Abstract
Guanethidine is commonly used as a drug to investigate adrenergic neurotransmission and, in combination with atropine, to realize non-adrenergic non-cholinergic (NANC) conditions. Previous studies suggested a nicotinic acetylcholine receptor blocking effect of guanethidine. Therefore, we investigated the effect of increasing concentrations of guanethidine (0.1–100 μM) on nicotine-induced relaxations of longitudinal muscle strips of rat gastric fundus.
In the presence of 1 μM atropine and 3 μM guanethidine, nicotine (30 μM) induces a fast and sustained relaxation which is partly inhibited by the nitric oxide synthase inhibitors Nω-nitro-L-arginine (L-NOARG) and Nω-nitro-L-arginine methyl ester (L-NAME) (both 30 and 100 μM). One μM tetrodotoxin (TTX) completely blocks this nicotine-induced relaxation.
High concentrations of guanethidine (⩾10 μM), but not adrenoceptor blockade by the α-adrenoceptor antagonist phentolamine in combination with the β-adrenoceptor antagonist nadolol (both 3 μM), inhibit the nicotine-induced relaxation.
Guanethidine (0.1–100 μM) has no effect on relaxations induced by electrical field stimulation (EFS; 1–8 Hz), nitric oxide (NO; 0.01–1 μM), vasoactive intestinal polypeptide (VIP; 0.1–10 nM) or isoprenaline (1–10 nM).
We conclude that high concentrations of guanethidine (⩾10 μM) block nicotine-induced NANC relaxations of longitudinal muscle strips of the rat gastric fundus most likely at the level of the nicotinic acetylcholine receptor.
Keywords: Rat gastric fundus, guanethidine; in vitro; L-NOARG; L-NAME; muscle strip; nicotine-induced relaxation; nicotinic acetylcholine receptor; nitric oxide; NANC
Introduction
Guanethidine is commonly used in pharmacological and physiological studies to evaluate the involvement of adrenergic neurotransmission. Most likely, it is taken up into adrenergic neurones and, thereby, blocks the nerve conduction at the level of preterminal axons and inhibits the re-uptake of noradrenaline into the nerve endings (Chang et al., 1965; Lundborg & Stitzel, 1968; Burnstock & Wong, 1980), abolishing the responses to post-ganglionic adrenergic nerve stimulation. Thirty years ago, however, Jaanus et al. (1968) had already suggested that guanethidine has a blocking side-effect on the nicotinic acetylcholine receptor. More recently, this idea was supported by the finding of Gandìa et al. (1991) who showed that guanethidine selectively blocks nicotinic acetylcholine receptor mediated Ca2+ uptake in cultured bovine chromaffin cells. Based on these studies, Villarroya et al. (1996) even used the guanethidinium moiety of guanethidine as a model to synthesize a novel selective nicotinic acetylcholine receptor antagonist.
Nicotinic acetylcholine receptors are present on striated muscle cells mediating cholinergic contractions. In addition, they are localized on postganglionic sympathetic adrenergic neurones and on postsynaptic cholinergic and inhibitory non-adrenergic non-cholinergic (NANC) neurones. These latter neurones are part of the enteric nervous system of the gastrointestinal tract and play an important role in the regulation of gastrointestinal motility. The inhibitory NANC neurones provide the main inhibitory neurotransmission of the gastrointestinal tract, mediating motility patterns like swallow-induced relaxations of the lower esophageal sphincter, the receptive relaxation of the gastric fundus during food intake and the recto-anal inhibitory reflex (Abrahamsson, 1986). In these in vivo and in vitro studies, guanethidine, in combination with atropine, is used to realize NANC conditions. However, the blocking effect of guanethidine on nicotinic acetylcholine receptors localized on myenteric neurones has not been investigated.
