Abstract
We characterized the regulation of cyclooxygenase-2 (COX-2) at the mRNA, protein and mediator level in two rat models of acute inflammation, carrageenan-induced paw ædema and mechanical hyperalgesia.
Carrageenan was injected in the hind paw of rat at low (paw ædema) and high doses (hyperalgesia). COX-2 and prostaglandin E2 (PGE2) levels were measured by RT–PCR and immunological assays. We also determined the distribution of COX-2 by immunohistochemistry.
The injection of carrageenan produced a significant and parallel induction of both COX-2 and PGE2. This induction was significantly higher in hyperalgesia than in paw ædema. This was probably due to the 9 fold higher concentration of carrageenan used to provoke hyperalgesia.
Immunohistochemical examination showed COX-2 immunoreactivity in the epidermis, skeletal muscle and inflammatory cells of rats experiencing hyperalgesia. In paw ædema however, only the epidermis showed positive COX-2 immunoreactivity.
Pretreatment with indomethacin completely abolished the induction of COX-2 in paw ædema but not in hyperalgesia.
These results suggest that multiple mechanisms regulate COX-2 induction especially in the more severe model. In carrageenan-induced paw ædema, prostanoid production have been linked through the expression of the COX-2 gene which suggest the presence of a positive feedback loop mechanism.
Keywords: Prostaglandin, cyclooxygenase, inflammation, antibodies
Introduction
Prostaglandin synthases, also known as cyclooxygenases (COX), are enzymes catalyzing the synthesis of prostaglandin H2 from arachidonic acid. Prostaglandin H2 is the common precursor for the synthesis of prostaglandins, prostacyclins and thromboxanes (Smith et al., 1991). Non-steroidal anti-inflammatory drugs (NSAIDs) are used as anti-inflammatory, anti-pyretic and analgesic agents and mediate their effect through the inhibition of COX activity (Herschman, 1996; Vane & Botting, 1995). Two isoforms of COX were cloned and characterized in mammals. The COX-1 enzyme is expressed in many tissues such as the platelets, the kidney and the gut (Funk et al., 1991; Kargman et al., 1996). This isoform was associated with many of the side effects related to NSAIDs therapy such as gastro-intestinal irritation and ulceration (Wallace et al., 1994) and kidney impairment (Murray & Brater, 1993). In contrast, the expression pattern of the COX-2 gene is very restricted. However, a wide variety of agents such as pro-inflammatory cytokines, lipopolysaccharides and growth factors induce expression of the COX-2 gene (Hemple et al., 1994; Jones et al., 1993; Lee et al., 1992). Many reports demonstrated that selective COX-2 inhibitors have anti-inflammatory and analgesic effects similarly to those of conventional NSAIDs but with a substantially improved side effect profile (Bjarnason et al., 1997; Furst, 1997; Lane, 1997).
The injection of carrageenan to the hind paw of rats is a common model to study inflammation and inflammatory pain. Carrageenan causes ædema, an increase in paw volume, and an exacerbated sensitivity to thermal and mechanical stimuli which is known as hyperalgesia. Conventional NSAIDs, COX-2 inhibitors and prostaglandin E2 (PGE2) monoclonal antibodies are effective anti-inflammatory agents in these models (Chan et al., 1995; Khanna et al., 1997; Riendeau et al., 1997; Zhang et al., 1997). In the paw ædema model, COX-2 levels are elevated with a concomitant increase in prostaglandin production (Kennedy et al., 1993; Portanova et al., 1996; Seibert et al., 1994; 1997; Zhang et al., 1997). However, a detailed biochemical and histological characterization of COX-2 induction in carrageenan-induced inflammation is lacking. Moreover, it is not known how NSAIDs affect the induction of COX-2 in these models. The concentration of carrageenan used to test for mechanical hyperalgesia needs to be substantially higher than in the ædema model. Moreover, carrageenan-induced paw ædema and hyperalgesia differ in their drug-dosing regimen. The paw ædema protocol is an assay in which the drug is given prophylactically before the inflammatory stimulus while hyperalgesia is a reversal assay.
In this paper, we investigated the regulation of COX-2 in carrageenan-induced paw ædema and hyperalgesia. We show that carrageenan increases COX-2 and PGE2 levels in both models but this induction is greater and more widespread in hyperalgesia than in ædema. We show that indomethacin blocks COX-2 induction in paw ædema but not in hyperalgesia suggesting that a positive feedback loop regulates COX-2 expression in the paw ædema model.
Methods
In vivo experiments
All in vivo experiments were approved by the Animal Care Committee at the Merck Frosst Center for Therapeutic Research according to guidelines established by the Canadian Council on Animal Care. The animals were fasted for 16–18 before each experiment. In carrageenan-induced paw ædema (Chan et al., 1995; Otterness & Moore 1988), male Sprague-Dawley rats (150–200 g) received oral administration of either the vehicle (1% methocel) or indomethacin (10 mg kg−1). One hour later, a water displacement plethysmometer (Ugo-Basile, Italy) measured the initial paw volume. Carrageenan (50 μl of a 1% solution in saline) was then administered by intraplantar injection in the left hind paw. The animals were then sacrificed at various time points between 1 and 3 h following carrageenan injection. Paw ædema was measured at the various time points by paw volume and the increase in paw volume due to ædema was calculated. In carrageenan-induced hyperalgesia (Chan et al., 1995; Randall & Selitto, 1957), carrageenan (150 μl of a 3% solution in saline) was administered by intraplantar injection to male Sprague-Dawley rats (90–110 g). The animals were sacrificed at various time-points between 0 and 3 h following the carrageenan injection. Vehicle (1% methocel) or indomethacin (10 mg kg−1) was administered orally 2 h. following the carrageenan injection. The vocalization response of the rat to increased pressure on the carrageenan-injected paw using an algesiometer (Ugo-Basile, Italy) was recorded at the 3-h time point.
