Abstract
Mice lacking the apolipoprotein E and low density lipoprotein receptor genes (E°×LDLR°) develop atherosclerosis. The aim of this study was to investigate changes in endothelium-dependent vasodilation and vasomotion in thoracic aortic rings of E°×LDLR° mice.
K+-induced contractions of the aorta from E°×LDLR° mice were stronger than those from control mice. The sensitivity of E°×LDLR° aorta to phenylephrine (PE) was decreased but the maximal contractions were increased. Acetylcholine-induced, but not sodium nitroprusside-induced, relaxations of E°×LDLR° aorta was decreased.
PE induced rhythmic activity in both E°×LDLR° and control aorta but the amplitude was larger in E°×LDLR° than in control mice. PE-induced rhythmic activity in both E°×LDLR° and control aorta was augmented by increase in extracellular Ca2+-concentration, but was abolished by removal of the endothelium, the nitric oxide (NO) synthase inhibitor N-nitro-L-arginine methyl ester, the guanylate cyclase inhibitor LY-83583, high K+ solution and ryanodine.
4-Aminopyridine, a voltage-dependent potassium (KV) channel blocker, increased basal tension and induced rhythmic activity in E°×LDLR° aorta but not in control aorta.
The Ca2+-activated potassium (KCa) channel blockers tetraethylammonium and charybdotoxin abolished PE-induced rhythmic activity in E°×LDLR° aorta.
In conclusion, opening of Kv channels in E°×LDLR° mice aorta is reduced and it is susceptible to be depolarized resulting in Ca2+ entry. The vascular smooth muscle is then dependent on compensatory mechanisms to limit Ca2+-entry. Such mechanisms may be decreased sensitivity to vasoconstrictors, or increased opening of KCa channels by NO via a cyclic GMP-dependent mechanism.
Keywords: Apolipoprotein E, endothelium, mouse, aorta, nitric oxide, potassium channels, calcium channels
Introduction
It has been well documented that endothelium-dependent vasodilation is impaired in both animal models of atherosclerosis (Freiman et al., 1986; Kolodgie et al., 1990; Ibengwe & Suzuki, 1986) and atherosclerotic human coronary arteries (Bossaler et al., 1987; Förstermann et al., 1988; Zeiher et al., 1991). Experiments performed on aorta from rabbits fed cholesterol for 6 months suggest that the impairment of endothelium-dependent vasodilation is related to reduced bioavailability of nitric oxide (NO) due to enhanced inactivation of NO by oxygen free radicals rather than a decrease in the production of NO (Minor et al., 1990).
NO is constitutively produced in vascular endothelial cells from the amino acid L-arginine (Palmer et al., 1988). The produced NO diffuses easily through the cell membrane of the vascular smooth muscle cells and causes vessel relaxation by activating guanylate cyclase which results in an increase in cyclic GMP levels (Arnold et al., 1977). Recent studies have further demonstrated that NO can activate both voltage-dependent and calcium-activated potassium channels (i.e., Kv and KCa channels) on the vascular smooth muscle cells through a cyclic GMP-dependent mechanism, thereby inhibiting the evoked membrane depolarization and increase in [Ca2+]i (Archer et al., 1994; Yuan et al., 1996; Zhao et al., 1997). This may be one mechanism by which NO contributes to maintenance of the normal vascular tone (Stamler et al., 1994). Hence, a reduction in the availability of NO, as in atherosclerosis, can result in vasomotor dysfunction (Giaid & Saleh, 1995; Morita et al., 1996).
Mice lacking the apolipoprotein E and/or low density lipoprotein (LDL) receptor genes have elevated total cholesterol and triglycerides and reduced level of high density lipoprotein (Zhang et al., 1992; Plump et al., 1992; Ishibashi et al., 1994; Reddick et al., 1994; Breslow, 1996). As a result, they develop massive lipid-filled lesions throughout the arterial tree by 3–4 months of age (Zhang et al., 1992; Nakashima et al., 1994). By 6 months of age, the vascular lesions in these mice resemble atherosclerotic lesions in humans, with a similar pattern of lesion distribution, microscopic appearance, and cellular composition (Zhang et al., 1992; Nakashima et al., 1994; Reddick et al., 1994). Therefore, the hypercholesterolemic mouse is a relevant animal model for studying cellular and functional consequences of atherosclerosis. The purpose of the present study was to investigate changes in endothelium-dependent vasodilation and abnormalities of vasomotion in atherosclerotic arteries by using the aorta of mice lacking apolipoprotein E and LDL receptors (E°×LDLR° mice).
Methods
Mice and diets
Male mice carrying targeted deletions of the apolipoprotein E and LDL receptor genes (Ishibashi et al., 1994) on the C57BL/6J background were put on a diet with 0.15% cholesterol (Zhou et al., 1996; 1998) at 6 weeks-of-age. At 8–9 months-of-age the animals were used for experiments. All experiments complied with national guidelines and were approved by the regional ethical committee.
Tissue preparation
The mice were anaesthetized with an intraperitoneal injection of sodium pentobarbital (150 mg kg−1) together with sodium heparin (100 IU kg−1, i.p.) to prevent intravascular coagulation. The thoracic aorta (TA) was quickly dissected free and placed in Krebs Henseleit solution at room temperature (22–23°C). By means of a dissecting microscope, adhering perivascular tissue was carefully removed and the descending TA was cut into 2 mm long rings. In some experiments, the endothelium was removed by gently rubbing the luminal surface of TA rings with a wooden stick. Functional removal of endothelium was confirmed by demonstrating almost 100% loss of relaxation induced by 10 μM acetylcholine (Ach).
Tension measurement
The vessels were mounted onto two thin stainless steel holders, one of which was connected to a force displacement transducer (Grass model FT03) and the other to a movable device that allowed the application of a passive tension of 650–700 mg, which were determined to be the optimal resting tension for obtaining the maximal active tension induced by 60 mM K+ solution. The mounted rings were kept in 2 ml organ baths containing Krebs Henseleit solution, kept at 37°C and continuously bubbled with a gas mixture of 95% O2 and 5% CO2 to maintain a pH of 7.4. The isometric tension was recorded on a polygraph (Grass model 7). After an equilibration period of 60 min the contractile function of the vessel was tested twice by replacing the Krebs Henseleit solution by 60 mM K+ solution, and the second contraction was taken as the reference contraction. Following washout, the vessel was contracted once with 10 μM phenylephrine (PE) for 6 min and then relaxed with 10 μM Ach for 2 min. After another washout period, cumulative dose-response curves for PE, Ach or sodium nitroprusside (SNP) were created. Ach- or SNP-induced vasorelaxation was tested after precontractions evoked by 10 μM PE to determine endothelium-dependent and endothelium-independent relaxation, respectively. Prolonged exposure to 10 μM PE evoked spontaneous rhythmic activity which was recorded for 15 min. The amplitude was measured as the mean of the last five oscillations. Each drug was investigated on TA segments from at least four mice.