The rat gastric fundus has a well defined inhibitory NANC innervation (Boeckxstaens et al., 1991; Takahashi & Owyang, 1995; Lefebvre, 1993). In the presence of guanethidine and atropine, electrical field stimulation (EFS) induced NANC relaxations of longitudinal muscle strips mediated by nitric oxide (NO) and vasoactive intestinal polypeptide (VIP) (Boeckxstaens et al., 1991; 1992; Li & Rand, 1990). These NANC neurones can also be stimulated by nicotine, again resulting in NO and VIP-mediated relaxations (Curro & Preziosi, 1997; McLaren et al., 1993).
In this study, the rat gastric fundus is used as a model to investigate the effects of increasing concentrations of guanethidine on nicotine-induced relaxations, in order to determine the nicotinic acetylcholine receptor blocking properties of guanethidine. Furthermore, the effects of the α-adrenoceptor antagonist phentolamine and the β-adrenoceptor antagonist nadolol on nicotine-induced relaxations are studied. To identify the site of action of guanethidine, the effects of increasing concentrations of guanethidine on electrical field stimulated relaxations and on NO-, VIP- and adrenoceptor-induced relaxations are investigated.
Methods
Tissue bath experiments
Tissue bath experiments were performed as described previously (Boeckxstaens et al., 1991). Briefly, after an overnight fast with free access to water, male Wistar rats (300–400 g) were sacrificed by decapitation, the stomach was removed and quickly transferred to modified Krebs-Ringer solution (composition in mM): NaCl 118.3, KCl 4.7, MgSO4 1.2, KH2PO4 1.2, CaCl2 2.5, NaHCO3 25.0 and glucose 11.1; pH 7.4, aerated with a mixture of 95% O2 and 5% CO2. The gastric fundus, characterized by its white mucosa, was separated from the distal stomach and cut open along the small curvature (longitudinal axis). Subsequently, it was spread open and full thickness longitudinal muscle strips of 1–1.5 cm long and 0.2–0.3 cm wide were prepared. The strips were mounted between parallel platina electrodes (0.75 cm2) in tissue baths (25 ml) filled with modified Krebs-Ringer (37°C, pH 7.4, 95% O2 and 5% CO2). One end of the muscle strip was fixed to a glass rod whereas the other end was connected to a strain gauge transducer (Model GM2/GM3, SCAIME S.A., New Jersey, U.S.A.) for continuous recording of isometric tension. To determine the optimal point of the length-tension relationship, the length of each muscle strip was increased stepwise and the strips were contracted with 5-hydroxytryptamine (5-HT; 0.1 μM). Once the optimal length point of length-tension relationship was reached, strips were washed three times with 165 ml Krebs-Ringer solution (37°C, pH 7.4) and then allowed to equilibrate at their optimal length for at least 45 min before the start of the actual experiment.
All experiments were performed during a 0.1 μM 5-HT-induced contraction and in presence of 1 μM atropine to block the muscarinic acetylcholine receptors. After each 5-HT-induced contraction, the muscle strips were washed three times with 165 ml Krebs-Ringer solution (37°C, pH 7.4) every 5 min. If nicotine was added, strips were washed six times every 5 min. During the experiments, the contractile responses remained stable and reproducible. However, despite repeated wash-outs to avoid desensitization of the nicotinic acetylcholine receptors, a decline in the nicotine-induced relaxations was observed. To correct for the decrease of the relaxation in time, control strips of the same fundus were used in parallel in each experiment (see also Data analysis and statistics).
The effect of Nω-nitro-L-arginine (L-NOARG; 30 and 100 μM), Nω-nitro-L-arginine methyl ester (L-NAME; 30 and 100 μM) and tetrodotoxin (TTX; 1 μM) was studied on the relaxations induced by 30 μM nicotine, in the presence of 1 μM atropine and 3 μM guanethidine. In order to confirm the neurogenic origin of the nicotine-induced relaxation, TTX was added to the strips at the end of the experiment. Due to the desensitization of the nicotinic receptors, however, the relaxations in the TTX experiments are smaller compared to the other experiments. L-NOARG, L-NAME and TTX were administered 10 min before the strips were contracted. Furthermore, the effect of phentolamine (3 μM) in combination with nadolol (3 μM) on nicotine- and isoprenaline-induced relaxations was studied. Strips were incubated with these compounds 20–30 min before they were contracted.