Paw sample preparation
Rat paws were removed below the ankle and degloved to remove the bone. The tissues were immediately frozen in liquid nitrogen and stored at −80°C until needed. The tissues were then ground to a powder using a mortar and a pestle under liquid nitrogen and used as described below.
COX-2 mRNA analysis
mRNA was purified using the Perkin Elmer mRNA purification kit following the manufacturer's instructions. COX-2 and β-Actin mRNA levels were determined by quantitative reverse transcription-polymerase chain reaction (RT–PCR) using the GeneAmp RNA–PCR kit (Perkin Elmer) using 50–100 ng of poly A+ RNA for each assay. Reverse transcription of mRNA was done in a 20 μl volume containing 1×PCR buffer II, 5 mM MgCl2, 1 mM dNTP, 1 U μl−1 RNase inhibitor, 2.5 U μl−1 MMLV reverse transcriptase and 2.5 μM random hexamers. The samples were incubated at 42°C for 15 min. and at 99°C for 5 min prior to the addition of 80 μl of PCR mix containing 1×PCR buffer II, 2 mM MgCl2, 2.5 U AmpliTaq DNA polymerase, 2 U TaqStart antibody (Clontech) and the appropriate primers at a final concentration of 0.15 μM. The primers for amplification of β-Actin were obtained from Clontech. For COX-2, the primer used were GACGATCAAGATAGTGATCGAAGAC and AAGCGTTTGCGGTACTCAATG. The PCR amplification was performed in a Perkin Elmer 9600 thermal cycler at 95°C for 60 s and at 60°C for 90 s. Aliquots (20 μl) were taken after 20, 25, 30 and 35 cycles and analysed by electrophoresis using an 1.8% agarose gel followed by transfer to a ZetaProbe nylon membrane (BioRad). The identity and intensity of the PCR products were determined by Southern blotting with a fluorescein-labelled (Amersham) probe (COX-2: AAAAGCAGCTCTGGGTCGAAC, β-Actin probe was obtained from Clontech) using a Storm FluorImager. Linearity of amplification was observed between 20–25 cycles for β-actin and 30–35 cycles for COX-2. COX-2 mRNA levels were then normalized to β-actin to correct for variations in mRNA concentrations.
COX-2 protein analysis
Pulverized paws were solubilized in extraction buffer (phosphate-buffered saline (PBS), 0.5% Tween-20, 100 μM leupeptin, 0.05 mg ml−1 pepstatin, 10 μM E-64, 50 μg ml−1 Pefabloc) for 1 h at 4°C with gentle agitation, then subjected to centrifugation at 2000 r.p.m. for 5 min. at 4°C. The protein extracts were routinely stored at −80°C. COX-2 protein was measured using a quantitative ELISA assay employing a rabbit polyclonal antibody raised against purified ovine(ov) COX-2 (Cayman) (Kargman et al., 1995; 1996). All incubations were conducted at room temperature with agitation. Nunc Immunosorb 96-well plates were coated with 200 μl per well of anti-COX-2 antibody diluted to 3 μg ml−1 in either PBS (ædema range) or PBS containing 10% SuperBlock Buffer (Pierce) (hyperalgesia range). The plates were then incubated for 1 h, emptied by inversion and washed three times with 200 μl per well of undiluted SuperBlock buffer. Samples (50 μl per well) were then added and the plates were incubated for a further 3 h. They were then washed three times with 200 μl per well of PBS containing 0.05% Tween 20 using a Skatron plate washer. Biotinylated second antibody (3 μg ml−1 in PBS, 0.05% Tween 20, 10% SuperBlock) was added (100 μl per well) and the plate incubated for 1 h. The plates were washed again as described above and incubated with horseradish peroxidases-linked streptavidin (Pierce) (100 μl of 0.3 μg ml−1 in PBS, 0.05% Tween 20, 10% SuperBlock) for 1 h. Finally, the plates were washed three times with 250 μl per well of PBS containing 0.05% Tween 20. Peroxidase activity was monitored at 450 nm using 100 μl TMB/Peroxide substrate solution (Pierce). Purified ovCOX-2 (Cayman) was used as the standard at a concentration ranging from 5–100 ng ml−1 (ædema) or 50–1250 ng ml−1 (hyperalgesia) in PBS, 0.5% Tween, 10% SuperBlock. This assay is linear with respect to increasing COX-2 immuno-reactivity at concentrations up to 1000 ng ml−1 of COX-2 and shows no detectable cross-reactivity with ovCOX-1 (Cayman).
PGE2 analysis
Pulverized paws were suspended in 1 ml acetone and incubated for 30 min at 4°C with gentle agitation. The samples were then centrifuged at 2000×g for 5 min at 4°C, the supernatants were removed and evaporated to dryness under vacuum. The resulting samples were then solubilized in sample buffer (from the manufacturer) and PGE2 levels measured using a commercial PGE2 EIA kit (Assay Design Inc.).
Statistical analysis
The data was analysed using the unpaired Student t-test method. Results were considered significant when P<0.05.