Solutions
The Krebs Henseleit solution consisted of (in mM): NaCl 118, KCl 4.7, CaCl2 2.5, KH2PO4 1.2, MgSO4(7H2O) 1.2, NaHCO3 25.2 and glucose 11.1. The high K+ solution (30 or 60 mM) was prepared by exchanging NaCl with an equimolar amount of KCl.
Drugs
Phenylephrine, Ach, SNP, tetraethylammonium (TEA), charybdotoxin (ChTX), 4-aminopyridine (4-AP), NG-nitro-L-arginine methyl ester (L-NAME), ouabain (Sigma Chemical, St. Louis, MO, U.S.A.), ryanodine (Wako Pure Chemical Industries, Japan) and N-(3-(Aminomethyl)benzyl)acetamidine (1400 W, Calbiochem, San Diego, U.S.A.) were dissolved in distilled water. Glibenclamide (Sigma) was dissolved in dimethyl sulphoxide to make a stock solution of 100 mM. 6-Anilino-5,8-quinolinequinone (LY-83583, Sigma) and indomethacin (Sigma) were dissolved in acetone and ethanol, respectively, to make a stock solution of 10 mM. All subsequent dilutions were made with Krebs Henseleit solution. Similar dilutions of the solvents into Krebs Henseleit solution were used as controls and had no effect on either the basal tension or the evoked tension of TA rings. All concentrations given are final molar concentrations in the organ chambers.
Statistics
Results are expressed as mean±s.e.mean. The number of observations indicated are the number of vessel segments studied. Since several segments were taken from the same animal the number of vessels were larger than the number of animals in some experiments. Statistical analyses were always performed using both the number of vessels and the number of animals. Differences were regarded statistically significant only if significant differences were found using both methods. Mean values of individual data points from the concentration-response curves and rhythmic activity within each strain were performed by analysis of variance followed by Newman-Keuls test to compare results in E°×LDLR° mice and age-matched control mice. The paired Student's t-test was used to compare results in treated and untreated aortas from each strain. Differences were considered to be significant when P<0.05.
Results
Responses to high K+ solution, PE, Ach and SNP
The contractile force induced by 60 mM K+ solution was significantly higher in TA rings of E°×LDLR° mice than in TA rings of age-matched control mice (398±9 mg, n=68 vs 327±10 mg, n=58, P<0.05). The sensitivity of TA rings of E°×LDLR° mice to PE was decreased whereas the maximal response to PE was increased (Figure 1A,B). Ach-induced relaxation was decreased in TA rings of E°×LDLR° mice (Figure 2A), whereas SNP-induced relaxation was not different from that in age-matched controls (Figure 2B).
Figure 1.
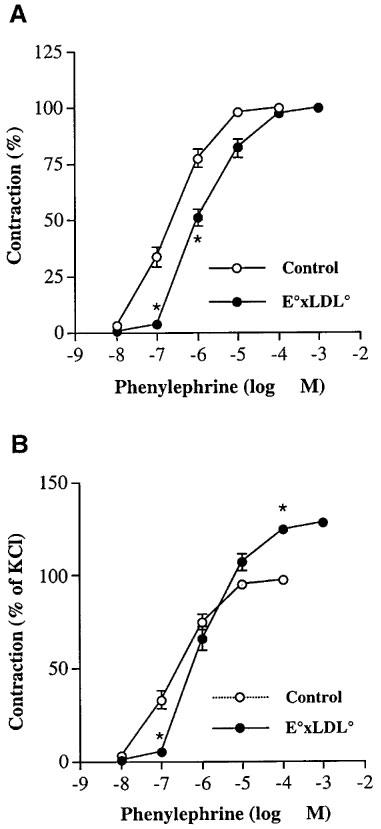
Cumulative concentration-response curves to phenylephrine (PE) in thoracic aortic rings of E°×LDLR° mice and age-matched controls. Data are expressed in per cent of maximal contraction evoked by (A) phenylephrine and (B) 60 mM K+ solution. Each point represents mean±s.e.mean, n=11–12. Significant differences from the control arteries are shown; *P<0.05.
Figure 2.
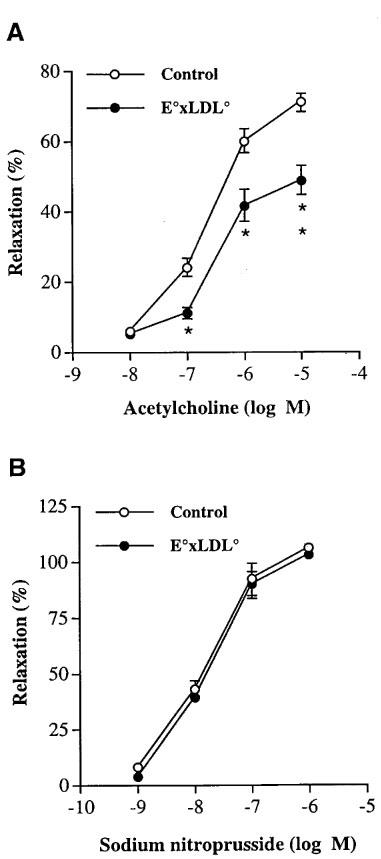
Concentration-response curves showing the relaxation elicited by increasing doses of (A) acetylcholine and (B) sodium nitroprusside in thoracic aortic rings of E°×LDLR° mice and age-matched controls. The relaxations are expressed in per cent of the precontractile tone induced by phenylephrine 10 μM. Each point represents mean±s.e.mean, n=20–26. Significant differences from the control arteries are shown; *P<0.05, **P<0.01.
PE-induced rhythmic activity
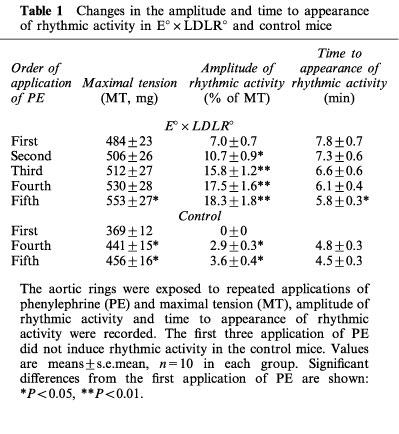
PE-induced rhythmic activity was observed in TA rings of both E°×LDLR° and age-matched control mice. Therefore a set of experiments was performed to study its time course. The contractions triggered by 10 μM PE were observed for 15 min without any intervention, and were repeated five times. The organ chamber was rinsed with Krebs Henseleit solution after each application of PE. In TA rings from E°×LDLR° mice, rhythmic activity usually appeared during the first application of PE and its amplitude was significantly increased with repeated application of PE. The rhythmic activity appeared sooner upon repetitive application of PE (Table 1). In TA rings of age-matched control mice, on the other hand, significant rhythmic activity did not appear until the fourth application of PE and the amplitude was much less than that observed in TA rings of E°×LDLR° mice (Table 1). After the fifth observation of PE-induced rhythmic activity, a solution containing 30 mM K+ was applied to trigger the contractions of TA rings. The contractile force evoked by 30 mM K+ solution was not significantly different from that induced by the last application of PE in each mouse strain while the high K+ solution-triggered contractions did not induce rhythmic activity. PE did not evoke rhythmic activity in TA rings from 4-month-old E°×LDLR° mice (n=5, data not shown).
