Abstract
The aim of the current study was to characterize which cannabinoid receptors, if any, are present on rat carotid artery smooth muscle. Additionally, the effects of cannabinoids on carotid artery tone, on cyclic AMP accumulation and on forskolin-induced relaxation were examined in the same tissue.
Stimulation of carotid arteries with forskolin (10 μM) significantly increased cyclic AMP accumulation, an effect that was inhibited in a concentration-dependent manner by the cannabinoid receptor agonist, methanandamide.
Similar inhibition was seen with the CB1 agonist HU-210 but this inhibition was not mimicked by the CB2 agonist, WIN 55,2212-2.
The inhibitory effect of methanandamide on cyclic AMP accumulation was prevented by incubation of the arteries with pertussis toxin and was significantly reduced by LY320135, a selective CB1 antagonist, but not by SR 144528, a CB2-selective antagonist.
Methanandamide failed to relax carotid arteries pre-contracted with phenylephrine, but inhibited forskolin-induced relaxation of these arteries. This functional inhibition of relaxation by methanandamide was inhibited by CB1-selective (LY320135 and SR 141716A), but not a CB2-selective antagonist (SR 144528).
These data demonstrate the presence of functional G protein-linked cannabinoid receptors of the CB1 subtype in the rat carotid artery, but show that these receptors inhibit cyclic AMP accumulation rather than cause relaxation.
Keywords: Cannabinoid receptors, vasorelaxation, cyclic AMP, CB-receptor antagonists, pertussis toxin
Introduction
The role of endocannabinoids in the regulation of smooth muscle tone, especially in the cardiovascular system, has recently become the subject of intense scrutiny, following reports that endocannabinoids, such as anandamide, and stereochemically pure synthetic cannabinoids for example (6aR) - trans - 3-(1,1-dimethylheptyl)-6a,7,10,10a-tetrahydro-1-hydroxy - 6,6 - dimethyl - 6H - dibenzo [b, d] pyran - 9 - methanol (HU-210), produce bradycardia and hypotension in vivo and vasorelaxation in vitro (Varga et al., 1995; 1996; Randall et al., 1996; Vidrio et al., 1996; Plane et al., 1997; Zygmunt et al., 1997). The fact that these cardiovascular effects mimic those seen during the recreational use of marijuana or hashish in humans has added to the interest in the mechanisms underlying this response. It has recently been reported (Wagner et al., 1997) that endogenous anandamide is also synthesized by macrophages and platelets in response to lipopolysaccharide and its production may underlie the hypotension associated with haemorrhagic shock. However, the powerful effects of endocannabinoids on the cardiovascular system in vivo are complex and poorly understood. Intravenous administration of anandamide, for example, produces a combination of pressor and depressor effects (Varga et al., 1995) while intravenous administration of Δ9-tetrahydrocannabinol has been shown to cause either pressor, or no effect on regional cerebral blood flow (Bloom et al., 1997). The origin of the pressor effect is currently unclear, but it is not thought to be mediated by cannabinoid receptors (Varga et al., 1995; Lake et al., 1997). The depressor effects, which occur particularly in the resistance vasculature, appear to involve two mechanisms. The first is inhibition of noradrenaline release from sympathetic nerve terminals (Malinowska et al., 1997; Niederhoffer & Szabo, 1999a), possibly by inhibition of Ca2+ entry through N-type Ca2+ channels (Mackie & Hille, 1992). The second, and potentially more important mechanism, is a direct relaxant effect on vascular smooth muscle (Randall et al., 1996; Plane et al., 1997; Zygmunt et al., 1997) which has been suggested to mimic the relaxations due to endothelium-derived hyperpolarizing factor (EDHF) (Randall et al., 1996; Harris et al., 1999). However, the identification of endocannabinoids as an EDHF has been controversial and many groups have disagreed with this classification (Plane et al., 1997; Zygmunt et al., 1997; Niederhoffer & Szabo, 1999b) with one report even suggesting that endocannabinoids were negatively coupled to the production of EDHF (Fleming et al., 1999). A number of studies have reported that the vasorelaxation/hypotension produced by endocannabinoids and synthetic cannabinoid receptor agonists is attenuated by the CB1 receptor antagonist, SR141716A (Varga et al., 1995; 1996; Randall et al., 1996; Vidrio et al., 1996; Zygmunt et al., 1997). However, other studies found no antagonism of cannabinoid-induced vasorelaxation by SR141716A (Plane et al., 1997) and there is almost no experimental evidence to indicate that cannabinoid receptors are present in vascular smooth muscle, or indeed how the direct effects of endocannabinoids on vascular smooth muscle are mediated. One mechanism proposed to account for the direct vasorelaxant effects of endocannabinoids is anandamide-induced inhibition of Ca2+-release from intracellular stores in arterial smooth muscle cells (Zygmunt et al., 1997). Whether this effect on Ca2+ homeostasis is CB1 receptor-mediated has not been determined, but a similar pertussis toxin-sensitive effect of anandamide has previously been reported in astrocytes (Venance et al., 1997).
The aim of the present study was to establish whether functional cannabinoid receptors exist on rat carotid artery smooth muscle, and if so which subtypes are present. Additionally, we aimed to investigate whether any cannabinoid receptors present were coupled via G proteins of the Gi/Go family to the inhibition of adenylyl cyclase as has been described in other cell types.
Methods
Tissue preparation
Male Wistar rats were killed by stunning followed by exsanguination. The carotid arteries were carefully removed, cleaned of any attached nervous and connective tissues and placed in Krebs Henseleit buffer (4°C). The vessels were then either used in biochemical studies or in studies of mechanical tone. Where de-endothelialized vessels were to be used the endothelium was removed by slowly passing 10 ml distilled water through the lumen.