Histology and COX-2 immuno-histochemistry
Rat paws were removed, degloved and fixed in 4% paraformaldehyde in PBS. The tissues were dehydrated, embedded in paraffin and sectioned in 5 μ-thin slices on silane-coated slides. Prior to immunohistochemical detection of COX-2, the paraffin was removed and the tissues were rehydrated by washing twice for 3 min in xylene, twice in ethanol, twice in 95% ethanol and once in water. The tissues were blocked in SuperBlock in PBS (Pierce) for 30 min. at room temperature (RT) and incubated for 1 h with a biotinylated rabbit anti-ovCOX-2 antibody (5 μg ml−1, see above) in PBS with 10% SuperBlock. The tissues were washed twice in PBS with 0.05% Tween 20 and treated for 15 min with peroxidase suppressor (Pierce). They were washed again as described above and incubated with horseradish peroxidase-linked streptavidin (Pierce) at 0.3 μg ml−1 in PBS with 10% SuperBlock for 30 min at RT. The COX-2 immuno-reactivity was revealed using Enhanced DAB substrate (Pierce). The tissue slides were counterstained with Gill's hematoxilin (Fisher) and mounted using an aqueous mounting media.
Results
COX-2 induction and PGE2 production in carrageenan-promoted inflammation
In both models of inflammation, carrageenan injection in the hind paw induced a significant increase in the expression of COX-2 mRNA (Figure 1a) and protein (Figure 1b) as well as production of PGE2 (Figure 1c). Maximal levels of COX-2 were observed at 1 h following carrageenan injection while PGE2 levels peaked at 2 h. The levels of COX-2 expression and the production of PGE2 were higher in hyperalgesia than in ædema, which is probably due to the higher concentration of carrageenan used in hyperalgesia.
Figure 1.
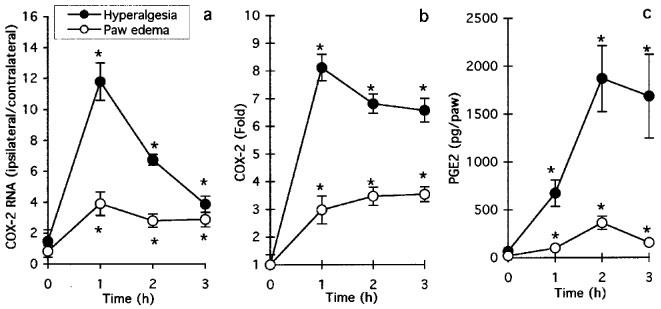
Regulation of COX-2 in carrageenan-induced paw ædema and hyperalgesia. (a) COX-2 mRNA levels as determined by RT–PCR (see Methods) under conditions of carrageenan-induced paw ædema and hyperalgesia. Data is expressed as fold of the intensity of COX-2-specific band obtained from ipsilateral (injected) paw over that of contralateral (non-injected) paw. Both sets of data have also been corrected for β-actin. Data are mean±s.e.mean of 12–24 determinations. (b) COX-2 protein levels as determined by ELISA under conditions of carrageenan-induced paw ædema and hyperalgesia. Data is expressed as fold of COX-2 levels in ipsilateral over contralateral paws and are mean±s.e.mean of 6–12 determinations. (c) PGE2 levels in paws under conditions of carrageenan-induced paw ædema and hyperalgesia. Data is expressed as pg per paw and are mean±s.e.mean of 6–12 determinations. *P<0.05 over value obtained at time zero.
Histological evaluation of inflamed paws at 3 h post-carrageenan show a disorganization of the connective tissues and an infiltration of inflammatory cells in both models. The inflammation observed in the hyperalgesia model appears more severe than in paw ædema. Numerous large eosin-labelled cells, potentially macrophages and eosinophils, are observed in the loose connective tissues of the inflamed paws. Although these cells are also present in control paws, they were prominently located between the loose connective tissue and the skeletal muscle and large blood vessels. In contrast, these cells were more widespread throughout the loose connective tissue of the inflamed paw. The localization of COX-2 immuno-reactivity (IR) indicates that there is a differential expression pattern of COX-2 in carrageenan-promoted paw ædema as compared to hyperalgesia (Figure 2). Under basal conditions, no COX-2 IR could be detected (Figure 2a,d,g). In paw ædema, COX-2 IR is observed in the stratum corneum of the epidermis (Figure 2b) but not in connective tissues or in the skeletal muscle (Figure 2e,h). In hyperalgesia, COX-2 IR was not only detected in the stratum corneum of the epidermis (Figure 2c) but also in small infiltrated cells in the connective tissues (Figure 2f) and in skeletal muscle cells (Figure 2i). These results suggest that the induction of COX-2 is larger and more widespread in hyperalgesia than in paw ædema.
Figure 2.