Subsequently, the effect of increasing concentrations of guanethidine (0.1–100 μM) on 30 μM nicotine-induced relaxations and on relaxations induced by electrical field stimulation (EFS; 1–8 Hz, rectangular pulses, width 1 msec, 9 V and duration 10 s), by NO (0.01–1 μM) and by cumulative administration of isoprenaline (1, 3 and 10 nM) and VIP (0.1–10 nM) was studied. Furthermore, the effect of increasing concentrations of hexamethonium (0.1–100 μM) on nicotine-, EFS-, NO- and isoprenaline-induced relaxation was studied. Strips were incubated with guanethidine (0.1–100 μM) or hexamethonium (0.1–100 μM) 20–30 min before they were contracted.
Drugs and materials
Atropine sulphate, guanethidine sulphate (2 : 1), 5-hydroxytryptamine hydrochloride (5-HT), (−)-isoprenaline hydrochloride, nadolol, nicotine hydrogen tartrate, Nω-nitro-L-arginine (L-NOARG), Nω-nitro-L-arginine methyl ester (L-NAME), phentolamine hydrochloride, tetrodotoxin (TTX) and vasoactive intestinal polypeptide (VIP) were purchased from Sigma Chemical Co. (St. Louis, U.S.A.). A standard aqueous nitric oxide (NO) solution was prepared according to Kelm et al. (1988). Degassed and deoxygenated water was saturated with purified NO gas (1–2 mM) and further diluted. Drugs used for the tissue bath experiments were dissolved in modified Krebs-Ringer solution and were prepared freshly. The salts and glucose (all pro analyse) used for the modified Krebs-Ringer solution were purchased from Merck (Darmstadt, Germany).
Data analysis and statistics
The data were digitized, stored and analysed with the use of the commercially available software Physiological Analysis Package POLY 5.0 (Inspector Research Systems BV, Amsterdam, The Netherlands). To correct for the effect of time, control strips of the same fundus were used in parallel in each experiment. The ‘time-factor' (TF) was calculated by dividing the relaxation of this control strip at certain time points during the experiment (t=q) by the relaxation of this strip at the beginning of the experiment (t=0):
Assuming that the effect of time was comparable between strips of the same fundus relaxations of each individual strip were corrected by the TF (1.4±0.1 (mean±s.e.mean; n=70)).
Data of tissue bath experiments are expressed as a percentage of the decrease of 5-HT-induced contraction. Values are shown as means±s.e.mean for the number rats indicated. For statistical analysis of the effect of guanethidine, a two-tailed Student's t-test for paired observations was used. For the statistical analysis of the effect of the NO synthase inhibitors and TTX, a one-tailed Student's t-test for paired observations was used. In both cases P values <0.05 were considered to be significantly different from time corrected controls.
Results
Effect of NO synthase inhibition and TTX on nicotine-induced relaxations of longitudinal muscle strips
In the presence of 1 μM atropine, 30 μM nicotine induced a fast and sustained relaxation of the longitudinal muscle of the rat gastric fundus (Figure 1A) with a mean of 80±4% (n=12). Under NANC conditions (i.e. 1 μM atropine plus 3 μM guanethidine), the NO synthase inhibitors L-NOARG (30 and 100 μM) and L-NAME (30 and 100 μM) both inhibited the relaxations induced by nicotine in a concentration dependent way, while 1 μM TTX completely abolished the nicotine-induced relaxation (Figure 2).
Figure 1.
