Abstract
The relationships between the density of dopamine D4.4 receptors and the agonist efficacies of L-745,870 (3-(4-[4-chlorophhenyl]piperazin-1-yl)-methyl-1H-pyrrolo [2,3-b]pyridine) and U-101958 ((1-benzyl-piperidin-4-yl)-(3-isopropoxy-pyridin-2-yl)-methyl-amine) were investigated in Chinese hamster ovary (CHO) cells, after treatment with the gene expression enhancer, sodium butyrate.
In CHO cells expressing D4.4 receptors (CHO/D4 cells), dopamine inhibited forskolin-stimulated cyclic AMP accumulation (Emax 56±1% inhibition, pEC50 7.4±0.1, n=10). U-101958 behaved as a partial agonist (39±7% the efficacy of dopamine, pEC50 8.1±0.3, n=4), whereas L-745,870 had no detectable agonist effect.
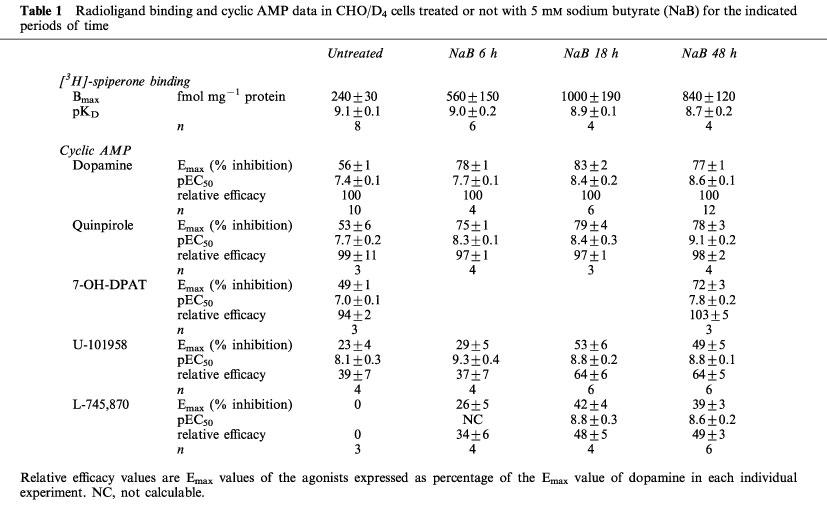
Receptor density, as estimated by [3H]-spiperone saturation binding was 240±30 fmol mg−1 protein (n=8) in CHO/D4 cell homogenates. It reached 560±150 (n=6), 1000±190 (n=4) and 840±120 (n=4) fmol mg−1 protein after treatment with sodium butyrate (5 mM) for 6, 18 and 48 h, respectively.
The increase in receptor density was associated with a gradual enhancement of the agonist effects (increased Emax and pEC50 values) of dopamine. The efficacy of U-101958 (relative to dopamine) doubled and L-745,870 was turned into a partial agonist (efficacy 49% relative to dopamine, pEC50 8.6±0.2, n=6, after 48 h treatment with sodium butyrate). These agonist effects of U-101958 and L-745,870 could be antagonized by spiperone (0.1 μM) but not by raclopride (10 μM).
The results show that U-101958 and L-745,870 are partial agonists at human dopamine D4.4 receptors expressed in CHO cells. Their efficacy is governed by receptor density. Agonist effects of these two compounds in vivo cannot be excluded under circumstances of increased receptor levels.
Keywords: L-745,870; U-101958; expression system; dopamine D4 receptor; cyclic AMP inhibition; [3H]-spiperone binding; schizophrenia
Introduction
Dopamine receptors have been originally classified into two pharmacological subtypes, namely D1 and D2 (Kebabian & Calne, 1979). However, recent molecular cloning studies have led to the discovery of multiple dopamine receptor genes and to their classification into D1-like and D2-like subfamilies (for a review, see Hartman & Civelli, 1997). The D1-like subfamily comprises D1 and D5 receptors, both positively coupled to cyclic AMP formation, whereas the D2-like subfamily consists of D2, D3 and D4 receptors, all negatively coupled to cyclic AMP formation.
D2-like receptors appear to play an important role in schizophrenia. Thus, all antipsychotics currently used to treat the symptoms of schizophrenia have the ability to antagonize D2-like receptors (Lahti et al., 1993; Seeman & Van Tol, 1995). Within the D2-like receptor family, D4 receptors have received particular attention for a number of reasons. Firstly, the atypical neuroleptic, clozapine has a small degree of selectivity for the D4 receptor over the D2 receptor (Van Tol et al., 1991). Secondly, mRNA expression of the dopamine D4 receptor, as well as the receptor protein itself, is found primarily in limbic and cortical regions of the brain, areas thought to be involved in emotional/affective behaviour and cognition (Meador-Woodruff et al., 1996; Primus et al., 1997). In addition, a selective increase in the density of dopamine D4 binding sites has been found in post-mortem brain tissue from schizophrenics (Seeman et al., 1993; Murray et al., 1995), although this remains controversial (Reynolds & Mason, 1995; Reynolds, 1996). All these findings strongly suggest that the dopamine D4 receptor is an interesting target for the treatment of schizophrenia and several pharmaceutical companies have therefore developed putative dopamine D4 receptor antagonists (see reviews by Hartman & Civelli, 1997; Wilson et al., 1998).
