Abstract
It has been reported that endothelium-dependent relaxation is impaired in pulmonary hypertensive vessels. The underlying mechanisms for this phenomenon, however, have not yet been identified. In this study, the mechanisms responsible for decreased endothelium-dependent relaxation in the pulmonary artery isolated from monocrotaline (MCT)-induced pulmonary hypertensive rat (MCT rat) were examined. MCT (60 mg kg−1), or its vehicle was administered by a single subcutaneous injection to 6-week-old male Sprague Dawley rats.
Endothelium-dependent relaxation induced by carbachol or ionomycin in the MCT rat artery was significantly smaller than that in vehicle-treated rat (control rat) artery. Cyclic GMP levels, measured by enzyme-immunoassay, under resting or stimulation with carbachol or ionomycin were also smaller in the MCT rat artery. However, sodium nitroprusside-induced cyclic GMP accumulation in the endothelium-denuded artery was similar in control and MCT rats. These results suggest that MCT treatment decreases endothelial nitric oxide (NO) production.
Resting endothelial Ca2+ levels ([Ca2+]i) in the fura-PE3-loaded MCT rat artery, were not different from those in the control rat. However, the increase in endothelial [Ca2+]i elicited by carbachol was attenuated in the MCT rat.
In quantitative RT–PCR analysis, the expression of mRNA encoding endothelial NO synthase was rather increased in the MCT rat artery, suggesting an up-regulation of eNOS expression.
These results provide evidence that impaired NO-mediated arterial relaxation in the MCT rat is due to dissociation between eNOS expression and NO production. This dissociation may be derived from an inhibition of receptor-mediated Ca2+ metabolism and also from the apparent decrease in Ca2+ sensitivity of eNOS.
Keywords: Pulmonary hypertension, endothelium, endothelium-dependent relaxation, nitric oxide, NOS mRNA, intracellular Ca2+
Introduction
Pulmonary hypertension is characterized by elevated pulmonary blood pressure, abnormally thickened pulmonary arteries, and right ventricular hypertrophy. Because single subcutaneous injection of monocrotaline (MCT), a plant toxin pyrolizine alkaloid, induces pulmonary hypertension and right ventricular hypertrophy (Hayashi et al., 1967; Meyrick et al., 1980; Ghodsi & Will, 1981), the MCT-treated rat (MCT rat) has been widely used as an experimental model of pulmonary hypertension.
Nitric oxide (NO) is produced from L-arginine by endothelial NO synthase (eNOS) and causes smooth muscle relaxation by the activation of soluble guanylate cyclase followed by cyclic GMP accumulation (Moncada et al., 1991). eNOS is stimulated by the increase in endothelial Ca2+ concentration (Luckhoff & Busse, 1990; Busse & Mulsche, 1990). It has been reported that endothelium-dependent vasodilators fail to relax the pulmonary arterial strips isolated from the MCT rat (Altiere et al., 1985; Ito et al., 1988; Mathew et al., 1995), suggesting the possibility of a reduction in endothelial NO release. The decrease in endothelium-dependent relaxation has also been reported in chronic hypoxia-induced pulmonary hypertensive rats (Adnot et al., 1991) and in patients with pulmonary hypertension in chronic obstructive lung disease (Dinh-Xuan et al., 1991; 1993). Although the loss of endothelium-dependent relaxation has been considered to be a pathogenesis of pulmonary hypertension (Dinh-Xuan, 1992), eNOS mRNA and/or protein expression in pulmonary arteries have been shown to be up-regulated in pulmonary hypertensive rats, suggesting the possibility of an increase in endothelial NO release (Le Cras et al., 1996; Resta et al., 1997). These reports suggest a low availability of bioactive NO in pulmonary hypertensive rats. To clarify the mechanisms responsible for the impaired endothelium-dependent relaxation in pulmonary hypertension, we examined the changes in endothelial NO synthesis in pulmonary artery isolated from the MCT rat.