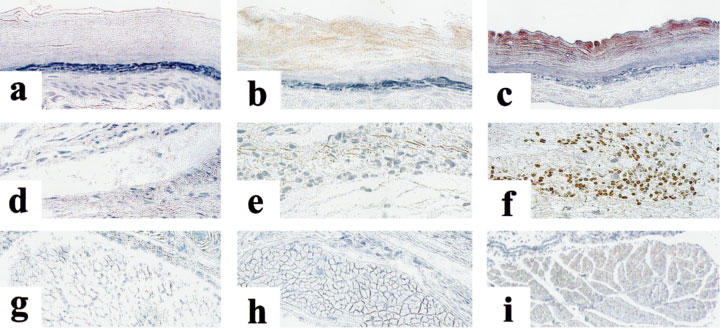
Photomicrographs of COX-2 immunoreactivity in rat paws under control conditions (Left column) and 3 h following injection of carrageenan under the paw ædema (centre column) and hyperalgesia protocols (right column). (a–c) Epidermis; (d–f) Loose connective tissue; (g–i) Skeletal muscle. Positive COX-2 immunoreactivity (brown staining) can be detected in the epidermis in paw ædema (b) and in the epidermis (c), loose connective tissue (f) and skeletal muscle (i) in hyperalgesia.
Effect of indomethacin on COX-2 induction and PGE2 production
We tested the effect of the non-selective COX inhibitor indomethacin on the induction of COX-2 and the production of PGE2 in both models. These two models differ in their drug-dosing regimen. In the paw ædema protocol, the drug is given 1 h before carrageenan. The hyperalgesia protocol, however, is a reversal assay with the drug given 2 h following the carrageenan provocation. As shown in Figure 3 and reported previously (Chan et al., 1995; Riendeau et al., 1997), indomethacin prevents the development of ædema and inhibited hyperalgesia as measured by paw volume and vocalization response. In agreement with these data, indomethacin significantly inhibited PGE2 production in both paw ædema and hyperalgesia (Figure 4a,b). Interestingly, however, the carrageenan-promoted COX-2 induction in paw ædema was also completely abrogated by the inhibitor (Figure 4c). However, COX-2 levels remained elevated in hyperalgesia following the administration of indomethacin (Figure 4d). To verify whether the different effects of indomethacin on COX-2 induction in the two models depended on the drug dosing regimen, we tested whether pretreatment for 1 h with indomethacin would inhibit the COX-2 induction occurring in hyperalgesia. As can be seen in Figure 5, indomethacin did not affect the COX-2 induction at 1 and 2 h post carrageenan injection although it completely blocked the production of PGE2 (not shown). At the 3 h time points, COX-2 levels were even significantly higher in the indomethacin-treated animals. In order to elucidate the mechanism of action of indomethacin in paw ædema, we assessed the regulation of COX-2 mRNA in a similar time-course study following oral dosing of indomethacin during carrageenan-induced paw ædema (Figure 6). In control animals, carrageenan induced a 5 fold increase in COX-2 mRNA in the ipsilateral (injected) paw. In the contralateral (non-injected) paw, COX-2 mRNA levels were not affected. In the treated group, a 1 h pre-treatment with indomethacin caused a significant reduction in COX-2 mRNA in both paws. The injection of carrageenan at time zero in the indomethacin-treated rats induced a significant (P<0.05) increase in COX-2 mRNA but those never went over the levels measured in the uninjected paws of the control animals. These results indicate that indomethacin inhibits COX-2 expression at the mRNA level.
Figure 3.
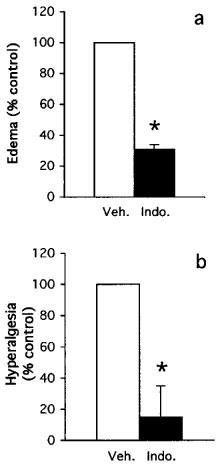
Effect of indomethacin on carrageenan-induced paw ædema and hyperalgesia. (a) paw ædema observed 3 h following the injection of carrageenan in vehicle or indomethacin-pretreated animal (10 mg kg−1 p.o.). Data are expressed as per cent of volume increase observed in control animals. (b) Hyperalgesia response observed 3 h following the injection of carrageenan in vehicle or indomethacin-treated animal (10 mg kg−1 p.o.). Data are expressed as percent of vocalization response observed in control animals. Data are mean±s.e.mean of 8–10 determinations. *P<0.05.
Figure 4.
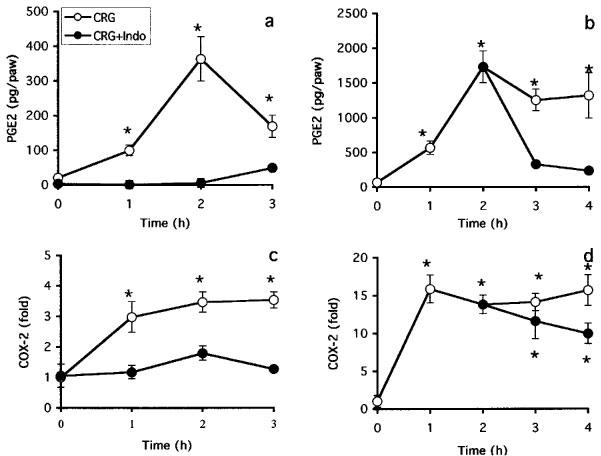
Effect of indomethacin on the regulation of COX-2 protein and PGE2 levels in carrageenan-induced paw ædema (a,c) and hyperalgesia (b,d). Indomethacin (10 mg kg−1 p.o.) or vehicle (methocel) was given 1 h prior (ædema) or 2 h after (hyperalgesia) carrageenan injection. Paws were taken at indicated time and PGE2 (a,b) and COX-2 protein (c,d) levels were analysed as described in Methods. PGE2 levels are expressed as pg per paw. COX-2 protein levels are expressed as fold of carrageenan-treated over control paws. Data are mean±s.e.mean of 8–12 determinations. *P<0.05 over value obtained at time zero.