We recently reported that two such compounds, L-745,870 (3-(4-[4-chlorophenyl]piperazin-1-yl)-methyl-1H-pyrrolo [2,3-b]pyridine; Patel et al., 1997) and U-101958 ((1-benzyl-piperidin - 4 - yl) - (3 - isopropoxy - pyridin - 2-yl)-methyl-amine; Schlachter et al., 1997) were in fact agonists at human recombinant dopamine D4.4 receptors stably expressed in human embryonic kidney (HEK)293 cells (Gazi et al., 1998). In these cells, L-745,870 and U-101958 mimicked dopamine at inhibiting forskolin-stimulated cyclic AMP accumulation, in contrast to spiperone and clozapine which were silent. Furthermore, the effects of L-745,870 and U-101958 could be antagonized by spiperone and clozapine but not by raclopride, consistent with a dopamine D4 receptor-mediated effect. In the absence of recognized functional model for native dopamine D4 receptors, these intriguing results prompted us to investigate the activities of L-745,870 and U-101958 in another expression system, namely Chinese hamster ovary (CHO) cells, using the same functional readout as previously (inhibition of cyclic AMP formation). We paid particular attention to the relationships between receptor density and ligand efficacy. Receptor density is known to be a factor strongly influencing ligand efficacy in both tissues and recombinant systems (Kenakin, 1997). Using sodium butyrate as an enhancer of receptor gene expression, we now show that the putative antipsychotics, L-745,870 and U-101958 are not silent antagonists at dopamine D4.4 receptors expressed in CHO cells. Their efficacy is a function of receptor density.
Methods
Cell line and culture
A mammalian expression vector containing a human dopamine D4.4 receptor cDNA (Gazi et al., 1998) was cotransfected with the pRSVNeo plasmid (in a 9 : 1 ratio) into CHO-K1 cells by the calcium phosphate precipitation method of Chen & Okayama (1987). Selection for stable integration was performed by adding 0.8 mg ml−1 of geneticin to the culture and transfectants were then tested for a cyclic AMP inhibitory response to dopamine. The best line (hereafter referred to as CHO/D4 cells) was propagated in Dulbecco's modified Eagle medium supplemented with 10% foetal calf serum, 100 iu ml−1 penicillin, 100 μg ml−1 streptomycin, non-essential aminoacids and 1 mg ml−1 geneticin. Cells were grown in a humidified atmosphere at 37°C in the presence of 5% CO2. They were split twice a week using a trypsin/EDTA solution. For radioligand binding experiments, cells were grown in 24.5 cm squared (‘bio-assay') dishes. For cyclic AMP measurements, cells were seeded at a density of 2−3×105 cells/well in 24-well plates.
Radioligand binding assay
CHO/D4 cells grown to confluence in bio-assay dishes were collected by scraping in 50 mM HEPES buffer, pH 7.4, containing 1 mM MgCl2, 2.5 mM CaCl2, 0.1% (w v−1) bovine serum albumin, 0.025% (w v−1) bacitracin and 0.025% (w v−1) sodium azide. They were then centrifuged at 1200 r.p.m. for 10 min at 4°C. After the removal of the supernatant, the cells were frozen at −70°C until the day of the experiment. For each binding assay experiment, the cells were resuspended in 50 mM Tris-HCl buffer (pH 7.4) containing (mM): NaCl 120, EDTA 5, MgCl2 1.5 and KCl 5, using a Polytron tissue homogenizer at setting 3–4 for 15 s. One hundred and 50 μl of the cell suspension (corresponding to ∼250,000 cells/assay) were added to 96-well microtitre plates containing 50 μl drug and 50 μl (∼40,000 c.p.m./assay) of [3H]-spiperone (110 Ci mmol−1, Amersham, Rahn AG, Zürich, Switzerland). The plates were incubated at room temperature for 120 min, rapidly filtered through Packard Unifilter-96, GF/C plates and washed three times with 300 μl ice-cold 10 mM Tris-HCl buffer containing 154 mM NaCl, pH 7.5. Filter-bound radioactivity was counted in 40 μl Microscint 40 in a Packard TopCount scintillation counter. Non specific binding was defined in the presence of 1 μM U-101958. Saturation experiments were performed using eight concentrations of the radioligand, ranging from approximately 0.4 to 25 nM. Assays were performed in triplicate and the determinations were replicated at least three times. The protein content was determined according to Bradford (1976).
Measurement of cyclic AMP accumulation
Cells grown to confluence in 24-well plates were washed with 1 ml of HEPES-buffered salt solution (in mM): NaCl 130, KCl 5.4, CaCl2 1.8, MgSO4 0.8, NaH2PO4 0.9, glucose 25, HEPES 20, pH 7.4, containing phenol red 5 mg l−1, and incubated with 6 μCi ml−1 of [8-3H]-adenine (23 Ci mmol−1, Anawa Trading SA, Wangen, Switzerland) at 37°C for 2 h in 0.5 ml of the same buffer. They were then washed twice with 1 ml of the buffer solution supplemented with 1 mM isobutylmethylxanthine. The cells were incubated in 1 ml of the same solution at 37°C, in the presence and absence of forskolin (10 μM) and of test compounds at the indicated concentrations. Experiments were conducted in duplicate. After 15 min, the medium was removed and replaced by 1 ml of 5% trichloroacetic acid solution containing cyclic AMP and ATP (both 0.1 mM). After 30 min at 4°C, the trichloroacetic acid extracts were directly subjected to sequential chromatography on Dowex AG 50W-X4 and alumina columns (Salomon, 1991). Cyclic AMP accumulation was calculated as the ratio [3H]-cyclic AMP/([3H]-cyclic AMP+[3H]-ATP). The recovery of cyclic AMP was 76%.