Methods
Monocrotaline (MCT)-induced pulmonary hypertensive rats
MCT (Sigma, U.S.A.) (300 mg) was dissolved in 1.8 ml 1 M HCl, and 3–4 ml of distilled water was added. This solution was adjusted to pH 7.4 with 1 M NaOH and filled up to 15 ml by distilled water (Hayashi et al., 1967). MCT (60 mg kg−1), or its vehicle was administered to 6-week-old male Sprague Dawley rats (180–210 g) as a single subcutaneous injection. Rats were housed with a 12 : 12-light-dark cycle and given water and standard rat chow ad libitum. All experiments were carried out 21 days after the administration. Animal care and treatment were conducted in conformity with institutional guidelines of The University of Tokyo.
Tissue preparations
Rats were killed by a sharp blow on the neck and exsanguination. Heart and lungs were removed en bloc, and right and left extrapulmonary arteries were dissected. After removal of the arteries, the right ventricle (RV) and left ventricle plus septum (LV+S) were separated and weighed.
Solutions
Physiological salt solution (PSS) contained (in mM): NaCl 136.9, KCl 5.4, CaCl2 1.5, MgCl2 1.0, glucose 5.5, and HEPES 5.0, and was saturated with 100% O2 at 37°C. The pH was adjusted to 7.3–7.4 with 1 M NaOH at 37°C. The endothelium-dependent relaxation was not changed in PSS buffered by NaHCO3 aerated with 95% O2 and 5% CO2. High K+ solution (72.7 mM) was prepared by replacing NaCl with equimolar KCl. Ca2+-free solution was made by omitting CaCl2 and adding 0.5 mM O, O′-bis (2-aminoethyl) ethyleneglycol-N, N, N′, N′-tetraacetic acid (EGTA). Ethylenediaminetetraacetic acid (EDTA, 0.1 mM) was added to all the solutions to remove contaminating heavy metal ions.
Measurements of muscle force
The pulmonary arteries were cut into rings approximately 2 mm wide. In some experiments, the intimal surface of the ring was gently rubbed with a glass rod moistened with PSS to remove the endothelium. Muscle tension was recorded isometrically under a resting tension of 10 mN. At the end of each experiment, 100 μM papaverine was added to determine the basal tone.
Measurement of endothelial Ca2+ level
Measurement of intracellular Ca2+ levels ([Ca2+]i) in endothelial cells in situ was performed according to the method described by Sato et al. (1990). Briefly, endothelium-intact pulmonary arterial tissue (1 mm wide) was incubated in PSS with 5 μM of the acetoxymethyl ester of fura-PE3 and 0.02% cremophor EL for 30 min at room temperature. The fluorescence was measured with a fluorimeter (CAF 110, Japan Spectroscopic, Japan). When fura-PE3 fluorescence was detected from the endothelial surface, carbachol, but not high K+, induced an increase in [Ca2+]i. Since smooth muscle cells but not endothelial cells have voltage-dependent Ca2+ channels (Hallam & Pearson, 1986; Colden-Stanfield et al., 1987; Johns et al., 1987), this result suggests that the fluorescence was derived only from endothelial cells. Absolute [Ca2+]i was calculated using the equation of Grynkiewicz et al. (1985). After observation of the [Ca2+]i response to carbachol at the 1.5 mM of Ca2+ concentration in PSS ([Ca2+]o) (normal PSS), ionomycin (1 μM), which induced a maximum increase in [Ca2+]i was added at 6.5 mM [Ca2+]o to obtain maximum [Ca2+]i levels (Rmax), and the external Ca2+ was then removed (with 4 mM EGTA) to obtain a minimum [Ca2+]i level (Rmin) (Figure 3A). At the end of each experiment, Mn2+ (5 mM) was added to quench fura-PE3 fluorescence and to determine the background fluorescence of the tissue. The fluorescence excited at 340 nm (F340) and at 380 nm (F380) was calculated after subtracting the background fluorescence. The dissociation constant of the fura-PE3-Ca2+ complex, KD, was assumed to be 290 nM (Vorndran et al., 1995).
Figure 3.