Measurement of cyclic nucleotide accumulation
Cyclic AMP accumulation was measured in tissues in the absence of phosphodiesterase inhibitors. The arteries were transferred into tubes containing 10 ml Krebs Henseleit buffer, cannabinoid receptor agonists and antagonists added, where indicated, and the tubes transferred to a waterbath and incubated (37°C) for 10–30 min. Forskolin was added to arteries to stimulate cyclic AMP accumulation, while control tissues were vehicle-treated. After 5 min stimulation the arteries were removed, rapidly blotted with damp filter paper and frozen in liquid nitrogen. 0.5 ml of cold (4°C) trichloroacetic acid (0.5 M) was added to each sample, which was homogenized (polytron, setting 6 for 5×15 s). Homogenized samples were kept on ice for 45 min to allow total extraction of cyclic AMP to occur. Following extraction, samples were centrifuged for 5 min at 4000×g at 4°C. The acid was extracted with 1.5 vol freon/tri-n-octylamine and the aqueous phase brought to pH 7 with NaHCO3. Cyclic AMP levels were determined using a standard binding protein assay (Brown et al., 1971) and the results expressed as pmol per mg of tissue protein.
Effects of pertussis toxin on methanandamide-induced effects on cyclic AMP accumulation
To further investigate whether cannabinoid receptors were present in rat carotid artery smooth muscle and were G protein-coupled, control arteries were incubated in minimum essential medium supplemented with 5% bovine serum albumin and 1% penicillin/streptomycin for 24 h at 37°C in a humidified air: 5% CO2 incubator. Test vessels were treated as control, but with the addition of pertussis toxin (PTX) at a concentration of 1 μg ml−1 for 24 h. Vessels which were treated in this way could still produce vasoconstriction and relaxation indicating that they had not been damaged by the period in culture medium.
Measurement of mechanical tone
Segments of mesenteric and carotid artery were mounted in a Mulvany-Halpern wire myograph in Krebs-Henseleit buffer at 37°C for the recording of isometric tension. Artery segments were stretched to the equivalent of a transmural pressure of 100 mmHg, the diameter was calculated and set to 90% of this value and the tissue allowed to equilibrate for 45 min. Tone was induced in the segments by the addition of the α1-adrenoceptor agonist, phenylephrine (10 μM). After a stable increase in tone was established, forskolin was added to the bath at a concentration that induced 40–60% relaxation of pre-contracted carotid artery segments (approximately 10 nM) and relaxation recorded. When assessing the effects of cannabinoid receptor agonists and antagonists paired tissues were set up as above and arbitrarily assigned to test or control groups. Methanandamide, a stable analogue of anandamide, was used to ensure that any observed effects were not due to the breakdown of anandamide to other vasoactive metabolites. Methanandamide (10 μM) or vehicle, either in the presence, or absence, of antagonist was added to the bath 10 min before tone was induced.
Materials
N -piperidino -5 - (4-chlorophenyl) -1- (2,4 - dichlorophenyl) - 4-methyl-3-pyrazole-carboxamide (SR 141716A) and N-([1S]-endo -1,3,3-trimethylbicyclo [2.2.1] heptan-2-yl)-5-(4-chloro-3-methylphenyl) - 1 - (4 - methylbenzyl) - pyrazole -3-carboxamide (SR 144528) were gifts from Sanofi Research (Montpellier, France). ([6-methoxy-2-(4-methoxyphenyl) benzo [b] thien-3-yl][4-cyanophenyl]methanone (LY320135) was a gift from Lilly Research Laboratories (Indianapolis, U.S.A.). Levcromakalim was a gift from SmithKline Beecham (Harlow, U.K.). Anandamide, methanandamide and R(+)-[2,3-dihydro-5-methyl -3-[(morpholinyl)methyl]- pyrrolo[1,2,3-de]-1,4-benzoxazin-yl]-(1-naphthalenyl)-methanone mesylate (WIN 55,2212-2) were purchased from RBI (Natick, U.S.A.). HU-210 was purchased from Tocris-Cookson (Bristol, U.K.). All other drugs and chemicals were obtained from Sigma.
Data analysis
All data are expressed as the means±s.e.mean, while n is the number of observations for the mechanical responses and the number of observations made in triplicate for the biochemical studies. The significance of the difference between means was assessed by means of one way analysis of variance or, where data was paired, by a paired Student's t-test. Results were accepted as significantly different when P<0.05.
Results
Conditions which produced a maximal cyclic AMP accumulation in response to forskolin (10 μM for 5 min) were used throughout and the cannabinoid agonists methanandamide, WIN 55,2212-2 or HU-210 were used to stimulate cannabinoid receptors.
Methanandamide inhibits forskolin-induced cyclic AMP accumulation
If cannabinoid receptors are present in vascular smooth muscle and are negatively-coupled to adenylyl cyclase, via Gi, then activation of the receptor should attenuate cyclic AMP accumulation. Figure 1 illustrates the effects of methanandamide on forskolin-induced cyclic AMP accumulation. Under control conditions, where carotid arteries were vehicle-treated with ethanol or DMSO (0.1%), the concentration of cyclic AMP was 18.5±2.5 pmol (mg protein)−1 (n=12). Following stimulation with forskolin (10 μM) for 5 min the concentration of cyclic AMP was significantly increased (P<0.001, Student's t-test) to 649±100 pmol (mg protein)−1 (n=12). Methanandamide significantly inhibited this increase in cyclic AMP accumulation in a concentration-dependent manner (Figure 1) with a maximum (56±7%) inhibition being observed at 100 nM methanandamide. Higher concentrations of methanandamide (1 and 10 μM) produced levels of inhibition that were not significantly different from those seen with 100 nM methanandamide. Methanandamide did not produce any contraction in the presence or absence of phenylephrine (n=20 and 4 respectively, data not shown).
Figure 1.