Table 1.
Changes in the amplitude and time to appearance of rhythmic activity in E°×LDLR° and control mice
Effects of endothelial removal, NOS inhibition and guanylate cyclase inhibition
Removal of the endothelium abolished PE-induced rhythmic activity in TA rings of both E°×LDLR° (n=6) and age-matched control (n=5) mice (data not shown). Pretreatment of TA rings from E°×LDLR° mice with 1 mM L-NAME for 10 min before administration of 10 μM PE abolished (10 of 13 samples) or decreased (three of 13 samples) PE-induced rhythmic activity and Ach-induced vasorelaxation (Figure 3A,B and Table 2). Similar results were observed in TA rings of age-matched control mice (Figure 4A,B and Table 3). L-NAME increased basal tension and enhanced the contractile force triggered by PE in both mouse strains (Tables 2 and 3). In contrast, 1400 W, a selective inhibitor of inducible NOS (Garvey et al., 1997), did not affect basal tension, the amplitude of PE-induced rhythmic activity or Ach-induced relaxation in TA rings of either E°×LDLR° or age-matched control mice (data not shown). Furthermore, pretreatment of TA rings from E°×LDLR° mice with 1 μM LY-83583, a guanylate cyclase inhibitor (Mülsch et al., 1988), for 10 min before administration of 10 μM PE abolished (eight of 13 samples) or decreased (five of 13 samples) PE-induced rhythmic activity and reduced Ach-induced vasorelaxation (Figure 3C and Table 2). Similar results were observed in TA rings of age-matched control mice (Figure 4C and Table 3).
Figure 3.
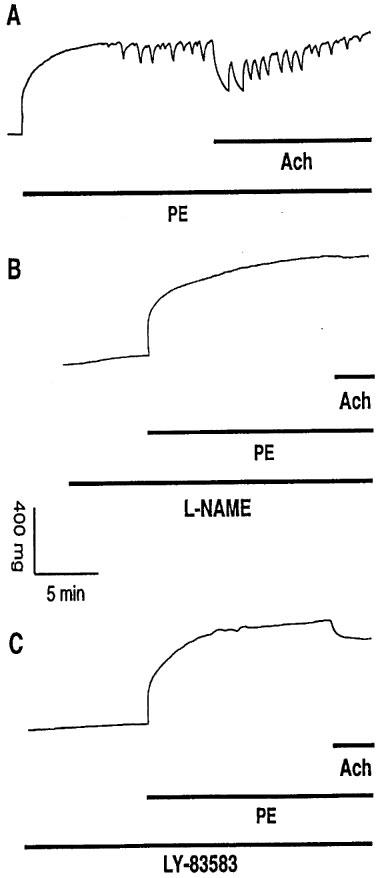
(A) Original registration showing the contractile effect and rhythmic activity induced by phenylephrine (PE, 10 μM) and relaxation induced by acetylcholine (Ach, 10 μM) in thoracic aortic rings of E°×LDLR° mice. Pretreatment of the vessels with (B) 1 mM L-NAME and (C) 1 μM LY-83583 for 10 min before addition of PE caused basal tension increase, abolished PE-induced rhythmic activity and reduced the relaxation to Ach.
Table 2.
Effects of L-NAME, LY-83583, 4-AP, TEA and ChTX on rhythmic activity (RA) and Ach-induced vasorelaxation in E°×LDLR° mice
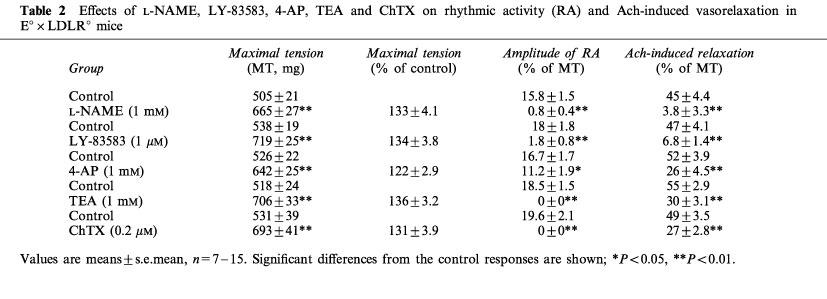
Figure 4.
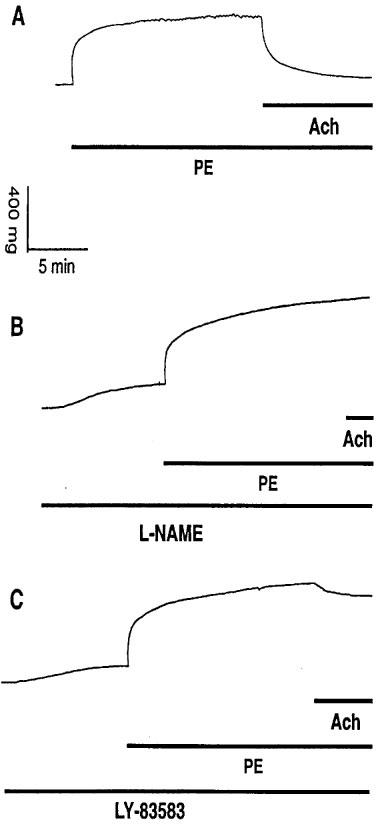
(A) Original registration showing the contractile effect and rhythmic activity induced by phenylephrine (PE, 10 μM) and relaxation induced by acetylcholine (Ach, 10 μM) in thoracic aortic rings of age-matched control mice. Pretreatment of the vessels with (B) 1 mM L-NAME and (C) 1 μM LY-83583 for 10 min before addition of PE caused basal tension increase, abolished PE-induced rhythmic activity and reduced the relaxation to Ach.
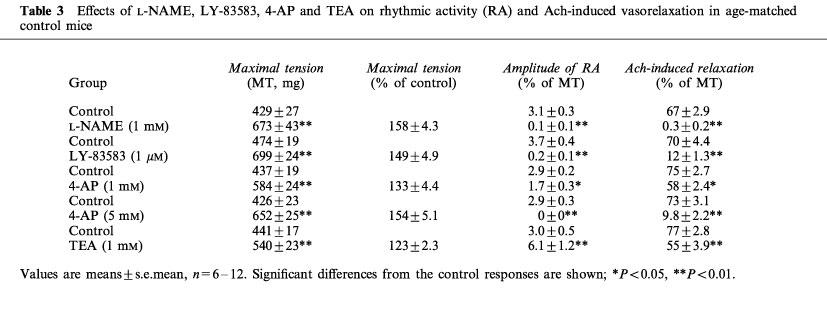
Table 3.
