Abstract
The localization of protease-activated receptor-2 (PAR2) and the effects of PAR2 activators were investigated in the mouse isolated ureter in order to test the hypothesis that PAR2 activation may initiate neuropeptide release from sensory nerve fibres and hence contribute to inflammation.
PAR2 was localized by fluorescence immunohistochemistry to both the smooth muscle and epithelium of the ureter. Macrophage-like cells in the adventitia of the ureter were also PAR2-immunoreactive. PAR2-immunoreactivity was not observed in mast cells or nerve fibres.
In circular muscle preparations of the ureter in which continuous rhythmic beating was induced by KCl (20 mM) and the thromboxane A2 mimetic U46619 (0.3 μM), trypsin (0.3 U ml−1) reduced beat frequency to 84.6±2.0% of control rates. The PAR2-selective peptide agonist SLIGRL-NH2 concentration-dependently (0.1–3.0 μM) slowed beat frequency to a maximum of 72.7±2.0%.
Histamine (1–300 μM) was more efficacious than SLIGRL-NH2 in inhibiting ureter beat frequency in a concentration-dependent manner to a maximum (at 300 μM) of 7.9±2.5% of the control rate.
Pretreatment of preparations with capsaicin (10 μM for 30 min) markedly attenuated the inhibitory effect of histamine, but not that of SLIGRL-NH2, indicating a role for sensory nerves in the inhibitory effect of histamine only.
The inhibitory effect of SLIGRL-NH2 on ureter beat frequency was unaffected by the nitric oxide (NO) synthase inhibitor, L-NOARG (100 μM) or the cyclo-oxygenase inhibitor, indomethacin (3 μM).
In conclusion, PAR2 activation causes inhibition of beating in the mouse ureter that is not mediated by axon reflex release of inhibitory neuropeptides. This inhibitory effect of PAR2 appears to be mediated directly on smooth muscle cells, although the contribution of non-NO, non-prostanoid epithelium-derived factors cannot be ruled out.
Keywords: Protease-activated receptor-2 (PAR2), ureter, mast cell, histamine, sensory nerve fibres
Introduction
Protease-activated receptors (PARs) are G-protein coupled receptors which require enzymatic cleavage of an extended N-terminal domain before a downstream ‘tethered ligand' can bind intramolecularly to initiate receptor activation (Déry et al., 1998; Böhm et al., 1998). This novel mode of self-activation allows PARs to act as sensors for serine proteases such as thrombin, trypsin and other tryptic enzymes. PARs can also be specifically activated by synthetic peptides mimicking the tethered ligand sequence of the receptor subtype concerned (Déry et al., 1998; Böhm et al., 1998). Four PARs have been cloned to date and are characterized by their sensitivity to activation by thrombin and trypsin. Thus, PAR1 and PAR3 are activated by thrombin, whilst PAR4 is activated by both thrombin and trypsin (Déry et al., 1998; Böhm et al., 1998). PAR2 is unique in that this receptor is only activated by trypsin and similar enzymes such as mast cell tryptase and coagulation Factor Xa (Déry et al., 1998; Böhm et al., 1998; Fox et al., 1997). Since the tissue distribution of PAR2 expression is not limited to the gastrointestinal system (D'Andrea et al., 1998; Molino et al., 1998), it has been suggested that PAR2 may be activated by mast cell tryptase, rather than pancreatic trypsin, and hence participate in inflammatory reactions (Déry et al., 1998; Böhm et al., 1998).