Analysis of data
Cyclic AMP data were expressed as percentage of forskolin-stimulated cyclic AMP accumulation. Radioligand binding saturation and inhibition curves, as well as concentration-response curves were fitted to the non linear logistic function of the Microcal Origin software package. Values of Bmax, KD, Ki, Emax (maximal effect) and EC50 (concentration producing half the maximal effect) were derived from this fit. The apparent pKB values of antagonists were calculated according to the formula: pKB=log[B]−log (CR-1) where [B] is the concentration of the antagonist used and CR (concentration-ratio) is the ratio of agonist EC50 measured in the presence of antagonist over that measured in the absence of antagonist. Results are given as mean±s.e.mean of the indicated n values.
Drugs and biochemicals
The substances were obtained from the following sources: forskolin, isobutylmethylxanthine, dopamine and sodium butyrate (n-butyric acid sodium salt, Sigma, Fluka, Buchs, Switzerland); quinpirole hydrochloride (Research Biochemicals International, Rahn, Zürich, Switzerland); 7-hydroxy-2-dipropylaminotetralin hydrobromide and L-745,870 (3-[[4-(4-chlorophenyl)piperazin - 1 - yl] - methyl] -1H-pyrrolo[2,3-b]pyridine trihydrochloride) (Tocris, Bristol, U.K.); raclopride tartrate (a gift from Astra, Sweden). U-101958 ((1-benzyl-piperidin-4-yl)-(3-isopropoxy-pyridin-2-yl)-methyl-amine; Dr R. Swoboda), spiperone and clozapine were from Novartis Pharma AG, Basel, Switzerland. The subtances were prepared daily at 40 mM, either in distilled water or in a mixture of 1-methyl-2-pyrrolidone:ethanol (1 : 1) containing 20 mg ml−1 ascorbic acid, and further diluted with water. Exceptions were forskolin (10 mM stock solution in ethanol) and sodium butyrate (directly dissolved at 5 mM in culture medium).
Results
Characterization of dopamine D4 receptor-mediated responses in CHO/D4 cells
Forskolin (10 μM) induced an average 15 fold stimulation of cyclic AMP accumulation in CHO/D4 cells. This effect was inhibited in a concentration-dependent fashion by dopamine (Emax 56±1% inhibition of forskolin-stimulated levels, pEC50 7.4±0.1, n=10; Figure 1a) and mimicked by the dopamine D2-like receptor agonists, quinpirole and 7-hydroxy-2-dipropylaminotetralin (7-OH-DPAT; see Table 1). U-101958 also inhibited forskolin-stimulated cyclic AMP accumulation in a concentration-dependent manner (Emax 23±4% inhibition of forskolin-stimulated levels, pEC50 8.1±0.3, n=4; Figure 1a). The Emax of U-101958 represented 39% of the Emax of dopamine (see Table 1). In contrast, L-745,870 did not inhibit forskolin-stimulated cyclic AMP accumulation (Figure 1a). At a concentration of 10 μM, there was even a trend for L-745,870 to enhance forskolin effects (not shown). Spiperone, clozapine and raclopride were silent in these cells up to 10 μM (n⩾3 each).
Figure 1.
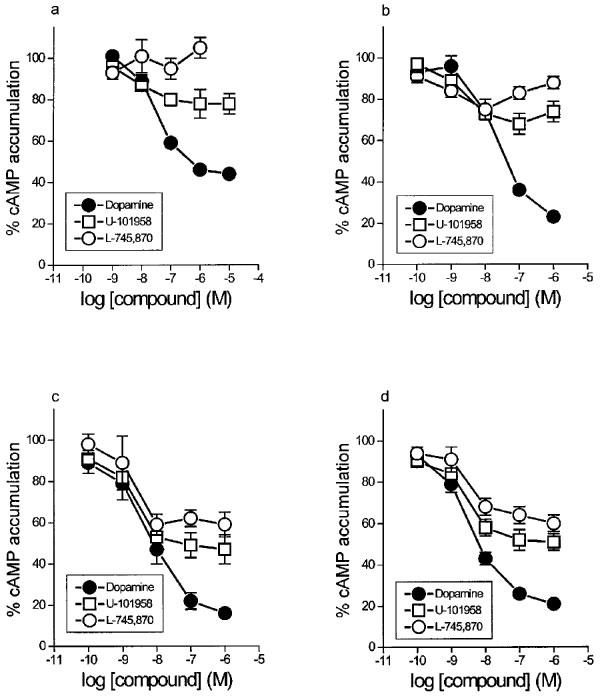
Concentration-response curves of dopamine, U-101958 and L-745,870 for inhibition of forskolin-stimulated cyclic AMP accumulation in (a) untreated CHO/D4 cells and CHO/D4 cells treated with 5 mM sodium butyrate for (b) 6 h, (c) 18 h and (d) 48 h. Data are means and vertical lines show s.e.mean from the number (n) of experiments indicated in Table 1.
Table 1.
Radioligand binding and cyclic AMP data in CHO/D4 cells treated or not with 5 mM sodium butyrate (NaB) for the indicated periods of time
Dopamine, quinpirole, 7-OH-DPAT, U-101958 and L-745,870 (1 nM–10 μM) did not inhibit forskolin-stimulated cyclic AMP accumulation in control, non-transfected CHO-K1 cells (n⩾3 each).
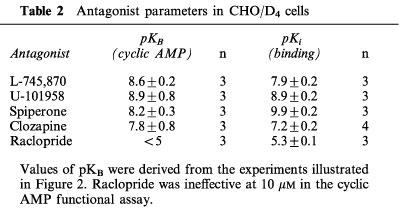
As illustrated in Figure 2, the concentration-response curve of dopamine was shifted to the right in a parallel manner in the presence of spiperone (0.1 μM), clozapine (1 μM), L-745,870 (0.1 μM) and the partial agonist U-101958 (0.1 μM) but not in the presence of raclopride (10 μM). Derived pKB values are to be found in Table 2.
Figure 2.