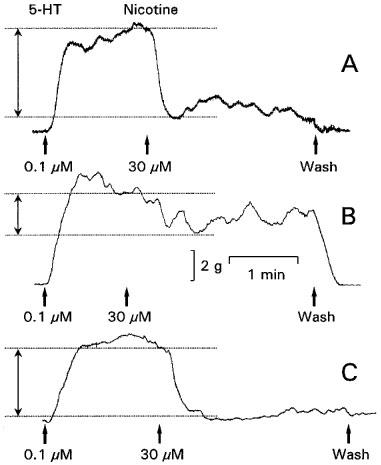
Representative isometric tension tracings of a longitudinal muscle strip of a rat gastric fundus showing the control (A), the effect of 10 μM guanethidine (B) and of 3 μM nadolol in combination with 3 μM phentolamine (C) on the relaxation induced by 30 μM nicotine. These experiments were performed during a 0.1 μM 5-HT-induced contraction in the presence of 1 μM atropine. The degree of the nicotine-induced relaxation is indicated by the double-headed arrow.
Figure 2.
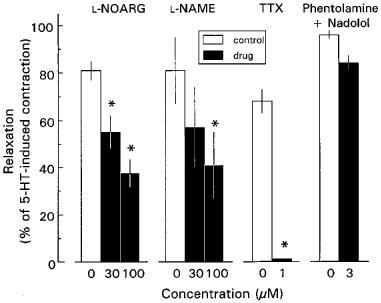
Effect of Nω-nitro-L-arginine (L-NOARG), Nω-nitro-L-arginine methyl ester (L-NAME), tetrodotoxin (TTX) and 3 μM nadolol in combination with 3 μM phentolamine on 30 μM nicotine-induced NANC relaxations of longitudinal muscle strips of rat gastric fundus. Experiments were performed in the presence of 1 μM atropine and 3 μM guanethidine (except for the phentolamine/nadolol experiments) during a 0.1 μM 5-HT-induced contraction. Open bars represent the control relaxations for each set of experiments. Results are shown as the means±s.e.mean and expressed as percentage of decrease of the 5-HT-induced contraction for n=3–5. *P value <0.05, significant different from time corrected controls.
Effect of guanethidine and adrenergic receptor blockade on nicotine-induced relaxations of longitudinal muscle strips
In the presence of 1 μM atropine, low concentrations of guanethidine (0.1–3 μM) had no effect on nicotine-induced relaxations (Figure 3), whereas higher concentrations of guanethidine (10 and 100 μM) significantly inhibited these relaxations in a concentration dependent way (Figures 1B and 3). After washing the muscle strips six times every 5 min, nicotine-induced relaxations returned to control values (not shown). In contrast, effective adrenergic receptor blockade by the α-adrenoceptor antagonist phentolamine in combination with the β-adrenoceptor antagonist nadolol (both 3 μM) had no effect on nicotine-induced relaxations (Figures 1C and 2).
Figure 3.
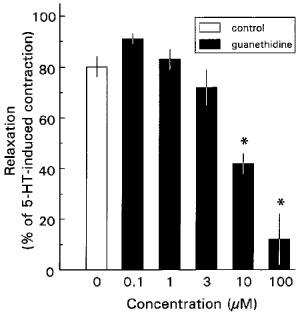
Effect of guanethidine on 30 μM nicotine-induced relaxations of longitudinal muscle strips of rat gastric fundus. The experiments were performed during a 0.1 μM 5-HT-induced contraction in the presence of 1 μM atropine. Results are shown as the means±s.e.mean and expressed as percentage of decrease of the 5-HT-induced contraction for n=6–12. *P value <0.05, significant different from time corrected controls.
Effect of guanethidine, adrenergic receptor blockade and hexamethonium on relaxations induced by EFS
In the presence of 1 μM atropine, EFS induced fast, transient and frequency-dependent relaxations (Figures 4A and 5A), which were reproducible in time. Guanethidine (0.1–100 μM) did not affect these relaxations, not even at a concentration of 100 μM (Figures 4B and 5A). Adrenoceptor blockade by 3 μM phentolamine in combination with 3 μM nadolol or nicotinic acetylcholine receptor blockade by hexamethonium (0.1–100 μM) also had no effect (Figures 4C and 5A, respectively).
