on the development of atherosclerosis has been reported previously (Steinberg et al., 1989; Witztum, 1994), while that of HDL has not been clearly elucidated. On the other hand, in the present study, ACE and renin activities in plasma were not affected in monkeys fed a high-cholesterol diet, while ACE activity in the aorta is significantly increased. Moreover, the angiotensin II level in the atherosclerotic aorta is increased when compared with that the normal aorta. This finding suggests that angiotensin II generation is increased in atherosclerotic lesions due to the activation of ACE activity in vascular tissue, but not in plasma. In this model, the development of atherosclerosis was thought to be induced by the increase of total cholesterol and LDL in plasma and angiotensin II generation due to the activation of vascular ACE.
In studying the effects of an ACE inhibitor and an angiotensin II receptor antagonist on atherosclerosis, it was found that trandolapril (10 mg kg−1 day−1) and HR 720 (20 mg kg−1 day−1) decreased significantly the atherosclerotic area in the aorta of monkeys fed a high-cholesterol diet. The doses of trandolapril and HR 720 did not reduce blood pressure in monkeys fed a high-cholesterol diet. However, both drugs significantly increased plasma renin activity, which is up-regulated under the blockade of angiotensin II function (Belz et al., 1988; Giannattasio et al., 1992). The differences in the effects of angiotensin II antagonists relative to the reduction of blood pressure and to the blockade of angiotensin II function in vascular tissue were suggested by some reports. For the blockade of angiotensin II-induced pressor responses, the dose of angiotensin II antagonist needed is more than 10–100 times that for angiotensin II-induced contraction in isolated vessels (Shibouta et al., 1993; Jin et al., 1997). The blood pressure of monkeys used in this study was normal; therefore the doses of these drugs might reduce the renin-angiotensin system in tissue without reducing the blood pressure. Previous reports have indicated that captopril decreases the development of atherosclerosis in rabbit and monkey along with a reduction of blood pressure (Chobanian et al., 1990; Aberg & Ferrer, 1990). Chobanian et al. (1995) reported that a high dose of trandolapril reduced the atherosclerotic area in WHHL rabbits with a reduction in blood pressure, but that a low dose of the drug neither decreased atherosclerosis nor blood pressure. However, in the present study, trandolapril and HR 720 significantly decreased the development of atherosclerosis without a reduction in blood pressure. Although blood pressure is a risk factor for development of atherosclerosis, the present model suggests that the anti-atherosclerotic activity of ACE inhibitors is not necessarily dependent on their anti-hypertensive effects.
Increases of cholesterol and LDL have been observed in patients with atherosclerosis (Miller, 1982), and cholesterol-lowering drugs may prevent the development of atherosclerosis (Watanabe et al., 1988; La Ville et al., 1989). However, trandolapril and HR 720 did not reduce the levels of cholesterol and LDL in plasma, while both of these drugs prevented the development of atherosclerosis. Previous reports also demonstrated that ACE inhibitors suppressed the development of atherosclerosis in minipig and monkey without changing plasma lipids (Jacobsson et al., 1994; Song et al., 1998).
The anti-atherosclerotic effect of ACE inhibitors may thus occur in the absence of reductions in plasma cholesterol, LDL or blood pressure. Angiotensin II directly stimulates cell proliferation via the activation of various growth factors (Naftilan et al., 1990; Gibbons et al., 1992). In previous reports, ACE activity was found to be increased in atherosclerotic lesions in hyperlipidemic rabbits and monkeys (Mitani et al., 1996; Song et al., 1998), and the present study demonstrated that the angiotensin II concentration in atherosclerotic lesions is increased as well. Trandolapril reduced ACE activity and the angiotensin II concentration in the aorta of monkeys fed a high-cholesterol diet, thus suggesting that the angiotensin II formation might be dependent on vascular ACE. The finding that HR 720 also reduced the angiotensin II concentration in the aorta of monkeys fed a high-cholesterol diet, was to some extent unexpected. Keidar (1998) reported that angiotensin II enhances scavenger receptor affinity to oxidized LDL via activation of AT1 receptors on the macrophage surface. Activated macrophages express ACE mRNA and protein (Kowala et al. 1998). Therefore, when HR 720 blocks AT1 receptors in the macrophage, the macrophage activation may be reduced, resulting in a decreased induction of ACE. In fact, HR 720 reduced ACE activity in monkeys fed a high-cholesterol diet, and this may result in a reduction of angiotensin II levels in the vascular tissues.