Some sensory nerve fibres with conduction velocities in the C-fibre range are thought to participate in inflammatory reactions by conducting antidromic action potentials along collateral fibres (axon reflexes) which subsequently release neuropeptide transmitters such as substance P and calcitonin gene-related protein (CGRP) (Lundberg, 1995; 1996). Therefore, these nerve fibres not only transduce visceral pain, but also respond and contribute to localized tissue responses to insult or injury. Some of the potent endogenous substances which can activate these sensory nerve fibres are mast cell mediators such as histamine and prostaglandins as well as other inflammatory mediators such as bradykinin (Lundberg, 1995; 1996). The subsequent release of inflammatory neuropeptides from these nerves is thought to participate in local vasodilatation and plasma extravasation as well as immune cell recruitment and activation, leading to progressive inflammation (Lundberg, 1996; Maggi, 1997). We reasoned that since release of tryptase from mast cells occurs during inflammatory reactions (Nadel, 1991), then tryptase, like other inflammatory mediators, might also activate C-fibre axon reflexes via PAR2 and thus contribute to inflammation.
The smooth muscle of ureters is densely innervated by capsaicin-sensitive sensory fibres which mediate inhibitory effects on rhythmic contractions via axonal reflex release of CGRP (Santicioli & Maggi, 1998). Therefore, we used this assay of sensory nerve activation in mouse isolated ureters to test our hypothesis that PAR2 may contribute to neurogenic inflammation.
Methods
Tissue preparation
Male BALB/c mice (20–25 g) were killed by cervical dislocation and the left and right ureters excised and placed in a Krebs solution (composition (mM): Na+ 143.1, K+ 5.9, Ca2+ 2.5, Mg2+ 1.2, Cl− 127.8, HCO3− 25.0, SO42− 1.2, H2PO4− 1.2 and glucose 11.0) at room temperature. Two preparations (2 mm in length) from the middle portion of each ureter were mounted on 40 μm stainless steel wires in small vessel myographs (J.P. Trading, Denmark) for isometric recording of circular smooth muscle activity. After a 30 min equilibration period at 37°C, the preparations were stretched to a passive tension of 2.5 mN and allowed to further equilibrate for 30 min. During this time, baseline tension gradually stabilized at approximately 1 mN. Because ureters do not beat spontaneously without artificial stimulation of latent pacemakers (Santicioli & Maggi, 1998), we developed the following procedure to initiate beating. In some preparations, latent pacemaking activity could be initiated by adding 20 mM KCl to the bathing medium; if no beating was initiated 0.3 μM of the thromboxane mimetic U46619 was added. We found that this procedure universally produced rhythmic contractions, the beat frequency of which was stable for at least 20 min.
Experimental design
After the induced beating rate had stabilized (5 min), SLIGRL-NH2, trypsin or histamine were added either cumulatively or as single concentrations (see Results). The influence of endogenous nitric oxide (NO) and prostaglandins was examined in preparations exposed to the NO synthase inhibitor L-NOARG (100 μM) or the cyclo-oxygenase inhibitor indomethacin (3 μM) for 30 min prior to the activation of pacemaking activity.
C-fibre mediator depletion
In order to deplete C-fibre sensory nerves of neuropeptides, some preparations were exposed to capsaicin (10 μM) for 30 min. The preparations were then thoroughly washed prior to the initiation of beating.
Immunohistochemistry
Freshly isolated ureters were fixed overnight in 4% paraformaldehyde in phosphate buffer at 4°C. After repeated washing in phosphate buffered saline (PBS), segments of ureter were either processed for paraffin embedding and subsequently sectioned (3 μm) or mounted on slides as wholemount preparations. Wholemounts or dewaxed sections were washed in PBS and exposed to a rabbit antiserum (RAB 9717; a generous gift of Professor Nigel Bunnett), directed against the carboxyl-terminal of PAR2 for 36 h at room temperature. After several washes in PBS, the sections or wholemounts were exposed to a biotinylated donkey anti-rabbit antiserum (Amersham) and subsequently labelled with a streptavidin-FITC complex (Amersham). Preparations were viewed under epifluorescence using a Zeiss Axioskop microscope and photographed on Kodak Ectachrome T160 slide film. Some wholemount preparations were subsequently stained with 0.03% toluidine blue for 10 min to stain adventitial mast cells and observed with conventional bright-field optics.