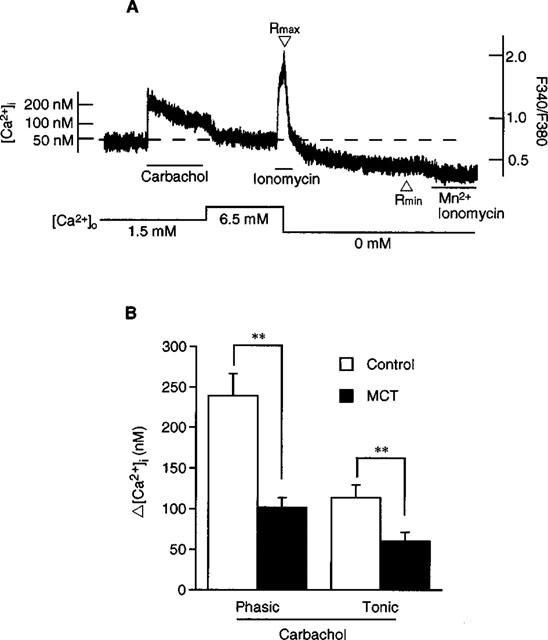
Effect of carbachol on endothelial [Ca2+]i. (A) shows a typical recording of the change in [Ca2+]i in endothelial cell measured in situ. After observation of [Ca2+]i response to carbachol at 1.5 mM [Ca2+]o, ionomycin (1 μM) was added at 6.5 mM [Ca2+]o to obtain a maximum [Ca2+]i level (Rmax), and external Ca2+ was removed (with 4 mM EGTA) to obtain a minimum [Ca2+]i level (Rmin). (B) shows the effect of carbachol on endothelial [Ca2+]i in control and MCT rats. Results are expressed as mean±s.e.mean. (n=6). **; significantly different between the control rat and MCT rat with P<0.01.
Measurement of cyclic GMP content
Subsequent to the incubation with or without test agent, vascular rings (approximately 5 mm wide) were immediately frozen in liquid nitrogen and homogenized in 6% trichloroacetic acid solution. After centrifugation at 2000×g for 15 min at 4°C, supernatant was applied to the cyclic GMP enzyme-immunoassay system (Amersham, U.K.) whereas pellet was used to determine the protein content by the method of Bradford (1976). Because weighting the tissue prior to incubation damaged endothelial functions, and because the rings were quickly frozen after incubation, it was not possible to measure the wet weight of the tissue before or after incubation. For these reasons, we determined the ratio of protein content to wet weight of the tissue in separate experiments. Using this ratio, the cyclic GMP contents were expressed as fmol mg−1 cell water. The tissue cell water content was determined as described previously (Urakawa et al., 1970). It has been reported that extracellular matrix proteins, such as collagen and elastin, are increased in the MCT rat artery (Todorovich-Hunter et al., 1988). Our preliminary experiment showed that the MCT rat artery had appriximately 2 fold higher protein content than control rat artery (unpublished observation). Therefore, we expressed cyclic GMP levels relative to cell water content rather than protein content, properly to reflect the cell volume.
Quantitative RT–PCR
Total RNA was extracted from endothelium-intact pulmonary artery by the acid-guanidinium isothiocynate phenol chloroform (AGPC) method (Chomczynski & Sacci, 1987), and the concentration of RNA was adjusted to 1 μg/μl with RNase-free distilled water. RT–PCR was performed using the Takara RNA PCR Kit Ver.2 (Takara Biomedicals, Japan). First-strand cDNA was synthesized using a random 9-mer primer and AMV Reverse Transcriptase XL at 30°C for 10 min, 55°C for 30 min, 99°C for 5 min, and 5°C for 2 min. PCR amplification was performed using Takara Taq polymerase. The oligonucleotide primers for eNOS designed from bovine eNOS (Sessa et al., 1992) were TAC CAG CCG GGG GAC CAC (forward) and CGA GCT GAC AGA GTA GTA (reverse). The oligonucleotide primers for inducible NO synthase (iNOS) and the rat glyceraldehyde 3-phosphate dehydrogenase (GAPDH) used have been described previously by Niwa et al. (1996). After initial denaturation at 94°C for 2 min, 28–43 cycles (5 cycle interval) of amplifications at 94°C for 0.5 min, 60°C for 0.5 min, and 72°C for 1.5 min were carried out using a thermal cycler (Takara PCR Thermal Cycler PERSONAL TP240, Takara Biomedicals, Japan). PCR-products in each of the cycles were electrophoresed on 2% agarose gel containing 0.1% ethidium bromide. The possible contamination of DNA was excluded by a PCR with total RNA without the reverse transcription step. Detectable fluorescent bands were visualized by a u.v.-transilluminator.