ACE is a bradykinin-degrading enzyme, and bradykinin may be increased in atherosclerotic lesions after treatment with an ACE inhibitor. In the rabbit model, the bradykinin antagonist icatibant (Hoe 140) abrogated the anti-atherosclerotic activity of the ACE inhibitor ramipril (Linz et al., 1993, 1995), suggesting that the increase of bradykinin upon ACE inhibition plays a role in the suppression of atherosclerosis. In the cholesterol fed rabbit, the cyclic GMP level, which assesses NO, was decreased in the atherosclerotic lesion, whereas ACE inhibitor treatment increased the cyclic GMP level, suggesting an increase of NO caused by accumulation of bradykinin due to ACE inhibition (Riezebos et al., 1994a,1994b). ACE inhibition has been shown to increase NO formation via accumulation of bradykinin in endothelial cells (Wiemer et al., 1991). In the rabbit model, doses of AT1 receptor antagonist which blocked most pressor effects of infused angiotensin II could not affect the development of atherosclerosis (Schuh et al., 1993), and the accumulation of bradykinin has been proposed to mediate the anti-atherosclerotic activity of ACE inhibition. However, in the present study, an AT1 receptor antagonist, HR 720, just like an ACE inhibitor, decreased the atherosclerotic area, suggesting that the inhibition of atherosclerotic lesions is dependent on the blockade of angiotensin II function in the monkey model.
ACE is known to convert angiotensin I to angiotensin II in vascular tissues, whereas we purified non-ACE angiotensin II-forming enzyme from human and monkey arteries and identified it as chymase (Takai et al., 1997a,1997b). Recently, we reported that dogs have a chymase in vascular tissues, and chymase activities were significantly increased in injured vessels and that an AT1 receptor antagonist was effective in preventing neointimal formation after balloon injury of vessels in dog, whereas an ACE inhibitor was ineffective (Miyazaki et al., 1999). These findings suggest that the chymase-dependent angiotensin II formation in vascular tissue may be closely related to promoting growth. In monkeys fed a high-cholesterol diet, chymase mRNA was increased significantly in atherosclerotic lesions of the aorta (Takai et al., 1997a). In the rat atherosclerotic model, the concentration of serum chymase was positively correlated with the intimal thickness (Nishizono et al., 1999). Moreover, in monkeys, the expression of AT1 receptors increased in atherosclerotic lesions and transforming growth factor β, which is induced by angiotensin II, was raised as well (Song et al., 1998). Based on these results, we expected that the AT1 receptor antagonist was more useful for preventing the development of atherosclerosis than the ACE inhibitor, because the AT1 receptor antagonist, unlike ACE inhibitors, would inhibit the effect of chymase-dependent angiotensin II formation. However, in the present study, both the AT1 receptor antagonist and the ACE inhibitor prevented atherosclerosis, to a similar extent. The functional significance for chymase in the development of atherosclerosis might be revealed in further studies with the use of specific chymase inhibitors.
In conclusion, in monkeys fed a high-cholesterol diet, atherosclerosis is induced along with increases of local angiotensin II concentration by vascular ACE; both an ACE inhibitor, trandolapril, and an AT1 receptor antagonist, HR 720, were effective in preventing the development of atherosclerosis with a reduction of angiotensin II, but without a reduction of blood pressure, cholesterol or LDL. These findings suggest that the therapeutic mechanism by an ACE inhibitor and an AT1 receptor antagonist depends on the blockade of angiotensin II function and angiotensin II may play an important role in the development of atherosclerosis.
Acknowledgments
We thank Dr Shokei Kim (Osaka City University Medical School) for the gift of anti-angiotensin II serum. This study was supported in part by a Grant-in-Aid for Scientific Research (B) 08457031 from the Ministry of Education, Science, Sports and Culture, Japan, and by a research grant (1998) from Jinsenkai, the Alumni of Osaka Medical College.