Drugs and their sources
Capsaicin (8-methyl-N-vanillyl-6-nonenamide), histamine dihydrochloride, L-NOARG (NG-nitro-L-arginine) and indomethacin were purchased from Sigma. The following other drugs were used: trypsin (high purity; Worthington Biochemical, NJ, U.S.A.), SLIGRL-NH2 (single letter amino acid code; custom synthesis by Auspep, Parkville, Australia) and U46619 (9-dideoxy-9α-methano-epoxy-prostaglandin F2α; Sapphire Bioscience, Sydney, Australia).
Data analysis
Responses are expressed as percentages (mean±s.e.mean) of the initial rate of beating. Comparisons between control and treatment groups were made using unpaired Student's t-tests; P<0.05 was accepted as significant.
Results
Immunohistochemistry
In transverse sections of ureter, strong PAR2-immunoreactivity was observed in the epithelium, whilst appreciably less immunoreactivity was observed in the muscle layers (Figure 1a). In cells in both layers intense punctate perinuclear staining was observed. No PAR2 immunoreactive nerve fibres were observed in sections or wholemount preparations, although PAR2-immunoreactive cells in the adventitia of the ureter were observed in wholemounts (Figure 1b). Subsequent toluidine blue staining of the same wholemount preparations revealed that these cells were not mast cells (not shown) and we suggest that they are macrophages based on their size and nuclear morphology. No specific immunoreactivity was observed in any cell type in experiments in which the primary antibody had been preabsorbed with 10 μM of the immunizing peptide.
Figure 1.

Immunohistochemical localization of PAR2 in the mouse ureter. (a) Transverse section (3 μm) of a ureter. Epithelial cells (arrows) are intensely stained, while smooth muscle cells contain less widespread, punctate fluorescence (arrowheads). Scale bar represents 10 μm. (b) Whole mount preparation of a ureter showing intense PAR2-immunoreactivity of macrophages (arrows) in the adventia. Asterisks indicate adipocytes. Scale bar represents 50 μm.
Effect of histamine, trypsin and SLIGRL-NH2 on beat frequency
The combination of KCl (20 mM) and U46619 (0.3 μM) initiated beating of mouse ureters with a mean beat frequency of 4.6±0.4 beats min−1 (n=45). Histamine (1–300 μM), trypsin (0.3 U ml−1) and the PAR2-activating peptide SLIGRL-NH2 (0.1–10 μM) each caused slowing of the induced beating of mouse ureters (Figure 2). Histamine showed the greatest efficacy, reducing beat frequency to 7.9±2.5% (n=5) of control rates at the highest concentration used (300 μM), while SLIGRL-NH2 (3 μM) had a maximum effect of reducing beat frequency to 72.7±2.0% (n=20). Trypsin, at a maximum effective concentration of 0.3 U ml−1, was less efficacious than SLIGRL-NH2 only reducing responses to 84.6±2.0% (n=5). The cumulative concentration-response curve for SLIGRL-NH2 tended to be biphasic with the highest concentration used (10 μM) showing a reversal of the inhibitory effect on beat frequency.
Figure 2.
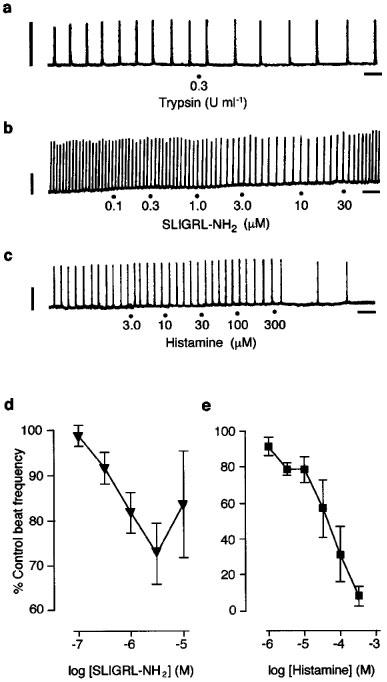
Effect of PAR2 activators and histamine on induced beating in the mouse isolated ureter. (a, b, c) Representative original traces of the inhibitory effects of trypsin, SLIGRL-NH2 and histamine respectively on beat frequency. (d) Cumulative concentration-effect curve to SLIGRL-NH2. (n=9), (e) Cumulative concentration-effect curve to histamine (n=5). Horizontal scale bars indicate 1 min; vertical scale bars indicate 0.5 mN.