Statistical analysis
The results of the experiments are expressed as mean±s.e.mean. Student's t-test was used for the statistical analysis. P values of less than 0.05 were considered to be significant.
Chemicals
The chemicals used were carbachol hydrochloride, phenylephrine hydrochloride, ionomycin calcium salt, verapamil hydrochloride, papaverine hydrochloride (Sigma, U.S.A.), sodium nitroprusside (Wako Pure Chemicals, Japan), and fura-PE3 (Teflabs, U.S.A.). Ionomycin calcium salt was dissolved in ethanol. Fura-PE3 was dissolved in dimethyl sulphoxide. Other chemicals were dissolved in distilled water.
Results
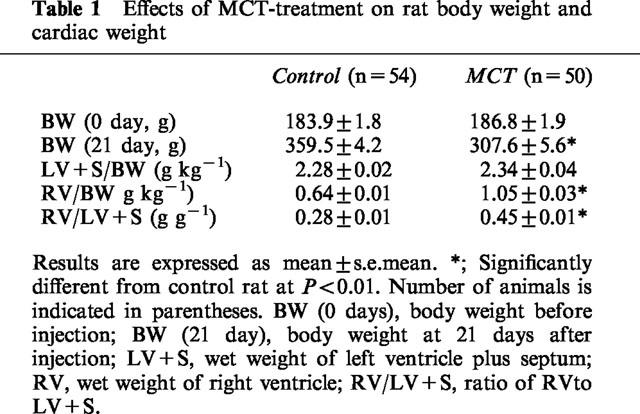
Effects of monocrotaline treatment on rat body weight and cardiac weight
As shown in Table 1, body weight measured after 21 days of administration was lower in the MCT rat than in the vehicle-treated rat (control rat). The ratios of RV weight to body weight and RV weight to LV+S weight were also increased in MCT rat, suggesting the occurrence of right ventricular hypertrophy. Right ventricular hypertrophy was shown to be a reliable index of pulmonary hypertension in this model (Wanstall & D'onnell, 1990).
Table 1.
Effects of MCT-treatment on rat body weight and cardiac weight
Endothelium-dependent relaxation
In the artery with endothelium, carbachol (3 nM–30 μM) inhibited the muscle contraction elicited by 1 μM phenylephrine in a concentration-dependent manner (Figure 1A). The carbachol-induced endothelium-dependent relaxation was significantly attenuated in the MCT rat. The maximum relaxation due to carbachol was 80.2±5.1% (n=7) in the control rat and 23.7±6.3% (n=6) in the MCT rat (P<0.01).
Figure 1.
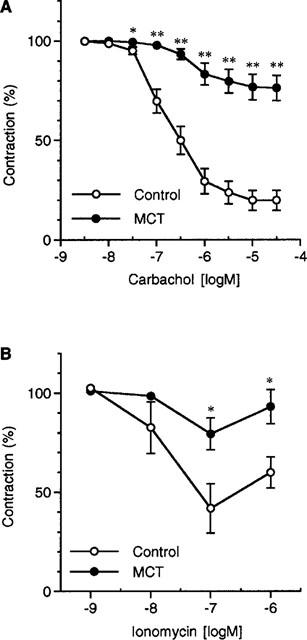
(A) Effects of carbachol on phenylephrine (1 μM)-induced contraction in the endothelium-intact pulmonary artery. (B) Effects of ionomycin on phenylephrine (1 μM)-induced contraction in the endothelium-intact pulmonary artery. Carbachol or ionomycin was added after the phenylephrine-induced contraction had reached a steady-state level. Results are expressed as mean±s.e.mean. (n=4–7). * and **; significantly different from the control rat with P<0.05 and P<0.01, respectively.
