Abstract
The VPAC2 and PAC1 receptors are closely related members of the Group II G protein-coupled receptor family. At the VPAC2 receptor, VIP is equipotent to PACAP-38 in stimulating cyclic AMP production, whereas at the PAC1 receptor PACAP-38 is many fold more potent than VIP. In this study, domains which confer this selectivity were investigated by constructing four chimaeric receptors in which segments of the VPAC2 receptor were exchanged with the corresponding segment from the PAC1 receptor.
When expressed in COS 7 cells all the chimaeric receptors bound the common ligand [125I]PACAP-27 and produced cyclic AMP in response to agonists.
Relative selectivity for agonists was determined primarily by the amino terminal extracellular domain of the PAC1 receptor and the VPAC2 receptor. The interchange of other domains had little effect on the potency of PACAP-38 or PACAP-27.
For chimaeric constructs with a PAC1 receptor amino terminal domain, the substitution of increasing portions of the VPAC2 receptor decreased the potency of VIP yet increased that of helodermin.
This suggests that the interaction of VIP/helodermin but not PACAP with the PAC1 receptor may be influenced (and differentially so) by additional receptor domains.
Keywords: VIP, PACAP, helodermin, receptor, chimaeric construct, cyclic AMP
Introduction
Vasoactive intestinal peptide (VIP) and pituitary adenylate cyclase activating polypeptide (PACAP) are structurally similar neuropeptides, with 68% conservation of amino acids. Receptors for VIP (which also recognise PACAP with equal affinity) are found in both the CNS and periphery (Arimura, 1992), and may mediate the peripheral actions of PACAP as well as VIP. High affinity PACAP receptors (where VIP is many fold less potent than PACAP in evoking receptor-mediated responses) are present mainly in the CNS, but also in the adrenal medulla (Arimura, 1992). Two genes encoding VIP/PACAP receptors have been cloned; formerly known as the VIP1 receptor (Ishihara et al., 1992), and the VIP2 receptor (Lutz et al., 1993). The gene encoding the PACAP selective receptor has been cloned by several laboratories (Hashimoto et al., 1993; Hosoya et al., 1993; Morrow et al., 1993; Pisegna & Wank, 1993; Spengler et al., 1993; Svoboda et al., 1993) and has been shown to undergo differential splicing, for instance five splice variants in the region encoding intracellular loop three have been reported (Spengler et al., 1993). Recently, a new nomenclature has been approved for these receptors, the VPAC1, the VPAC2 and the PAC1 receptor respectively (Harmar et al., 1998).
The VPAC2 and PAC1 receptor are 50% identical at the amino acid level, and have 60% identity within the transmembrane spanning domains (the receptor trunk). These receptors belong to the secretin (Group II) G protein-coupled receptor family (Segre & Goldring, 1993), which does not have the consensus amino acid motifs which have been defined for the rhodopsin/β-adrenergic (Group I) G protein-coupled receptor family (Wess, 1997). Ligands for the secretin receptor family are all relatively large peptide hormones (⩾27 amino acids); all members couple to stimulation of adenylyl cyclase (AC), apparently through the heterotrimeric G protein Gs; many also couple to phospholipase C (PLC) stimulation through the Gq family (Hezareh et al., 1996; Offermanns et al., 1996). These receptors are highly conserved at the amino acid level (Segre & Goldring, 1993) suggesting that there may be common principles to the molecular mechanisms by which these receptors transduce agonist signals from the extracellular surface to the intracellular second messenger systems. It is likely that these receptors have evolved from a common ancestral gene, with strong selection pressure to maintain certain key molecular features which are necessary for this mechanism, but diverging in ligand specificity.
We have found marked functional differences in respect to ligand selectivity, levels of expression and second messenger coupling for the VPAC2 and PAC1 receptors when transiently expressed in COS 7 cells. The VPAC2 and PAC1 receptors not only stimulate AC (Lutz et al., 1993; Morrow et al., 1993) but couple to other signal transduction pathways as well (MacKenzie et al., 1996; McCulloch et al., 1995; Spengler et al., 1993). In the present study we have begun to characterize receptor domains which confer the characteristics of agonist recognition for the PAC1 and the VPAC2 receptors by exchanging equivalent regions between the two types of receptor (see Figure 1), with divisions at the extracellular boundary of transmembrane region 1 (tm1) and within transmembrane region 5 (tm5). Chimaeric receptors were designated according to a scheme describing amino N-terminal portion/junction site/Carboxyl C-terminal portion (for example, the construct consisting of NH2-PAC1 receptor/junction site in TM5/VPAC2–COOH was designated P5V). Four chimaeric receptors thus were constructed between the rat VPAC2 and the rat PAC1 receptor. Wild-type and chimaeric receptors were transiently expressed in COS 7 cells. Levels of expression at the plasma membrane were determined by whole cell ligand binding. The ability of PACAP-38, PACAP-27, VIP and helodermin to activate cyclic AMP production at each receptor was then compared. All chimaeric receptors were functionally expressed at levels lower than those found for the PAC1 receptor, but were 2–5 fold higher than that of the VPAC2 receptor. Some of these results have been published in preliminary form (Lutz et al., 1994; 1996).
Figure 1.