Effect of capsaicin pretreatment on responses to histamine, CGRP, trypsin and SLIGRL-NH2
Addition of capsaicin (10 μM) abolished induced beating of mouse ureters (Figure 3a). In preparations which had been previously exposed to capsaicin for 30 min and subsequently washed, this inhibitory effect of capsaicin on induced beating was abolished (Figure 3a). Also, capsaicin pretreatment greatly attenuated the inhibitory effect of histamine (300 μM) on induced beating, but had no effect on the inhibitory response to SLIGRL-NH2 (3 μM; Figure 3b).
Figure 3.

(a) Representative traces of two preparations of a ureter from the same animal. In the control preparation (left panel), addition of capsaicin (3 μM; at dot) abolishes beating, whilst in the capsaicin pretreated preparation (right panel), addition of capsaicin has no effect. Horizontal scale bars indicate 1 min; vertical scale bars indicate 0.5 mN. (b) Group data illustrating the effect of capsaicin pretreatment on responses to SLIGRL-NH2 (3 μM) and histamine (300 μM). Asterisk indicates P<0.05 (n=5; unpaired t-test).
Lack of effect of NOARG and indomethacin on responses to SLIGRL-NH2
Pretreatment of the preparations with either L-NOARG (100 μM) or indomethacin (3 μM) had no effect on the inhibition of beat frequency by SLIGRL-NH2 (3 μM; Figure 4).
Figure 4.

Lack of effect of L-NOARG (100 μM) and indomethacin (3 μM) on the inhibition of beating in the mouse ureter induced by SLIGRL-NH2 (3 μM). Data from five preparations.
Discussion
This is the first study of effects of PAR2 activation on upper urinary tract smooth muscle. The results show that, unlike exogenous histamine, activation of PAR2 did not initiate axon reflex release of inhibitory neuropeptides from capsaicin-sensitive nerve fibres. However, PAR2 activation did inhibit the rate of beating in this preparation. Thus, while activation of PAR2 shares similarities with other inflammatory mediators in terms of smooth muscle function in the ureter, it is unlikely that the effects of PAR2 activation involve multicellular inflammatory pathways.
Whilst trypsin may activate PAR1 and PAR4 in addition to PAR2 (Vu et al., 1991; Xu et al., 1998), there is no evidence to suggest that the PAR2 selective peptide mimetic SLIGRL-NH2 activates other receptors apart from PAR2. However, the existence of PAR2 subtypes has been suggested by others on the basis of the relative efficacies of a number of synthetic peptide agonists (Saifeddine et al., 1998; Vergnolle et al., 1998). Since these agonists were not used in the present study, we cannot precisely define the receptor involved as the cloned PAR2 or a novel receptor subtype.
The conclusion that the inhibitory effect of PAR2 activation on beating of the ureter does not involve sensory nerve or mast cell activation is supported by our immunohistochemical data which shows that PAR2 are predominantly located on both epithelial and smooth muscle cells of the mouse ureter. No PAR2-immunoreactivity was observed on nerve fibres in this preparation, although some large cells in the adventitia stained positively for PAR2. These cells were unlikely to have been mast cells since they did not stain with the metachromatic dye toluidine blue. Based on their location and slightly lobular nuclear morphology, we believe that these PAR2-immunoreactive cells were most likely macrophages. Thus, we found no evidence in the mouse ureter to suggest that PAR2 are located on cell types likely to contribute to acute inflammatory responses. This was a surprising finding because mast cell tryptase, an activator of PAR2 (Déry et al., 1998; Böhm et al., 1998), is known to degranulate human mast cells (He et al., 1998). Furthermore, activation of PAR2 by SLIGRL-NH2 causes paw oedema after subplantar injection in rats via a partially mast cell-dependent pathway (Kawabata et al., 1998). Since the mast cell degranulating compound 48/80 had no effect on beat frequency in our hands (Moffatt & Cocks, unpublished observation), we consider it unlikely that mast cell-derived histamine could explain the inhibitory effect of PAR2 activation on beat frequency in the mouse ureter. An explanation for the difference between our findings and previous studies of mast cell-dependent effects of PAR2 activation (He et al., 1998; Kawabata et al., 1998) may be that phenotypic differences occur between mast cell populations in different tissues (Galli, 1997).
