Abstract
We have extended previous investigations of four analogues of Δ8-tetrahydrocannabinol (Δ8-THC): 6′-azidohex-2′-yne-Δ8-THC (O-1184), 6′-azidohex-cis-2′-ene-Δ8-THC (O-1238) and octyl-2′-yne-Δ8-THC (O-584) and its 1-deoxy-analogue (O-1315).
O-1184, O-1238 and O-584 displaced [3H]-CP55940 from specific binding sites on Chinese hamster ovary (CHO) cell membranes expressing CB1 or CB2 cannabinoid receptors, with pKi values of 8.28 to 8.45 (CB1) and 8.03 to 8.13 (CB2). The pKi values of O-1315 were significantly less, 7.63 (CB1) and 7.01 (CB2).
All the analogues inhibited forskolin-stimulated cyclic AMP production by CB1-transfected CHO cells (pEC50=9.16 to 9.72). Only O-1238 behaved as a full agonist in this cell line.
In mouse vasa deferentia, O-1238 inhibited electrically-evoked contractions (pEC50=10.18 and Emax=70.5%). Corresponding values for O-1184 were 9.08 and 21.1% respectively. At 1 nM, O-1184 produced surmountable antagonism of the cannabinoid receptor agonist, CP55940. However, at 0.1 nM, O-1184 did not attenuate CP55940-induced inhibition of cyclic AMP production by CB1-transfected CHO cells.
In CB2-transfected CHO cells, cyclic AMP production was inhibited by CP55940 (pEC50=8.59), enhanced by O-1184 and O-584 (pEC50=8.20 and 6.86 respectively) and not significantly affected by O-1238 or O-1315.
At 100 nM, O-1184 and O-1238 produced surmountable antagonism of CP55940 in CB2 cells, decreasing the pEC50 of CP55940 from 8.61 to 7.42 (O-1184) or from 8.54 to 7.44 (O-1238).
These data support the hypothesis that increasing the degree of unsaturation of the aliphatic side-chain of Δ8-THC analogues has little effect on CB1 or CB2 receptor affinity but can reduce CB1 receptor efficacy and reverse the direction of responses elicited at CB2 receptors.
Keywords: Cannabinoids, cannabinoid CB1 receptors, cannabinoid CB2 receptors, inverse agonism, 6′-azidohex-2′-yne-Δ8-tetrahydrocannabinol, O-1184, mouse vas deferens
Introduction
In previous experiments we have shown that the synthetic cannabinoid, 6′-azidohex-2′-yne-Δ8-tetrahydrocannabinol (O-1184, Figure 1) behaves as a potent and selective cannabinoid receptor antagonist in the myenteric plexus-longitudinal muscle preparation of guinea-pig small intestine and that it readily binds to cannabinoid receptors both on membranes obtained from this tissue and on guinea-pig forebrain membranes (Ross et al., 1998). More recently, results from binding experiments with rat cerebellar membranes using [35S]-GTPγS or the radiolabelled cannabinoid, [3H]-CP55940, led Griffin et al. (1999) to conclude that O-1184 is a low-efficacy agonist at CB1 receptors. Their data also suggest that replacement of the carbon–carbon triple bond in the aliphatic side-chain of O-1184 with a cis double bond to form 6′-azidohex-cis-2′-ene-Δ8-tetrahydrocannabinol (O-1238; Figure 1) enhances CB1 efficacy, whilst replacement of N3 on the terminal carbon of this side-chain with C2H5 to form octyl-2′-yne-Δ8-tetrahydrocannabinol (O-584; Figure 1) has little effect either on CB1 efficacy or affinity.
Figure 1.
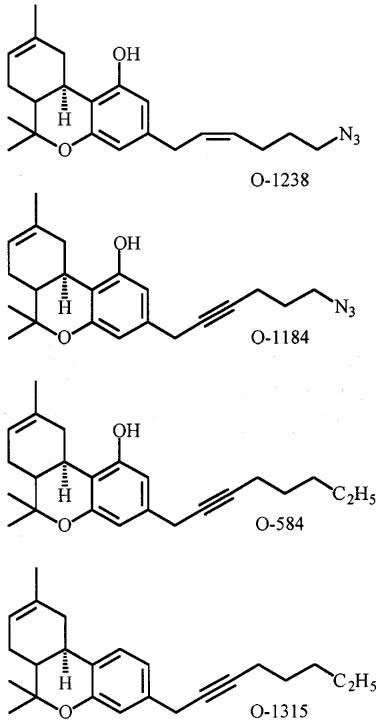
Structures of O-1238, O-1184, O-584 and O-1315.
The present investigation was directed at characterizing the pharmacological properties of O-1184, O-1238 and O-584 more completely using Chinese hamster ovary (CHO) cells expressing human CB1 or CB2 receptors. This was achieved by determining the affinities of these compounds for CB1 and CB2 receptors and by addressing the question of whether they share the ability of the established CB1-CB2 cannabinoid receptor agonist, CP55940, to inhibit forskolin-stimulated cyclic AMP production by CB1 or CB2 transfected cells (Pertwee, 1997). Additional experiments were performed to establish whether O-1184 or O-1238 oppose the ability of CP55940 to inhibit forskolin-stimulated cyclic AMP production in these cell lines. As there are reports that the CB2 receptor affinity of certain analogues of Δ8-tetrahydrocannabinol can be selectively enhanced by removing the 1-hydroxyl group (Huffman et al., 1996; 1998), it was also of interest to compare O-584 with its 1-deoxy-analogue, 1-deoxy-octyl-2′-yne-Δ8-tetrahydrocannabinol (O-1315; Figure 1). Finally, in view of the evidence that O-1184 is a partial agonist (see above), experiments were carried out to establish whether its ability to exhibit cannabinoid receptor antagonist properties in the myenteric plexus-longitudinal muscle preparation of guinea-pig small intestine (Ross et al., 1998) extends to the mouse isolated vas deferens, another cannabinoid receptor-containing preparation (Griffin et al., 1997; Pertwee, 1997).