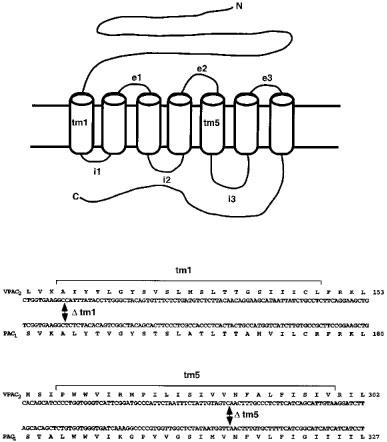
Exchange sites for construction of the tm1 and tm5 VPAC2/PAC1 chimaeric receptors. Schematic diagram of the seven transmembrane-spanning receptor. Junction sites for making the chimaeric receptor constructs are shown below and are labelled with the transmembrane domain in which they occur.
Methods
Drugs and chemicals
Tissue culture media were obtained from Life Technologies, Paisley, UK; DEAE dextran was from Pharmacia Biotech Ltd., St Albans, UK. [125I]PACAP-27 (2200 Cimmol−1) and [125I]VIP (2200 Cimmol−1) were obtained from DuPont (UK) Ltd, Stevenage, UK. Peptides were supplied by Calbiochem-Novabiochem (UK) Ltd, Nottingham, UK. Standard laboratory chemicals of Analar grade were obtained from Sigma-Aldrich Chemical Company, Poole, UK. Oligonucleotide primers were synthesized by Cruachem Ltd, Glasgow, UK or Oswel DNA Service, Southampton, UK.
Construction of chimaeric receptors
Chimaeric receptors were made by exchanging the equivalent regions between the VPAC2 receptor and the short intracellular loop 3 (i3) splice variant of the PAC1 receptor, at exchange sites within tm1 and tm5 (Figure 1). The tm5 chimaeric receptors V5P: VPAC2(1–294)PAC1(319–467) and P5V: PAC1(1–318)VPAC2(295–437) were made by utilizing a conserved restriction (HincII) site in the region encoding the fifth transmembrane region within the VPAC2 and PAC1 receptor cDNA sequences. The N-terminal V1P: VPAC2 (1–127)PAC1(155–467) and P1V: PAC1(1–154)VPAC2(128–437) chimaeric receptors were made by overlap extension polymerase chain reaction (PCR) mutagenesis (Huang et al., 1995). For each junction site a set of four oligonucleotide primers was used, two external primers derived from flanking sequences of the vector and two internal primers one of which (the overlap primer) contained sequences derived from both receptor encoding cDNAs and which spanned the junction site of the chimaeric construct [PAC1(155–467): 5′-CG TTTT ATAT TCTG GTGA AG GCTC TCTA CACAG TC; VPAC2 (128–437) 5′-GAT TATT ACTA CCT GTC GGT GAAGG CCATT TATACC TTG G). The second internal primer (cDNA specific primer) contained sequences complementary to the 5′ end of the overlap primer and corresponding to the cDNA encoding the receptor portion 5′ of the junction site [VPAC2 (1–127): 5′-CA CCA GA ATAT AAA ACGTG ATCTT AC); PAC1 (1–154); 5′-CACCGACAGGTAGTAATAATCCTG). In the first round of PCR amplification the 5′ region encoding the N-terminal end of the chimaeric receptor was amplified with the cDNA specific primer along with the corresponding flanking external primer while the 3′ region encoding the C-terminal end was amplified with the overlap primer and corresponding flanking external primer. PCR reactions were set up in 100 μl volumes containing 15 ng cDNA, 15 pmol of 5′ and 3′ primer, in PCR buffer with 2 mM MgCl2, 100 nM dNTPs and 10% DMSO and overlaid with mineral oil. The reaction was heated to 95°C for 5 min, then maintained at 80°C while adding 2.5U Pfu polymerase (Stratagene), after which the reaction was put through 30 cycles with denaturing at 94°C (1 min), annealing at 57°C (1 min) and extension at 72°C (3 min). After the first round of PCR, 10 μl samples were analysed by electrophoresis. The remaining PCR reactions were purified by extracting with Wizard cDNA purification system (Promega), then in the second round of PRC amplification 1 μl of each appropriate extract were mixed and amplified using the flanking pBluescript primers under the same conditions as the first round of amplification. The polymerase enzyme was removed by Wizard cDNA purification system and the reaction digested with either EcoRI or EcoRI+XhoI, then run on agarose gels for size selection. These were ligated into pBluescript for selection of appropriate clones by sequence analysis, then inserted into the expression vector pcDNA 1 (InVitrogen, R&D Systems Europe Ltd., Abingdon, UK) for functional expression in COS 7 cells.
Transfection of COS cells
COS 7 cells were transfected using DEAE dextran as described previously (Morrow et al., 1993) and allowed to recover for 24 h. Transfected cells were then trypsinized and plated into 12 well plates (for whole cell binding) or 24 well plates (for cyclic AMP assays). Assays were performed 48 h after plating.
Receptor binding assay
Transfected cells in 12 well plates were washed twice and incubated at 0°C for 1 h in ice-cold medium 199 containing 0.2% BSA with [125I]PACAP-27 (14,000 c.p.m. per well) in the absence or presence of increasing concentrations of unlabelled PACAP-27 or with [125I]VIP (30,000 c.p.m. per well) in the absence or presence of increasing concentrations of unlabelled VIP. Non-specific binding was defined with 300 nM PACAP-27 or 3 μM VIP respectively as preliminary experiments showed that higher concentrations began to displace ligand binding non-specifically from COS 7 cells transfected with empty vector. Unbound radioactivity was removed by washing cells three times with Earle's Balanced Salt solution (EBSS) containing 0.1% BSA. Bound radioactivity was removed by acid wash (0.2 M acetic acid, 0.5 M NaCl) for 5 min on ice, and measured by γ-counting.