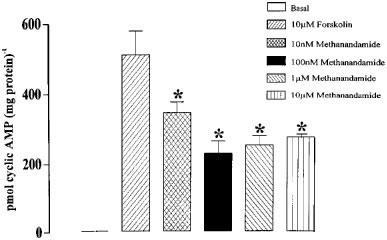
Effects of the cannabinoid agonist methanandamide, 10 nM–10 μM, on forskolin-stimulated cyclic AMP accumulation in rat carotid arteries. The asterisks denote statistically significant differences from forskolin-stimulated cyclic AMP accumulation in control arteries (P<0.05). The results obtained with 100 nM, 1 μM and 10 μM methanandamide, while not significantly different from each other, are significantly different from those obtained with 10 nM methanandamide. Data are shown as means±s.e.mean for 5–8 artery segments from ten animals.
CB1, but not CB2, receptor agonists reduce forskolin-induced cyclic AMP accumulation
To determine whether the effects of methanandamide were receptor-mediated or could be explained by some other non-specific effect, we assessed the effects of the synthetic cannabinoid agonists HU-210 and WIN 55,2212-2 on forskolin-stimulated cyclic AMP accumulation. HU-210 is somewhat more selective for CB1 receptors while WIN 55,2212-2 is more potent at CB2 receptors (Felder et al., 1995), although the degree of specificity of both compounds is concentration-dependent. HU-210 (1 μM) significantly (P<0.05) inhibited cyclic AMP accumulation (forskolin control, 253±31; plus HU-210, 190±17 pmol cyclic AMP (mg protein)−1) while WIN 55,2212-2 (10 μM) failed to inhibit forskolin-induced increases in cyclic AMP (forskolin control, 265±46; plus WIN 55,2212-2, 257±72 pmol cyclic AMP (mg protein)−1).
The vessels used in the above experiments possessed an intact endothelium and so it was not clear whether this inhibition was due to a direct effect on the smooth muscle layer or was endothelium-dependent. Experiments were therefore performed on arteries in which the endothelium had been removed. This treatment prevented endothelium-dependent relaxation in vessels subsequently mounted in a myograph, but had no effect on phenylephrine-induced contractions, or relaxation due to sodium nitroprusside or pinacidil (data not shown). Removal of the endothelium from these vessels had no effect on basal cyclic AMP levels. Forskolin-induced increases in cyclic AMP were not significantly different from endothelium intact vessels, nor was the inhibition of this increase by methanandamide (Figure 2), indicating that the cannabinoid receptors responsible for inhibition were on the smooth muscle.
Figure 2.
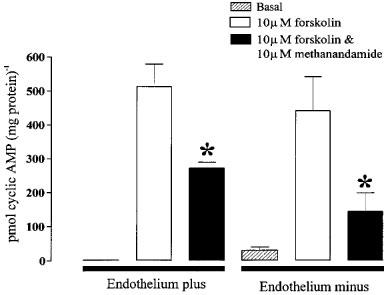
Effects of methanandamide are not endothelium-dependent. Data were obtained in vessels with an intact endothelium or vessels from which the endothelium had been removed. The asterisks denote a statistically significant difference (P<0.05) between forskolin-stimulated cyclic AMP accumulation in the presence and absence of methanandamide. Data are shown as means±s.e.mean (n=8). Please note that the endothelium plus and minus data were not obtained on paired tissues and so no statistical comparisons have been made between these groups.
Cannabinoid antagonists inhibit the actions of methanandamide
Incubation for ⩾15 min with LY320135 (10 μM), a recently developed, selective CB1 antagonist, had no effect on forskolin-stimulated cyclic AMP accumulation in the carotid artery (forskolin control, 280±98 pmol (mg protein)−1; forskolin plus LY320135, 234±20 pmol (mg protein)−1). However, pre-treatment of the artery with LY320135 (10 μM) partially reversed the effect of methanandamide such that methanandamide failed to significantly inhibit forskolin-stimulated cyclic AMP accumulation (Figure 3a). Surprisingly, incubation with another, prototypic, CB1 antagonist SR141716A caused a 47% reduction (P<0.05 Student's t-test n=8) of forskolin-stimulated cyclic AMP-accumulation in rat carotid arteries (Figure 3b). Since this effect is similar, in magnitude and direction, to that of the cannabinoid agonists, this antagonist could not be used to try to reverse the inhibitory effects of anandamide or methanandamide on the accumulation of cyclic AMP. Unlike SR 141716A, SR144528 (10 μM), a CB2 selective antagonist had no effect on either forskolin-induced cyclic AMP accumulation (data not shown) or on the inhibition of this effect by methanandamide (Figure 3c).
Figure 3.
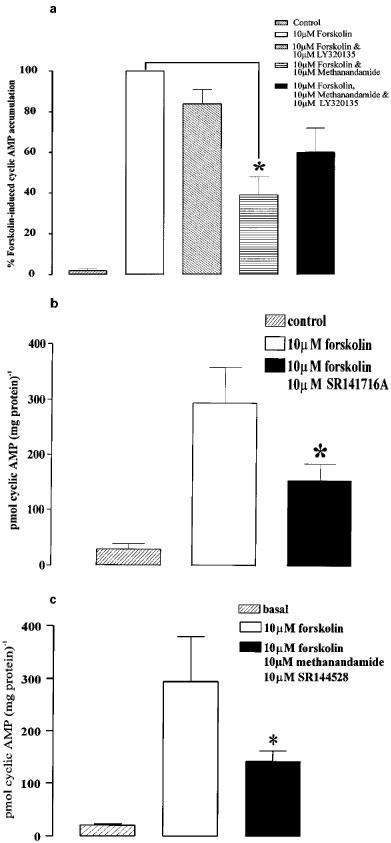
Effects of CB1 receptor antagonists on forskolin-stimulated cyclic AMP accumulation in rat carotid arteries. (a) Shows the basal level of cyclic AMP, cyclic AMP following stimulation with forskolin, effect of 10 μM LY320135 on forskolin-induced increases in cyclic AMP, the forskolin-induced cyclic AMP accumulation following incubation with methanandamide, and the effect of LY320135 (10 μM) on methanandamide-induced inhibition of forskolin-stimulated cyclic AMP accumulation. The presence of LY320135 reduced the inhibitory effect of methanandamide on cyclic AMP accumulation such that it was not significantly different from the forskolin control. (b) Shows the basal level of cyclic AMP, forskolin-induced increase in cyclic AMP, and the inhibition of cyclic AMP accumulation following incubation with 10 μM SR141716A. (c) Shows the basal level of cyclic AMP, the forskolin-induced increase in cyclic AMP, and the lack of effect of 10 μM SR144528 on methanandamide-induced inhibition of forskolin-induced cyclic AMP accumulation. The asterisks denote statistically significant differences (P<0.05) from forskolin-induced cyclic AMP accumulation in control arteries. Data are shown as means±s.e.mean (n=5).