Figure 5.
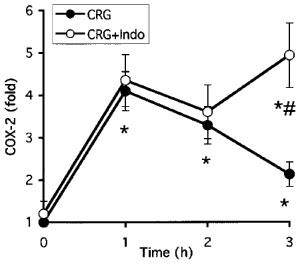
Effect of indomethacin pre-treatment on the regulation of COX-2 protein and PGE2 levels in carrageenan-induced hyperalgesia. Indomethacin (10 mg kg−1 p.o.) or vehicle (methocel) was given 1 h prior to carrageenan injection. Paws were taken at indicated time and COX-2 protein levels were analysed as described in Methods. COX-2 protein levels are expressed as fold of carrageenan-injected over saline-injected paws. Data are mean±s.e.mean of eight determinations. *P<0.05 over value obtained at time zero. #P<0.05 between indomethacin-treated and untreated curves.
Figure 6.
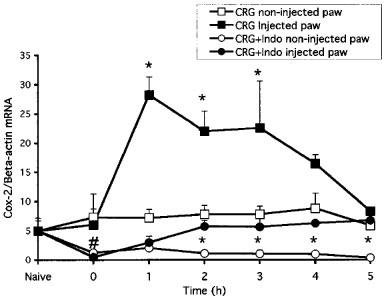
Effect of indomethacin on the regulation of COX-2 mRNA in carrageenan-induced paw ædema. Indomethacin (10 mg kg−1 p.o.) or vehicle (methocel) was given orally 1 h prior to intraplantar injection of carrageenan. Ipsilateral (injected) and contralateral (non-injected) paws were taken at indicated time, mRNA was isolated and both COX-2 and β-actin mRNA levels analysed by RT–PCR. Data are expressed as fold±s.e.mean of the intensity of the COX-2-specific band over that of β-actin obtained from the injected and non-injected paws of eight animals. *P<0.05 between ipsilateral and contralateral curves. #P<0.05 between data obtained at time zero and in naive animals.
Discussion
Carrageenan injection in the hind paw of the rat is one of the most commonly used models of inflammation and inflammatory pain. Multiple physical and behavior responses can be followed to assess the extent of injury and its prevention or reversal by NSAIDs. The doses of carrageenan used in paw ædema and thermal hyperalgesia assays are similar (Chan et al., 1995; Higgs et al., 1980; Khanna et al., 1997; Penning et al., 1997; Portanova et al., 1996; Riendeau et al., 1997; Seibert et al., 1994; Zhang et al., 1997). In this study, we show that ædema is accompanied by an induction of COX-2 in the epidermis. The onset of mechanical hyperalgesia, however, requires a much higher dose of carrageenan (Chan et al., 1995; Randall & Selitto, 1957; Riendeau et al., 1997) and the hallmarks of inflammation in this model are more severe. The induction of COX-2 in hyperalgesia is more widespread than in ædema since COX-2 IR could be detected not only in the epidermis, but also in skeletal muscle and inflammatory cells. In agreement with the COX-2 induction data, higher levels of PGE2 are produced with the more severe provocation. An infiltration of inflammatory cell was also observed in both assays which is in agreement with the previously reported infiltration of leukocytes and neutrophils by carrageenan in the pleural cavity (Almeida et al., 1980; Higgs et al., 1980; Vinegar et al., 1987).
The pivotal role of PGE2 in carrageenan-induced ædema and thermal hyperalgesia was suggested by other groups (Mnich et al., 1995; Portanova et al., 1996; Zhang et al., 1997). A neutralizing monoclonal antibody against PGE2 was shown to inhibit carrageenan-induced ædema and thermal hyperalgesia as efficiently as indomethacin. Although many components have been implicated in the inflammatory response, these studies placed prostanoid synthesis either as an early element or as an important link in the chain of events leading to carrageenan-invoked inflammatory responses.
Our data extend these findings and suggest that distinct and multiple mechanisms regulate the expression of COX-2. These depend on the initial extent of inflammatory stimuli such as the dose of carrageenan. Moreover, the present study on the COX-2 induction in carrageenan-induced paw ædema suggests the presence of a prostanoid-dependent positive feedback loop since this induction is inhibited by indomethacin. This positive feedback mechanism was also proposed in a chronic model of inflammation, the rat adjuvant arthritis. In this model, therapeutic administration of a selective COX-2 inhibitor, SC-58125, reduced the expression of both COX-2 mRNA and protein levels (Anderson et al., 1996). Further evidence supporting the importance of PGE2 in this feedback loop comes from recent papers describing the induction of COX-2 expression by prostaglandins in human and mouse cell lines (Murakami et al., 1997; Tjandrawinata et al., 1997). In these studies, a stable analogue of PGE2, 16, 16-dimethyl PGE2, induces the expression of COX-2 mRNA in human PC-3 and mouse MC3T3-E1 cells. In the MC3T3-E1 cells, a selective EP1 receptor agonist also induced COX-2 mRNA expression implicating at least the EP1 receptor in this autoamplification loop (Suda et al., 1998). Together, these studies and ours suggest that the regulation of COX-2 by prostanoids occurs at the transcriptional level. The contribution of other prostanoid in the induction of COX-2 remains to be clearly established. We have observed that the injection of PGE2 in the paw does lead to increased levels of COX-2 protein (D. Denis, unpublished observation). From the data presented in our study, it would appear that the prostanoid-dependent regulation of COX-2 in carrageenan-induced paw ædema occurs in the epidermis. The reasons indomethacin had such a limited effect on the induction of COX-2 in hyperalgesia probably relates to the higher levels of inflammatory mediators produced. This would also account for the wider distribution of COX-2 in this model. Under these conditions, the feedback loop may still operate but the levels of the other inflammatory mediators are high enough to drive the expression of COX-2. It would also appear that prostanoids may have an inhibitory effect on COX-2 induction. This is suggested by the observation that the levels of COX-2 in hyperalgesia are higher at the 3 h time point if the animals are pretreated with indomethacin. There is precedent to that effect when one considers that PGE2 has been shown to inhibit tumor necrosis factor-α production by human monocytes (Brideau et al., 1999). Since this cytokine is known to induce COX-2 (Arias-Negrete et al., 1995) and there is a large infiltration of monocytes to the site of inflammation, this suggest that this mediator and others are involved in the sustained action of COX-2 in this model. Therefore, it is possible that prostanoids are instrumental in the induction of COX-2 at early time points and in its down-regulation later on. What remains to be determined is the implication of each individual prostanoid receptor in this pathway. Distribution studies and the use of specific receptor antagonists will help to clarify this issue.