Methods
Mouse vas deferens
Vasa deferentia were obtained from albino MF1 mice weighing 35–52 g. Each tissue was mounted in a 4 ml organ bath at an initial tension of 0.5 g. The baths contained Krebs solution which was kept at 37°C and bubbled with 95% O2 and 5% CO2. The composition of the Krebs solution was (mM): NaCl 118.2; KCl 4.75; KH2PO4 1.19; NaHCO3 25.0; glucose 11.0 and CaCl2.6H2O 2.54. Isometric contractions were evoked by stimulation with 0.5 s trains of three pulses of 110% maximal voltage (train frequency 0.1 Hz; pulse duration 0.5 ms) through a platinum electrode attached to the upper end and a stainless steel electrode attached to the lower end of each bath. Stimuli were generated by a Grass S48 stimulator, then amplified (Med-Lab channel attenuator) and divided to yield separate outputs to four organ baths (Med-Lab StimuSplitter). Contractions were monitored by computer (Apple Macintosh LCIII and Performa 475) using a data recording and analysis system (MacLab) that was linked via preamplifiers (Macbridge) to Dynamometer UF1 transducers (Pioden Controls). Each tissue was subjected to several 5-min periods of stimulation, the first of these beginning after the tissue had equilibrated but before drug administration. The stimulator was switched off for 25 min between each 5-min stimulation period. Concentration-response curves for O-1184 or O-1238 were constructed cumulatively by adding one or other drug immediately after each 5-min stimulation period. In some experiments, concentration-response curves for CP55940 were constructed in tissues that had been pretreated with O-1184 or O-1238. In these experiments, O-1184, O-1238 or vehicle were administered after the first 5-min stimulation period and CP55940 after the second and all subsequent stimulation periods. For addition to organ baths, each cannabinoid was mixed with two parts of Tween 80 by weight and dispersed in a 0.9% aqueous solution of NaCl (saline) as described previously for Δ9-tetrahydrocannabinol (Pertwee et al., 1992). Drug additions were made in a volume of 10 μl. In control experiments, Tween 80 was added instead of a cannabinoid. The control dose of Tween 80 was the same as the dose added in combination with the highest cannabinoid dose used.
CHO cells
These were stably transfected with cDNA encoding human CB1 (Bmax=3.41 pmol mg−1 protein) or CB2 receptors (Bmax=31.4 pmol mg−1 protein). They were provided by Drs G. Disney and A. Green, GlaxoWellcome R & D, Medicines Research Centre, Stevenage, U.K. and were the same clones as those used in the sPAP reporter assay described by Green et al. (1999). Untransfected CHO cells were obtained from the Centre for Applied Microbiology and Research, Porton Down, U.K. Cells were maintained at 37°C and 5% CO2 in DMEM/f-12 HAM with 2 mM glutamine, geneticin (600 μg ml−1) and hygromycin (300 μg ml−1). For binding experiments, CHO cells were removed from flasks by scraping and pellets frozen at −80°C for up to 1 month. Once defrosted the cell membranes were diluted to 1 mg ml−1 in 50 mM Tris buffer (pH 7.4) and resuspended using a hand-held homogenizer.
Binding experiments
A modification of the filtration procedure described by Compton et al. (1993) was used. Assays were performed with [3H]-CP55940, 1 mM MgCl2, 1 mM EDTA, 1 mg ml−1 bovine serum albumin (BSA) and 50 mM Tris buffer, total assay volume 500 μl. Binding was initiated by the addition of CB1 (100 μg protein) or CB2 cell membranes (50 μg protein). Assays were carried out at 37°C for 30 min before termination by addition of ice-cold wash buffer (50 mM Tris buffer, 1 mg ml−1 BSA) and vacuum filtration using a 12-well sampling manifold (Brandel Cell Harvester) and Whatman GF/B glass-fibre filters that had been soaked in wash buffer at 4°C for 24 h. Each reaction tube was washed three times with a 4 ml aliquot of buffer. The filters were oven-dried for 60 min and then placed in 5 ml of scintillation fluid (Ultima Gold XR, Packard). Radioactivity was quantified by liquid scintillation spectrometry. Specific binding was defined as the difference between the binding that occurred in the presence and absence of 1 μM unlabelled CP55940. Protein assays were performed using a Bio-Rad Dc kit. Unlabelled cannabinoids were each added in a volume of 50 μl after serial dilution from a 1 mg ml−1 ethanolic stock solution using assay buffer (50 mM Tris buffer containing 5 mg ml−1 BSA). [3H]-CP55940 was also added in a 50 μl volume following dilution in assay buffer. The concentration of [3H]-CP55940 used in displacement assays was 0.5 nM. The concentrations of cannabinoids that produced a 50% displacement of radioligand from specific binding sites (IC50 values) were calculated using GraphPad Prism (GraphPad Software, San Diego, U.S.A.) Dissociation constant (Ki) values were calculated using the equation of Cheng & Prusoff (1973) and KD values for [3H]-CP55940 of 1.62 (CB1) or 0.99 nM (CB2). These KD values were obtained in saturation binding assays using membranes from CB1 or CB2-transfected CHO cells as described previously (Ross et al., 1999).
Cyclic AMP assay
Cells (4–6×105 cells ml−1) were preincubated for 20 min at 37°C with cannabinoid agonist and 3-isobutyl-1-methylxanthine (IBMX; 50 μM) in phosphate buffered saline containing 1 mg ml−1 BSA (assay buffer) followed by a further 20 min incubation with 2 μM forskolin in a total volume of 500 μl. For antagonist studies, cells were preincubated for 10 min with the antagonist before agonist incubation. The reaction was terminated by addition of 0.1 M HCl and centrifugation performed to remove cell debris. The pH was brought to 8–9 using 1 M NaOH and cyclic AMP content was then measured using a radioimmunoassay kit (Biotrak, Amersham, U.K.). Cannabinoids were dissolved in ethanol as 1 mg ml−1 stock solutions and diluted in assay buffer. Forskolin and IBMX were dissolved in DMSO. Cyclic AMP concentrations in CB1-transfected cells were 21.80±6.50 pmol ml−1 in the presence of 2 μM forskolin and 2.83±1.01 pmol ml−1 in the absence of forskolin (n=11). Corresponding values in CB2-transfected cells were 18.24±5.81 and 3.00±1.27 pmol ml−1 respectively (n=11). In some experiments, CB2-transfected cells were incubated with 100 ng ml−1 pertussis toxin for 18 h prior to cyclic AMP measurement in order to uncouple the CB2 receptors from Gi/o proteins (Pertwee, 1997).