In the artery with endothelium, ionomycin (1 nM–1 μM) inhibited the muscle contraction elicited by 1 μM phenylephrine in a concentration-dependent manner (Figure 1B). The effect of ionomycin was significantly attenuated in the MCT rat; the maximum relaxation due to ionomycin was 67.3±6.5% (n=4) in the control rat and 20.7±8.0% (n=4) in the MCT rat (P<0.01).
Cyclic GMP content in pulmonary artery
In the artery without endothelium (Figure 2A), the resting cyclic GMP content in the MCT rat did not differ from that in the control rat. SNP increased the cyclic GMP content in a time-dependent manner. MCT-treatment did not affect the SNP-induced increase in cyclic GMP content. In the artery with endothelium (Figure 2B), the resting cyclic GMP content was greater in the control rat than in the MCT rat (P<0.05). In the control rat artery, 2 min treatment of both carbachol and ionomycin increased the cyclic GMP content. In the MCT rat artery, however, carbachol but not ionomycin increased the cyclic GMP content. The increase in cyclic GMP due to carbachol was smaller in the MCT rat artery than in the control rat artery (P<0.01).
Figure 2.
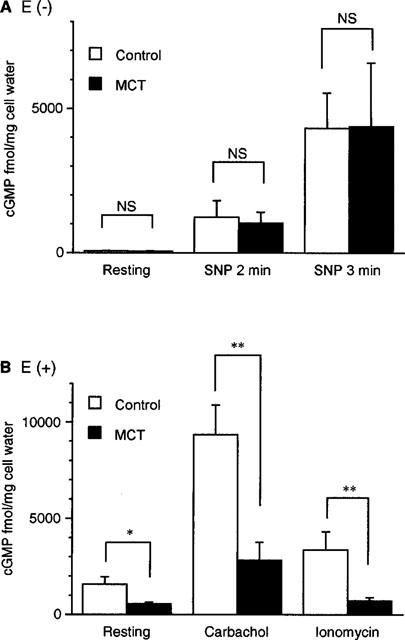
Changes in cyclic GMP content in the pulmonary artery of control and MCT rats. (A) shows the effect of SNP (1 μM) in the endothelium-denuded artery of control and MCT rats. (B) shows the effects of carbachol and ionomycin in the endothelium-intact pulmonary artery of control and MCT rats. Results are expressed as mean±s.e.mean. (n=6–21). * and **; significantly different between the control rat and MCT rat with P<0.05 and P<0.01, respectively. NS, not significant.
Cytosolic Ca2+ levels in endothelial cells
Endothelial [Ca2+]i at resting condition was 66.2±11.5 nM (n=6) in the control rat artery; this concentration was not significantly different from that in the MCT rat artery (82.1±11.0 nM, n=6). As shown in Figure 3A, carbachol (10 μM) induced a transient increase in [Ca2+]i followed by a sustained increase. The peak amplitude of the increase in [Ca2+]i was lower in the MCT rat (238.9±27.5 nM in the control rat and 101.4±12.3 nM in the MCT rat, n=6 each, P<0.01). The carbachol-induced increase in [Ca2+]i at sustained phase was also lower in the MCT rat (113.9±15.2 nM in the control rat and 60.3±11.0 nM in the MCT rat, n=6 each, P<0.01) (Figure 3B).
Quantitative RT–PCR for NO synthase mRNA
As shown in Figure 4, the expression of RT–PCR products encoding GAPDH (308 basepairs) and iNOS (442 basepairs) were identical in the MCT and the control rats. In the quantitative analysis for eNOS, in contrast, the detectable RT–PCR product encoding eNOS (459 basepairs) could be observed from 33 cycles of PCR amplification in the MCT rat but not in the control rat. Similar results were obtained in five out of seven experiments. In the other two experiments, however, expression of eNOS mRNA in the MCT rat artery was not different from that in the control rat artery.