Another aspect which has not been covered in the current study is the exact contribution of COX-1 and COX-2 in the production of PGE2 and the induction of COX-2 in these models. The inhibitor used in the current study, indomethacin, is equally effective at inhibiting both COX-1 and COX-2. It has been reported that part of the PGE2 produced following carrageenan injection in the foot pad may be produced by the COX-1 enzyme (Smith et al., 1998). This would be true especially at the early time points before COX-2 protein is present. A complete characterization using selective COX-1 and COX-2 inhibitors would help to clarify this point.
In conclusion, we showed that carrageenan-induced paw ædema and mechanical hyperalgesia present distinct patterns of expression and regulation of COX-2. The induction of COX-2 can be blocked by the non-selective NSAID indomethacin in paw ædema but not in mechanical hyperalgesia. This result implies that prostanoids, possibly acting through a positive feedback loop, are implicated in the COX-2 induction observed in paw ædema.
Acknowledgments
We wish to acknowledge Mark Abramovitz, Donald W. Nicholson, Philip Tagari, Christine Brideau, Denis Riendeau, Brian P. Kennedy and Stacia Kargman for comments, discussions and gift of material. The help of Kevin Clark with the color prints is also acknowledged.
Abbreviations
- COX-1
cyclooxygenase-1
- COX-2
cyclooxygenase-2
- IR
immunoreactivity
- NSAID
non-steroidal anti-inflammatory drug
- PBS
phosphate-buffered saline
- PCR
polymerase chain reaction
- PGE2
prostaglandin E2
- RT–PCR
reverse transcription-polymerase chain reaction
References
- ALMEIDA A.P., BAYER B.M., HORAKOVA Z., BEAVEN M.A. Influence of indomethacin and other anti-inflammatory drugs on mobilization and production of neutrophils: studies with carrageenan-id inflammation in rats. J. Pharmacol. Exp. Ther. 1980;214:74–79. [PubMed] [Google Scholar]
- ANDERSON G.D., HAUSER S.D., MCGARITY K.L., BREMER M.E., ISAKSON P.C., GREGORY S.A. Selective inhibition of cyclooxygenase (COX)-2 reverses inflammation and expression of COX-2 and interleukin 6 in rat adjuvant arthritis. J. Clin. Invest. 1996;97:2672–2679. doi: 10.1172/JCI118717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ARIAS-NEGRETE S., KELLER K., CHADEE K. Proinflammatory cytokines regulate cyclooxygenase-2 mRNA expression in human macrophages. Biochem. Biophys. Res. Commun. 1995;208:582–589. doi: 10.1006/bbrc.1995.1378. [DOI] [PubMed] [Google Scholar]
- BJARNASON I., MACPHERSON A., ROTMAN H., SCHUPP J., HAYLLAR J. A randomized, double-blind, crossover comparative endoscopy study on the gastroduodenal tolerability of a highly specific cyclooxygenase-2 inhibitor, flosulide, and naproxen. Scand. J. Gastroenterol. 1997;32:126–130. doi: 10.3109/00365529709000182. [DOI] [PubMed] [Google Scholar]
- BRIDEAU C., VAN STADEN C., STYHLER A., RODGER I.W., CHAN C.C. The effects of phosphodiesterase type 4 inhibitors on tumor necrosis factor-a and leukotriene B4 in a novel human whole blood assay. Brit. J. Pharmacol. 1999;126:979–988. doi: 10.1038/sj.bjp.0702387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CHAN C.C., BOYCE S., BRIDEAU C., FORD-HUTCHINSON A.W., GORDON R., GUAY D., HILL R.G., LI C.S., MANCINI J., PENNETON M., PRASIT P., RASORI R., RIENDEAU D., ROY P., TAGARI P., VICKERS P., WONG E., RODGER I.W. Pharmacology of a selective cyclooxygenase-2 inhibitor, L-745,337: a novel nonsteroidal anti-inflammatory agent with an ulcerogenic sparing effect in rat and non human primate stomach. J. Pharmacol. Exp. Ther. 1995;274:1531–1537. [PubMed] [Google Scholar]
- FUNK C.D., FUNK L.B., KENNEDY M.E., PONG A.S., FITZGERALD G.A. Human platelet/erythroleukemia cell prostaglandin G/H synthase: cDNA cloning, expression, and gene chromosomal assignment. FASEB J. 1991;5:2304–2312. [PubMed] [Google Scholar]