Analysis of data
Values have been expressed as means and variability as s.e.mean or as 95% confidence limits. Mean values have been compared using Student's unpaired t-test or analysis of variance followed by Dunnett's test or by the Newman-Keuls test. A P value <0.05 was considered to be significant. Effects of test compounds on forskolin-stimulated cyclic AMP production have been expressed in percentage terms. This was calculated from the equation [100×(f′−b)]/(f−b) where f′, f and b are values of cyclic AMP production (pmol ml−1), f′ in the presence of forskolin and the test compound, f in the presence of forskolin only and b in the absence of both forskolin and the test compound (Ross et al., 1999). Values for EC50, IC50 and maximal effects (Emax) and the s.e.mean or 95% confidence limits of these values have been calculated by non-linear regression analysis using the equation for a sigmoidal concentration-response curve (GraphPad Prism). In organ bath experiments, the degree of drug-induced inhibition of evoked contractions has been expressed in percentage terms. This was calculated by comparing the amplitude of the twitch response after each addition of a twitch inhibitor with its amplitude immediately before the first addition of the inhibitor.
The KB value of O-1184 for antagonism of CP55940 in the vas deferens was calculated by substituting a single concentration ratio value into the equation (x−1)=B/KB, where x (the ‘concentration ratio') is the concentration of CP55940 that produced a particular size of effect in the presence of O-1184 at a concentration, B, divided by the concentration of CP55940 that produced an identical effect in the absence of O-1184 (Tallarida et al., 1979). Values of the concentration ratio and its 95% confidence limits were determined by symmetrical (2+2) dose parallel line assays (Colquhoun, 1971), using responses to pairs of CP55940 concentrations located on the steepest part of each log concentration-response curve. This method was also used to establish whether the log concentration-response curves of CP55940 in the presence and absence of O-1184 deviated significantly from parallelism.
Drugs
CP55940 {(−)-3-[2-hydroxy-4-(1,1-dimethylheptyl)phenyl]-4-(3-hydroxypropyl)cyclohexan-1-ol} was supplied by Pfizer, SR144528 {N-[(1S)-endo-1,3,3-trimethyl bicyclo [2.2.1] heptan-2-yl]-5 -(4-chloro-3-methylphenyl)-1-(4-methylbenzyl)-pyrazole-3-carboxamide} by Sanofi Recherche and pertussis toxin by Sigma. O-1184, O-1238, O-1315 and O-584 (Figure 1) were synthesized in the laboratory of Dr Razdan. [3H]-CP55940 (126 Ci mmol−1) was obtained from NEN Life Science Products.
Results
Effects of O-1184, O-1238, O-1315 and O-584 on [3H]-CP55940 binding and on forskolin-stimulated cyclic AMP production
As shown in Table 1 and Figure 2, O-1184, O-1238, O-584 and CP55940 were equipotent in displacing [3H]-CP55940 from specific binding sites on both CB1 and CB2 CHO cell membranes. O-1315 also displaced [3H]-CP55940 from these binding sites but was significantly less potent than the other agents investigated (Table 1).
Table 1.
Effects on forskolin-stimulated cyclic AMP production and on [3H]-CP55940 binding in experiments with CB1- and CB2-transfected CHO cells or CHO cell membranes
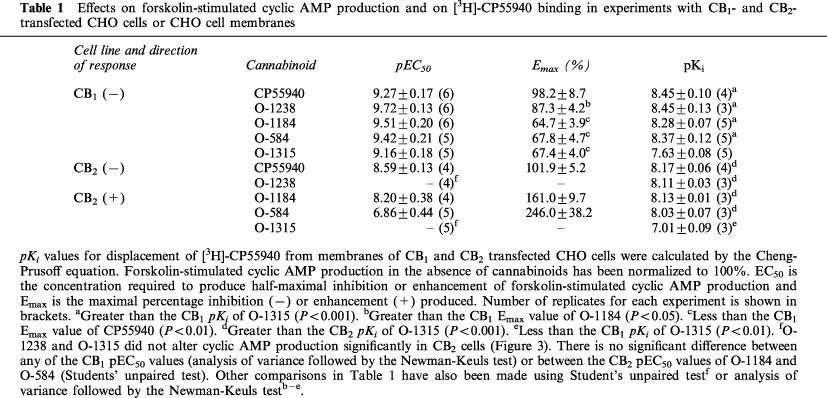
Figure 2.
Displacement of bound [3H]-CP55940 (0.5 nM) by O-1184 and O-1238, (a) in CB1- and (b) in CB2-transfected CHO cells and by O-584 and O-1315, (c) in CB1- and (d) in CB2-transfected CHO cells. Each symbol represents the mean per cent displacement±s.e.mean (n=3 or 5).
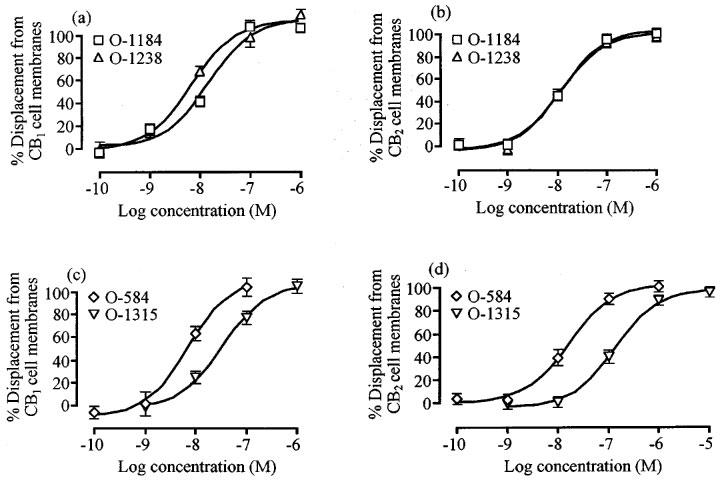
Cyclic AMP production in CB1-transfected cells was potently inhibited by CP55940, O-1184, O-1238, O-1315 and O-584 (Table 1 and Figure 3). O-1184, O-584 and O-1315 were equipotent with CP55940. However, they behaved as CB1 receptor partial agonists, their maximal inhibitory effects (Emax values) being significantly less than the Emax of CP55940 (Table 1 and Figure 3). O-1238 was also equipotent with CP55940 in CB1-transfected cells and its Emax did not deviate significantly from that of CP55940 (Table 1 and Figure 3). Neither O-1184 nor O-1238 affected forskolin-stimulated cyclic AMP production in untransfected CHO cells at concentrations ranging from 1 nM to 1 μM (n=3; data not shown).