Figure 4.
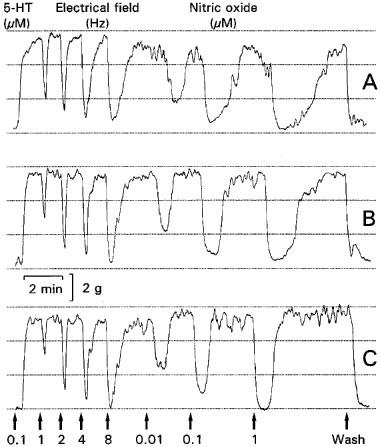
Representative isometric tension tracings of longitudinal muscle strips of a rat gastric fundus showing the control (A), the effect of 10 μM guanethidine (B) and of 3 μM nadolol in combination with 3 μM phentolamine (C) on the relaxations induced by electrical field stimulation (1–8 Hz, pulse width 1 msec, 9 V and duration 10 s) and nitric oxide (0.01–1 μM). This experiment was performed during a 0.1 μM 5-HT-induced contraction in the presence of 1 μM atropine.
Figure 5.
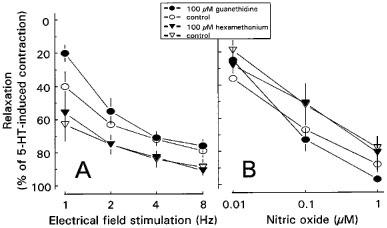
Effect of 100 μM guanethidine and 100 μM hexamethonium on EFS- (1–8 Hz, pulse width 1 msec, 9 V and duration 10 s (A) and NO- (B) induced relaxations of longitudinal muscle strips of rat gastric fundus. The experiments were performed during a 0.1 μM 5-HT-induced contraction in the presence of 1 μM atropine. Results are shown as the means±s.e.mean and are expressed as percentage of decrease of the 5-HT-induced contraction for n=5.
Effect of guanethidine, adrenergic receptor blockade and hexamethonium on relaxations induced by NO, isoprenaline or VIP
In the presence of 1 μM atropine, NO (0.01–1 μM) induced fast transient and concentration-dependent relaxations (Figures 4A and 5B). Guanethidine (0.1–100 μM), phentolamine in combination with nadolol (both 3 μM) and hexamethonium (0.1–100 μM) did not influence these relaxations (Figures 4B,C and 5B, respectively). Isoprenaline (1–10 nM) induced concentration-dependent relaxations (Figures 6A and 7A) which were not affected by guanethidine or hexamethonium (Figures 6B and 7A). Adrenergic receptor blockade by 3 μM phentolamine in combination with 3 μM nadolol completely blocked the 1 and 3 nM isoprenaline-induced relaxation, while the 10 nM isoprenaline-induced relaxation was blocked by 80±1% (n=3) (Figure 6C and 7A). Cumulative administration of VIP (0.1–10 nM) induced sustained concentration-dependent relaxations which were not affected by guanethidine (0.1–100 μM) (Figure 7B).
Figure 6.
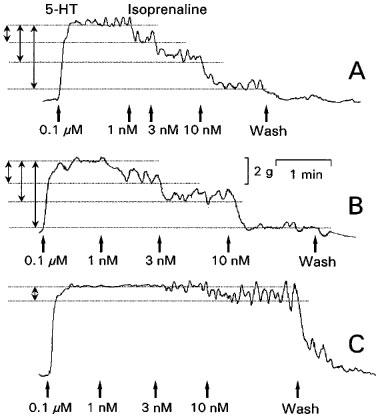
Representative isometric tension tracings of longitudinal muscle strips of the rat gastric fundus showing the control (A), the effect of 10 μM guanethidine (B) and of 3 μM nadolol in combination with 3 μM phentolamine (C) on isoprenaline-induced (1–10 nM) relaxations. This experiment was performed during a 0.1 μM 5-HT-induced contraction in the presence of 1 μM atropine. The degree of the isoprenaline-induced relaxations are indicated by the double-headed arrows.