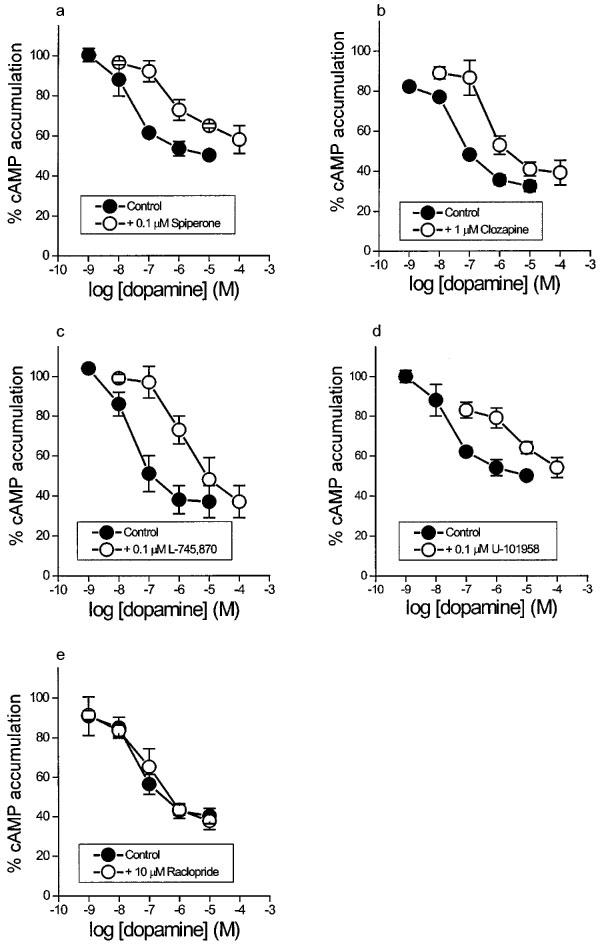
Concentration-response curves of dopamine for inhibition of forskolin-stimulated cyclic AMP accumulation in CHO/D4 cells, in the absence and in the presence of (a) spiperone (0.1 μM), (b) clozapine (1 μM), (c) L-745,870 (0.1 μM), (d) U-101958 (0.1 μM) and (e) raclopride (10 μM). Data are means and vertical lines show s.e.mean from three experiments.
Table 2.
Antagonist parameters in CHO/D4 cells
[3H]-spiperone binding
In saturation binding experiments, [3H]-spiperone was found to label a homogeneous and saturable population of specific binding sites in CHO/D4 cell membrane homogenates. The Bmax values amounted to 240±30 fmol mg−1 protein and the pKD value was 9.1±0.1 (n=8). The specific binding of [3H]-spiperone was inhibited in a monophasic manner by L-745,870, U-101958, spiperone and clozapine with high affinity, but much less so by raclopride (see Table 2). After treatment with sodium butyrate (5 mM) for 6, 18 and 48 h, the Bmax value reached 560±150, 1000±190 and 840±120 fml mg−1 protein, respectively, without major changes in the pKD value (see Table 1).
Influence of sodium butyrate treatment on the efficacy and potency of D4 receptor ligands
Figure 1b, c and d shows the effects of dopamine, U-101958 and L-745,870 on CHO/D4 cells treated with 5 mM sodium butyrate for 6, 18 and 48 h, respectively. These results are summarized in Table 1, along with data on quinpirole and 7-OH-DPAT. There were no noticeable changes in basal and forskolin-stimulated cyclic AMP levels after sodium butyrate treatment. A 6 h treatment with sodium butyrate resulted in an increased Emax of dopamine (from 56±1% to 78±1% inhibition of forskolin-stimulated cyclic AMP levels), with a minimal increase in the pEC50 value (from 7.4±0.1 to 7.7±0.1). Prolonged treatment up to 48 h did not increase the Emax value further, whereas the pEC50 value increased to 8.4±0.2 and 8.6±0.1 after 18 and 48 h treatment, respectively (Table 1). The same trend was observed with quinpirole and 7-OH-DPAT (Table 1). Under the same conditions, the Emax of U-101958 approximately doubled (from 23±4% in untreated cells to 53±6% and 49±5% after 18 and 48 h treatment), along with a slight increase in the pEC50 value (from 8.1±0.3 to 8.8±0.1). The relative efficacy of U-101958 (compared to dopamine) thus changed from 39% in untreated cells to 64% in sodium butyrate-treated cells. In addition, treatment with sodium butyrate revealed an agonist effect of L-745,870. After 6 h treatment, this effect reached 26±5% inhibition, but the concentration-response curve of L-745,870 tended to be biphasic, preventing any accurate determination of the EC50 value (Figure 1b). In cells treated for 18 and 48 h with sodium butyrate, L-745,870 induced concentration-dependent inhibitions of forskolin-stimulated cyclic AMP accumulation, with Emax values of 42±4% and 39±3% (48 and 49% efficacy relative to dopamine) and pEC50 values of 8.8±0.3 and 8.6±0.2. No such effects were observed with spiperone and clozapine after 48 h treatment with sodium butyrate (n=4 each, not shown).
The agonist effects of U-101958 and L-745,870 in cells treated with sodium butyrate for 48 h could be antagonized by spiperone (0.1 μM, Figure 3a, b), but not by raclopride (10 μM; Figure 3c, d). Spiperone pKB values were 8.8±0.1 and 9.1±0.1 (n=3 each) against U-101958 and L-745,870, respectively.
Figure 3.