Abbreviations
- ACE
angiotensin converting enzyme
- HDL
high density lipoprotein
- HHL
hippuryl-His-Leu
- LDL
low-density lipoprotein
- NO
nitric oxide
- WHHL
Watanabe heritable hyperlipidemic
References
- ABERG G., FERRER P. Effects of captopril on atherosclerosis in cynomolgus monkeys. J. Cardiovasc. Pharmacol. 1990;15 Suppl:S65–S72. [PubMed] [Google Scholar]
- BELZ G.G., ESSIG J., KLEINBLOESEM C.H., HOOGKAMER J.F., WIEGAND U.W., WELLSTEIN A. Interactions between cilazapril and propranolol in man; plasma drug concentrations, hormone and enzyme responses, haemodynamics, agonist dose-effect curves and baroreceptor reflex. Br. J. Clin. Pharmacol. 1988;26:547–556. doi: 10.1111/j.1365-2125.1988.tb05294.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BLUMLEIN S.L., SIEVERS R., KIDD P., PARMLEY W.W. Mechanism of protection from atherosclerosis by verapamil in the cholesterol-fed rabbit. Am. J. Cardiol. 1984;54:884–889. doi: 10.1016/s0002-9149(84)80226-x. [DOI] [PubMed] [Google Scholar]
- BROWN NL., BADEL M.Y., BENZONI F., ZANIRATO J., VINCENT J.C., FICHELLE J., WORCEL M. Angiotensin-converting enzyme inhibition, anti-hypertensive activity and hemodynamic profile of trandolapril (RU 44570) Eur. J. Pharmacol. 1988;148:79–91. doi: 10.1016/0014-2999(88)90456-6. [DOI] [PubMed] [Google Scholar]
- BRUNEVAL P., HINGLAIS N., ALHENC-GELAS F., TRICOTTET V., CORVOL P., MENARD J., CAMILLERI J.P., BARIETY J. Angiotensin I converting enzyme in human intestine and kidney. Ultrastructual immunohistochemical locarization. Histochemistry. 1986;85:73–80. doi: 10.1007/BF00508656. [DOI] [PubMed] [Google Scholar]
- CATALANO R.A., LILLIE R.D. Elimination of precipitates in oil red O fat stain by adding dextrin. Stain Technol. 1975;50:297–299. doi: 10.3109/10520297509117078. [DOI] [PubMed] [Google Scholar]
- CHOBANIAN A.V., HAUDENSCHILD C.C., NICKERSON C., DRAGO R. Antiatherogenic effect of captopril in the Watanabe heritable hyperlipidemic rabbit. Hypertension. 1990;15:327–331. doi: 10.1161/01.hyp.15.3.327. [DOI] [PubMed] [Google Scholar]
- CHOBANIAN A.V., HAUDENSCHILD C.C., NICKERSON C., HOPE S. Trandolapril inhibits atherosclerosis in the Watanabe heritable hyperlipidemic rabbits. Hypertension. 1992;20:473–477. doi: 10.1161/01.hyp.20.4.473. [DOI] [PubMed] [Google Scholar]
- CHOBANIAN A.V., HOPE S., BRECHER P. Dissociation between the antiatherosclerotic effect of trandolapril and suppression of serum and aortic angiotensin-converting enzyme activity in the Watanabe heritable hyperlipidemic rabbit. Hypertension. 1995;25:1306–1310. doi: 10.1161/01.hyp.25.6.1306. [DOI] [PubMed] [Google Scholar]
- FINTA K.M., FISCHER M.J., LEE L., GORDON D., PITT B., WEBB R.C. Ramipril prevents impaired endothelium-dependent relaxation in arteries from rabbits fed an atherogenic diet. Atherosclerosis. 1993;100:149–156. doi: 10.1016/0021-9150(93)90201-5. [DOI] [PubMed] [Google Scholar]
- GIANNATTASIO C., CATTANEO B.M., OMBONI S., SERAVALLE G., BOLLA G., TUROLO L., MORGANTI A., GRASSI G., ZANCHETTI A., MANCIA G. Sympathomoderating influence of benazepril in essential hypertension. J. Hypertens. 1992;10:373–378. doi: 10.1097/00004872-199204000-00009. [DOI] [PubMed] [Google Scholar]