Cyclic AMP formation assay
Transfected cells were treated essentially as described previously (Morrow et al., 1993). Forty-eight hours after trypsinisation cells were washed twice in MEM containing 0.25% BSA, and preincubated at 37°C for 15 min in the presence of 0.5 mM isobutyl methylxanthine (IBMX). Peptides were directly added, and incubations were continued at 37°C for 5 min. The reaction was stopped by adding an equal volume of ice-cold 0.2 M HCl and frozen. Cyclic AMP levels were measured by radioimmunosassay with antibodies to cyclic AMP kindly provided by Dr Ian Gow, Department of Physiology, University of Edinburgh, UK.
Data analysis
Curve fitting and standard error calculation was performed using a non-linear regression program, P-fit (Elsevier Biosoft, Cambridge, UK).
Results
Cell surface expression of wild-type and chimaeric receptors in COS 7 cells
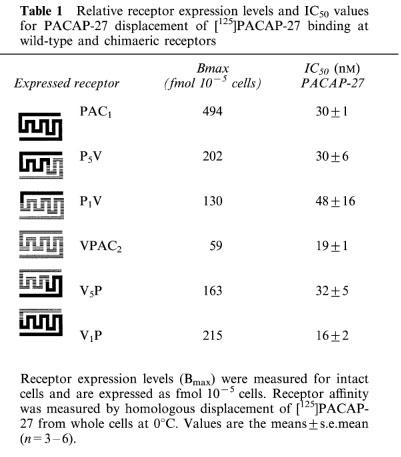
To determine cell surface receptor levels and binding characteristics for the wild-type and chimaeric VPAC2 and PAC1 receptors, Bmax and IC50 values were measured by homologous displacement of [125I]PACAP-27 binding (at 0°C) to whole cells expressing VPAC2 or PAC1 receptors (Table 1). Ligand affinities measured by this in vivo method for cell surface binding are routinely lower than those found in vitro membrane binding (presumably as a result of different assay constituents and conditions). Nevertheless, this approach gives information on cell surface expression of receptors, rather than the entire cellular complement, and allows direct internal comparisons between the different constructs in this study. Receptor expression levels monitored in this way varied between 59 fmol10−5 cells (the VPAC2 receptor) and 494 fmol10−5 cells (the PAC1 receptor). The chimaeric receptors displayed levels 130–215 fmol10−5 cells (between 26 and 44% of the levels found for the PAC1 receptor). Table 1 also shows the IC50 values for PACAP 27 displacement of [125I]PACAP-27 binding in whole cells expressing wild-type and chimaeric receptors. Values for wild-type receptors were 19±1 nM for VPAC2 and 30±1 nM for PAC1. The affinity of wild-type receptors and chimaeric constructs for PACAP-27 was very similar in all cases with IC50 values at the chimaeric receptors differing by less than 2 fold from their corresponding wild-type controls (Table 1). This indicates, in general terms, that the ability of the chimaeras to recognise an appropriate agonist ligand is not grossly perturbed by the presence of exchanged domains. Both the best fit slope values from curve fitting (ranging from 0.81–1.22) and Scatchard-type plots of the data gave no cause to suggest the presence of multiple components in [125I]PACAP-27 binding under these conditions. Pilot experiments were carried out using [125I]VIP as a ligand in a similar protocol. Binding that was displaceable with high affinity by unlabelled VIP was observed in each case, but the computed Bmax values varied considerably from those obtained with the broad specificity ligand [125I]PACAP-27 probably as a result of the heterogeneous affinity of [125I]VIP for VPAC2/PAC1 receptors. Since results would not be directly comparable with those obtained using [125I]PACAP-27 (Table 1), these studies were not pursued any further. It was possible to confirm however that [125I]VIP can label, with relatively high affinity (39±9 nM), a subpopulation of the PAC1 receptors identified by [125I]PACAP-27 binding (approximately 44% in our hands compared to 32% in the previous report of Hashimoto et al., (1993). The nature of this subpopulation is unclear.
Table 1.
Relative receptor expression levels and IC50 values for PACAP-27 displacement of [125]PACAP-27 binding at wild-type and chimaeric receptors
Agonist activation of cyclic AMP production mediated by wild-type and chimaeric receptors
We have previously shown that the wild-type VPAC2 and PAC1 receptors expressed in COS 7 cells show clear differences in agonist specificity. VIP and PACAP-38 are equipotent in stimulating cyclic AMP production at the VPAC2 receptor (Lutz et al., 1993), whereas VIP is 50 fold less potent than PACAP-38 at the PAC1 receptor (Morrow et al., 1993). In order to determine which receptor domains were involved in agonist recognition, transiently-transfected COS 7 cells were stimulated with VIP, PACAP-38, PACAP-27 and helodermin before cyclic AMP levels were measured (Table 2). All peptides caused a concentration-dependent increase in cyclic AMP levels in COS 7 cells transfected with the wild-type and chimaeric receptors. As predicted from the [125I]PACAP-27 binding data, both PACAP-27 and PACAP-38 showed very similar EC50 values for cyclic AMP production at wild-type PAC1 and VPAC2 receptors and at all the chimaeric constructs (Table 2). This confirms that viable coupling to second messenger production is not in itself impaired by the presence of non-matching domains in the construct. Responses to VIP and helodermin however showed a number of differences between the receptors. The most striking difference correlated closely with the presence of particular N-terminal domains. All receptors with the VPAC2 N-terminal domain (wild-type VPAC2, V5P and V1P) displayed high affinities for VIP and helodermin at sub-nM concentrations similar to those shown for PACAP-38 and PACAP-27. All receptors with the PAC1 N-terminal domain (wild-type PAC1, P5V and P1V) showed lower affinities for VIP and helodermin. The clear segregation of pharmacological characteristics according to the presence of VPAC2 or PAC1 receptor N-terminal domain strongly suggests that this domain acts as the primary determinant of agonist recognition in these receptors. In general terms, VPAC2-like or PAC1-like characteristics are conferred by the presence of a VPAC2- or PAC1-receptor N-terminal domain respectively.