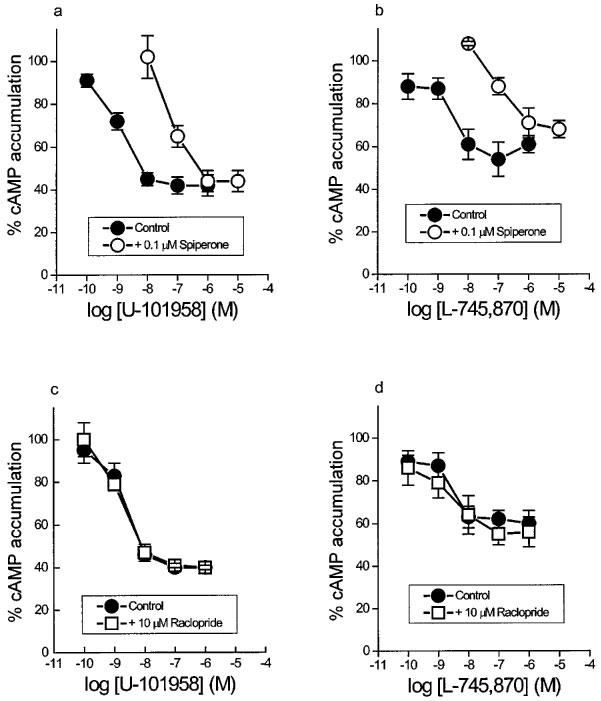
Concentration-response curves of (a,c) U-101958 and (b,d) L-745,870 for inhibition of forskolin-stimulated cyclic AMP accumulation in the absence and in the presence of (a,b) spiperone (0.1 μM) and (c,d) raclopride (10 μM) in CHO/D4 cells treated with 5 mM sodium butyrate for 48 h. Data are means and vertical lines show s.e.mean from three or four experiments.
Dopamine, quinpirole, U-101958 and L-745,870 did not inhibit forskolin-stimulated cyclic AMP accumulation in non-transfected CHO-K1 cells treated with sodium butyrate for 6, 18 or 48 h (not shown).
Discussion
U-101958 and L-745,870 have originally been introduced as dopamine D4 receptor antagonists (Patel et al., 1997; Schlachter et al., 1997). However, we recently found them to be agonists at human recombinant dopamine D4.4 receptors expressed in HEK293 cells (Gazi et al., 1998). In the latter cells, U-101958 acted as a quasi-full agonist, compared to dopamine, and L-745,870 had a substantial efficacy (71% relative to dopamine). Since most of the previous in vitro information on these two compounds had been obtained in CHO cells, we transfected this type of cells with the same cDNA as was used for HEK293 cells (Gazi et al., 1998). Inhibition of forskolin-stimulated cyclic AMP accumulation was used as a functional readout. The resulting pharmacological profile was confirmed as being dopaminergic D4 in nature, since: (1) dopamine, quinpirole and 7-OH-DPAT induced concentration-dependent inhibitions of forskolin-stimulated cyclic AMP accumulation in CHO/D4 cells but not in non-transfected CHO-K1 cells; and (2) the effect of dopamine could be potently antagonized by spiperone and clozapine, but not by raclopride. In addition, radioligand binding studies confirmed the presence of sites with the same profile. Nevertheless, the present data notably differ from our previous results obtained with HEK293 cells in that, in CHO/D4 cells U-101958 behaved as a partial agonist whereas L-745,870 was a pure antagonist. It is also noteworthy that dopamine and the full agonists, quinpirole and 7-OH-DPAT were less potent in CHO/D4 cells than in human embryonic kidney 293 cells by more than one order of magnitude (see Gazi et al., 1998). As a whole, these findings hint toward a higher receptor density and/or a more efficient receptor-effector coupling (for some reason) in the latter cells than in CHO/D4 cells. It is well known that functional responses mediated by recombinant receptors may be affected by such factors (see Kenakin, 1997).
In the present study, we addressed the possible influence of receptor density on functional responses in CHO/D4 cells by treating the cells with sodium butyrate. Sodium butyrate is an enhancer of gene expression, acting by inducing mammalian promoters like the cytomegalovirus promoter (Cockett et al., 1990) which controlled the expression of human dopamine D4.4 receptors in our cells (see Gazi et al., 1998). For instance, this agent has been successfully used for increasing the expression of 5-HT1B and 5-HT1D receptors in C6 glioma cells and HEK293 cells (Lesage et al., 1998). In CHO/D4 cells, the Bmax value of dopamine D4 receptors, as estimated by [3H]-spiperone saturation binding, was 240 fmol mg−1 protein. This value was multiplied by a factor 2 after 6 h and by a factor 4 after 18 h of treatment with sodium butyrate. This level of expression was not increased further after 48 h of treatment.