- GIBBONS G.H., PRATT R.E., DZAU V.J. Vascular smooth muscle cell hypertrophy vs hyperplasia. Autocrine transforming growth factor-beta 1 expression determines growth response to angiotensin II. J. Clin. Invest. 1992;90:456–461. doi: 10.1172/JCI115881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ITOH H., MUKOYAMA M., PRATT R.E., GIBBONS G.H., DZAU V.J. Multiple autocrine growth factors modulate vascular smooth muscle cell growth response to angiotensin II. J. Clin. Invest. 1993;91:2268–2274. doi: 10.1172/JCI116454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- JACOBSSON L.S., PERSSON K., ABERG G., ANDERSSON R.G., KARLBERG B.E., OLSSON A.G. Antiatherosclerotic effects of the angiotensin-converting enzyme inhibitors captopril and fosinopril in hypercholesterolemic minipigs. J. Cardiovasc. Pharmacol. 1994;24:670–677. doi: 10.1097/00005344-199410000-00019. [DOI] [PubMed] [Google Scholar]
- JIN D., SONG K., OKA Y., TAKAI S., SHIOTA N., MIYAZAKI M. Pharmacological Profiles of a novel non-peptide angiotensin II type I receptor antagonist HR720 in vitro and in vivo. Jpn. J. Pharmacol. 1997;75:259–266. doi: 10.1254/jjp.75.259. [DOI] [PubMed] [Google Scholar]
- KEIDAR S. Angiotensin, LDL peroxidation and atherosclerosis. Life Sci. 1998;63:1–11. doi: 10.1016/s0024-3205(98)00014-9. [DOI] [PubMed] [Google Scholar]
- KIM S., TOKUYAMA M., HOSOI M., YAMAMOTO K. Adrenal and circulating renin-angiotensin system in stroke-prone hypertensive rats. Hypertension. 1992;20:280–291. doi: 10.1161/01.hyp.20.3.280. [DOI] [PubMed] [Google Scholar]
- KOWALA M.C., VALENTINE M., RECCE R., BEYER S., GOLLER N., DURHAM S., ABERG G. Enhanced reduction of atherosclerosis in hamsters treated with pravastatin and captopril: ACE in atheromas provides cellular targets for captopril. J. Cardiovasc. Pharmacol. 1998;32:29–38. doi: 10.1097/00005344-199807000-00005. [DOI] [PubMed] [Google Scholar]
- LAIBERTE F., LAIBERTE M.F., ALHENC-GELAS F., CHEVILLARD C. Cellular and subcellular immunohistochemical in the rat adrenal gland. Lab. Invest. 1987;56:364–371. [PubMed] [Google Scholar]
- LA VILLE A.E., SEDDON A., SHAIKH M., ROWLES P.M., WOOLF N., LEWIS B. Primary prevention of atherosclerosis by lovastatin in a genetically hyperlipidaemic rabbit strain. Atherosclerosis. 1989;78:205–210. doi: 10.1016/0021-9150(89)90224-4. [DOI] [PubMed] [Google Scholar]
- LINDPAINTNER K., JIN M., WILHELM M., TOTH M., GANTEN D. Aspects of molecular biology and biochemistry of the cardiac renin-angiotensin system. Br. J. Clin. Pharmacol. 1989;27 Suppl:S159–S165. doi: 10.1111/j.1365-2125.1989.tb03477.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LINZ W., WIEMER G., GOHLKE P., UNGER T., SCHOLKENS B.A. Contribution of kinins to the cardiovascular actions of angiotensin-converting enzyme inhibitors. Pharmacol. Rev. 1995;47:25–49. [PubMed] [Google Scholar]
- LINZ W. , WIEMER G., SCHOLKENS B.A. Contribution of bradykinin to the cardiovascular effects of ramipril. J. Cardiovasc. Pharmacol. 1993;22 Suppl:S1–S8. [PubMed] [Google Scholar]
- MIDDEKE M., HOLZGREVE H. Review of major intervention studies in hypertension and hyperlipidemia: focus on coronary heart disease. Am. Heart. J. 1998;116:1708–1712. doi: 10.1016/0002-8703(88)90219-0. [DOI] [PubMed] [Google Scholar]