Figure 7.
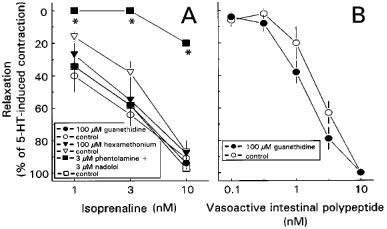
Effect of 100 μM guanethidine, 3 μM nadolol in combination with 3 μM phentolamine and 100 μM hexamethonium on isoprenaline-induced (1–10 nM) relaxations (A) on longitudinal muscle strips of rat gastric fundus. The effect of 100 μM guanethidine on VIP-induced (0.1–10 nM) relaxations is shown in B. The experiments were performed during a 0.1 μM 5-HT-induced contraction in the presence of 1 μM atropine. Results are shown as the means±s.e.mean and expressed as percentage of decrease of the 5-HT-induced contraction for n=3–5.
Discussion
In (patho)physiological and pharmacological studies, guanethidine is one of the most commonly used drugs to block adrenergic neurotransmission and as a consequence many functions have been ascribed to the adrenergic nervous system based on these studies (Chang et al., 1965; Burnstock & Wong, 1980; Nelson et al., 1988; Curro & Preziosi, 1997). Furthermore, in combination with atropine, guanethidine is often used to realise NANC conditions. In the present study, however, we demonstrate that high concentrations of guanethidine (⩾10 μM) have nicotinic acetylcholine receptor blocking side effects. This finding may have consequences for both the correct interpretation of earlier and design of future studies
Nicotine-induced relaxations of muscle strips of the rat gastric fundus are fast and sustained. They are completely blocked by TTX and partly inhibited by the NO synthase inhibitors L-NOARG and L-NAME indicating that they result from activation of nicotinic acetylcholine receptors located on nitrergic neurones. Since the effect of both NO synthase inhibitors was of the same magnitude, we can exclude any inhibitory effect of L-NAME on cholinergic neural responses, as has been suggested previously (Buxton et al., 1993).
The nicotine-induced relaxations were also concentration-dependent inhibited by guanethidine, an effect which can not be attributed to an aspecific side effect. More specifically, even at a concentration of 100 μM, no effects of guanethidine on relaxations induced by nerve stimulation or smooth muscle relaxants like NO, VIP and isoprenaline, are observed. Furthermore, the inhibitory effect is reversible after wash out, excluding permanent neural or muscular damage. Since it has been suggested that part of the nicotine-induced relaxation was adrenergic (Curro & Preziosi, 1997), the inhibitory effect of guanethidine could be due to its well-accepted anti-adrenergic properties. However, α- and β-adrenoceptor blockade with phentolamine and nadolol, at concentration effectively blocking isoprenaline-induced relaxations, had no effect on nicotine-induced relaxations, illustrating that under our experimental condition the nicotine-induced relaxations are completely non-adrenergic.
Although the exact site of interaction can not be determined using the current experimental set-up, our results clearly indicate that guanethidine has nicotinic acetylcholine receptor blocking effects. A direct interaction of guanethidine with the relaxing capacity of the smooth muscle cells is most unlikely, since well known receptor mediated (VIP and isoprenaline) and non-receptor mediated (NO) relaxations of the muscle are not affected at all by guanethidine even at high concentrations. In addition, guanethidine does not interfere with the release of the NANC neurotransmitters NO and VIP involved in NANC nerve mediated relaxations of the rat gastric fundus, since it has no effect on relaxations induced by EFS. Thus, as the inhibitory effect of guanethidine can not be attributed to a direct effect on the smooth muscle cell or a presynaptic effect on the NANC nerve terminals, guanethidine should interact at the nicotinic acetylcholine receptor. A previous study from Jaanus et al. (1968) already suggested that in the adrenal medulla of the cat guanethidine (4–80 μM) has a blocking side-effect on acetylcholine-induced catecholamine release, suggesting a nicotinic acetylcholine receptor blocking side effect. Moreover, in a study with cultured bovine chromaffin cells Gandía et al. (1991) indeed showed nicotinic acetylcholine receptor blocking effects at a concentration of 30 μM guanethidine (Gandia et al., 1991). Furthermore, Villarroya et al. (1996) even used the guanethidinium moiety of guanethidine as a model to synthesize a novel selective nicotinic acetylcholine receptor antagonist, Therefore, we conclude that inhibition of the nicotine-induced NANC relaxation by ⩾10 μM guanethidine is due to a direct interaction of guanethidine with the nicotinic acetylcholine receptor.