Effects of L-NAME, LY-83583, 4-AP and TEA on rhythmic activity (RA) and Ach-induced vasorelaxation in age-matched control mice
Effects of an increase in extracellular Ca2+ concentration
Fifteen minutes after 10 μM PE was administered, an increase in extracellular Ca2+ concentration from 2.5 to 5 mM enhanced the amplitude of PE-induced rhythmic activity in TA rings of E°×LDLR° mice (from 38±5 to 79±8 mg, n=7, P<0.05). In another group, an increase in extracellular Ca2+ concentration from 2.5 mM to 7.5 mM strikingly augmented the amplitude of PE-induced rhythmic activity (from 44±5 to 116±12 mg, n=7, P<0.01). A similar increase in PE-induced rhythmic activity was obtained in TA rings of age-matched control mice by elevation of extracellular Ca2+ concentration from 2.5 to 5 mM (the amplitude increased from 17±2 to 32±5 mg, n=6, P<0.05). The increase in extracellular Ca2+ concentration did not cause any further increase in the maximal tension in TA rings from either group.
Effects of ryanodine
In a group of TA rings from E°×LDLR° mice, 10 μM ryanodine (an inhibitor of Ca2+-release channels in sarcoplasmic reticulum) was added 15 min after administration of 10 μM PE. Approximately 5 min after application of ryanodine, the contractions of the vessels were reduced (from 532±31 to 432±15 mg, n=7, P<0.01) and PE-induced rhythmic activity was abolished, subsequently, contractile tension gradually recovered to the level prior to application of ryanodine (Figure 5A). Similar results were obtained in TA rings of age-matched control mice (Figure 5B).
Figure 5.
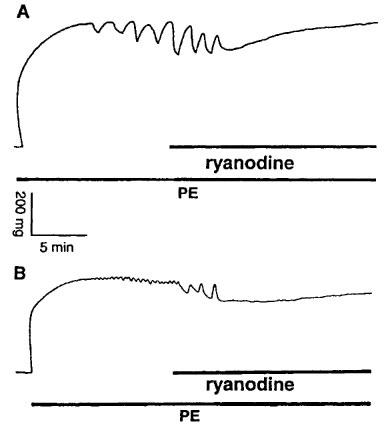
Original registration showing effects of 10 μM ryanodine on 10 μM phenylephrine (PE)-induced rhythmic activity in thoracic aortic rings (A) from E°×LDLR° mice and (B) age-matched controls. Ryanodine abolished PE-induced rhythmic activity.
Effects of Kv blocker 4-AP
Application of 1 mM 4-AP, a concentration that selectively blocks Kv channels (Gelband & Hume, 1992; Nelson & Quayle, 1995; Knot & Nelson, 1995), increased basal tension in all TA rings of E°×LDLR° mice and also induced rhythmic activity in five of 15 samples (Figure 6A,B and Table 2). When the 4-AP-induced increase in tension reached a plateau, administration of 10 μM PE caused further contractions. The amplitude of PE-induced rhythmic activity and Ach-induced vasorelaxation was decreased in the presence of 4-AP (Figure 6A,B and Table 2). In contrast to the results in TA rings of E°×LDLR° mice, 1 mM 4-AP did not increase basal tension but attenuated PE-induced rhythmic activity and Ach-induced vasorelaxation in TA rings of age-matched control mice (Figure 7A,B and Table 3). A higher concentration of 4-AP (5 mM) increased basal tension but did not induce rhythmic activity, and abolished PE-induced rhythmic activity in all TA rings of age-matched control mice. Ach-induced vasorelaxation was almost abolished by 5 mM 4-AP (Figure 7C and Table 3).
Figure 6.
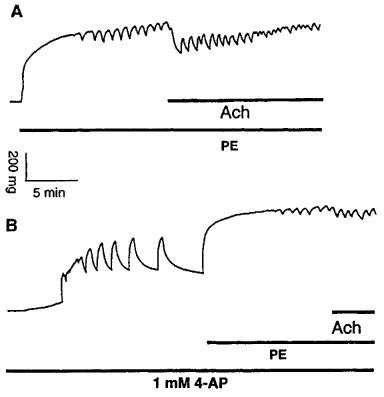
(A) Original registration showing the contraction and rhythmic activity induced by phenylephrine (PE, 10 μM) and relaxation induced by acetylcholine (Ach, 10 μM) in thoracic aortic rings of E°×LDLR° mice. (B) pretreatment of the vessels with 1 mM 4-AP before addition of PE caused basal tension increase and induced rhythmic activity. 4-AP decreased the amplitude of PE-induced rhythmic activity and the relaxation to Ach.
Figure 7.
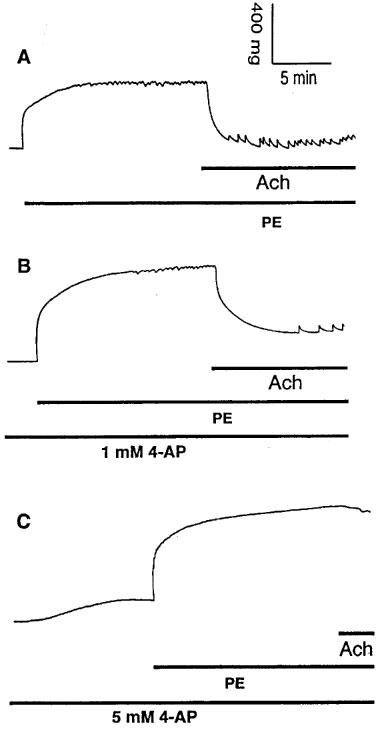
(A) Original registration showing the contraction and rhythmic activity induced by phenylephrine (PE, 10 μM) and relaxation induced by acetylcholine (Ach, 10 μM) in thoracic aortic rings of age-matched control mice. (B) pretreatment of the vessels with 1 mM 4-AP for 10 min before addition of PE decreased the amplitude of PE-induced rhythmic activity and the relaxation to Ach without affecting basal tension. (C) pretreatment of the vessels with 5 mM 4-AP for 10 min before addition of PE caused basal tension increase without inducing rhythmic activity, abolished PE-induced rhythmic activity and reduced the relaxation to Ach.
Effects of KCa blockers TEA and ChTX
TEA at 1 mM, a concentration that selectively blocks KCa channels (Langton et al., 1991), did not affect basal tension but abolished PE-induced rhythmic activity and reduced Ach-induced vasorelaxation in TA rings of E°×LDLR° mice (Figure 8A,B and Table 2). Similar results were obtained with 200 nM ChTX (Table 2). By contrast, in TA rings of age-matched control mice 1 mM TEA augmented the amplitude of PE-induced rhythmic activity without affecting basal tension. Ach-induced vasorelaxation was decreased in the presence of TEA (Figure 9A,B and Table 3). The effects of TEA on PE-induced rhythmic activity in TA rings of age-matched control mice was reversible after washing the vessels.