Figure 3.
Changes of forskolin-stimulated cyclic AMP production induced by CP55940, O-1184 and O-1238, (a) in CB1- and (b) in CB2-transfected CHO cells and by O-584 and O-1315, (c) in CB1- and (d) in CB2-transfected CHO cells. Each symbol represents mean percentage change in forskolin-stimulated cyclic AMP production±s.e.mean. Forskolin-stimulated cyclic AMP production in the absence of any cannabinoid has been normalized to 100%. See Table 1 for further details. None of the concentrations of O-1238 or O-1315 used caused cyclic AMP production by CB2-transfected CHO cells to deviate significantly from 100% (P>0.05; one-sample t-test).
In CB2-transfected cells, CP55940 potently inhibited cyclic AMP production whereas O-1184 and O-584 each produced a concentration-related enhancement of this effect of forskolin (Table 1 and Figure 3). As has been reported previously (Rinaldi-Carmona et al., 1998), we found that the CB2-selective ligand, SR144528, also enhanced forskolin-stimulated cyclic AMP production in CB2-transfected cells with an Emax of 1764±468% (n=4). This value is significantly greater than the CB2 Emax values of O-1184 and O-584 shown in Table 1 (P<0.01; analysis of variance followed by Dunnett's test). In the same cell line, mean responses to forskolin were increased by concentrations of O-1315 in the low nanomolar range and decreased by micromolar concentrations of O-1238. However neither of these effects was statistically significant (Figure 3). At concentrations ranging from 1 nM to 10 μM, O-1184 and O-584 did not alter forskolin-stimulated cyclic AMP production in CB2-transfected cells that had been treated for 18 h with 100 ng ml−1 pertussis toxin (n=2; data not shown). Nor did concentrations of 1 nM to 1 μM O-1184 or O-584 affect cyclic AMP production in CB2 cells in the absence of forskolin (data not shown).
Effect of O-1184 and O-1238 on CP55940-induced inhibition of forskolin-stimulated cyclic AMP production by CB1 or CB2 transfected cells
Initial experiments with CB1 transfected cells were performed with a concentration of O-1184 that had little effect on cyclic AMP production when administered alone (Figure 3). This concentration of O-1184 (0.1 nM) had no effect on the position of the concentration response curve of CP55940 for inhibition of cyclic AMP production (Figure 4). Further experiments showed that at higher concentrations, O-1184 did not reverse the inhibitory effect of 10 nM CP55940 on cyclic AMP production in this cell line (Figure 4).
Figure 4.
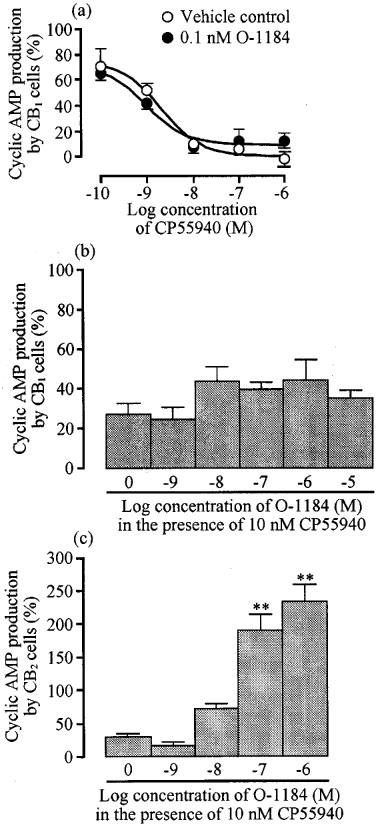
The upper panel (a) shows the effect of 0.1 nM O-1184 on CP55940-induced inhibition of forskolin-stimulated cyclic AMP production in CB1-transfected CHO cells. Each symbol in (a) represents mean percentage change in forskolin-stimulated cyclic AMP production±s.e.mean (n=3). Forskolin-stimulated cyclic AMP production in the absence of CP55940 and O-1184 has been normalized to 100%. The pEC50 values of CP55940 in the absence and presence of O-1184 (8.69±0.25 and 9.03±0.25 respectively) did not differ significantly (unpaired t-test). The lower panels (b) and (c) show the effect of O-1184 on inhibition of forskolin-stimulated cyclic AMP production by 10 nM CP55940 in CHO cells transfected with CB1 (n=11) or CB2 receptors (n=6). Each column represents mean percentage change in forskolin-stimulated cyclic AMP production±s.e.mean. Forskolin-stimulated cyclic AMP production in the absence of CP55940 and O-1184 has been normalized to 100%. Asterisks indicate significant differences from forskolin-stimulated cyclic AMP production in the presence of CP55940 alone (**P<0.01; one-way analysis of variance followed by Dunnett's test).