- FURST D.E. Meloxicam: selective COX-2 inhibition in clinical practice. Semin. Arthritis Rheum. 1997;26:21–27. doi: 10.1016/s0049-0172(97)80049-2. [DOI] [PubMed] [Google Scholar]
- HEMPLE S.L., MONICK M.M., HUNNINGHAKE G.W. Lipopolysaccharide induces prostaglandin H synthase-2 protein and mRNA in human alveolar macrophages and blood monocytes. J. Clin. Invest. 1994;93:391–396. doi: 10.1172/JCI116971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HERSCHMAN H.R. Prostaglandin synthase 2. Biochim. Biophys. Acta. 1996;1299:125–140. doi: 10.1016/0005-2760(95)00194-8. [DOI] [PubMed] [Google Scholar]
- HIGGS G.A., EAKINS K.E., MUGRIDGE K.G., MONCADA S., VANE J.R. The effects of non-steroid anti-inflammatory drugs on leukocyte migration in carrageenan-induced inflammation. Eur. J. Pharmacol. 1980;66:81–86. doi: 10.1016/0014-2999(80)90297-6. [DOI] [PubMed] [Google Scholar]
- JONES D.A., CARLTON D.P., MCINTYRE T.M., ZIMMERMAN G.A., PRESCOTT S.M. Molecular cloning of human prostaglandin H synthase type II and demonstration of expression in response to cytokines. J. Biol. Chem. 1993;268:9049–9054. [PubMed] [Google Scholar]
- KARGMAN S., CHARLESON S., CARTWRIGHT M., FRANK J., RIENDEAU D., MANCINI J., EVANS J., O'NEILL G. Characterization of Prostaglandin G/H Synthase 1 and 2 in rat, dog, monkey, and human gastrointestinal tracts. Gastroenterology. 1996;111:445–454. doi: 10.1053/gast.1996.v111.pm8690211. [DOI] [PubMed] [Google Scholar]
- KARGMAN S.L., O'NEILL G.P., VICKERS P.J., EVANS J.F., MANCINI J.A., JOTHY S. Expression of prostaglandin G/H synthase-1 and -2 protein in human colon cancer. Cancer Res. 1995;55:2556–2559. [PubMed] [Google Scholar]
- KENNEDY B.P., CHAN C.C., CULP S.A., CROMLISH W.A. Cloning and expression of rat prostaglandin endoperoxide synthase (cyclooxygenase)-2 cDNA. Biochem. Biophys. Res. Commun. 1993;197:494–500. doi: 10.1006/bbrc.1993.2506. [DOI] [PubMed] [Google Scholar]
- KHANNA I.K., WEIER R.M., YU Y., COLLINS P.W., MIYASHIRO J.M., KOBOLDT C.M., VEENHUIZEN A.W., CURRIE J.L., SEIBERT K., ISAKSON P.C. 1,2-Diarylpyrroles as potent and selective inhibitors of cyclooxygenase-2. J. Med. Chem. 1997;40:1619–1633. doi: 10.1021/jm970036a. [DOI] [PubMed] [Google Scholar]
- LANE N.E. Pain management in osteoarthritis: the role of COX-2 inhibitors. J. Rheumatol. 1997;24:20–24. [PubMed] [Google Scholar]
- LEE S.H., SOYOOLA E., CHANMUNGAM P., HART S., SUN W., ZHONG H., LIOU S., SIMMONS D., HWANG D. Selective expression of mitogen-inducible cyclooxygenase in macrophage stimulated with lipopolysaccharide. J. Biol. Chem. 1992;267:25934–25938. [PubMed] [Google Scholar]
- MNICH S.J., VEENHUIZEN A.W., MONAHAN J.B., SHEEHAN K.C., LYNCH K.R., ISAKSON P.C., PORTANOVA J.P. Characterization of a monoclonal antibody that neutralizes the activity of prostaglandin E2. J. Immunol. 1995;155:4437–4444. [PubMed] [Google Scholar]
- MURAKAMI M.K.H., AMAKASU Y., SHIMBARA S., NAKATANI Y., ATSUMI G.-I., KUDO I. Prostaglandin E2 amplifies cytosolic phospholipase A2- and cyclooxygenase-2-dependent delayed prostaglandin E2 generation in mouse osteblastic cells. J. Biol. Chem. 1997;272:19891–19897. doi: 10.1074/jbc.272.32.19891. [DOI] [PubMed] [Google Scholar]
- MURRAY M.D., BRATER D.C. Renal toxicity of the nonsteroidal anti-inflammatory drugs. Annu. Rev. Pharmacol. Toxicol. 1993;33:435–465. doi: 10.1146/annurev.pa.33.040193.002251. [DOI] [PubMed] [Google Scholar]
- OTTERNESS I.G., MOORE P.F.Carrageenan foot edema test Methods in Enzymology 1988Academic Press: San Diego, California; pp, 320–327.Sabato, G.D. (ed.) [DOI] [PubMed] [Google Scholar]
- PENNING T.D., TALLEY J.J., BERTENSHAW S.R., CARTER J.S., COLLINS P.W., DOCTER S., GRANETO M.J., LEE L.F., MALECHA J.W., MIYASHIRO J.M., ROGERS R.S., ROGIER D.J., YU S.S., ANDERSON G.D., BURTON E.G., COGBURN J.N., GREGORY S.A., KOBOLDT C.M., PERKINS W.E., SEIBERT K., VEENHUIZEN A.W., ZHANG Y.Y., ISAKSON P.C. Synthesis and biological evaluation of the 1,5-diarylpyrazole class of cyclooxygenase-2 inhibitors: identification of 4-[5-(4-methylphenyl)-3- (trifluoromethyl)-1H-pyrazol-1-yl]benze nesulfonamide (SC-58635, celecoxib) J. Med. Chem. 1997;40:1347–1365. doi: 10.1021/jm960803q. [DOI] [PubMed] [Google Scholar]