Figure 8.
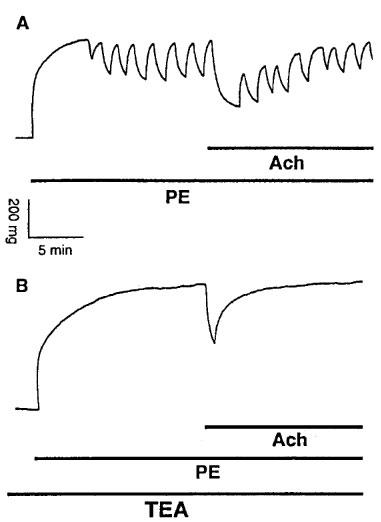
(A) Original registration showing the contraction and rhythmic activity induced by phenylephrine (PE, 10 μM) and relaxation induced by acetylcholine (Ach, 10 μM) in thoracic aortic rings of E°×LDLR° mice. (B) pretreatment of the vessels with 1 mM TEA for 10 min before addition of PE abolished PE-induced rhythmic activity and reduced the relaxation to Ach without affecting basal tension.
Figure 9.
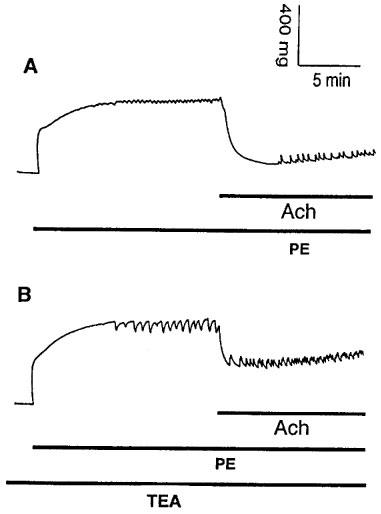
(A) Original registration showing the contraction and rhythmic activity induced by phenylephrine (PE, 10 μM) and relaxation induced by acetylcholine (Ach, 10 μM) in thoracic aortic rings of age-matched control mice. (B) pretreatment of the vessels with 1 mM TEA for 10 min before addition of PE enhanced PE-induced rhythmic activity without affecting basal tension but reduced the relaxation to Ach.
Effects of KATP channel blocker glibenclamide, cyclo-oxygenase inhibitor indomethacin and Na+-K+-ATPase inhibitor ouabain
Pretreatment of TA rings from E°×LDLR° mice or age-matched control mice with 10 μM glibenclamide for 10 min, 10 μM indomethacin for 25 min or 10 μM ouabain for 15 min before administration of 10 μM PE did not significantly influence PE-induced rhythmic activity (n=5–6 in each group, data not shown).
Discussion
In the present study, the vasomotion and endothelial function were investigated in a murine experimental model of atherosclerosis that closely resembles the human atherosclerotic disease. Aorta of E°×LDLR° mice showed enhanced contractile responses to K+ and PE but reduced endothelium-dependent relaxation. Moreover, the experimental results demonstrated that: (1) PE induced rhythmic activity in TA rings of both E°×LDLR° and age-matched control mice but the amplitude of PE-induced rhythmic activity was considerably larger in E°×LDLR° mice than in age-matched control mice and (2) 1 mM 4-AP, a concentration that selectively blocks Kv channels, evoked basal tension increase in all TA rings of E°×LDLR° mice and induced rhythmic activity in approximately 35% of samples, a phenomenon that was not seen in TA rings of age-matched control mice.
Vasoconstrictor-induced rhythmic activity in vascular smooth muscles has previously been observed in both physiological (Gustafsson et al., 1993; Jackson et al., 1991; Stein & Driska, 1984) and pathological (Myers et al., 1985; Ross et al., 1980) states. Ca2+-free solution of Ca2+-channel blockers have been shown to abolish vasoconstrictor-induced rhythmic activity in vascular smooth muscle (Jackson et al., 1991; Stein & Driska, 1984; Myers et al., 1985; Ross et al., 1980). Moreover, two studies have demonstrated that vasoconstrictor-induced rhythmic activity in vascular smooth muscle results from cyclic GMP-dependent activation of K+ channels by endothelium-derived NO (Gustafsson et al., 1993; Jackson et al., 1991). The rhythmic activity observed in the present study was abolished by 30 mM K+, removal of the endothelium, the NOS inhibitor L-NAME, the guanylate cyclase inhibitor LY-83583 and ryanodine (an inhibitor of Ca2+-release channels in sarcoplasmic reticulum). On the other hand, it was augmented by an increase in extracellular Ca2+ concentration and repeated administration of PE. Furthermore, the KCa channel blockers TEA and ChTX abolished the PE-induced rhythmic activity in TA rings of E°×LDLR° mice. Taken together, these results suggest that enhanced PE-induced rhythmic activity observed in the aorta of E°×LDLR° mice results from an increase in activation of KCa channels through a NO- and cyclic GMP-dependent mechanism.
Kv channels, i.e., 4-AP-sensitive K+ channels, are considered as the major K+ channels controlling resting membrane potential and [Ca2+]i in vascular smooth muscle cells (Knot & Nelson, 1995; Yuan, 1995; Leblanc et al., 1994; Volk et al., 1991) whereas KCa channels mainly contribute to the repolarization following increased [Ca2+]i (Brayden & Nelson, 1992). NO can activate both Kv and KCa channels on vascular smooth muscle cells through a cyclic GMP-dependent mechanism, thereby inhibiting the evoked membrane depolarization and increase in [Ca2+]i (Archer et al., 1994; Yuan et al., 1996; Zhao et al., 1997), and basal release of endothelium-derived NO contributes to maintenance of normal vascular tone in humans (Stamler et al., 1994). Obviously, a reduction in the availability of NO will result in changes in the function of Kv and KCa channels on vascular smooth muscle cells and vasomotor dysfunction. Consistent with the results obtained from other animal models of atherosclerosis (Freiman et al., 1986; Kolodgie et al., 1990; Ibengwe & Suzuki, 1986) and atherosclerotic human coronary arteries (Bossaler et al., 1987; Förstermann et al., 1988), the data from the present study showed that Ach-induced vasorelaxation was reduced in TA rings of E°×LDLR° mice while SNP-induced vasorelaxation was unchanged. This suggests that endothelial function was impaired whereas the relaxatory capacity of the vascular smooth muscle was intact. More importantly, in the present study it was found that treatment of TA rings from E°×LDLR° mice with 1 mM 4-AP resulted in basal tension increase and induced rhythmic activity. These results indicate that: (1) the sensitivity of the aortic smooth muscle cells of E°×LDLR° mice to the Kv channel blocker is enhanced compared with age-matched control mice; (2) the sarcolemmal depolarization and consequent increase in [Ca2+]i caused by 1 mM 4-AP activate KCa channels on the aortic smooth muscle cells of E°×LDLR° mice; (3) an increase in the opening of KCa channels observed in the aorta of E°×LDLR° mice is a consequence of a decrease in the opening of Kv channels and (4) a decrease in the availability of endothelium-derived NO rather than a reduction of the number of Kv channels or change in the structure of Kv channels is the main factor causing an increase in the sensitivity of the aortic smooth muscle cells of E°×LDLR° mice to Kv channel blocker.