The effect of methanandamide is pertussis toxin-sensitive
Having demonstrated that CB1-cannabinoid receptors can affect forskolin-stimulated cyclic AMP accumulation in carotid artery, it was also important to determine whether their effect in inhibiting forskolin-stimulated cyclic AMP accumulation was mediated via a Gi protein-coupled to the inhibition of adenylyl cyclase. Figure 4 shows that under control conditions, where vessels were incubated for 24 h in tissue culture medium at 37°C the basal level of cyclic AMP was somewhat lower than that observed in freshly-isolated vessels. However, in agreement with the data shown in Figure 1 stimulation with forskolin significantly increased cyclic AMP levels (approximately 50 fold), while incubation with methanandamide (10 μM) characteristically reduced cyclic AMP levels by approximately 50% (n=4). These data suggest that 24 h incubation of the vessels did not detrimentally affect their responses to either forskolin or methanandamide. When PTX, (1 μg ml−1), was included in the tissue culture medium and the vessels incubated at 37°C for 24 h the basal level of cyclic AMP was slightly increased relative to control tissues. Stimulation of PTX-treated vessels with forskolin significantly increased cyclic AMP accumulation (n=4; P<0.01) while incubation of similarly treated vessels with 10 μM methanandamide failed to reduce forskolin-stimulated cyclic AMP levels (see Figure 4). These results strongly suggest that the receptor activated by methanandamide couples, via a pertussis toxin-sensitive G protein, to the inhibition of cyclic AMP accumulation.
Figure 4.
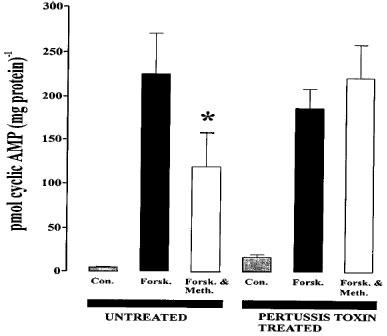
Effects of pertussis toxin treatment (1 μg ml−1 for 24 h) on methanandamide-induced attenuation of cyclic AMP-accumulation in rat carotid arteries. Carotid arteries were maintained in minimum essential medium supplemented with 10% bovine serum albumin for 24 h in the absence of pertussis toxin. The columns show the basal level of cyclic AMP, the level of forskolin-induced cyclic AMP accumulation and the inhibition of this effect of forskolin by methanandamide in pertussis toxin-treated and untreated arteries. The asterisk denotes a statistically significant difference (P<0.05) from forskolin-stimulated cyclic AMP accumulation in control arteries. Data are shown as means±s.e.mean (n=4).
Effects of methanandamide on vascular tone
Figure 5 illustrates the effects of methanandamide on rat mesenteric and carotid arteries. Methanandamide produced concentration-dependent relaxation of mesenteric arteries, yielding an EC50 value of 158 nM (125–199, 95% confidence limits) (n=16). However, concentrations of methanandamide, 20 fold greater than the EC50 value for methanandamide in mesenteric arteries failed to relax phenylephrine-constricted carotid arteries. In contrast, pinacidil, an opener of ATP-dependent K+ channels in vascular smooth muscle, produced nearly full relaxation of the rat carotid artery (Figure 5) (81% relaxation, EC50 250 nM (223–281) (n=4)), indicating that hyperpolarization per se can cause relaxation of the rat carotid artery. Similar data were obtained using a structurally dissimilar KATP channel opener, levcromakalim (data not shown).
Figure 5.
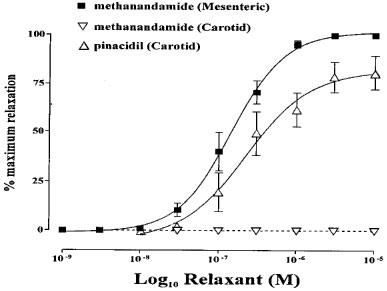
Methanandamide- and pinacidil-induced vasorelaxation. Methanandamide caused relaxation of phenylephrine-induced tone (approximately 70% of phenylephrine maximum) in mesenteric, but not carotid arteries (n=16 and 20 respectively). Pinacidil, a hyperpolarizing vasodilator, induced relaxation of carotid artery rings (n=4).
The observation that methanandamide failed to relax carotid arteries enabled a functional assay to be devised which further demonstrated the presence of cannabinoid receptors in arterial smooth muscle. Because cannabinoid receptors negatively couple, via Gi/Go, to adenylyl cyclase in rat carotid artery, we assessed the effects of methanandamide on concentration-effect curves to forskolin. Figure 6a shows the concentration-relaxation relationship in response to forskolin in rat carotid arteries. Relaxation to forskolin (3, 10 and 30 nM) was significantly reduced (P<0.05) by methanandamide (10 μM). Figure 6b illustrates the results from separate experiments where a single concentration of forskolin (10 nM) was used, allowing paired data to be obtained. Under control conditions forskolin (10 nM) produced a 57±7% relaxation of arterial rings pre-contracted with phenylephrine (10 μM). This was significantly reduced (P<0.05 Student's t-test) to 35±2% following incubation with methanandamide (10 μM, 15 min), but unchanged when the CB1-selective antagonists SR141716A (10 μM) or LY320135 (10 μM) were added prior to methanandamide challenge (54±6 and 51±3% respectively). SR141716A or LY320135 alone did not influence the effects of forskolin. The selective CB2 antagonist, SR 144528 (10 μM), had no effect on either forskolin-stimulated cyclic AMP accumulation or on the inhibition of this effect by methanandamide (Figure 6b).