Table 2.
EC50 values for PACAP-38, PACAP-27, VIP and helodermin-stimulated cAMP production at wild-type and chimaeric receptors
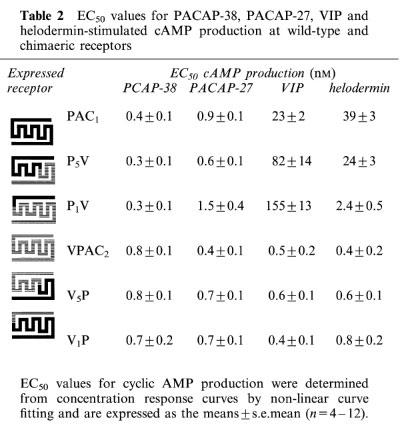
However, the VPAC2 and PAC1 receptors clearly differ in the extent to which additional receptor elements affect agonist potency. The potency of VIP and helodermin at receptors with the VPAC2 N-terminal domain was unaltered (no more than 2 fold changes) by replacement of the tm5–C-terminal domain (in V5P) or the tm1–C-terminal domain (in V1P). In receptors with the PAC1 N-terminal domain however, the progressive replacement of tm5–C-terminal (in P5V) and tm1–C-terminal (in P1V) with corresponding VPAC2 receptor domains led to a decline in VIP potency by 3.6 fold and 6.7 fold respectively.
In contrast, the potency of helodermin was increased when the tm1–C-terminal segment of the PAC1 receptor were replaced with homologous VPAC2 receptor domains (in P5V and P1V). In the case of the tm5–C-terminal substitution the effect was marginal (1.6 fold) but when the tm1–tm5 segments were additionally replaced, a very marked (16 fold) increase in helodermin potency was apparent. The concentration-response data for the key shifts in potency of VIP and helodermin (but not PACAP-38) between wild-type PAC1 receptors and the P1V chimaeric construct are shown in Figure 2. There was no evidence that any agonist at any receptor investigated produced a maximal response less than that of PACAP-38.
Figure 2.
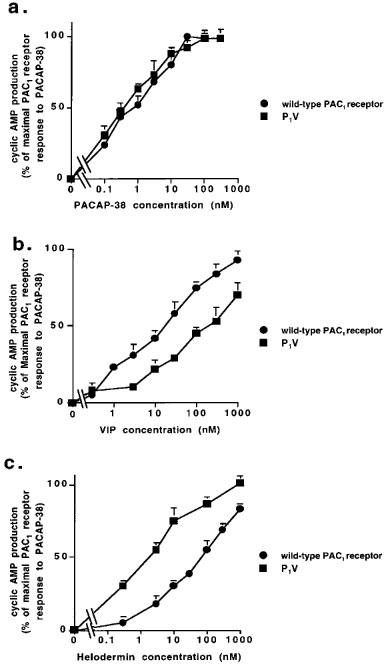
Comparison of agonist-evoked cyclic AMP responses at the wild-type PAC1 receptor and the P1V chimaeric receptor construct. COS 7 cells transiently expressing the wild-type PAC1 receptor or the P1V chimaeric construct were preincubated with IBMX for 15 min before stimulation for 5 min with agonists: (a) PACAP-38; (b) VIP; (c) helodermin. All values are means ±s.e.mean (n=4–12). Error bars not shown fall within the dimensions of the symbol.
Discussion
The present data accord closely with the general idea that the main determinant of agonist response pharmacology at the VPAC2 and PAC1 receptors is within the N-terminal extracellular domain of the receptors. This broadly matches conclusions on the influence of the PAC1 receptor N-terminal domain derived from experiments with PAC1/VPAC1 receptor chimaeric constructs (Hashimoto et al., 1997; Van Rampelbergh et al., 1996). This issue has not been previously addressed in the VPAC2 receptor, but chimaeric and mutant constructs of the VPAC1 receptor suggest that elements in the N-terminal domain are also key determinants of its agonist selectivity (Couvineau et al., 1995; Hashimoto et al., 1997; Holtmann et al., 1995; Van Rampelbergh et al., 1996).