When the opening of Kv channels on the aortic smooth muscle cells of E°×LDLR° mice is reduced due to lack of stimulation by NO, the cells are susceptible to be depolarized resulting in Ca2+ entry via voltage-gated Ca2+ channels. As a result, the maximal contractile response of TA rings of E°×LDLR° mice to high K+ solution and PE was increased, which has been observed in the thoracic aorta of cholesterol-fed rabbits (Ibengwe & Suzuki, 1986) and diabetic mice (Piercy & Taylor, 1998) as well as the abdominal aorta of human apolipoprotein A-I transgenic rabbits (Lebuffe et al., 1997). However, no change in maximal contractile response to PE or noradrenaline has been reported in cholesterol-fed rabbits (Kawachi et al., 1984) or Watanabe heritable hyperlipidemic rabbits (Miwa et al., 1994; Tagawa et al., 1993), respectively. In fact, a decrease in maximal contractile response to PE has also been reported in the latter model (Kolodgie et al., 1990). Use of different concentrations of vasoconstrictors and K+ besides differences in the models and stage of atherosclerosis may be important reasons for the apparently different results.
Owing to a reduction in the opening of Kv channels, the aortic smooth muscle cells of E°×LDLR° mice are dependent on compensatory mechanisms to limit Ca2+ entry via voltage-gated Ca2+ channels. One such mechanism may be a decrease in sensitivity of the cells to endogenous and exogenous vasoconstrictors such as the α-adrenoceptor agonists noradrenaline (Ibengwe & Suzuki, 1986) and PE. Another is an increase in the opening of KCa channels, which was observed as enhanced PE-induced rhythmic activity in the present study.
Since endothelium-dependent vasorelaxation is decreased in TA rings of E°×LDLR° mice, an increase in NO- and cyclic GMP-dependent activation of KCa channels on the aortic smooth muscle cells of E°×LDLR° mice might result from production of NO by inducible NOS expressed by smooth muscle cells (Buttery et al., 1996; Hansson et al., 1994). However, the selective inhibitor of inducible NOS, 1400 W, did not affect basal tension, PE-induced rhythmic activity or Ach-induced relaxation. These results further indicate that the inhibitory effect of L-NAME on PE-induced rhythmic activity was related to blockade of endothelial NOS. Therefore, the most possible mechanism underlying an increase in NO- and cyclic GMP-dependent activation of KCa channels in the aorta of E°×LDLR° mice is that expression of KCa channels on smooth muscle cells is augmented. An increase in the currents of KCa channels (England et al., 1993) and the expression of KCa channels (Liu et al., 1997) has recently been observed in the aortic smooth muscle cells of spontaneously hypertensive rats.
The mechanism by which TEA enhanced PE-induced rhythmic activity in TA rings of age-matched control mice was unclear. The finding that both TEA and 4-AP increased maximal tension induced by PE but only TEA enhanced rhythmic activity suggests that the effect was unrelated to the developed tension of the vessel. One possibility is that the opening of other K+ channels is compensatorily increased when KCa channels were blocked by TEA and this hypothesis needs to be established in further studies.
In conclusion, the present results indicate that the opening of Kv channels on aortic smooth muscle cells of E°×LDLR° mice is reduced so that the cells are susceptible to be depolarized resulting in Ca2+ entry via voltage-gated Ca2+ channels. In this situation, the cells are dependent on compensatory mechanisms to limit Ca2+ entry. One such mechanism may be a decrease in sensitivity of the cells to endogenous and exogenous vasoconstrictors, another is an increase in the opening of KCa channels which are activated by endothelium-derived NO via a cyclic GMP-dependent mechanism.
Acknowledgments
The present study was supported by grants from the Swedish Medical Research Council (10857, 4764 and 6816), the Swedish Heart and Lung Foundation, Loo and Hans Osterman Foundation, King Gustav and Queen Victoria Foundation and the Karolinska Institute.
Abbreviations
- Ach
acetylcholine
- 4-AP
4-aminopyridine
- ChTX
charybdotoxin
- E°×LDLR°
apolipoprotein E and low density lipoprotein receptor knockout
- KCa
Ca2+-activated potassium channels
- Kv
voltage-dependent potassium channels
- L-NAME
NG-nitro-L-arginine methyl ester
- NO
nitric oxide
- PE
phenylephrine
- RA
rhythmic activity
- SNP
sodium nitroprusside
- TA
thoracic aorta
- TEA
tetraethylammonium
References
- ARCHER S.L., HANG J.M.C., HAMPLE V., NELSON D.P., SCHULTZ P.J., WEIR E.K. Nitric oxide and cGMP cause vasorelaxation by activation of a charybdotoxin-sensitive K+ channel by cGMP-dependent protein kinase. Proc. Natl. Acad. Sci. U.S.A. 1994;91:7583–7587. doi: 10.1073/pnas.91.16.7583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ARNOLD W.P., MITTEL C., KATSUKI S., MURAD F. Nitric oxide activates guanylate cyclase and increases guanosine 3′ : 5′-cyclic monophosphate levels in various tissue preparations. Proc. Natl. Acad. Sci. U.S.A. 1977;74:3203–3207. doi: 10.1073/pnas.74.8.3203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BOSSALER C., HABIB G.B., YAMOMOTO H., WILLIAMS C., WELLS S., HENRY P.D. Impaired muscarinic endothelial-dependent relaxation and cyclic guanosine 5-monophosphate formation in atherosclerotic human coronary artery and rabbit aorta. J. Clin. Invest. 1987;79:170–174. doi: 10.1172/JCI112779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BRAYDEN J.E., NELSON M.T. Regulation of arterial tone by activation of calcium-dependent potassium channels. Science. 1992;256:532–535. doi: 10.1126/science.1373909. [DOI] [PubMed] [Google Scholar]