Figure 6.
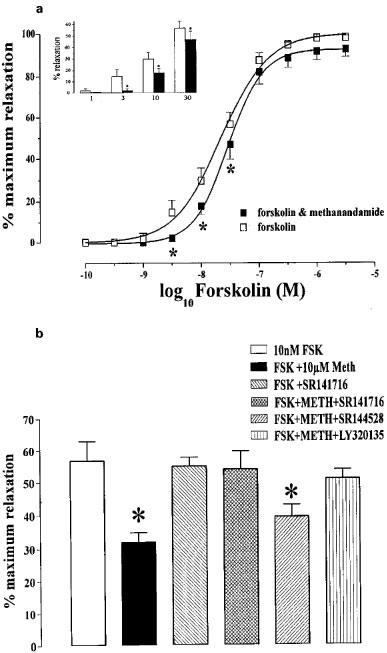
Inhibition by methanandamide of relaxation of the carotid artery by forskolin. (a) Concentration-effect curves for carotid artery rings to forskolin in the presence and absence of methanandamide (1 μM) (n=10). The insert shows data for 1–30 nM forskolin in more detail. The asterisks denote statistically significant differences (P<0.05) from relaxations induced by forskolin alone. (b) Relaxation of carotid artery rings to single concentrations of forskolin (10 nM). The 10 nM forskolin column represents pooled control data from paired experiments. The asterisks denote statistically significant differences (P<0.05) from relaxations induced by 10 nM forskolin in paired tissues. Data are shown as means±s.e.mean (n=5).
Discussion
Although it is now clear that cannabinoids can cause relaxation of arterial smooth muscle (Randall et al., 1996; Plane et al., 1997; Zygmunt et al., 1997; White & Hiley, 1998) the precise mechanism underlying this relaxation is unknown. CB1 receptors have been shown, mainly in tissues other than smooth muscle, to couple to three signalling pathways that potentially could explain their vasorelaxant actions. These are inhibition of cyclic AMP accumulation, inhibition of N-type calcium channels and activation of inwardly rectifying K+ channels (KIR) (Felder et al., 1998). However, there are reasons to doubt that the second and third of these mechanisms are important in vascular smooth muscle. Cannabinoids have not been shown to have a direct inhibitory effect on L-type Ca2+ channels which form the main voltage-sensitive Ca2+ channel population in smooth muscle. Since there is no evidence for N-type Ca2+ channels in vascular smooth muscle it is very unlikely that direct inhibition of Ca2+ channels underlies cannabinoid-induced relaxation of arterial smooth muscle. However, indirect inhibition of voltage-sensitive Ca2+ channels by cellular hyperpolarization might occur as suggested by Randall et al. (1996). Therefore, the activation of KIR is more appealing as a relaxant mechanism especially since cannabinoids produce hyperpolarization/repolarization (Randall et al., 1996; Plane et al., 1997) of mesenteric resistance arteries. However, there is no evidence that KIR channels are present on mesenteric or carotid artery smooth muscle (Quayle et al., 1997), and what electrophysiological evidence there is, suggests that cannabinoids do not activate K+ channels of any kind in vascular smooth muscle (Plane et al., 1997; Zygmunt et al., 1997). In the present study, methanandamide and HU-210 failed to relax the carotid artery, while hyperpolarizing vasodilators produced greater than 80% relaxation of carotid arteries. Taken together these data strongly suggest that cannabinoids do not function as hyperpolarizing factors. Zygmunt et al. (1997) put forward the hypothesis that anandamide may produce vasorelaxation by interfering with intracellular Ca2+ handling. One possible explanation for this result might be the prevention of cyclic AMP accumulation by cannabinoids acting on cell surface receptors. However, it might be expected that such a mechanism would cause relaxation of rat carotid artery smooth muscle, and so alternative mechanisms to explain (endo)cannabinoid-induced relaxation may still have to be examined.
One possible explanation for the failure of cannabinoids to induce relaxation of the carotid artery is the contractile agonist used to induce tone. Phenylephrine was used throughout the current study while others have routinely used methoxamine. However, anandamide and methanandamide induce concentration-dependent relaxation of phenylephrine-induced tone in both mesenteric artery segments (Plane et al., 1997) and isolated, perfused mesenteric beds (Gardner, Holland and Boyle, unpublished observation). Other studies have also reported that cannabinoids do not relax all vascular beds. Chataigneau et al. (1998) reported that anandamide did not relax porcine coronary arteries. The effects of anandamide and other cannabinoids may therefore depend on the species and arterial bed used, with these compounds being more effective relaxants of resistance arteries.
Another difference between the effects of cannabinoids on mesenteric and carotid arteries would appear to be the potency of the cannabinoids at inducing their functional effects (maximum effects at 0.1 and 10 μM respectively). However, it should be noted that where anandamide or methanandamide induce vasodilatation this is a two component relaxation. There is a phasic component, particularly at low concentrations, and a maintained relaxation that is seen at medium to high concentrations. The data in the current study, as with most other studies, was based on the maintained or steady state relaxation. However, if the data are derived from the phasic component then the discrepancy between relaxation of mesenteric artery and inhibition of cyclic AMP accumulation in the carotid artery is much less (EC50 10 nM, maximum 300 nM; EC50 approximately 5 nM, maximum 100 nM, respectively (see also Plane et al., 1997). The investigation of whether this two-phase relaxation is due to two entirely different mechanisms is beyond the scope of the current study but is an intriguing possibility.