Although it is presently unclear which G-proteins PAR2 is coupled to, activation of this receptor is known to increase the intracellular generation of inositol 1,4,5-triphosphate and diacylglycerol and subsequently mobilize intracellular calcium stores (Böhm et al., 1998). Consequently, the expected effect of activation of smooth muscle PAR2 would be excitation, rather than inhibition of spontaneous activity or tone as reported in some vascular (Moffatt & Cocks, 1998) and gastrointestinal smooth muscle preparations (Saifeddine et al., 1996). However, in the vasculature (Moffatt & Cocks, 1998; Sobey & Cocks, 1998; Hwa et al., 1996; Saifeddine et al., 1996) and airways (Cocks et al., 1999a), PAR2 activation has a predominantly inhibitory effect on smooth muscle via paracrine release of inhibitory mediators such as NO and prostaglandins from the endothelium or epithelium. Since we observed intense PAR2-immunoreactivity in epithelial cells of the ureter, we considered the possibility that PAR2-mediated inhibition of beat frequency in this preparation is indirectly mediated by similar mechanisms. However, responses to SLIGRL-NH2 were unaffected by both the NO synthase inhibitor L-NOARG and the cyclo-oxygenase inhibitor indomethacin. While we were unable to mechanically remove the epithelium in these preparations without damaging the smooth muscle layer, the negative results with L-NOARG and indomethacin argue against a role for NO and prostanoids released not only from the epithelium but also from PAR2- immunoreactive macrophages. In a previous study of the effects of PAR1 and PAR2 activation in the mouse gastric fundus, we found that previously undescribed smooth muscle relaxant responses to activation of both receptors were similarly unaffected by L-NOARG and indomethacin (Cocks et al., 1999b). In that study, however, we found that the PAR-mediated relaxant responses were blocked by both the small conductance Ca2+-sensitive K+ channel (SKCa) inhibitor apamin and by inhibition of intracellular calcium store release with ryanodine. From these results we hypothesized that PAR-mediated relaxation of the mouse gastric fundus was due to focal increases in Ca2+, or ‘calcium sparks', which activated SKCa to cause membrane hyperpolarization and smooth muscle relaxation (Cocks et al., 1999b). Similar mechanisms may explain the PAR2-mediated inhibitory responses observed in the present study, as has been suggested for the inhibitory effects of CGRP on contractility in the isolated guinea-pig ureter (Maggi et al., 1996; Santicioli & Maggi, 1998).
In conclusion, activation of PAR2 by trypsin or SLIGRL-NH2 caused inhibition of beating in the mouse isolated ureter. The immunohistochemical data suggest that this effect is mediated either by an unknown epithelium-derived inhibitory factor or by a direct effect of smooth muscle PAR2. We found no evidence that PAR2 may mediate effects via mast cell- or sensory nerve-derived mediators. Therefore, in contrast to histamine, PAR2 is unlikely to activate complex inflammatory pathways in the mouse ureter. It remains to be determined, however, whether PAR2 play any role during inflammation of the upper urogenital tract.
Acknowledgments
This study was supported by the NHMRC of Australia and the Western Healthcare Network (Victoria). We gratefully acknowledge the generous gift of the anti-PAR2 antiserum by Professor Nigel Bunnett and the technical assistance of Ms Vitina Sozzi and Mrs Janet Rogers.