Although guanethidine has only nicotinic acetylcholine blocking effects at high concentrations (⩾10 μM), our findings may have important implications for the interpretation of many physiological and pharmacological studies. In preliminary experiments, we determined that 2 h after a single i.v. dose of 5 mg kg−1 of guanethidine the concentration in rat gastric fundic tissue is approximately 3 μM. For comparison, 2 h after a single i.v. dose of 7 and 28 mg kg−1 of guanethidine the concentration in heart tissue is even as high as 30 and 94 μM, respectively (Chang et al., 1965). Therefore, in in vivo studies in which 20–50 mg kg−1 of guanethidine is given 2–5 days a week during 2–5 weeks to destroy peripheral sympathetic neurones, the tissue concentration of guanethidine must be at least 100 μM, a concentration at which guanethidine undoubtedly has major nicotinic acetylcholine receptor blocking effects.
In conclusion, guanethidine (⩾10 μM) inhibits nicotine-induced NANC relaxations in the rat gastric fundus, most likely via blocking the nicotinic acetylcholine receptor. Therefore, the use of high concentrations of guanethidine should be avoided and the results of both in vivo and in vitro studies, in which high doses of guanethidine have been used, should be interpreted with appropriate caution.
Abbreviations
- EFS
electrical field stimulation
- 5-HT
5-hydroxytryptamine
- L-NAME
Nω-nitro-L-arginine methyl ester
- L-NOARG
Nω-nitro-L-arginine
- NANC
non-adrenergic non-cholinergic
- NO
nitric oxide
- TTX
tetrodotoxin
- VIP
vasoactive intestinal polypeptide
References
- ABRAHAMSSON H. Non-adrenergic non-cholinergic nervous control of gastrointestinal motility patterns. [Review] Arch. Int. Pharmacodyn. Ther. 1986;280:50–61. [PubMed] [Google Scholar]
- BOECKXSTAENS G.E., PELCKMANS P.A., BOGERS J.J., BULT H., DE MAN J.G.O.L., HERMAN A.G., VAN MAERCKE Y.M. Release of nitric oxide upon stimulation of nonadrenergic noncholinergic nerves in the rat gastric fundus. J. Pharmacol. Exp. Ther. 1991;256:441–447. [PubMed] [Google Scholar]
- BOECKXSTAENS G.E., PELCKMANS P.A., DE MAN J.G., BULT H., HERMAN A.G., VAN MAERCKE Y.M. Evidence for a differential release of nitric oxide and vasoactive intestinal polypeptide by nonadrenergic noncholinergic nerves in the rat gastric fundus. Arch. Int. Pharmacodyn. Ther. 1992;318:107–115. [PubMed] [Google Scholar]
- BURNSTOCK G., WONG H.Systemic pharmacology of adrenergic agonists and antagonists: effect on the digestive system Handbook experimental pharmacology: Adrenergic activators and inhibitors 1980Berlin: Springer-Verlag; 129–159.Szekeres, L. (ed)54 ed [Google Scholar]
- BUXTON I.L., CHEEK D.J., ECKMAN D., WESTFALL D.P., SANDERS K.M., KEEF K.D. NG-nitro-L-arginine methyl ester and other alkyl esters of arginine are muscarinic receptor antagonists. Circ. Res. 1993;72:387–395. doi: 10.1161/01.res.72.2.387. [DOI] [PubMed] [Google Scholar]