Figure 4.
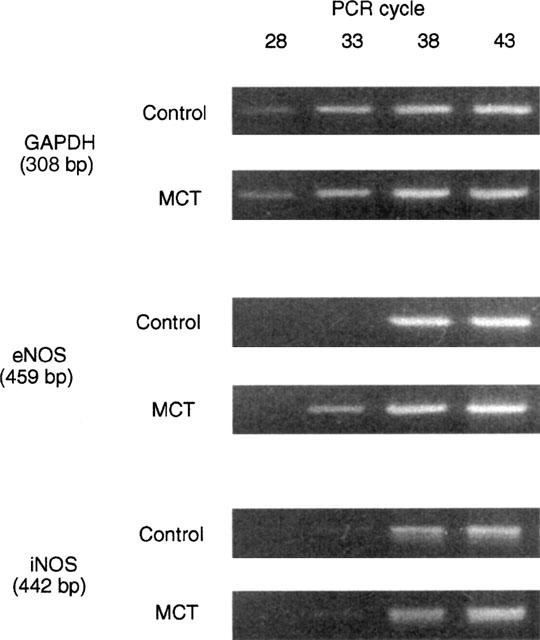
Quantitative RT–PCR for determination of mRNA of eNOS and iNOS in pulmonary artery with endothelium. Agarose-gel electrophoresis of RT–PCR products for eNOS, iNOS, and GAPDH after 28, 33, 38, and 43 cycles of amplification. Total RNA was isolated from the endothelium-intact artery of control and MCT rats. Agarose-gel electrophoresis demonstrated RT–PCR products of expected size corresponding to mRNA encoding the iNOS (442 base pairs), eNOS (459 base pairs), and GAPDH (308 base pairs).
Discussion
In the present results, endothelium-dependent relaxation induced by carbachol or ionomycin was attenuated in the pulmonary artery of the MCT rat. These results are consistent with previous reports in which endothelium-dependent relaxation caused by receptor agonists (Altiere et al., 1985; Ito et al., 1988; Adnot et al., 1991; Mathew et al., 1995) or by calcium ionophore (Mathew et al., 1995) was shown to be impaired in the pulmonary artery of pulmonary hypertensive rats. In the light microscopic observation, endothelial excoriation was not observed in the MCT rat artery (n=6) (data not shown). Therefore, we hypothesized and examined the possibility that the MCT-induced impairment of endothelium-dependent relaxation might result from (1) decreased endothelial NO production and/or (2) decreased cyclic GMP production ability in smooth muscle cells.
Endothelial NO production was examined by measuring the cyclic GMP content, an indicator of endothelial NO production. Resting cyclic GMP content was greater in the presence of endothelium in both control and MCT rat arteries. In the presence of endothelium, however, the resting cyclic GMP content in the MCT rat artery was significantly smaller than that in the control rat artery. Furthermore, the increase in cyclic GMP content elicited by carbachol or ionomycin was again significantly smaller in the MCT rat artery. On the other hand, SNP elicited a similar increase in cyclic GMP in control and MCT rat arteries. These results suggest that the guanylate cyclase/cyclic GMP pathway in smooth muscle cells is not changed by MCT and that decreased cyclic GMP levels in the MCT rat artery might be due to decreased NO production. It has been reported that endothelin is involved in the MCT-induced pulmonary hypertension (Takahashi et al., 1998; Frasch et al., 1999). Furthermore, when rats were chronically treated with an endothelin ET-A receptor antagonist, LU135252, during the application of MCT, MCT-induced impairment of NO-dependent vasodilations were partly inhibited, suggesting that impairment of NO-dependent vasodilation is at least partly mediated by endothelin system (Prie et al., 1998). Further investigations are necessary to evaluate the contribution of the endothelin system to the reduced NO production and/or impairment of endothelium-dependent relaxation.