There is increasing evidence that the effective potency of agonists at some receptors in the family is not always a simple reflection of docking affinity (Hjorth et al., 1996; Stroop et al., 1995). For example, it has been recently demonstrated that VIP binds with high affinity to the rat secretin (Holtmann et al., 1996) and PAC1 receptors (Hashimoto et al., 1997), although it has much lower potency in activating cyclic AMP production at these receptors. Similarly, a report on a chimaeric construct between the N-terminal domain of the PAC1 receptor and the body of the VPAC1 receptor described its high affinity binding of [125I]VIP although binding to the wild-type PAC1 receptor was not noted (Van Rampelbergh et al., 1996). This suggests the idea that some agonists, for example VIP at the PAC1 receptor, may bind strongly (at least to a subpopulation of sites) but display a reduced molecular efficiency in causing the conformational changes that elicit signal transduction. Together with results from the literature, our data suggest that at the VPAC2 receptor, PACAP-38, PACAP-27 and VIP dock strongly and efficiently activate cyclic AMP production. In contrast at the PAC1 receptor, while PACAP-38 and PACAP-27 both dock and activate strongly, VIP appears to show rather low potency of cyclic AMP production (25 fold less than PACAP-27) despite labelling almost half of the PAC1 receptors identified by [125I]PACAP-27 binding with a very similar affinity (39±9 nM for VIP; 30±1 μM for PACAP-27). This is consistent with the hypothesis that VIP may show a reduced activation efficiency at the PAC1 receptor compared to PACAP.
Chimaeric VPAC1/PAC1 receptor studies have shown that the VPAC1 receptor contains auxiliary sites within the extracellular loops (el–3) of the receptor trunk which facilitate VIP activation of cyclic AMP production (Hashimoto et al., 1997; Van Rampelbergh et al., 1997). Unlike chimaeric VPAC1/secretin receptors where the N-terminal domain was largely sufficient to confer VPAC1-like pharmacology to the secretin receptor trunk (Holtmann et al., 1995; 1996), chimaeric VPAC1/PAC1 receptors also required a segment encompassing e3 (Hashimoto et al., 1997). When either the VPAC1 N-terminal–tm1 segment or the e3 segment (tm5–tm7) was inserted into the corresponding region of the PAC1 receptor, VIP potency in activating cyclic AMP production was increased 21 or 16 fold respectively, compared to wild-type PAC1 receptors (Hashimoto et al., 1997). The combination of both regions caused an additional 11 fold increment in VIP potency. Conversely the replacement of segments containing e1 and then e2 plus e3 domains in the VPAC1 receptor with those of the PAC1 receptor, progressively reduced the ability of VIP to activate cyclic AMP production for chimaeric VPAC1/PAC1 receptors compared to the wild-type VPAC1 receptor (Hashimoto et al., 1997). Site-directed mutagenesis of the VPAC1 receptor has also indicated that, in addition to elements within the N-terminal domain, sites within e1 and tm3 are important in ligand recognition and selectivity (Couvineau et al., 1996; Du et al., 1997). Differences in the structure–function relationships of agonist recognition by VPAC1 and VPAC2 receptors have recently been emphasised in experiments to mutate corresponding residues in the two receptors (Nicole et al., 1998). In the present study with VPAC2/PAC1 chimaeric constructs, we found no evidence that the ability of the VPAC2 receptor to respond to PACAP-38, PACAP-27, VIP or helodermin was reduced by the replacement of tm5–C-terminal or tm1–C-terminal segments by equivalent domains of the PAC1 receptor. This could mean either that agonist recognition and activation of the cyclic AMP production response by the VPAC2 receptor is little influenced by sequence motifs outwith the N-terminal domain, or that any requisite motifs were perfectly replaced by the homologous domains of the PAC1 receptor.
Although receptors with a VPAC2 N-terminal domain were insensitive to changes in other regions of the receptor, the same was not true of those with a PAC1 N-terminal domain. For these receptors, recognition and action of PACAP-38 and PACAP-27 was unaltered by substitutions from tm1 to the C-terminal, indicating that the PAC1 N-terminal domain alone is fully sufficient for recognition of PACAP-38 and PACAP-27 and their activation of cyclic AMP production. It is possible that the VPAC2 receptor sequences fully replace the requisite motifs in the non-N-terminal extracellular domains. The ligands VIP and helodermin however were differentially recognised by wild-type and chimaeric PAC1-N-terminal receptors. The reduction in [125I]VIP binding affinity and in VIP potency of cyclic AMP production as tm5–C-terminal and tm1–C-terminal of the PAC1 receptor were progressively replaced with VPAC2 sequences suggests that recognition of VIP by the PAC1 receptor (and consequent cyclic AMP responses) optimally requires elements in the tm1–C-terminal segment as well as the N-terminal domain. The presence of equivalent domains substituted from the VPAC2 receptor does not enhance VIP potency even though the wild-type VPAC2 receptor is potently activated by VIP. This indicates that the substitutions do not make the chimaeric constructs more VPAC2-like but instead remove the supportive influence provided by tm1–C-terminal tail segment in the PACAP receptor. The lack of alteration in PACAP-27 and PACAP-38 recognition by the same chimaerics shows that a generalized disruption of recognition and function is not the reason for reduced VIP potency in the receptors. Although directly comparable constructs were not made in the study of chimaeric VPAC1/PAC1 receptors (Hashimoto et al., 1997) PAC1 receptor constructs with the tm3–tm5 segment and tm5–tm7 segment of the VPAC1 receptor showed 1.6 fold and 16 fold increases in VIP potency at cyclic AMP in the face of minimal changes in [125I]VIP binding affinity. Thus, while substitutions of VPAC1 receptor sequences in these segments can enhance VIP action but not docking, substitution of VPAC2 receptor sequences results in reduced binding affinity for VIP and corresponding reduced potency in cyclic AMP production. This implies that the tm1–C-terminal tail segments of VPAC1 and VPAC2 receptors make very different contributions to the recognition of VIP (at least in the context of a PAC1 receptor N-terminal domain).