In CB2-transfected cells, O-1184 induced a concentration-related reversal of the inhibitory effect of 10 nM CP55940 on cyclic AMP production (Figure 4), its pEC50 for reversal being 7.93±0.07 (n=6). It was also found that O-1238 and O-1184 could both produce dextral shifts in the log concentration-response curve of CP55940 (Figure 5). At 100 nM, O-1238 decreased the pEC50 of CP55940 in CB2-transfected cells from 8.54±0.23 to 7.44±0.23, a 12.6 fold potency decrease (P<0.05; unpaired t-test; n=3). pEC50 values of CP55940 in the absence and presence of 100 nM O-1184 were 8.61±0.16 and 7.42±0.17 respectively, a 15.6 fold potency decrease (P<0.01; unpaired t-test; n=3). At this concentration, neither O-1238 nor O-1184 significantly affected the position of the upper or lower asymptotes of the CP55940 log concentration-response curve (unpaired t-test; n=3). However, at 1 μM, O-1184 caused the concentration-response curve of CP55940 to become steeper and to shift upwards as well as to the right (Figure 5). This upward shift presumably reflects the ability of O-1184 to enhance forskolin-stimulated cyclic AMP production in the CB2 transfected cells.
Figure 5.
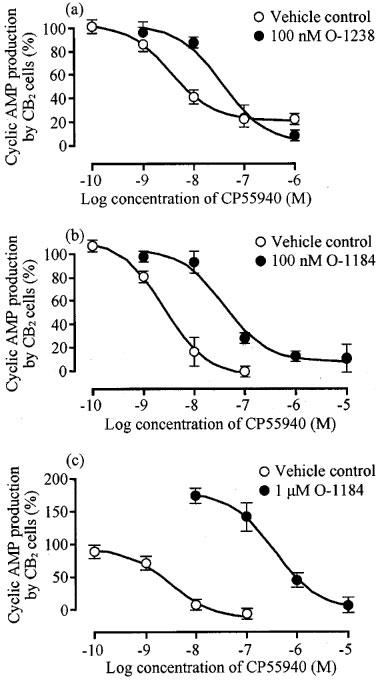
Effect (a) 100 nM O-1238, (b) 100 nM O-1184 and (c) 1 μM O-1184 on CP55940-induced inhibition of forskolin-stimulated cyclic AMP production in CB2-transfected CHO cells. Each symbol represents mean percentage change in forskolin-stimulated cyclic AMP production±s.e.mean (n=3). Forskolin-stimulated cyclic AMP production in the absence of CP55940, O-1238 and O-1184 has been normalized to 100%. The upper asymptote of the log concentration response curve of CP55940 was significantly greater in the presence of 1 μM O-1184 (178.8±14.3%) than in its absence (94.2±9.3%; P<0.01; unpaired t-test).
Effects of O-1238 and O-1184 on CP55940-induced inhibition of electrically-evoked contractions of the mouse vas deferens
As shown in Figure 6, O-1238 decreased the amplitude of evoked contractions in a concentration-related manner, with a pEC50 of 10.18±0.20. O-1184 also induced detectable inhibition of evoked contractions with a pEC50 of 9.08±0.46 (Figure 6). However, its Emax (21.1±3.54%) was significantly less than that of O-1238 (70.5±3.9%) which, in turn, was less than that of CP55940 (95.1±5.3%; Figure 6) (P<0.01; analysis of variance followed by the Newman-Keuls test). Electrically evoked contractions were not significantly inhibited by Tween 80 (n=6; data not shown). At 1 nM, O-1184 produced a 17.3 fold dextral shift in the log concentration-response curve of CP55940 for inhibition of evoked contractions that did not deviate significantly from parallelism (Figure 6). Insertion of this value (concentration ratio, x) and its 95% confidence limits into the equation (x−1)=B/KB (see Methods) gave a pKB value for O-1184 of 10.21±0.38. This value is significantly greater than the CB1 and CB2 pKi values of O-1184 shown in Table 1 (P<0.01; analysis of variance followed by Dunnett's test).
Figure 6.
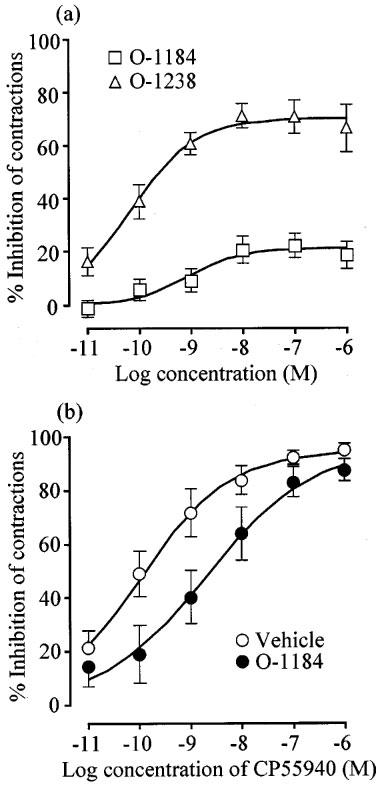
The upper panel (a) shows mean concentration-response curves for O-1238 and O-1184 in the mouse isolated vas deferens. Each symbol in (a) represents the mean value±s.e.mean for inhibition of electrically-evoked contractions expressed as a percentage of the amplitude of the twitch response measured immediately before the first addition to the organ bath of O-1238 (n=7) or O-1184 (n=20). The lower panel (b) shows the effect of pretreatment with 1 nM O-1184 on the mean concentration-response curve for CP55940 in the vas deferens (n=7). Each symbol in (b) represents the mean values±s.e.mean for inhibition of electrically-evoked contractions expressed as a percentage of the amplitude of the twitch response measured immediately before the first addition of CP55940 to the organ bath. O-1184 or Tween 80 were added 30 min before the first addition of CP55940.
Discussion
O-1184 displayed a similar potency to the established cannabinoid receptor agonist, CP55940, both in its affinity for CB1 binding sites and in its ability to inhibit forskolin-stimulated cyclic AMP production by CB1-transfected CHO cells. However, its maximal effect for inhibition of cyclic AMP production was less than that of CP55940, suggesting it to be a partial agonist. In line with this pharmacological classification, O-1184 exhibited mixed agonist-antagonist properties in the mouse vas deferens. Thus whilst it shared the ability of CP55940 to inhibit electrically-evoked contractions of this tissue at low nanomolar concentrations, its maximal effect was well below that of CP55940. Moreover, at a concentration of 1 nM, which induced only marginal inhibition of evoked contractions (Figure 6), O-1184 behaved as a cannabinoid receptor antagonist with a KB value against CP55940 in the picomolar range. These results are consistent with an earlier observation that O-1184 can antagonize CP55940-induced inhibition of electrically-evoked contractions of the myenteric plexus-longitudinal muscle preparation of guinea-pig small intestine, again with a KB value in the picomolar range (Ross et al., 1998).