These changes in receptor density were accompanied by an enhancement of the agonist activities of dopamine, quinpirole and 7-OH-DPAT (both in terms of maximal inhibition of forskolin-stimulated cyclic AMP accumulation and of pEC50 values). More specifically, after 6 h treatment with sodium butyrate (when receptor density had doubled), the Emax of dopamine increased in a substantial manner (from 56±1% to 78±1% inhibition of forskolin-stimulated cyclic AMP levels). The pEC50 value was slightly changed from 7.4±0.1 to 7.7±0.1 under these conditions. A much more dramatic increase in the potency of dopamine was observed after 18 h of treatment with butyrate (pEC50 8.4±0.2), i.e. when receptor density had been four times higher than in untreated cells. By contrast, the Emax of dopamine was not altered in a significant manner between 6 and 18 h of treatment (83±2% versus 78±1%). Such data are consistent with classical receptor theory which predicts that increasing the receptor number will first result in an increase of the maximal responsiveness to agonists, until a ceiling is reached (i.e., the maximal achievable cell or tissue responses). Further increases in receptor number should then lead to higher agonist potencies, with no more changes in the maximal effect (Kenakin, 1997). Classical receptor theory also predicts that variations in receptor number will have a larger impact on the intrinsic activity of partial agonists than for full agonists. Examples that this is really the case in practice have been reported (e.g., Hermans et al., 1999). In the present study, marked increases in the efficacies of U-101958 and L-745,870 were found after 18 h of treatment with sodium butyrate (i.e., concomittantly with receptor density being four times higher than in untreated cells). The Emax of U-101958 more than doubled (from 23±4% to 53±6% inhibition of forskolin-stimulated cyclic AMP accumulation) and L-745,870 was turned into an agonist under these conditions. In antagonist studies using spiperone and raclopride, the effects of both U-101958 and L-745,870 were characterized as being mediated by dopamine D4 receptors. Thus, both compounds were partial agonists at dopamine D4.4 receptors expressed in CHO cells. In particular, for L-745,870, a relatively high threshold level of receptor number appears to be required for significant agonism to be manifest. This level must lie between 560 and 1000 fmol mg−1 protein, a range in which the agonist effects of L-745,870 became apparent in CHO/D4 cells. This may provide an explanation as to why Patel et al. (1997) did not observe agonist effects of L-745,870. Their CHO cells expressed 710 fmol mg−1 protein, which might have been insufficient to see clearcut agonist effects. As far as U-101958 is concerned, there was no mention of receptor number in the study by Schlachter et al. (1997), who reported it to be a dopamine D4 receptor antagonist, using a mitogenesis assay in CHO cells.
The present findings show that increases in the efficacy of the dopamine D4 receptor ligands, U-101958 and L-745,870 are associated with increases in receptor density in CHO cells treated with sodium butyrate for various periods of time. The parallelism between these changes as well as the fact that similar findings have been described for other receptors (Esbenshade et al., 1995; Hermans et al., 1999) strongly suggest that receptor density is a factor governing the efficacy of dopamine D4 receptor agonists. Nevertheless, other effects of the sodium butyrate treatment cannot be totally excluded. Because sodium butyrate has been incubated for varying periods of time in the present study, it might have had a different impact on cell proliferation and functioning in these various situations. However, we did not observe any change in the morphology of the cells during the treatment with sodium butyrate. In addition, in separate, strictly time-controlled experiments, the yield of protein was not altered by sodium butyrate treatment. Another possible consequence of this treatment, likely to impinge on the efficiency of stimulus-response coupling, might have been an alteration of the density of the G-proteins involved in the transduction of dopamine D4 receptor-mediated effects. With respect to this possibility, we measured the number of dopamine-activated G-proteins in CHO cells treated or not with sodium butyrate for 18 h, using [35S]-GTPγS isotopic dilution saturation binding (according to Newman-Tancredi et al., 1997). The number of G-proteins activated per receptor tended to be reduced after the treatment with sodium butyrate (3 versus 5 in untreated cells), making it unlikely that changes at the level of G-proteins could account for the observed increases in efficacy.
Even though receptor density is a determinant of the efficacy of dopamine D4 receptor ligands, it is probably not the sole one. Comparing the present data in CHO cells with those obtained previously in HEK293 cells (Gazi et al., 1998) also indicates an influence of the expression system. At similar receptor densities, 560 fmol mg−1 protein in CHO cells treated with sodium butyrate for 6 h (this study) and 505 fmol mg−1 protein in HEK293 cells (Gazi et al., 1998), dopamine was more potent by one order of magnitude in the latter cells (pEC50 8.7 versus 7.7). In addition, the efficacies of U-101958 and L-745,870 were much more marked in HEK293 cells (93 and 71% relative to dopamine; Gazi et al., 1998). Even under conditions of high receptor densities (1000 and 840 fmol mg−1 protein after 18 and 48 h treatment with sodium butyrate), and even though the pEC50 value of dopamine approached that in human embryonic kidney cells, U-101958 and L-745,870 remained less efficacious in CHO cells (64 and 49% relative to dopamine). Such differences suggest a more efficient receptor-effector coupling in HEK293 than in CHO cells. Tentative explanations are different repertoires of G protein subunits, of adenylate cyclase subtypes and the receptor/G protein/effector stoichiometry. For instance, a greater number of G proteins may be activated per receptor unit in HEK293 than in CHO cells.
As a whole, the present data confirm our previous view that U-101958 and L-745,870 are not silent antagonists but can behave as agonists at dopamine D4 receptors. In the absence of any recognized model of native dopamine D4 receptor activation, the relevance of these findings to the in vivo situation is difficult to ascertain. It is worth noting, nevertheless, that U-101958 exhibited dopaminergic agonist activity at inhibiting potassium currents in rat posterior pituitary (Wilke et al., 1998) and at inducing phospholipid methylation in human neuroblastoma SK-N-MC cells (Sharma et al., 1999). L-745,870 was found to behave as a partial agonist at dopamine D2-like receptors mediating inhibition of serotonin N-acetyltransferase in chick retina (Zawilska & Nowak, 1997). That putative dopamine D4 receptor antagonists may have agonist properties under some circumstances may have clinical implications. These compounds have been conceived as potential antipsychotics, in an effort to mimic the blocking effect of clozapine at dopamine D4 receptors. Whereas U-101958 has not been developed further because of poor metabolic stability and bioavailability (Schlachter et al., 1997), clinical studies with L-745,870 have been completed (Kramer et al., 1997). The compound was found to be ineffective in schizophrenic patients and intriguingly, some signs of aggravation of the illness were noticed. If the density of dopamine D4 binding sites is really increased by several fold in the brain of schizophrenics as has been reported (Seeman et al., 1993, 1995; Murray et al., 1995; Sumiyoshi et al., 1995; Marzella et al., 1997), L-745,870 might have acted as an agonist in these patients and this might possibly provide an explanation to the negative outcome of these trials.