A different mode of action is revealed in the same receptors by the use of helodermin as agonist rather than VIP or PACAP. In this case, substitution of the tm5–C-terminal and tm1–C-terminal segments of the PAC1 receptor by equivalent VPAC2 receptor domains lead to increased potency of helodermin in cyclic AMP production. This might suggest that elements in the tm1–C-terminal segment of the VPAC2 receptor facilitate helodermin recognition and action. However, this is unlikely, since in VPAC2 N-terminal receptors, the replacement of tm5–C-terminal and tm1–C-terminal segments with PAC1 receptor sequences had no detectable effect on helodermin potency. It is possible that influences are context-specific in that with a VPAC2 N-terminal domain, no further contributions are needed for helodermin recognition, whereas with a PAC1 N-terminal domain auxiliary sites are supportive and can be supplied by a VPAC2 but not PAC1 receptor body. Instead it seems most likely that elements in the PAC1 receptor tm1–C-terminal segment exert a negative effect on the recognition of helodermin by the PAC1 N-terminal domain or its subsequent cyclic AMP response. Such negative regulatory elements have been described in the PTH, secretin and VPAC1 receptors (Couvineau et al., 1996; Holtmann et al., 1996; Turner et al., 1996). Corresponding elements from the VPAC2 receptor would appear to lack this influence. It is not yet clear whether this negative gating influence is exerted at the level of helodermin binding affinity or its subsequent effectiveness in signal transduction.
In conclusion, we have provided evidence that ligand recognition and action at the rat VPAC2 receptor is largely determined by the N-terminal segment of the receptor. Additional motifs in other parts of the receptor appear to make relatively little contribution to modulation of this, in contrast to observations with the VPAC1 receptor (Hashimoto et al., 1997). While agonist recognition and activation of the PAC1 receptor is primarily influenced by the N-terminal domain, this receptor also shows important auxiliary influences of other domains in the recognition of and activation by the agonists VIP and helodermin (but not PACAP). Hashimoto et al., (1997) indicated that a subpopulation of PAC1 receptor sites can bind [125I]VIP with high affinity but only weakly activate cyclic AMP production in response to VIP. Our data (Tables 1 and 2) are consistent with this although the differing usage of in vivo/in vitro binding assays means that the ligand affinity values are not directly comparable between the two studies. The findings suggest the presence of elements that normally restrict effectiveness of, but not affinity for, VIP. However, VIP appears to benefit from the influence of auxiliary sites in the tm1–C-terminal of the PAC1 receptor, in its activation of cyclic AMP production. Replacement of these domains with corresponding VPAC2 receptor sequences further reduces the potency of VIP at cyclic AMP production and reduces the affinity with which this ligand binds to the receptor. The actions of helodermin at the PAC1 receptor appear to be quite differently regulated. Replacement of the tm1–C-terminal segment with VPAC2 receptor sequences appears to remove a selective inhibitory influence which normally suppresses the potency of helodermin. These observations strongly emphasise that the structure–activity relationships for agonist docking and effectiveness in the PAC1 receptor but perhaps not the VPAC2 receptor are agonist-dependent and complex.
Acknowledgments
We wish to thank Marianne Eastwood for helping prepare the manuscript, Elma Clark for help with sequencing the constructs, and John Bennie and Sheena Carroll for help with the radioimmunoassays. R Mitchell and M Johnson are members of the Membrane Biology Group of the University of Edinburgh.
Abbreviations
- AC
adenylate cyclase
- BSA
bovine serum albumin
- C terminal
carboxyl terminal
- DMSO
dimethyl sulphoxide
- e
extracellular loop
- EBSS
Earle's Balanced Salt solution
- i
intracellular loop
- IBMX
isobutyl methylxanthine
- MEM
Minimal Essential Medium
- N terminal
amino terminal
- PACAP
pituitary adenylate cyclase-activating polypeptide
- PCR
polymerase chain reaction
- PLC
phospholipase C
- PTH
parathyroid hormone
- tm
transmembrane domain
- VIP
vasoactive intestinal peptide
References
- ARIMURA A. Receptors for pituitary adenylate cyclase activating polypeptide. Comparison with vasoactive intestinal peptide receptors. Trends Endocrinol. Metab. 1992;3:288–294. doi: 10.1016/1043-2760(92)90139-r. [DOI] [PubMed] [Google Scholar]