The hypothesis that relaxation to cannabinoids was dependent on activation of cannabinoid receptors, probably of the CB1 subtype, was based on the ability of the selective cannabinoid antagonist, SR141716A (1–10 μM), to antagonize the vasorelaxant effects of methacholine and anandamide in rat mesenteric and hepatic arteries (Randall et al., 1996; Zygmunt et al., 1997; White & Hiley, 1997). Conversely, another study reported that SR141716A did not significantly affect anandamide-induced vasorelaxation in rat mesenteric arteries, and that other cannabinoid receptor agonists were relatively ineffective vasorelaxants (Plane et al., 1997). The present study supports the idea that interaction of cannabinoids with cannabinoid receptors does not lead to vasorelaxation and once again raises the question of how cannabinoids produce relaxation of some arteries.
Methanandamide inhibits forskolin-induced cyclic AMP accumulation
Forskolin stimulated a large increase in cyclic AMP accumulation in rat carotid artery. This varied from approximately 20 fold over basal to greater than 50 fold. If cannabinoid receptors were present on these smooth muscle cells then, by analogy with results obtained using other tissue preparations, these increases would be expected to be attenuated by cannabinoid agonists. The significant inhibition of forskolin-induced increases in cyclic AMP accumulation by methanandamide and HU-210 is in good agreement with data obtained from a variety of tissues including smooth muscle, and from studies where recombinant cannabinoid receptors have been expressed in mammalian cell lines (Slipetz et al., 1995; Pertwee et al., 1996; Rinaldi-Carmona et al., 1996; Shire et al., 1996). Our data strongly suggest that cannabinoid receptors are not only present in carotid artery smooth muscle but that they are functional, and couple negatively to adenylyl cyclase. Further evidence that these receptors are coupling to adenylyl cyclase via G proteins of the Gi/Go family, in a similar way to that described on other tissues and on cloned CB1 and CB2 receptors, was the sensitivity of the inhibitory effects of methanandamide to treatment with pertussis toxin. Cloned human and mouse CB1 and CB2 receptors expressed in a variety of mammalian cell lines have been described as coupling to the inhibition of adenylyl cyclase through the Gi/Go family of G proteins (Slipetz et al., 1995; Rinaldi-Carmona et al., 1996; Shire et al., 1996). The results of pertussis toxin treatment in rat carotid artery show that the receptors present are coupling through the Gi family to inhibit forskolin-induced cyclic AMP accumulation. Pertussis toxin treatment of Chinese hamster ovary (CHO) cells transfected with CB1 receptors has been shown to unmask a stimulatory effect of anandamide on adenylyl cyclase activated by forskolin (Felder et al., 1998) and it was suggested that this might represent regulation of adenylyl cyclase activity by βγ subunits of G proteins. Although it was noted, in the present study, that in pertussis toxin treated vessels the combination of forskolin and methanandamide seemed to produce greater cyclic AMP accumulation than forskolin alone (see Figure 4) this effect did not reach significance. Therefore, it seems that positive coupling of CB1 receptors to adenylyl cyclase is unlikely to be important in the regulation of smooth muscle tone in the carotid artery. The lack of effect of cannabinoid agonists on resting cyclic AMP levels in the carotid artery or on the tone of these vessels in the presence or absence of phenylephrine would tend to support this observation. However, care should be taken when interpreting these observations since Felder et al. (1998) reported that stimulation of cyclic AMP accumulation only occurred when adenylyl cyclase had already been activated by forskolin.
These results make it difficult to understand the mechanism by which activation of cannabinoid receptors by agonists such as anandamide could lead to relaxation of smooth muscle. It seems anomalous that the same receptor would couple, in the same cell, to a mechanism that causes relaxation and, at the same time, to another mechanism impeding relaxation. The data obtained concerning the effects of methanandamide on forskolin-induced relaxation in the rat carotid artery reinforces this point and illustrates that activation of the cannabinoid receptor can inhibit smooth muscle relaxation, at least in this artery.
Which cannabinoid receptor sub-types are present in smooth muscle?
Both subtypes of cloned cannabinoid receptor have been described as coupling via Gi/Go to the inhibition of adenylyl cyclase and therefore neither pertussis toxin sensitivity, nor inhibition of cyclic AMP accumulation provides any information on which receptor subtypes are present on these smooth muscle cells. The presence of membrane-delimited receptors is usually detected using radioligand binding methods. Cannabinoids, however, are extremely lipophilic and when radiolabelled cannabinoids have been used to determine CB1/CB2-specific binding in vascular smooth muscle cell membranes the level of non-specific binding is so high as to ‘mask' specific binding (Kendall D.A., personal communication). Because of these practical problems we devised an alternative method to assay for cannabinoid receptors in intact vascular smooth muscle. Both CB1 and CB2 receptors, although differentially distributed (see Howlett (1996) and Randall & Kendall (1998) for recent reviews), share a similar signal transduction pathway such that occupation of either receptor, by a cannabinoid receptor agonist, leads, via the activation of Gi/Go, to inhibition of adenylyl cyclase. We made use of this inhibition of a cell-signalling pathway to assay for cannabinoid receptors by examining the effects of cannabinoids on forskolin-induced cyclic AMP accumulation in the rat carotid artery. Additionally we examined the effects of a number of cannabinoid receptor agonists and antagonists to define which receptors, if any, are present in the rat carotid artery.
All compounds which inhibited cyclic AMP accumulation have a higher affinity for CB1 than CB2 receptors (Felder et al., 1995; Slipetz et al., 1995), while WIN55,2212-2, which did not antagonize the effects of forskolin, has a higher affinity for CB2 receptors. Since a previous study using cloned CB1 and CB2 receptors reported that the Ki values for anandamide for inhibition of cyclic AMP accumulation were 0.5 and 2 μM, respectively (Felder et al., 1998), the observation that low concentrations of methanandamide (100 nM and 1 μM) significantly attenuated forskolin-stimulated cyclic AMP accumulation in the carotid artery provides further supportive evidence that the cannabinoid receptor mediating this inhibition is probably of the CB1 subtype.