The classification of O-1184 as a partial agonist at CB1 receptors is supported by results from recent experiments with rat cerebellar membranes (Griffin et al., 1999). These showed O-1184 to lack detectable agonist activity in the GTPγS binding assay and yet, as expected for a ligand that possesses at least some efficacy, to be less effective in displacing [3H]-CP55940 in the presence than in the absence of GDP, GTPγS and added sodium ions. Further support for this classification comes from evidence that 6′-cyanohex-2′-yne-Δ8-tetrahydrocannabinol (O-823), which differs from O-1184 only in having CN rather than N3 on the terminal carbon of the aliphatic side-chain, is a partial agonist at cannabinoid receptors in the mouse vas deferens (Pertwee et al., 1996).
O-1184 did not show mixed agonist-antagonist properties in CB1-transfected cells, sub-inhibitory concentrations of this compound having no antagonistic effect on CP55940-induced inhibition of forskolin-stimulated cyclic AMP production in this cell line. This apparent discrepancy may be an indication that there were fewer cannabinoid receptors in the vas deferens than in the transfected cell line. Thus, it is generally accepted that the agonist-antagonist properties of a partial agonist show tissue-dependence, the agonist component dominating in tissues that are well-endowed with receptors for the partial agonist and the antagonist component dominating in tissues that are more sparsely populated with such receptors (Kenakin, 1993). In previous experiments with the myenteric plexus-longitudinal muscle preparation (Ross et al., 1998), we found O-1184 to behave as a cannabinoid receptor antagonist at a concentration at which it behaved as an agonist in the mouse vas deferens (100 nM). This too may reflect an inter-tissue difference in receptor density, there already being some evidence that the myenteric plexus-longitudinal muscle preparation expresses fewer cannabinoid receptors than the mouse vas deferens (Pertwee et al., 1996).
The mean pKi values of O-1184 for displacement of [3H]-CP55940 from CB1 and CB2 binding sites shown in Table 1 (8.28 and 8.13 respectively) are of the same order as a previously reported pKB value of this compound for antagonism of CP55940-induced enhancement of [35S]GTPγS binding to rat cerebellar membranes, the mean value of which is 8.53 (Griffin et al., 1999). However, these pKi and pKB values are markedly less than the mean pKB of O-1184 for antagonism of CP55940-induced inhibition of electrically-evoked contractions of the mouse vas deferens (10.21; see Results). This difference may stem from the use of different vehicles, Tween 80 in the vas deferens experiments and ethanol in the [3H]-CP55940 and [35S]-GTPγS binding assays. However, it also remains possible that in the vas deferens, O-1184 and CP55940 interact at least in part through an as yet unidentified cannabinoid receptor. Indeed, each of these cannabinoids binds avidly to both CB1 and CB2 receptors, demonstrating a lack of selectivity for any one cannabinoid receptor type, and there is already evidence for the presence of a novel CB2-like receptor type in the mouse vas deferens (Griffin et al., 1997).
Like its affinity for CB1 binding sites, the affinity of O-1184 for CB2 binding sites matched that of CP55940. However, O-1184 differed from CP55940 in that it enhanced rather than inhibited forskolin-stimulated cyclic AMP production by CB2-transfected cells. In this respect O-1184 resembles the CB2-selective ligands AM630 (6-iodopravadoline) and SR144528 (Rinaldi-Carmona et al., 1998; Ross et al., 1999), although the maximum degree of the enhancement it produced (1.6 fold) is somewhat less than that produced in the same cell line by AM630 (4.9 fold; Ross et al., 1999) and SR144528 (17.6 fold; Results). Also like AM630 and SR144528 (Rinaldi-Carmona et al., 1998; Ross et al., 1999), O-1184 attenuated CP55940-induced inhibition of forskolin-stimulated cyclic AMP production by CB2-transfected cells.
Modification of the aliphatic side-chain of O-1184 by replacing the carbon–carbon triple bond with a cis double bond (O-1238) did not produce detectable changes in CB1 or CB2 receptor affinity or in the pEC50 value for inhibition of forskolin-stimulated cyclic AMP production by CB1 transfected cells. However, this structural change did cause an apparent increase in CB1 efficacy, the Emax of O-1238 exceeding that of O-1184 for inhibition both of cyclic AMP production by CB1-transfected cells and of evoked contractions of the vas deferens. The Emax of O-1238 was similar to that of CP55940 in the cyclic AMP assay but not in the isolated tissue assay in which O-1238 behaved as a partial agonist with an Emax less than that of CP55940. Again, this may reflect the presence of fewer cannabinoid receptors in the vas deferens than in the CB1 cell line (see above). Evidence that O-1238 is a partial agonist at CB1 receptors with greater efficacy than O-1184 has also been obtained by Griffin et al. (1999) from experiments in which the measured effect was agonist-induced stimulation of GTPγS binding to rat cerebellar membranes.
Although O-1238 retained the ability of O-1184 to attenuate CP55940-induced inhibition of forskolin-stimulated cyclic AMP production in CB2-transfected cells without any reduction in potency, it did not share the ability of O-1184 to enhance forskolin-stimulated cyclic AMP production in this cell line when administered alone. Other drugs that are known to block CB2 receptor-mediated responses at concentrations in the nanomolar range, for example SR144528 and AM630 (see above), resemble O-1184 in producing ‘inverse cannabimimetic' effects when administered by themselves. Consequently, our O-1238 data demonstrate for the first time that it is possible for an agent to antagonize a CB2 receptor-mediated response even when it lacks the ability to produce inverse cannabimimetic effects. Because O-1184 and O-1238 can both antagonize CP55940 in CB2-transfected cells but only O-1184 enhances forskolin-stimulated cyclic AMP production in this cell line, it is unlikely that O-1184 produces this enhancement by blocking the action of endogenous cannabinoids released by these cells. This conclusion is supported by evidence that no such endogenous cannabinoid release takes place in CHO cells expressing human CB1 or CB2 receptors (Bouaboula et al., 1997; MacLennan et al., 1998). A more likely explanation for our results is that the potentiation of forskolin produced by O-1184 in CB2-transfected cells reflects the presence of a mixed population of constitutively active and inactive CB2 receptors and that these two sets of receptors are in equilibrium with each other. As has already been proposed for SR144528 and AM630 (Rinaldi-Carmona et al., 1998; Ross et al., 1999), O-1184 might then enhance forskolin-stimulated cyclic AMP production because it is an inverse agonist that reduces the proportion of receptors in the constitutively active state by binding preferentially to the inactive receptors.