- COUVINEAU A., GAUDIN P., MAORET J-J., ROUYER-FESSARD C., NICOLE P., LABURTHE M. Highly conserved aspartate 68, tryptophan 73 and glycine 109 in the N-terminal extracellular domain of the human VIP receptor are essential for its ability to bind VIP. Biochem. Biophys. Res. Commun. 1995;206:246–252. doi: 10.1006/bbrc.1995.1034. [DOI] [PubMed] [Google Scholar]
- COUVINEAU A., ROUYER-FESSARD C., MAORET J.J., GAUDIN P., NICOLE P., LABURTHE M. Vasoactive intestinal peptide (VIP)1 receptor. Three nonadjacent amino acids are responsible for species selectivity with respect to recognition of peptide histidine isoleucineamide. J. Biol. Chem. 1996;271:12795–12800. doi: 10.1074/jbc.271.22.12795. [DOI] [PubMed] [Google Scholar]
- DU K., NICOLE P., COUVINEAU A., LABURTHE M. Aspartate 196 in the first extracellular loop of the human VIP1 receptor is essential for VIP binding and VIP-stimulated cAMP production. Biochem. Biophys. Res. Commun. 1997;230:289–292. doi: 10.1006/bbrc.1996.5949. [DOI] [PubMed] [Google Scholar]
- HARMAR A.J., ARIMURA A., GOZES I., JOURNOT L., LABURTHE M., PISEGNA J.R., RAWLINGS S.R., ROBBERECHT P., SAID S.I., SREEDHARAN S.P., WANK S.A., WASCHEK J.A. International Union of Pharmacology. XVIII. Nomenclature of receptors for vasoactive intestinal peptide and pituitary adenylate cyclase-activating polypeptide. Pharmacol. Rev. 1998;50:265–270. [PMC free article] [PubMed] [Google Scholar]
- HASHIMOTO H., ISHIHARA T., SHIGEMOTO R., MORI K., NAGATA S. Molecular cloning and tissue distribution of a receptor for pituitary adenylate cyclase-activating polypeptide. Neuron. 1993;11:333–342. doi: 10.1016/0896-6273(93)90188-w. [DOI] [PubMed] [Google Scholar]
- HASHIMOTO H., OGAWA N., HAGIHARA N., YAMAMOTO K., IMANISHI K., NOGI H., NISHINO A., FUJITA T., MATSUDA T., NAGATA S., BABA A. Vasoactive intestinal polypeptide and pituitary adenylate cyclase- activating polypeptide receptor chimeras reveal domains that determine specificity of vasoactive intestinal polypeptide binding and activation. Mol. Pharmacol. 1997;52:128–135. doi: 10.1124/mol.52.1.128. [DOI] [PubMed] [Google Scholar]
- HEZAREH M., SCHLEGEL W., RAWLINGS S.R. PACAP and VIP stimulate Ca2+ oscillations in rat gonadotrophs through the PACAP/VIP type 1 receptor (PVR1) linked to a pertussin toxin-insensitive G-protein and activation of phospholipase C-b. J. Neuroendocrinol. 1996;8:367–374. doi: 10.1046/j.1365-2826.1996.04645.x. [DOI] [PubMed] [Google Scholar]
- HJORTH S.A., ØRSKOV C., SCHWARTZ T.W. Two-domain structure of the glucagon receptor: peptide binding and signal transduction. Regul. Pept. 1996;64:70. [Google Scholar]
- HOLTMANN M.H., GANGULI S., HADAC E.M., DOLU V., MILLER L.J. Multiple extracellular loop domains contribute critical determinants for agonist binding and activation of the secretin receptor. J. Biol. Chem. 1996;271:14944–14949. doi: 10.1074/jbc.271.25.14944. [DOI] [PubMed] [Google Scholar]
- HOLTMANN M.H., HADAC E.M., MILLER L.J. Critical contributions of amino-terminal extracellular domains in agonist binding and activation of secretin and vasoactive intestinal polypeptide receptors. J. Biol. Chem. 1995;270:14394–14398. doi: 10.1074/jbc.270.24.14394. [DOI] [PubMed] [Google Scholar]
- HOSOYA M., ONDA H., OGI K., MASUDA Y., MIYAMOTO Y., OHTAKI T., OKAZAKI H., ARIMURA A., FUJINO M. Molecular cloning and functional expression of rat cDNAs encoding the receptor for pituitary adenylate cyclase activating polypeptide (PACAP) Biochem. Biophys. Res. Commun. 1993;194:133–143. doi: 10.1006/bbrc.1993.1795. [DOI] [PubMed] [Google Scholar]
- HUANG Z., CHEN Y., NISSENSON R.A. The cytoplasmic tail of the G-protein-coupled receptor for parathyroid hormone and parathyroid hormone-related protein contains positive and negative signals for endocytosis. J. Biol. Chem. 1995;270:151–156. doi: 10.1074/jbc.270.1.151. [DOI] [PubMed] [Google Scholar]
- ISHIHARA T., SHIGEMOTO R., MORI K., TAKAHASHI K., NAGATA S. Functional expression and tissue distribution of a novel receptor for vasoactive intestinal polypeptide. Neuron. 1992;8:811–819. doi: 10.1016/0896-6273(92)90101-i. [DOI] [PubMed] [Google Scholar]
- LUTZ E.M., MACKENZIE C.J., MORROW J., MITCHELL R., BENNIE J., CARROLL S., CLARK E., HARMAR A.J. Chimaeric VIP2/PACAP receptors reveal that agonist pharmacology but not signal transduction is determined by extracellular domain 1. Ann. N.Y. Acad. Sci. 1996;805:574–578. doi: 10.1111/j.1749-6632.1996.tb17522.x. [DOI] [PubMed] [Google Scholar]