Abbreviations
- CGRP
calcitonin gene-related peptide
- L-NOARG
NG-nitro-L-arginine
- NO
nitric oxide
- PAR
protease-activated receptor
References
- BÖHM S.K., MCCONALOGUE K., KONG W., BUNNETT N.W. Proteinase-activated receptors: new functions for old enzymes. News Physiol. Sci. 1998;13:231–240. doi: 10.1152/physiologyonline.1998.13.5.231. [DOI] [PubMed] [Google Scholar]
- COCKS T.M., FONG B., CHOW J.M., ANDERSON G.P., FRAUMAN A.G., GOLDIE R.G., HENRY P.J., CARR M.J., HAMILTON J.R., MOFFATT J.D. A protective role for protease-activated receptors in the airways. Nature. 1999a;398:156–160. doi: 10.1038/18223. [DOI] [PubMed] [Google Scholar]
- COCKS T.M., SOZZI V., MOFFATT J.D., SELEMIDIS S. Protease-activated receptors mediate apamin-sensitive relaxation of mouse and guinea pig gastrointestinal smooth muscle. Gastroenterology. 1999b;116:586–592. doi: 10.1016/s0016-5085(99)70180-0. [DOI] [PubMed] [Google Scholar]
- D'ANDREA M.R., DERIAN C.K., LETURCQ D., BAKER S.M., BRUNMARK A., LING P., DARROW A.L., SANTULLI R.J., BRASS L.F., ANDRADE-GORDON P. Characterization of protease-activated receptor-2 immunoreactivity in normal human tissues. J. Histochem. Cytochem. 1998;46:157–164. doi: 10.1177/002215549804600204. [DOI] [PubMed] [Google Scholar]
- DÉRY O., CORVERA C.U., STEINHOFF M., BUNNETT N.W. Proteinase-activated receptors: novel mechanisms of signalling by serine proteases. Am. J. Physiol. 1998;274:C1429–C1542. doi: 10.1152/ajpcell.1998.274.6.C1429. [DOI] [PubMed] [Google Scholar]
- FOX M.T., HARRIOTT P., WALKER B., STONE S.R. Identification of potential activators of proteinase-activated receptor-2. FEBS Lett. 1997;417:267–269. doi: 10.1016/s0014-5793(97)01298-2. [DOI] [PubMed] [Google Scholar]
- GALLI S.J. The mast cell: a versatile effector cell for a challenging world. Int. Arch. Allergy Immunol. 1997;113:14–22. doi: 10.1159/000237497. [DOI] [PubMed] [Google Scholar]
- HE S., GAÇA M.D.A., WALLS A.F. A role for tryptase in the activation of human mast cells: modulation of histamine release by tryptase and inhibitors of tryptase. J. Pharmacol. Exper. Ther. 1998;286:289–297. [PubMed] [Google Scholar]
- HWA J.J., GHIBAUDI L., WILLIAMS P., CHINTALA M., ZHANG R., CHATTERJEE M., SYBERTZ E. Evidence for the presence of a proteinase-activated receptor distinct from the thrombin receptor in vascular endothelial cells. Circ. Res. 1996;78:581–588. doi: 10.1161/01.res.78.4.581. [DOI] [PubMed] [Google Scholar]
- KAWABATA A., KURODA R., MINAMI T., KATAOKA K., TANEDA M. Increased vascular permeability by a specific agonist of protease-activated receptor-2 in rat hindpaw. Br. J. Pharmacol. 1998;125:419–422. doi: 10.1038/sj.bjp.0702063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LUNDBERG J.M. Tachykinins, sensory nerves, and asthma–an overview. Can. J. Physiol. Pharmacol. 1995;73:908–914. doi: 10.1139/y95-125. [DOI] [PubMed] [Google Scholar]
- LUNDBERG J.M. Pharmacology of cotransmission in the autonomic nervous system: integrative aspects on amines, neuropeptides, adenosine triphosphate, amino acids and nitric oxide. Pharmacol. Rev. 1996;48:113–178. [PubMed] [Google Scholar]