Abbreviations
- CHO
Chinese hamster ovary
- CHO/D4 cells
CHO cells expressing dopamine D4.4 receptors
- HEK
human embryonic kidney
- L-745,870
(3-(4-[4-chlorophenyl]piperazin-1-yl)-methyl-1H-pyrrolo [2,3-b]pyridine)
- 7-OH-DPAT
7-hydroxy-2-dipropylaminotetralin
- U-101958
((1-benzyl-piperidin-4-yl)-(3-isopropoxy-pyridin-2-yl)-methyl-amine)
References
- BRADFORD M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- CHEN C., OKAYAMA H. High efficiency transformation of mammalian cells by plasmid DNA. Mol. Cell. Biol. 1987;7:2745–2752. doi: 10.1128/mcb.7.8.2745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- COCKETT M.I., BEBBINGTON C.R., YARRANTON G.T. High level expression of tissue inhibitor of metalloproteinases in Chinese hamster ovary cells using glutamine synthetase gene amplification. Biotechnology. 1990;8:662–667. doi: 10.1038/nbt0790-662. [DOI] [PubMed] [Google Scholar]
- ESBENSHADE T.A., WANG X., WILLIAMS N.G., MINNEMAN K.P. Inducible expression of α1B-adrenoceptors in DDT1MF-2 cells: comparison of receptor density and response. Eur. J. Pharmacol. 1995;289:305–310. doi: 10.1016/0922-4106(95)90108-6. [DOI] [PubMed] [Google Scholar]
- GAZI L., BOBIRNAC I., DANZEISEN M., SCHÜPBACH E., BRUINVELS A.T., GEISSE S., SOMMER B., HOYER D., TRICKLEBANK M., SCHOEFFTER P. The agonist activities of the putative antipsychotic agents, L-745,870 and U-101958 in HEK293 cells expressing the human dopamine D4.4 receptor. Br. J. Pharmacol. 1998;124:889–896. doi: 10.1038/sj.bjp.0701921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HARTMAN D.S., CIVELLI O.Dopamine receptor diversity: molecular and pharmacological perspectives Progress in Drug Research 19974Basel: Birkhäuser; 173–194.ed. Jucker, E. pp [DOI] [PubMed] [Google Scholar]
- HERMANS E., CHALLISS R.A.J., NAHORSKI S.R. Effects of varying the expression level of recombinant human mGlu1α receptors on the pharmacological properties of agonists and antagonists. Br. J. Pharmacol. 1999;126:873–882. doi: 10.1038/sj.bjp.0702359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KEBABIAN J.W., CALNE D.B. Multiple receptors for dopamine. Nature. 1979;277:93–96. doi: 10.1038/277093a0. [DOI] [PubMed] [Google Scholar]
- KENAKIN T.P.Efficacy Pharmacological analysis of drug-receptor interaction 1997Philadelphia-New York: Lippincott-Raven; 289–330.ed. Kenakin, T.P. pp [Google Scholar]
- KRAMER M.S., LAST B., GETSON A., REINES S. The effects of a selective D4 dopamine receptor antagonist (L-745,870) in acutely psychotic inpatients with schizophrenia. Arch. Gen. Psychiatry. 1997;54:567–572. doi: 10.1001/archpsyc.1997.01830180085011. [DOI] [PubMed] [Google Scholar]
- LAHTI R.A., EVANS D.L., STRATMAN N.C., FIGUR L.M. Dopamine D4 versus D2 receptor selectivity of dopamine receptor antagonists: possible therapeutic implications. Eur. J. Pharmacol. 1993;236:483–486. doi: 10.1016/0014-2999(93)90488-4. [DOI] [PubMed] [Google Scholar]
- LESAGE A.S., WOUTERS R., VAN GOMPEL P., HEYLEN L., VANHOENACKER P., HAEGEMAN G., LUYTEN W.H.M.L., LEYSEN J.E. Agonistic properties of alniditan, sumatriptan and dihydroergotamine on human 5-HT1B and 5-HT1D receptors expressed in various mammalian cell lines. Br. J. Pharmacol. 1998;123:1655–1665. doi: 10.1038/sj.bjp.0701766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MARZELLA P.L., HILL C., KEKS N., SINGH B., COPOLOV D. The binding of both [3H]nemonapride and [3H]raclopride is increased in schizophrenia. Biol. Psychiatry. 1997;42:648–654. doi: 10.1016/s0006-3223(96)00471-4. [DOI] [PubMed] [Google Scholar]
- MEADOR-WOODRUFF J.H., DAMASK B.S., WANG J., HAROUTUNIAN V., DAVIS K.L., WARSON S.J. Dopamine receptor mRNA expression in human striatum and neocortex. Neuropsychopharmacology. 1996;15:17–29. doi: 10.1016/0893-133X(95)00150-C. [DOI] [PubMed] [Google Scholar]
- MURRAY A.M., HYDE T.M., KNABLE M.B., HERMAN M.M., BIGELOW L.B., CARTER J.M., WEINBERGER D.R., KLEINMAN J.E. Distribution of putative D4 dopamine receptors in postmortem striatum from patients with schizophrenia. J. Neurosci. 1995;15:2186–2191. doi: 10.1523/JNEUROSCI.15-03-02186.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NEWMAN-TANCREDI A., AUDINOT V., CHAPUT C., VERRIELE L., MILLAN M.J. [35S]Guanosine-5′-O-(3-thio)triphosphate binding as a measure of efficacy at human recombinant dopamine D4.4 receptors: actions of antiparkinsonian and antipsychotic agents. J. Pharmacol. Exp. Ther. 1997;282:181–191. [PubMed] [Google Scholar]