- MILLER N.E. Coronary atherosclerosis and plasma lipoproteins: epidemiology and pathophysiologic considerations. J. Cardiovasc. Pharmacol. 1982;4 Suppl:S190–S195. [PubMed] [Google Scholar]
- MITANI H., BANDOH T., KIMURA M., TOTSUKA T., HAYASHI S. Increased activity of vascular ACE related to atherosclerotic lesions in hyperlipidemic rabbits. Am. J. Physiol. 1996;271:H1065–H1071. doi: 10.1152/ajpheart.1996.271.3.H1065. [DOI] [PubMed] [Google Scholar]
- MIYAZAKI M., OKUNISHI H., NISHIMURA K., TODA N. Vascular angiotensin-converting enzyme activity in man and other species. Clin. Sci. 1984;66:39–45. doi: 10.1042/cs0660039. [DOI] [PubMed] [Google Scholar]
- MIYAZAKI M., WADA T., SHIOTA N., TAKAI S. Effect of an angiotensin II receptor antagonist, candesartan cilexetil, on canine intima hyperplasia after balloon injury. J. Hum. Hypertens. 1999;12 Suppl:S21–S25. doi: 10.1038/sj.jhh.1000745. [DOI] [PubMed] [Google Scholar]
- NAFTILAN A.J., GILLILAND G.K., ELDRIDGE C.S., KRAFT A.S. Induction of the proto-oncogene c-jun by angiotensin II. Mol. Cell. Biol. 1990;10:5536–5540. doi: 10.1128/mcb.10.10.5536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NISHIZONO S., KUSABA M., ADAN Y., IMAIZUMI K. Induction of atherosclerosis in brown Norway rats by immunization with ovalbumin. Biosci. Biotechnol. Biochem. 1999;63:379–383. doi: 10.1271/bbb.63.379. [DOI] [PubMed] [Google Scholar]
- NOMA A., NEZU-NAKAYAMA K., KITA M., OKABE H. Simultaneous determination of serum cholesterol in high- and low-density lipoproteins with use of heparin, Ca2+, and an anion-exchange resin. Clin. Chem. 1978;24:1504–1508. [PubMed] [Google Scholar]
- RICHMOND W. Use of cholesterol oxidase for assay of total and free cholesterol in serum by continuous-flow analysis. Clin. Chem. 1976;22:1579–1588. [PubMed] [Google Scholar]
- RIEZEBOS J., VLEEMING W., BEEMS R.B., VAN AMSTERDAM J.G., MEIJER G.W., DE WILDT D.J., PORSIUS A.J., WEMER J. Comparison of the antiatherogenic effects of isradipine and ramipril in cholesterol-fed rabbits: I. Effect on progression of atherosclerosis and endothelial dysfunction. J. Cardiovasc. Pharmacol. 1994a;23:415–423. [PubMed] [Google Scholar]
- RIEZEBOS J., VLEEMING W., BEEMS R.B., VAN AMSTERDAM J.G., MEIJER G.W., DE WILDT D.J., PORSIUS A.J., WEMER J. Comparison of the antiatherogenic effects of isradipine and ramipril in cholesterol-fed rabbits: II. Effect on regression of atherosclerosis and restoration of endothelial function. J. Cardiovasc. Pharmacol. 1994b;23:424–431. [PubMed] [Google Scholar]
- SASAKI S., YONEDA Y., FUJITA H., UCHIDA A., TAKENAKA K., TAKESAKO T., ITOH H., NAKATA T., TAKEDA K., NAKAGAWA M. Association of blood pressure variability with induction of atherosclerosis in cholesterol-fed rats. Am. J. Hypertens. 1994;7:453–459. doi: 10.1093/ajh/7.5.453. [DOI] [PubMed] [Google Scholar]
- SCHUH J.R., BLEHM D.J., FRIERDICH G.E., MCMAHON E.G., BLAINE E.H. Differential effects of renin-angiotensin system blockade on atherogenesis in cholesterol-fed rabbits. J. Clin. Invest. 1993;91:1453–1458. doi: 10.1172/JCI116350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SEIGLER L., WU W.T. Separation of serum high-density lipoprotein for cholesterol determination: ultracentrifugation vs precipitation with sodium phosphotungstate and magnesium chloride. Clin. Chem. 1981;27:838–841. [PubMed] [Google Scholar]