- LUTZ E.M., MENDELSON S., WEST K., MITCHELL R., HARMAR A.J. Molecular characterisation of novel receptors for PACAP and VIP. Biochem. Soc. Trans. 1994;22:88S. doi: 10.1042/bst023083s. [DOI] [PubMed] [Google Scholar]
- LUTZ E.M., SHEWARD W.J., WEST K.M., MORROW J.A., FINK G., HARMAR A.J. The VIP2 receptor: molecular characterisation of a cDNA encoding a novel receptor for vasoactive intestinal peptide. FEBS Lett. 1993;334:3–8. doi: 10.1016/0014-5793(93)81668-p. [DOI] [PubMed] [Google Scholar]
- MACKENZIE C.J., LUTZ E.M., MCCULLOCH D.A., MITCHELL R., HARMAR A.J. Phospholipase C activation by VIP1 and VIP2 receptors expressed in COS 7 cells involves a pertussis toxin-sensitive mechanism. Ann. N.Y. Acad. Sci. 1996;805:579–584. doi: 10.1111/j.1749-6632.1996.tb17523.x. [DOI] [PubMed] [Google Scholar]
- MCCULLOCH D., GRIEVE S., JOHNSON M., LUTZ E., MITCHELL R. Activation of phospholipase D by the VIP2 receptor and evidence for its attenuation by protein kinase A. Proc. Intnl Conf Second Messengers Phosphoproteins. 1995;9:414P. [Google Scholar]
- MORROW J.A., LUTZ E.M., WEST K.M., FINK G., HARMAR A.J. Molecular cloning and expression of a cDNA encoding a receptor for pituitary adenylate cyclase activating polypeptide (PACAP) FEBS Lett. 1993;329:99–105. doi: 10.1016/0014-5793(93)80202-6. [DOI] [PubMed] [Google Scholar]
- NICOLE P., DU K., COUVINEAU A., LABURTHE M. Site-directed mutagenesis of human vasoactive intestinal peptide receptor subtypes VIP1 and VIP2: evidence for difference in the structure-function relationship. J. Pharmacol. Exp. Ther. 1998;284:744–750. [PubMed] [Google Scholar]
- OFFERMANNS S., IIDA-KLEIN A., SEGRE G.V., SIMON M.I. Gαq family members couple parathyroid hormone (PTH)/PTH-related peptide and calcitonin receptors to phospholipase C in COS-7 cells. Mol. Endocrinol. 1996;10:566–574. doi: 10.1210/mend.10.5.8732687. [DOI] [PubMed] [Google Scholar]
- PISEGNA J.R., WANK S.A. Molecular cloning and functional expression of the pituitary adenylate cyclase-activating polypeptide type I receptor. Proc. Natl. Acad. Sci. U.S.A. 1993;90:6345–6349. doi: 10.1073/pnas.90.13.6345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SEGRE V., GOLDRING S.R. Receptors for secretin, calcitonin, parathyroid hormone (PTH)/PTH-related peptide, vasoactive intestinal peptide, glucagon-like peptide 1, growth hormone-releasing hormone, and glucagon belong to a newly discovered G-protein-linked receptor family. Trends Endocrinol. Metab. 1993;4:309–314. doi: 10.1016/1043-2760(93)90071-l. [DOI] [PubMed] [Google Scholar]
- SPENGLER D., WAEBER C., PANTALONI C., HOLSBOER F., BOCKAERT J., SEEBURG P.H., JOURNOT L. Differential signal transduction by 5 splice variants of the PACAP receptor. Nature. 1993;365:170–175. doi: 10.1038/365170a0. [DOI] [PubMed] [Google Scholar]
- STROOP S.D., KUESTNER R.E., SERWOLD T.F., CHEN L., MOORE E.E. Chimaeric human calcitonin and glucagon receptors reveal two dissociable calcitonin interaction sites. Biochemistry. 1995;34:1050–1057. doi: 10.1021/bi00003a040. [DOI] [PubMed] [Google Scholar]
- SVOBODA M., TASTENOY M., CICCARELLI E., STIEVENART M., CHRISTOPHE J. Cloning of a splice variant of the pituitary adenylate cyclase- activating polypeptide (PACAP) type I receptor. Biochem. Biophys. Res. Commun. 1993;195:881–888. doi: 10.1006/bbrc.1993.2127. [DOI] [PubMed] [Google Scholar]
- TURNER P.R., BAMBINO T., NISSENSON R.A. A putative selectivity filter in the G-protein-coupled receptors for parathyroid hormone and secretin. J. Biol. Chem. 1996;271:9205–9208. doi: 10.1074/jbc.271.16.9205. [DOI] [PubMed] [Google Scholar]
- VAN RAMPELBERGH J., GOURLET P., DE NEEF P., ROBBERECHT P., WAELBROECK M. Properties of the pituitary adenylate cyclase-activating polypeptide I and II receptors, vasoactive intestinal peptide 1, and chimaeric amino-terminal pituitary adenylate cyclase-activating polypeptide/vasoactive intestinal peptide 1 receptors: evidence for multiple receptor states. Mol. Pharmacol. 1996;50:1596–1604. [PubMed] [Google Scholar]
- VAN RAMPELBERGH J., POLOCZEK P., FRANCOYS I., DELPORTE C., WINAND J., ROBBERECHT P., WAELBROECK M. The pituitary adenylate cyclase activating polypeptide (PACAP I) and VIP (PACAP II VIP1) receptors stimulate inositol phosphate synthesis in transfected CHO cells through interaction with different G proteins. Biochim. Biophys. Acta. 1997;1357:249–255. doi: 10.1016/s0167-4889(97)00028-1. [DOI] [PubMed] [Google Scholar]
- WESS J. G-protein-coupled receptors: molecular mechanisms involved in receptor activation and selectivity of G-protein recognition. FASEB J. 1997;11:346–354. [PubMed] [Google Scholar]