- PATEL S., FREEDMAN S., CHAPMAN K.L., EMMS F., FLETCHER A.E., KNOWLES M., MARWOOD R., MCALLISTER G., MYERS J., PATEL S., CURTIS N., KULAGOWSKI J.J., LEESON P.D., RIDGILL M., GRAHAM M., MATHESON S., RATHBONE D., WATT A.P., BRISTOW L.J., RUPNIAK N.M.J., BASKIN E., LYNCH J.J., RAGAN C.I. Biological profile of L-745,870, a selective antagonist with high affinity for the dopamine D4 receptor. J. Pharmacol. Exp. Ther. 1997;283:636–647. [PubMed] [Google Scholar]
- PRIMUS R.J., THURKAUF A., XU J., YEVICH E., MCINERNAY S., SHAW K., TALLMAN J.F., GALLAGER D.W. Localization and characterization of dopamine D4 binding sites in rat and human brain by use of the novel, D4 receptor-selective ligand [3H]NGD 94-1. J. Pharmacol. Exp. Ther. 1997;282:1020–1027. [PubMed] [Google Scholar]
- REYNOLDS G.P. The importance of dopamine D4 receptors in the action and development of antipsychotic agents. Drugs. 1996;51:7–11. doi: 10.2165/00003495-199651010-00002. [DOI] [PubMed] [Google Scholar]
- REYNOLDS G.P., MASON S.L. Absence of detectable striatal dopamine D4 receptors in drug-treated schizophrenia. Eur. J. Pharmacol. 1995;281:R5–R6. doi: 10.1016/0014-2999(95)00408-d. [DOI] [PubMed] [Google Scholar]
- SALOMON Y. Cellular responsiveness to hormones and neurotransmitters: conversion of [3H]adenine to [3H]cAMP in cell monolayers, cell suspension, and tissue slices. Methods Enzymol. 1991;195:22–28. doi: 10.1016/0076-6879(91)95151-9. [DOI] [PubMed] [Google Scholar]
- SCHLACHTER S.K., POEL T.J., LAWSON C.F., DINH D.M., LAJINESS M.E., ROMERO A.G., REES S.A., DUNCAN J.N., SMITH M.W. Substituted 4-aminopiperidines having high in vitro affinity and selectivity for the cloned human dopamine D4 receptor. Eur. J. Pharmacol. 1997;322:283–286. doi: 10.1016/s0014-2999(97)00013-7. [DOI] [PubMed] [Google Scholar]
- SEEMAN P., GUAN H.-C., VAN TOL H.H.M. Dopamine D4 receptors elevated in schizophrenia. Nature. 1993;365:441–445. doi: 10.1038/365441a0. [DOI] [PubMed] [Google Scholar]
- SEEMAN P., GUAN H.-C., VAN TOL H.H.M. Schizophrenia: elevation of dopamine D4-like sites, using [3H]nemonapride and [125I]epidepride. Eur. J. Pharmacol. 1995;286:R3–R5. doi: 10.1016/0014-2999(95)00677-d. [DOI] [PubMed] [Google Scholar]
- SEEMAN P., VAN TOL H.H.M. Deriving the therapeutic concentrations for clozapine and haloperidol: the apparent dissociation constant of a neuroleptic at the dopamine D2 or D4 receptor varies with the affinity of the competing radioligand. Eur. J. Pharmacol. Mol. Pharmacol. Section. 1995;291:59–66. doi: 10.1016/0922-4106(95)90125-6. [DOI] [PubMed] [Google Scholar]
- SHARMA A., KRAMER M.L., WICK P.F., LIU D., CHARI S., SHIM S., TAN W., OUELLETTE D., NAGATA M., DURAND C.J., KOTB M., DETH R.C. D4 dopamine receptor-mediated phospholipid methylation and its implications for mental illnesses such as schizophrenia. Mol. Psychiatry. 1999;4:235–246. doi: 10.1038/sj.mp.4000522. [DOI] [PubMed] [Google Scholar]
- SUMIYOSHI T., STOCKMEIER C.A., OVERHOLSER J.C., THOMPSON P.A., MELTZER H.Y. Dopamine D4 receptors and effects of guanine nucleotides on [3H]raclopride binding in postmortem caudate nucleus of subjects with schizophrenia or major depression. Brain Res. 1995;681:109–116. doi: 10.1016/0006-8993(95)00301-6. [DOI] [PubMed] [Google Scholar]
- VAN TOL H.H.M., BUNZOW J.R., GUAN H.-C., SUNAHARA R.K., SEEMAN P., NIZNIK H.B., CIVELLI O. Cloning of the gene for a human dopamine D4 receptor with high affinity for the antipsychotic clozapine. Nature. 1991;350:610–614. doi: 10.1038/350610a0. [DOI] [PubMed] [Google Scholar]
- WILKE R.A., HSU S.-F., MEYER B.J. Dopamine D4 receptor mediated inhibition of potassium current in neurophysial nerve terminals. J. Pharmacol. Exp. Ther. 1998;284:542–548. [PubMed] [Google Scholar]
- WILSON J.M., SANYAL S., VAN TOL H.H.M. Dopamine D2 and D4 receptor ligands: relation to antipsychotic action. Eur. J. Pharmacol. 1998;351:273–286. doi: 10.1016/s0014-2999(98)00312-4. [DOI] [PubMed] [Google Scholar]
- ZAWILSKA J.B., NOWAK J.Z. Dopamine D4-like receptors in vertebrate retina: does the retina offer a model for the D4-receptor analysis. Pol. J. Pharmacol. 1997;49:201–211. [PubMed] [Google Scholar]