- SHIBOUTA Y., INADA Y., OJIMA M., WADA T., NODA M., SANADA T., KUBO K., KOHARA Y., NAKA T., NISHIKAWA K. Pharmacological profile of a highly potent and long-acting angiotensin II receptor antagonist, 2-ethoxy-1-[ [2′-(1H- tetrazol -5- yl) biphenyl -4- yl] methyl]-1H -benzimidazole-7-carb-oxylic acid (CV-11974), and its prodrug, (+/-)-1-(cyclohexyloxycarbonyloxy)-ethyl 2-ethoxy-1-[ [2′-(1H-tetrazol-5- yl) biphenyl-4-yl] methyl]-1H-benzimidazole-7-carboxylate (TCV-116) J. Pharmacol. Exp. Ther. 1993;266:114–120. [PubMed] [Google Scholar]
- SONG K., SHIOTA N., TAKAI S., TAKASHIMA H., IWASAKI H., KIM S., MIYAZAKI M. Induction of angiotensin converting enzyme and angiotensin II receptors in the atherosclerotic aorta of high-cholesterol fed Cynomolgus monkeys. Atherosclerosis. 1998;138:171–182. doi: 10.1016/s0021-9150(98)00021-5. [DOI] [PubMed] [Google Scholar]
- SPENCE J.D., PESOUT A.B., MELMON K.L. Effects of antihypertensive drugs on blood velocity in rhesus monkeys. Stroke. 1977;8:589–594. doi: 10.1161/01.str.8.5.589. [DOI] [PubMed] [Google Scholar]
- STEINBERG D., CAREW T.E., FIELDING C., FOGELMAN A.M., MAHLEY R.W., SNIDERMAN A.D., ZILVERSMIT D.B. Lipoproteins and the pathogenesis of atherosclerosis. Circulation. 1989;80:719–723. doi: 10.1161/01.cir.80.3.719. [DOI] [PubMed] [Google Scholar]
- TAKAI S., SHIOTA N., KOBAYASHI S., MATSUMURA E., MIYAZAKI M. Induction of chymase that forms angiotensin II in the monkey atherosclerotic aorta. FEBS Lett. 1997a;412:86–90. doi: 10.1016/s0014-5793(97)00752-7. [DOI] [PubMed] [Google Scholar]
- TAKAI S., SHIOTA N., SAKAGUCHI M., MURAGUCHI H., MATSUMURA E., MIYAZAKI M. Characterization of chymase from human vascular tissues. Clin. Chim. Acta. 1997b;265:13–20. doi: 10.1016/s0009-8981(97)00114-9. [DOI] [PubMed] [Google Scholar]
- UJHELYI M.R., FERGUSON R.K., VLASSES P.H. Angiotensin-converting enzyme inhibitors: mechanistic controversies. Pharmacotherapy. 1989;9:351–362. doi: 10.1002/j.1875-9114.1989.tb04149.x. [DOI] [PubMed] [Google Scholar]
- WATANABE Y., ITO T., SHIOMI M., TSUJITA Y., KURODA M., ARAI M., FUKAMI M., TAMURA A. Preventive effect of pravastatin sodium, a potent inhibitor of 3-hydroxy-3-methylglutaryl coenzyme A reductase, on coronary atherosclerosis and xanthoma in WHHL rabbits. Biochim. Biophys. Acta. 1988;960:294–302. [PubMed] [Google Scholar]
- WIEMER G., SCHOLKENS B.A., BECKER R.H., BUSSE R. Ramiprilat enhances endothelial autacoid formation by inhibiting breakdown of endothelium-derived bradykinin. Hypertension. 1991;18:558–563. doi: 10.1161/01.hyp.18.4.558. [DOI] [PubMed] [Google Scholar]
- WITZTUM J.L. The role of oxidized LDL in the atherogenic process. J. Atheroscler. Thromb. 1994;1:71–75. doi: 10.5551/jat1994.1.71. [DOI] [PubMed] [Google Scholar]
- YANG H.Y., ERDOS E.G., LEVIN Y. Characterization of a dipeptide hydrolase (kininase II: angiotensin I converting enzyme) J. Pharmacol. Exp. Ther. 1971;177:291–300. [PubMed] [Google Scholar]