The selective CB1 antagonist, LY320132, inhibited the effects of methanandamide such that it no longer significantly inhibited forskolin-induced cyclic AMP accumulation. In functional experiments LY320132 also reversed the inhibition of forskolin-induced relaxation by methanandamide, as did the prototypic CB1 antagonist SR141716A. LY320132 did not itself have any affect on forskolin-induced cyclic AMP accumulation or relaxation. The CB2 antagonist SR144528 did not share this inhibition of the effects of methanandamide. Taken together these data strongly suggest that the inhibitory effect of methanandamide on cyclic AMP accumulation occurred via the activation of CB1 receptors in this tissue.
The CB1 antagonist SR141716A inhibited forskolin-induced cyclic AMP accumulation per se, making it impossible to use in the biochemical studies, and suggesting that perhaps this antagonist may either exhibit agonist properties or have non-specific effects on the adenylyl cyclase/cyclic AMP system in some tissues. This observation that SR141716A has an effect on forskolin-stimulated cyclic AMP accumulation which is similar to that of cannabinoid agonists suggests it may be acting as a low intrinsic activity agonist, rather than a pure antagonist at CB1 receptors. Further evidence in support of this idea can be found by examining data obtained on CB1 receptors in myenteric neurones (López-Redondo et al., 1997), or stably transfected into mammalian cell lines (Felder et al., 1995).
The data presented in this study demonstrates that functional, Gi-linked cannabinoid receptors of the CB1 receptor subtype are present in the rat carotid artery. However, in this tissue, these receptors inhibit cyclic AMP accumulation rather than cause relaxation via hyperpolarization.
Acknowledgments
This study was supported by the Medical Research Council (Grant No. G9609076). We acknowledge the expert technical assistance of Mr Raj Mistry with some of the cyclic AMP measurements.
References
- BLOOM A.S., TERSHNER S., FULLER S.A., STEIN E.A. Cannabinoid-induced alterations in regional cerebral blood flow in the rat. Pharmacol. Biochem. Behav. 1997;57:625–631. doi: 10.1016/s0091-3057(96)00475-3. [DOI] [PubMed] [Google Scholar]
- BROWN B.L., ALBANO J.D.M., SGHERZI A.M., TAMPION W. A simple and sensitive saturation assay method for the measurement of adenosine 3′, 5′-monophosphate. Biochem. J. 1971;121:561–563. doi: 10.1042/bj1210561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CHATAIGNEAU T., FELETOU M., THOLLON C., VILLENEUVE N., VILAINE J.P., DUHAULT J., VANHOUTTE P.M. Cannabinoid CB1 receptor and endothelium-dependent hyperpolarization in guinea-pig carotid, rat mesenteric and porcine coronary arteries. Br. J. Pharmacol. 1998;123:968–974. doi: 10.1038/sj.bjp.0701690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FELDER C.C., JOYCE K.E., BRILEY E.M., GLASS M., MACKIE K.P., FAHEY K.J., CULLINAN G.J., HUNDEN D.C., JOHNSON D.W., CHANEY M.O., KOPPEL G.A., BROWNSTEIN M. LY320135, a novel cannabinoid CB1 receptor antagonist, unmasks coupling of the CB1 receptor to stimulation of cAMP accumulation. J. Pharmacol. Exp. Ther. 1998;284:291–297. [PubMed] [Google Scholar]
- FELDER C.C., JOYCE K.E., BRILEY E.M., MANSOURI J., MACKIE K., BLOND O., LAI Y., MA A.L., MITCHELL R.L. Comparison of the pharmacology and signal-transduction of the human cannabinoid CB1 and CB2 receptors. Mol. Pharmacol. 1995;48:443–450. [PubMed] [Google Scholar]
- FLEMING I., SCHERMER B., POPP R., BUSSE R. Inhibition of the production of endothelium-derived hyperpolarizing factor by cannabinoid receptor agonists. Br. J. Pharmacol. 1999;126:949–960. doi: 10.1038/sj.bjp.0702381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HARRIS D., KENDALL D.A., RANDALL M.D. Characterization of cannabinoid receptors coupled to vasorelaxation by endothelium-derived hyperpolarizing factor. Naunyn-Schmiedeberg's Arch. Pharmacol. 1999;359:48–52. doi: 10.1007/pl00005322. [DOI] [PubMed] [Google Scholar]
- HOWLETT A.C. Pharmacology of cannabinoid receptors. Annu. Rev. Pharmacol. Toxicol. 1996;35:607–634. doi: 10.1146/annurev.pa.35.040195.003135. [DOI] [PubMed] [Google Scholar]
- LAKE K.D., COMPTON D.R., VARGA K., MARTIN B.R., KUNOS G. Cannabinoid-induced hypotension and bradycardia in rats is mediated by CB1-like cannabinoid receptors. J. Pharmacol. Exp. Ther. 1997;281:1030–1037. [PubMed] [Google Scholar]
- LÓPEZ-REDONDO F., LEES G.M., PERTWEE R.G. Effects of cannabinoid receptor ligands on electrophysiological properties of myenteric neurones of the guinea-pig ileum. Br. J. Pharmacol. 1997;122:330–334. doi: 10.1038/sj.bjp.0701393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MACKIE K., HILLE B. Cannabinoids inhibit N-type calcium channels in neuroblastoma glioma-cells. Proc. Natl. Acad. Sci. U.S.A. 1992;89:3825–3829. doi: 10.1073/pnas.89.9.3825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MALINOWSKA B., GODLEWSKI G., BUCHER B., SCHLICKER E. Cannabinoid CB1 receptor-mediated inhibition of the neurogenic vasopressor response in the pithed rat. Naunyn-Schmiedeberg's Arch. Pharmacol. 1997;356:197–202. doi: 10.1007/pl00005041. [DOI] [PubMed] [Google Scholar]