- BRESLOW J.L. Mouse models of atherosclerosis. Science. 1996;272:685–688. doi: 10.1126/science.272.5262.685. [DOI] [PubMed] [Google Scholar]
- BUTTERY L., SPRINGALL D.R., CHESTER A.H., EVANS T.J., STANDFIELD N., PARUMS D.V., YACOUB M.H., POLAK J.M. Inducible nitric oxide synthase is present within human atherosclerotic lesions and promotes the formation and activity of peroxynitrite. Lab. Invest. 1996;75:77–85. [PubMed] [Google Scholar]
- ENGLAND S.K., WOOLDRIGE T.A., STEKIEL W.J., RUSCH N.J. Enhanced single-channel K+ current in arterial membranes from genetically hypertensive rats. Am. J. Physiol. 1993;264:H1337–H1345. doi: 10.1152/ajpheart.1993.264.5.H1337. [DOI] [PubMed] [Google Scholar]
- FÖRSTERMANN U., MÜGGE A., ACHEID U., HAVERICH A., FRÖLICH J.C. Selective attenuation of endothelial-mediated vasodilation atherosclerotic human coronary arteries. Circ. Res. 1988;62:185–190. doi: 10.1161/01.res.62.2.185. [DOI] [PubMed] [Google Scholar]
- FREIMAN P.C., MITCHELL G.C., HEISTAD D.D., ARMSTRONG M.L., HARRISON D.G. Atherosclerosis impairs endothelial-dependent vascular relaxation to acetylcholine and thrombin in primates. Circ. Res. 1986;58:783–789. doi: 10.1161/01.res.58.6.783. [DOI] [PubMed] [Google Scholar]
- GARVEY E.P., OPLINGER J.A., FURFINE E.S., KIFF R.J., LASZLO F., WHITTLE B.J.R., KNOWLES R.G. 1400W is a slow, tight binding, and highly selective inhibitor of inducible nitric-oxide synthase in vitro and vivo. J. Biol. Chem. 1997;272:4959–4963. doi: 10.1074/jbc.272.8.4959. [DOI] [PubMed] [Google Scholar]
- GELBAND C.H., HUME J.R. Ionic currents in single smooth muscle cells from canine renal artery. Circ. Res. 1992;71:745–758. doi: 10.1161/01.res.71.4.745. [DOI] [PubMed] [Google Scholar]
- GIAID A., SALEH D. Reduced expression of endothelial nitric oxide synthase in the lungs of patients with pulmonary hypertension. N. Engl. J. Med. 1995;333:214–221. doi: 10.1056/NEJM199507273330403. [DOI] [PubMed] [Google Scholar]
- GUSTAFSSON H., MULVANY M.J., NILSSON H. Rhythmic contractions of isolated small arteries from rat: influence of the endothelium. Acta. Physiol. Scand. 1993;148:153–163. doi: 10.1111/j.1748-1716.1993.tb09545.x. [DOI] [PubMed] [Google Scholar]
- HANSSON G.K., GENG Y.J., HOLM J., HÅRDHAMMAR P., WENNMALM Å., JENNISCHE E. Arterial smooth muscle cells express nitric oxide synthase in response to endothelial injury. J. Exp. Med. 1994;180:733–738. doi: 10.1084/jem.180.2.733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- IBENGWE J.K., SUZUKI H. Changes in mechanical responses of vascular smooth muscles to acetylcholine, noradrenaline and high-potassium solution in hypercholesterolemic rabbits. Br. J. Pharmacol. 1986;78:395–402. doi: 10.1111/j.1476-5381.1986.tb10829.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ISHIBASHI S., HERZ J., MAEDA N., GOLDSTEIN J.L., BROWN M.S. The two-receptor model of lipoprotein clearance: Tests of the hypothesis in ‘knockout' mice lacking the low density lipoprotein receptor, apolipoprotein E, or both proteins. Proc. Natl. Acad. Sci. U.S.A. 1994;93:10489–10494. doi: 10.1073/pnas.91.10.4431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- JACKSON W.F., MÜLSCH A., BUSSE R. Rhythmic smooth muscle activity in hamster aortas is mediated by continuous release of NO from the endothelium. Am. J. Physiol. 1991;260:H248–H253. doi: 10.1152/ajpheart.1991.260.1.H248. [DOI] [PubMed] [Google Scholar]
- KAWACHI Y., TOMOIKE H., MARUOKA Y., KIKUCHI Y., ARAKI H., ISHII Y., TANAKA K., NAKAMURA M. Selective hypercontraction caused by ergonovine in the canine coronary artery under conditions of induced atherosclerosis. Circulation. 1984;69:441–450. doi: 10.1161/01.cir.69.2.441. [DOI] [PubMed] [Google Scholar]
- KNOT H.J., NELSON M.T. Regulation of membrane potential and diameter by voltage-dependent K+ channels in rabbit myogenic cerebral arteries. Am. J. Physiol. 1995;269:H348–H355. doi: 10.1152/ajpheart.1995.269.1.H348. [DOI] [PubMed] [Google Scholar]
- KOLODGIE F., VIRMANI R., RICE H.E., MERGNER W.J. Vascular reactivity during the progression of atherosclerotic plaque: a study in Watanabe heritable hyperlipidemic rabbits. Circ. Res. 1990;66:1112–1116. doi: 10.1161/01.res.66.4.1112. [DOI] [PubMed] [Google Scholar]
- LANGTON P.D., NELSON M.T., HUANG Y., STANDEN N.B. Block of calcium-activated potassium channels in mammalian arterial myocytes by tetraethylammonium ions. Am. J. Physiol. 1991;260:H927–H934. doi: 10.1152/ajpheart.1991.260.3.H927. [DOI] [PubMed] [Google Scholar]
- LEBLANC N., WAN X., LEUNG P.M. Physiological role of Ca2+-activated and voltage-dependent K+ currents in rabbit coronary myocytes. Am. J. Physiol. 1994;262:C1523–C1537. doi: 10.1152/ajpcell.1994.266.6.C1523. [DOI] [PubMed] [Google Scholar]
- LEBUFFE G., BOULLIER A., TAILLEUX A., DELFLY B., DEPUIS B., FRUCHART J.C., DUVERGER N., EMMANUEL F., DENEFLE P., VALLET B., DURIEZ P. Endothelial derived vasorelaxation is impaired in human APO A-I transgenic rabbits. Biochem. Biophys. Res. Comm. 1997;241:205–211. doi: 10.1006/bbrc.1997.7790. [DOI] [PubMed] [Google Scholar]
- LIU P, , PLEYTE K., KNAUS H.G., RUSCH N.J. Increased expression of Ca2+-sensitive K+ channels in aorta of hypertensive rats. Hypertension. 1997;30:1403–1409. doi: 10.1161/01.hyp.30.6.1403. [DOI] [PubMed] [Google Scholar]