In agreement with results obtained by Griffin et al. (1999) in experiments with rat cerebellar membranes, we found that replacement of N3 with C2H5 on the terminal carbon of the aliphatic side-chain of O-1184 to form O-584 had no effect on CB1 receptor affinity. We also found O-584 and O-1184 to have the same affinity for CB2 binding sites and the same ability to alter forskolin-stimulated cyclic AMP production in CHO cells transfected with CB1 receptors (inhibition) or CB2 receptors (enhancement). Neither of these compounds enhanced forskolin-stimulated cyclic AMP production in CB2-transfected cells that had been pretreated with pertussis toxin, pointing to an involvement of Gi/o proteins and hence of CB2 receptors (Pertwee, 1997). That the effects of O-584 and O-1184 on cyclic AMP production by CB2- and, indeed, by CB1-transfected cells were cannabinoid receptor-mediated, is also supported by our finding that these compounds lacked detectable activity in untransfected cells. A further structural modification, in which the phenolic 1-hydroxyl group of O-584 was removed to form O-1315 did not affect either the pEC50 or Emax for inhibition of cyclic AMP production by CB1-transfected cells. However, this structural change did abolish the ability of the molecule to induce any significant enhancement of forskolin-stimulated cyclic AMP production by CB2-transfected cells. It also caused reductions in CB1 and CB2 receptor affinity of 5.0 and 10.6 fold respectively. The nature of these affinity changes was unexpected as removal of the 1-hydroxyl group from another analogue of Δ8-THC, 11-hydroxy-Δ8-THC-dimethylheptyl, or from Δ8-THC itself, has no detectable effect on CB1 receptor affinity but enhances CB2 receptor affinity, thereby conferring a significant degree of CB2-selectivity (Pertwee, 1999; Huffman et al., 1996; 1998).
In conclusion, comparison of the pharmacological properties of O-1184 and O-1238 suggests that although increasing the degree of unsaturation of the aliphatic side-chain has little effect on CB1 or CB2 receptor affinity, it can markedly reduce CB1 receptor efficacy and reverse the direction of responses elicited at CB2 receptors. Thus at CB1 receptors, the less saturated of these two agents, O-1184, appears to have the lower efficacy and to possess the mixed agonist-antagonist properties of a partial agonist. At CB2 receptors the effect of Δ8-THC analogues seems to change from agonism when the side-chain is fully saturated as in 11-hydroxy-Δ8-THC-dimethylheptyl (Slipetz et al., 1995), through weak partial agonism/antagonism when the side-chain contains a cis-double bond (O-1238) to inverse agonism when the side-chain contains a carbon–carbon triple bond (O-1184). Because O-1238 has relatively high affinity for CB2 receptors but lacks significant CB2 receptor efficacy or inverse cannabimimetic properties, at least in the cyclic AMP assay, it may be an important lead compound from which to develop the first pure cannabinoid receptor antagonist. Accordingly, further experiments are needed to establish whether the structure of O-1238 can be modified to yield a pure CB1-CB2 antagonist through elimination of CB1 efficacy or to yield a pure CB2-selective antagonist through elimination of CB1 affinity. Further experiments are also needed to determine the extent to which the properties exhibited by O-1184 and its analogues in CB2-transfected cells extend to tissues in which these receptors are expressed naturally. Such studies must, however, await the development of a suitable CB2 receptor bioassay.
Acknowledgments
This work was supported by grant 047980 from the Wellcome Trust (to R.G. Pertwee and R.A. Ross), by grants DA09789 (to R.G. Pertwee) and DA05488 (to R.K. Razdan) from the National Institute on Drug Abuse (NIDA) and by Pfizer. We thank Sanofi Recherche for SR144528.