- MAGGI C.A. The effects of tachykinins on inflammatory and immune cells. Regul. Pept. 1997;70:75–90. doi: 10.1016/s0167-0115(97)00029-3. [DOI] [PubMed] [Google Scholar]
- MAGGI C.A., GIULIANI S., SANTICIOLI P., BRADING A.F. Role of intracellular Ca2+ in the K+ channel opener action of CGRP in the guinea-pig ureter. Br. J. Pharmacol. 1996;118:1493–1503. doi: 10.1111/j.1476-5381.1996.tb15565.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MOFFATT J.D., COCKS T.M. Endothelium-dependent and -independent responses to protease-activated receptor-2 (PAR-2) activation in mouse isolated renal arteries. Br. J. Pharmacol. 1998;125:591–594. doi: 10.1038/sj.bjp.0702157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MOLINO M., RAGHUNATH P.N., KUO A., AHUJA M., HOXIE J.A., BRASS L.F., BARNATHAN E.S. Differential expression of functional protease-activated receptor-2 (PAR-2) in human vascular smooth muscle cells. Arterioscler. Thromb. Vasc. Biol. 1998;18:825–832. doi: 10.1161/01.atv.18.5.825. [DOI] [PubMed] [Google Scholar]
- NADEL J.A. Biology of mast cell tryptase and chymase. Ann. N.Y. Acad. Sci. 1991;629:319–331. doi: 10.1111/j.1749-6632.1991.tb37986.x. [DOI] [PubMed] [Google Scholar]
- SAIFEDDINE M., AL-ANI B., CHENG C.H., WANG L., HOLLENBERG M.D. Rat proteinase-activated receptor-2 (PAR-2): cDNA sequence and activity of receptor-derived peptides in gastric and vascular tissue. Br. J. Pharmacol. 1996;118:521–530. doi: 10.1111/j.1476-5381.1996.tb15433.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SAIFEDDINE M., ROY S.S., AL-ANI B., TRIGGLE C.R., HOLLENBERG M.D. Endothelium-dependent contractile actions of proteinase-activated receptor-2-activating peptides in human umbilical vein: release of a contracting factor via a novel receptor. Br. J. Pharmacol. 1998;125:1445–1454. doi: 10.1038/sj.bjp.0702213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SANTICIOLI P., MAGGI C.A. Myogenic and neurogenic factors in the control of pyeloureteral motility and ureteral peristalsis. Pharmacol. Rev. 1998;50:683–721. [PubMed] [Google Scholar]
- SOBEY C.G., COCKS T.M. Activation of protease-activated receptor-2 (PAR-2) elicits nitric oxide-dependent dilatation of the basilar artery in vivo. Stroke. 1998;29:1439–1444. doi: 10.1161/01.str.29.7.1439. [DOI] [PubMed] [Google Scholar]
- VERGNOLLE N., MACNAUGHTON W.K., AL-ANI B., SAIFEDDINE M., WALLACE J.L., HOLLENBERG M.D. Proteinase-activated receptor-2 (PAR2)-activating peptides: identification of a receptor distinct from PAR2 that regulates intestinal transport. Proc. Natl. Acad. Sci. U.S.A. 1998;95:7766–7771. doi: 10.1073/pnas.95.13.7766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VU T.K., HUNG D.T., WHEATON V.I., COUGHLIN S.R. Molecular cloning of a functional thrombin receptor reveals a novel proteolytic mechanism of receptor activation. Cell. 1991;64:1057–1068. doi: 10.1016/0092-8674(91)90261-v. [DOI] [PubMed] [Google Scholar]
- XU W.F., ANDERSEN H., WHITMORE T.E., PRESNELL S.R., YEE D.P., CHING A., GILBERT T., DAVIE E.W., FOSTER D.C. Cloning and characterization of human protease-activated receptor 4. Proc. Natl. Acad. Sci. U.S.A. 1998;95:6642–6646. doi: 10.1073/pnas.95.12.6642. [DOI] [PMC free article] [PubMed] [Google Scholar]