- CHANG C.C., COSTA E., BRODIE B.B. Interaction of guanethidine with adrenergic neurons. J. Pharmacol. Exp. Ther. 1965;147:303–312. [PubMed] [Google Scholar]
- CURRO D., PREZIOSI P. Involvement of vasoactive intestinal polypeptide in nicotine- induced relaxation of the rat gastric fundus. Br. J. Pharmacol. 1997;121:1105–1112. doi: 10.1038/sj.bjp.0701245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GANDIA L., CASADO L.F., LOPEZ M.G., GARCIA A.G. Separation of two pathways for calcium entry into chromaffin cells. Br. J. Pharmacol. 1991;103:1073–1078. doi: 10.1111/j.1476-5381.1991.tb12302.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- JAANUS S.D., MIELE E., RUBIN R.P. The action of guanethidine on the adrenal medulla of the cat. Br. J. Pharmacol. 1968;33:560–569. doi: 10.1111/j.1476-5381.1968.tb00505.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KELM M., FEELISCH M., SPAHR R., PIPER H.M., NOACK E., SCHRADER J. Quantitative and kinetic characterization of nitric oxide and EDRF released from cultured endothelial cells. Biochem. Biophys. Res. Commun. 1988;154:236–244. doi: 10.1016/0006-291x(88)90675-4. [DOI] [PubMed] [Google Scholar]
- LEFEBVRE R.A. Non-adrenergic non-cholinergic neurotransmission in the proximal stomach. [Review] Gen. Pharmacol. 1993;24:257–266. doi: 10.1016/0306-3623(93)90301-d. [DOI] [PubMed] [Google Scholar]
- LI C.G., RAND M.J. Nitric oxide and vasoactive intestinal polypeptide mediate non-adrenergic, non-cholinergic inhibitory transmission to smooth muscle of the rat gastric fundus. Eur. J. Pharmacol. 1990;191:303–309. doi: 10.1016/0014-2999(90)94162-q. [DOI] [PubMed] [Google Scholar]
- LUNDBORG P., STITZEL R.E. Studies on the dual action of guanethidine in sympathetic nerves. Acta Phys. Scand. 1968;72:100–107. doi: 10.1111/j.1748-1716.1968.tb03831.x. [DOI] [PubMed] [Google Scholar]
- MCLAREN A., LI C.G., RAND M.J. Mediators of nicotine-induced relaxations of the rat gastric fundus. Clin. Exp. Pharmacol. Physiol. 1993;20:451–457. doi: 10.1111/j.1440-1681.1993.tb01723.x. [DOI] [PubMed] [Google Scholar]
- NELSON D.K., SERVICE J.E., STUDELSKA D.R., BRIMIJOIN S., GO V.L. Gastrointestinal neuropeptide concentrations following guanethidine sympathectomy. J. Auton. Nerv. Sys. 1988;22:203–210. doi: 10.1016/0165-1838(88)90108-7. [DOI] [PubMed] [Google Scholar]
- TAKAHASHI T., OWYANG C. Vagal control of nitric oxide and vasoactive intestinal polypeptide release in the regulation of gastric relaxation in rat. J. Physiol. 1995;484:481–492. doi: 10.1113/jphysiol.1995.sp020680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VILLARROYA M., GANDIA L., LOPEZ M.G., GARCIA A.G., CUETO S., GARCIA-NAVIO J.L., ALVAREZ-BUILLA J. Synthesis and pharmacology of alkanediguanidinium compounds that block the neuronal nicotinic acetylcholine receptor. Bioorg. Med. Chem. 1996;4:1177–1183. doi: 10.1016/0968-0896(96)00108-3. [DOI] [PubMed] [Google Scholar]