Abbreviations
- AM630
6-iodopravadoline
- BSA
bovine serum albumin
- CHO
Chinese hamster ovary
- CP55940
(−)-3-[2-hydroxy-4-(1,1-dimethylheptyl)phenyl]-4-(3-hydroxypropyl)cyclohexan-1-ol
- IBMX
3-isobutyl-1-methylxanthine
- O-584
octyl-2′-yne-Δ8-tetrahydrocannabinol
- O-1184
6′-azidohex-2′-yne-Δ8-tetrahydrocannabinol
- O-1238
6′-azidohex-cis-2′-ene-Δ8-tetrahydrocannabinol
- O-1315
1-deoxy-octyl-2′-yne-Δ8-tetrahydrocannabinol
- SR144528
N-[(1S)-endo-1,3,3-trimethyl bicyclo [2.2.1] heptan-2-yl]-5-(4-chloro-3-methylphenyl)-1-(4-methylbenzyl)-pyrazole-3-carboxamide
References
- BOUABOULA M., PERRACHON S., MILLIGAN L., CANAT X., RINALDI-CARMONA M., PORTIER M., BARTH F., CALANDRA B., PECCEU F., LUPKER J., MAFFRAND J.-P., LE FUR G., CASELLAS P. A selective inverse agonist for central cannabinoid receptor inhibits mitogen-activated protein kinase activation stimulated by insulin or insulin-like growth factor 1. Evidence for a new model of receptor/ligand interactions. J. Biol. Chem. 1997;272:22330–22339. doi: 10.1074/jbc.272.35.22330. [DOI] [PubMed] [Google Scholar]
- CHENG Y.-C., PRUSOFF W.H. Relationship between the inhibition constant (KI) and the concentration of inhibitor which causes 50 per cent inhibition (IC50) of an enzymatic reaction. Biochem. Pharmacol. 1973;22:3099–3108. doi: 10.1016/0006-2952(73)90196-2. [DOI] [PubMed] [Google Scholar]
- COLQUHOUN D. Lectures on Biostatistics. Oxford: Oxford University Press; 1971. [Google Scholar]
- COMPTON D.R., RICE K.C., DE COSTA B.R., RAZDAN R.K., MELVIN L.S., JOHNSON M.R., MARTIN B.R. Cannabinoid structure-activity relationships: correlation of receptor binding and in vivo activities. J. Pharmacol. Exp. Ther. 1993;265:218–226. [PubMed] [Google Scholar]
- GREEN A., O'SHAUGHNESSY C., DISNEY G., MARSHALL F. Reporter assays for human cannabinoid CB1 and CB2 receptors for the identification of novel agonists. Br. J. Pharmacol. (Proc. Suppl.) 1999;126:112P. [Google Scholar]
- GRIFFIN G., FERNANDO S.R., ROSS R.A., MCKAY N.G., ASHFORD M.L.J., SHIRE D., HUFFMAN J.W., YU S., LAINTON J.A.H., PERTWEE R.G. Evidence for the presence of CB2-like cannabinoid receptors on peripheral nerve terminals. Eur. J. Pharmacol. 1997;339:53–61. doi: 10.1016/s0014-2999(97)01336-8. [DOI] [PubMed] [Google Scholar]
- GRIFFIN G., WRAY E.J., RORRER W.K., CROCKER P.J., RYAN W.J., SAHA B., RAZDAN R.K., MARTIN B.R., ABOOD M.E. An investigation into the structural determinants of cannabinoid receptor efficacy. Br. J. Pharmacol. 1999;126:1575–1584. doi: 10.1038/sj.bjp.0702469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HUFFMAN J.W., YU S., LIDDLE J., WILEY J.L., ABOOD M., MARTIN B.R., AUNG M.M.1-Deoxy-1′,1′-dimethylalkyl-Δ8-THC derivatives: selective ligands for the CB2 receptor Symposium on the Cannabinoids, Burlington, Vermont, International Cannabinoid Research Society 1998. p. 10
- HUFFMAN J.W., YU S., SHOWALTER V., ABOOD M.E., WILEY J.L., COMPTON D.R., MARTIN B.R., BRAMBLETT R.D., REGGIO P.H. Synthesis and pharmacology of a very potent cannabinoid lacking a phenolic hydroxyl with high affinity for the CB2 receptor. J. Med. Chem. 1996;39:3875–3877. doi: 10.1021/jm960394y. [DOI] [PubMed] [Google Scholar]
- KENAKIN T. Pharmacologic analysis of drug-receptor interaction. Raven Press, New York; 1993. [Google Scholar]
- MACLENNAN S.J., REYNEN P.H., KWAN J., BONHAUS D.W. Evidence for inverse agonism of SR141716A at human recombinant cannabinoid CB1 and CB2 receptors. Br. J. Pharmacol. 1998;124:619–622. doi: 10.1038/sj.bjp.0701915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PERTWEE R.G. Pharmacology of cannabinoid CB1 and CB2 receptors. Pharmacol. Ther. 1997;74:129–180. doi: 10.1016/s0163-7258(97)82001-3. [DOI] [PubMed] [Google Scholar]
- PERTWEE R.G. Pharmacology of cannabinoid receptor ligands. Curr. Med. Chem. 1999;6:617–646. [PubMed] [Google Scholar]
- PERTWEE R.G., FERNANDO S.R., GRIFFIN G., RYAN W., RAZDAN R.K., COMPTON D.R., MARTIN B.R. Agonist-antagonist characterization of 6′-cyanohex-2′-yne-Δ8-tetrahydrocannabinol in two isolated tissue preparations. Eur. J. Pharmacol. 1996;315:195–201. doi: 10.1016/s0014-2999(96)00631-0. [DOI] [PubMed] [Google Scholar]
- PERTWEE R.G., STEVENSON L.A., ELRICK D.B., MECHOULAM R., CORBETT A.D. Inhibitory effects of certain enantiomeric cannabinoids in the mouse vas deferens and the myenteric plexus preparation of guinea-pig small intestine. Br. J. Pharmacol. 1992;105:980–984. doi: 10.1111/j.1476-5381.1992.tb09088.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RINALDI-CARMONA M., BARTH F., MILLAN J., DEROCQ J.-M., CASELLAS P., CONGY C., OUSTRIC D., SARRAN M., BOUABOULA M., CALANDRA B., PORTIER M., SHIRE D., BRELIÈRE J.-C., LE FUR G. SR144528, the first potent and selective antagonist of the CB2 cannabinoid receptor. J. Pharmacol. Exp. Ther. 1998;284:644–650. [PubMed] [Google Scholar]
- ROSS R.A., BROCKIE H.C., FERNANDO S.R., SAHA B., RAZDAN R.K., PERTWEE R.G. Comparison of cannabinoid binding sites in guinea-pig forebrain and small intestine. Br. J. Pharmacol. 1998;125:1345–1351. doi: 10.1038/sj.bjp.0702204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ROSS R.A., BROCKIE H.C., STEVENSON L.A., MURPHY V.L., TEMPLETON F., MAKRIYANNIS A., PERTWEE R.G. Agonist inverse agonist characterization at CB1 and CB2 cannabinoid receptors of L759633, L759656 and AM630. Br. J. Pharmacol. 1999;126:665–672. doi: 10.1038/sj.bjp.0702351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SLIPETZ D.M., O'NEILL G.P., FAVREAU L., DUFRESNE C., GALLANT M., GAREAU Y., GUAY D., LABELLE M., METTERS K.M. Activation of the human peripheral cannabinoid receptor results in inhibition of adenylyl cyclase. Mol. Pharmacol. 1995;48:352–361. [PubMed] [Google Scholar]
- TALLARIDA R.J., COWAN A., ADLER A.W. pA2 and receptor differentiation: a statistical analysis of competitive antagonism. Life Sci. 1979;25:637–654. doi: 10.1016/0024-3205(79)90505-8. [DOI] [PubMed] [Google Scholar]