- MINOR R.L., MYERS P.R., GUERRA R., JR, BATES J.N., HARRISON D.G. Diet-induced atherosclerosis increases the release of nitrogen oxides from rabbit aorta. J. Clin. Invest. 1990;86:2109–2116. doi: 10.1172/JCI114949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MIWA Y., HIRATA K.-I., MATSUDA Y., SUEMATSU M., KAWASHIMA S., YOKOYAMA M. Augmented receptor-mediated Ca2+ mobilization causes supersensitivity of contractile response to serotonin in atherosclerotic arteries. Circ. Res. 1994;75:1096–1102. doi: 10.1161/01.res.75.6.1096. [DOI] [PubMed] [Google Scholar]
- MORITA K., IHNKEN K., BUCKBERG G.D., SHERMAN M.P., IGNARRO L.J. Pulmonary vasoconstriction due to impaired nitric oxide production after cardiopulmonary bypass. Annals of Thoracic Surgery. 1996;61:1775–1780. doi: 10.1016/0003-4975(96)00146-4. [DOI] [PubMed] [Google Scholar]
- MÜLSCH A., BUSSE R., LIEBAU S., FÖRSTERMANN U. LY-83583 interferes with the release of endothelium-derived relaxing factor and inhibits soluble guanylate cyclase. J. Pharmacol. Exp. Ther. 1988;247:283–288. [PubMed] [Google Scholar]
- MYERS J.H., LAMB F.S., WEBB R.C. Norepinephrine-induced phasic activity in tail arteries from genetically hypertensive rats. Am. J. Physiol. 1985;248:H419–H423. doi: 10.1152/ajpheart.1985.248.3.H419. [DOI] [PubMed] [Google Scholar]
- NELSON M.T., QUAYLE J.M. Physiological roles and properties of potassium channels in arterial smooth muscle. Am. J. Physiol. 1995;268:C799–C822. doi: 10.1152/ajpcell.1995.268.4.C799. [DOI] [PubMed] [Google Scholar]
- NAKASHIMA Y., PLUMP A.S., RAINES E.W., BRESLOW J.L., ROSS R. ApoE-deficient mice develop lesions of all phases of atherosclerosis throughout the arterial tree. Arterioscler. Thromb. 1994;14:133–140. doi: 10.1161/01.atv.14.1.133. [DOI] [PubMed] [Google Scholar]
- PALMER R.M., ASHTON D.S., MONCADA S. Vascular endothelial cells synthesize nitric oxide from L-arginine. Nature. 1988;333:664–666. doi: 10.1038/333664a0. [DOI] [PubMed] [Google Scholar]
- PIERCY V., TAYLOR S.G. A comparison of spasmogenic and relaxant responses in aortae from C57/BL/KsJ diabetic mice with those from their non-diabetic litter mates. Pharmacology. 1998;56:267–275. doi: 10.1159/000028208. [DOI] [PubMed] [Google Scholar]
- PLUMP A.S., SMITH J.D., HAYEK T., AALTO, SETÄLÄ K., WALSH A., VERSTUYFT J.G., RUBIN E.M., BRESLOW J.L. Severe hypercholesterolemia and atherosclerosis in apolipoprotein E-deficient mice created by homologous recombination in ES cells. Cell. 1992;71:343–353. doi: 10.1016/0092-8674(92)90362-g. [DOI] [PubMed] [Google Scholar]
- REDDICK R.L., ZHANG S.H., MAEDA N. Atherosclerosis in mice lacking apo E: evaluation of lesional development and progression. Arterioscler. Thromb. 1994;14:141–147. doi: 10.1161/01.atv.14.1.141. [DOI] [PubMed] [Google Scholar]
- ROSS G., SCHROEDER E.S.J., GINSBURG R. Spontaneous phasic activity of isolated human coronary arteries. Cardiovasc. Res. 1980;14:613–618. doi: 10.1093/cvr/14.10.613. [DOI] [PubMed] [Google Scholar]
- STAMLER J.S., LOH E., RODDY M.-A., CURRIE K.E., CREAGER M.A. Nitric oxide regulates basal systemic and pulmonary vascular resistance in healthy humans. Circulation. 1994;89:2035–2040. doi: 10.1161/01.cir.89.5.2035. [DOI] [PubMed] [Google Scholar]
- STEIN P.G., DRISKA S.P. Histamine-induced rhythmic contraction of hog carotid artery smooth muscle. Circ. Res. 1984;55:480–485. doi: 10.1161/01.res.55.4.480. [DOI] [PubMed] [Google Scholar]
- TAGAWA H., TOMOIKE H., MITSOKA W., SATOH S., KUGA T., SHIMOKAWA H., NAKAMURA M., TAKESHITA A. Hyperreactivity of aortic smooth muscle to serotinin is related to the presence of atheroma in Watanabe heritable hyperlipidemic rabbits. Cardiovas. Res. 1993;27:2164–2169. doi: 10.1093/cvr/27.12.2164. [DOI] [PubMed] [Google Scholar]
- VOLK K.A., MATSUDA J.J., SHIBADA E.F. A voltage-dependent potassium current in rabbit coronary artery smooth muscle cells. J. Physiol. 1991;439:751–768. doi: 10.1113/jphysiol.1991.sp018691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- YUAN X.-J. Voltage-gated K+ currents regulate resting membrane potential and [Ca2+]i in pulmonary arterial myocytes. Circ. Res. 1995;77:370–378. doi: 10.1161/01.res.77.2.370. [DOI] [PubMed] [Google Scholar]
- YUAN X.-J., TOD M.L., RUBIN L.J., BLAUSTEIN M.P. NO hyperpolarizes pulmonary artery smooth muscle cells and decreases the intracellular Ca2+ concentration by activating voltage-gated K+ channels. Proc. Natl. Acad. Sci. U.S.A. 1996;93:10489–10494. doi: 10.1073/pnas.93.19.10489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ZEIHER A.M., DREXLER H., WOLLSCHLÄGER H., JUST H. Endothelial dysfunction of the coronary microvasculature is associated with coronary blood flow regulation in patients with early atherosclerosis. Circulation. 1991;84:1984–1992. doi: 10.1161/01.cir.84.5.1984. [DOI] [PubMed] [Google Scholar]
- ZHANG S.H., REDDICK R.L., PIEDRAHITA J.A., MAEDA N. Spontaneous hypercholesterolemia and arterial lesions in mice lacking apolipoprotein E. Science. 1992;258:468–471. doi: 10.1126/science.1411543. [DOI] [PubMed] [Google Scholar]
- ZHAO Y.-J., WANG J., RUBIN L.J., YUAN X.J. Inhibition of Kv and KCa channels antagonizes NO-induced relaxation in pulmonary artery. Am. J. Physiol. 1997;272:H904–H912. doi: 10.1152/ajpheart.1997.272.2.H904. [DOI] [PubMed] [Google Scholar]
- ZHOU X., PAULSSON G., STEMME S., HANSSON G.K. Hypercholesterolemia is associated with a Th1/Th2 switch of the autoimmune response in atherosclerotic apo E-knockout mice. J. Clin. Invest. 1998;101:1717–1725. doi: 10.1172/JCI1216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ZHOU X., STEMME S., HANSSON G.K. Evidence for a local immune response in atherosclerosis. CD4+ T cells infiltrate lesions of apo E-deficient mice. Am. J. Pathol. 1996;149:359–366. [PMC free article] [PubMed] [Google Scholar]