- NIEDERHOFFER N., SZABO B. Effect of the cannabinoid receptor agonist WIN55212-2 on sympathetic cardiovascular regulation. Br. J. Pharmacol. 1999a;126:457–456. doi: 10.1038/sj.bjp.0702337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NIEDERHOFFER N., SZABO B. Involvement of CB1 cannabinoid receptors in the EDHF-dependent vasorelaxation in rabbits. Br. J. Pharmacol. 1999b;126:1383–1386. doi: 10.1038/sj.bjp.0702452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PERTWEE R.G., JOE-ADIGWE G., HAWKSWORTH G.M. Further evidence for the presence of cannabinoid CB1 receptors in mouse vas deferens. Eur. J. Pharmacol. 1996;296:169–172. doi: 10.1016/0014-2999(95)00790-3. [DOI] [PubMed] [Google Scholar]
- PLANE F., HOLLAND M., WALDRON G.J., GARLAND C.J., BOYLE J.P. Evidence that anandamide and EDHF act via different mechanisms in rat isolated mesenteric arteries. Br. J. Pharmacol. 1997;121:1509–1511. doi: 10.1038/sj.bjp.0701361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- QUAYLE J.M., NELSON M.T., STANDEN N.B. ATP-sensitive and inwardly rectifying potassium channels in smooth muscle. Physiol. Rev. 1997;77:1165–1232. doi: 10.1152/physrev.1997.77.4.1165. [DOI] [PubMed] [Google Scholar]
- RANDALL M.D., ALEXANDER S.P.H., BENNETT C.F., BOYD E.A., FRY J.R., GARDINER S.M., KEMP P.A., MCCULLOCH A.I., KENDALL D.A. An endogenous cannabinoid as an endothelium-derived vasorelaxant. Biochem. Biophys. Res. Commun. 1996;229:114–120. doi: 10.1006/bbrc.1996.1766. [DOI] [PubMed] [Google Scholar]
- RANDALL M.D., KENDALL D.A. Endocannabinoids: a new class of vasoactive substances. Trends Pharmacol. Sci. 1998;19:55–58. doi: 10.1016/s0165-6147(97)01161-9. [DOI] [PubMed] [Google Scholar]
- RINALDI-CARMONA M., CALANDRA B., SHIRE D., BOUABOULA M., OUSTRIC D., BARTH F., CASELLAS P., FERRARA P., LEFUR G. Characterization of 2 cloned human CB1 cannabinoid receptor isoforms. J. Pharmacol. Exp. Ther. 1996;278:871–878. [PubMed] [Google Scholar]
- SHIRE D., CALANDRA B., RINALDI-CARMONA M., OUSTRIC D., PESSEGUE B., BONNINCABANNE O., LEFUR G., CAPUT D., FERRARA P. Molecular-cloning, expression and function of the murine cb2 peripheral cannabinoid receptor. Biochim. Biophys. Acta. 1996;1307:132–136. doi: 10.1016/0167-4781(96)00047-4. [DOI] [PubMed] [Google Scholar]
- SLIPETZ D.M., ONEILL G.P., FAVREAU L., DUFRESNE C., GALLANT M., GAREAU Y., GUAY D., LABELLE M., METTERS K.M. Activation of the human peripheral cannabinoid receptor results in inhibition of adenylyl cyclase. Mol. Pharmacol. 1995;48:352–361. [PubMed] [Google Scholar]
- VARGA K., LAKE K.D., HUANGFU D., GUYENET P.G., KUNOS G. Mechanism of the hypotensive action of anandamide in anesthetized rats. Hypertension. 1996;28:682–686. doi: 10.1161/01.hyp.28.4.682. [DOI] [PubMed] [Google Scholar]
- VARGA K., LAKE K., MARTIN B.R., KUNOS G. Novel antagonist implicates the CB1 cannabinoid receptor in the hypotensive action of anandamide. Eur. J. Pharmacol. 1995;278:279–283. doi: 10.1016/0014-2999(95)00181-j. [DOI] [PubMed] [Google Scholar]
- VENANCE L., SAGAN S., GIAUME C. (R)-methanandamide inhibits receptor-induced calcium responses by depleting internal calcium stores in cultured astrocytes. Pflügers Arch. 1997;434:147–149. doi: 10.1007/s004240050376. [DOI] [PubMed] [Google Scholar]
- VIDRIO H., SANCHEZSALVATORI M.A., MEDINA M. Cardiovascular effects of (−)-11-Oh-delta(8)-Tetrahydrocannabinol- dimethylheptyl in rats. J. Cardiovasc. Pharmacol. 1996;28:332–336. doi: 10.1097/00005344-199608000-00022. [DOI] [PubMed] [Google Scholar]
- WAGNER J.A., VARGA K., ELLIS E.F., RZIGALINSKI B.A., MARTIN B.R., KUNOS G. Activation of peripheral CB1 cannabinoid receptors in haemorrhagic shock. Nature. 1997;390:518–521. doi: 10.1038/37371. [DOI] [PubMed] [Google Scholar]
- WHITE R., HILEY C.R. A comparison of EDHF-mediated and anandamide-induced relaxations in the rat isolated mesenteric artery. Br. J. Pharmacol. 1997;122:1573–1584. doi: 10.1038/sj.bjp.0701546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WHITE R., HILEY C.R. The actions of some cannabinoid receptor ligands in the rat isolated mesenteric artery. Br. J. Pharmacol. 1998;125:533–541. doi: 10.1038/sj.bjp.0702111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ZYGMUNT P.M., HÖGESTÄTT E.D., WALDECK K., EDWARDS G., KIRKUP A., WESTON A.H. Studies on the effects of anandamide in rat hepatic artery. Br. J. Pharmacol. 1997;122:1679–1686. doi: 10.1038/sj.bjp.0701601. [DOI] [PMC free article] [PubMed] [Google Scholar]