- PORTANOVA J.P., ZHANG Y., ANDERSON G.D., HAUSER S.D., MASFERRER J.L., SEIBERT K., GREGORY S.A., ISAKSON P.C. Selective neutralization of prostaglandin E2 blocks inflammation, hyperalgesia, and interleukin 6 production in vivo. J. Exp. Med. 1996;184:883–891. doi: 10.1084/jem.184.3.883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RANDALL L.O., SELITTO J.J. A method for measurement of analgesic activity on inflamed tissue. Arch. Intl. Pharmacodyn. 1957;111:409–419. [PubMed] [Google Scholar]
- RIENDEAU D., PERCIVAL M.D., BOYCE S., BRIDEAU C., CHARLSTON S., CROMLISH W., ETHIER D., EVANS J., FALGUEYRET J.P., FORD-HUTCHINSON A.W., GORDON R., GREIG G., GRESSER M., GUAY J., KARGMAN S., LEGER S., MANCINI J.A., O'NEILL G., OUELLET M., RODGER I.W., THERIEN M., WANG Z., WEBB J.K., WONG E., CHAN C.C. Biochemical and pharmacological profile of a tetrasubstituted furanone as a highly selective COX-2 inhibitor. Br. J. Pharmacol. 1997;121:105–117. doi: 10.1038/sj.bjp.0701076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SEIBERT K., ZHANG Y., LEAHY K., HAUSER S., MASFERRER J., ISAKSON P. Distribution of COX-1 and COX-2 in normal and inflamed tissues. Adv. Exp. Med. Biol. 1997;400A:167–170. doi: 10.1007/978-1-4615-5325-0_24. [DOI] [PubMed] [Google Scholar]
- SEIBERT K., ZHANG Y., LEAHY K., HAUSER S., MASFERRER J., PERKINS W., LEE L., ISAKSON P. Pharmacological and biochemical demonstration of the role of cyclooxygenase 2 in inflammation and pain. Proc. Natl. Acad. Sci. USA. 1994;91:12013–12017. doi: 10.1073/pnas.91.25.12013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SMITH C.J., ZHANG Y., KOBOLDT C.M., MUHAMMAD J., ZWEIFEL B.S., SHAFFER A., TALLEY J.J., MASFERRER J.L., SEIBERT K., ISAKSON P.C. Pharmacological analysis of cyclooxygenase-1 in inflammation. Proc. Natl. Acad. Sci. USA. 1998;95:13313–13318. doi: 10.1073/pnas.95.22.13313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SMITH W.L., MARNETT L.J., DEWITT D.L. Prostaglandin and thromboxane biosynthesis. Pharmacol. Ther. 1991;49:153–179. doi: 10.1016/0163-7258(91)90054-p. [DOI] [PubMed] [Google Scholar]
- SUDA M., TANAKA K., YASODA A., NATSUI K., SAKUMA Y., TANAKA I., USHIKUBI F., NARUMIYA S., NAKAO K. Prostaglandin E2 (PGE2) autoamplifies its production through EP1 subtype of PGE receptor in mouse osteoblastic MC3T3-E1 cells. Calcif Tissue Int. 1998;62:327–331. doi: 10.1007/s002239900440. [DOI] [PubMed] [Google Scholar]
- TJANDRAWINATA R.R., DAHIYA R., HUGHES-FULFORD M. Induction of cyclo-oxygenase-2 mRNA by prostaglandin E2 in human prostatic carcinoma cells. Brit. J. Cancer. 1997;75:1111–1118. doi: 10.1038/bjc.1997.192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VANE J.R., BOTTING R.M. New insights into the mode of action of anti-inflammatory drugs. Inflamm. Res. 1995;44:1–10. doi: 10.1007/BF01630479. [DOI] [PubMed] [Google Scholar]
- VINEGAR R., TRUAX J.F., SELPH J.L., JOHNSTON P.R., VENABLE A.L., MCKENZIE K.K. Pathway to carrageenan-induced inflammation in the hind limb of the rat. Federation Proc. 1987;46:118–126. [PubMed] [Google Scholar]
- WALLACE J.L., REUTER B., CICALA C., MCKNIGHT W., GRISHAM M.B., CIRINO G. Novel nonsteroidal anti-inflammatory drug derivatives with markedly reduced ulcerogenic properties in the rat. Gastroenterology. 1994;107:173–179. doi: 10.1016/0016-5085(94)90074-4. [DOI] [PubMed] [Google Scholar]
- ZHANG Y., SHAFFER A., PORTANOVA J., SEIBERT K., ISAKSON P.C. Inhibition of cyclooxygenase-2 rapidly reverses inflammatory hyperalgesia and prostaglandin E2 production. J. Pharmacol. Exp. Ther. 1997;283:1069–1075. [PubMed] [Google Scholar]