An increase in endothelial [Ca2+]i activates NO synthesis in endothelial cells (Luckhoff & Busse, 1990; Busse & Mulsche, 1990). We found that an increase in endothelial [Ca2+]i stimulated by the maximally effective concentration of carbachol is significantly smaller in the MCT rat than in the control rat at both transient and tonic phases. This result suggests that abnormalities in Ca2+ handling may at least in part be responsible for the decreased activity of carbachol in producing NO in the MCT rat artery. Consistently, in cultured vascular endothelial cells of the aldosterone-salt hypertensive rat, it has been shown that [Ca2+]i response to acetylcholine was attenuated (Liu et al., 1995). In addition, in cultured aortic endothelial cells of the spontaneously hypertensive rat, bradykinin-induced increases in [Ca2+]i have been shown to be impaired (Wang et al., 1995).
Ionomycin causes endothelium-dependent relaxation without any direct activation of receptor-mediated signal transduction. Ionomycin-induced relaxation was also weaker in the MCT rat artery than in the control rat artery. Moreover, although the resting endothelial [Ca2+]i was not changed by MCT-treatment, resting cyclic GMP level in artery with endothelium was significantly lower in the MCT rat than in the control rat. These results suggest that MCT rat endothelial cells synthesize less NO at a given [Ca2+]i. The apparent decrease in Ca2+ sensitivity may be due to the changes in eNOS protein in such a manner to inhibit the interaction with Ca2+-calmodulin-caveolin. Since caveolin has been reported to inhibit eNOS activity, whereas Ca2+-calmodulin complex attenuates its inhibitory effect (Shaul et al., 1996; Michel et al., 1997a, 1997b), it is also possible that the interaction between eNOS and caveolin may be changed. Another possibility is that, in the MCT rat endothelium, Ca2+ may be localized in a compartment apart from eNOS. The existence of Ca2+ compartments has been discussed in smooth muscle cell (Karaki et al., 1997). However, Ca2+ localization may not explain the attenuated effect of ionomycin, which is believed to elicit a global increase in cellular Ca2+. Further experiments are necessary to evaluate these possibilities.
The change in the amount of eNOS was examined by RT–PCR analysis, which revealed that eNOS mRNA expression in endothelium was increased in the MCT rat artery. These results suggest that impairment of NO production may not be due to decreased eNOS expression. Others have also reported that eNOS mRNA and/or protein expression was rather increased in pulmonary vessels prepared from hypoxia- and MCT-induced, and fawn-hooded pulmonary hypertensive rats (Le Cras et al., 1996; Resta et al., 1997; Tyler et al., 1999). In this study, eNOS expression was up-regulated, although NO production was decreased in the MCT rat endothelium. Endothelial cells may up-regulate eNOS expression to compensate for the endothelial dysfunction. In contrast, Resta et al. (1997) reported that eNOS expression was upregulated and that the arginine vasopressin-induced endothelium-dependent vasodilation was enhanced in isolated perfused rat lung isolated from the MCT-treated rat compared to that of the control rat. The difference between these results might be due to the difference in the preparations used; i.e. conduit artery in our study versus resistance artery in Resta et al. (1997).
We also found that there was no difference in the iNOS mRNA expression between the control and MCT rat arteries. However, others have reported that iNOS mRNA and protein expression were up-regulated in lung homogenate and small intrapulmonary vessels from chronic hypoxic rats (Xue & Johns, 1996; Le Cras et al., 1996). These differences may be due to the difference in arteries and/or animal models.
It has been reported that impaired endothelium-dependent relaxation in isolated MCT rat pulmonary artery was not restored by the addition of a NO precursor, L-arginine, or a superoxide scavenger, superoxide dimustase (Mathew et al., 1995), and we confirmed this (unpublished observation). Thus, the change in the availability of L-arginine and the elimination of NO by superoxide might not be involved in the impaired endothelium-dependent relaxation in the MCT rat artery.
In summary, our study has demonstrated that, in the MCT rat pulmonary artery, eNOS is up-regulated, although the total NO production is reduced at resting condition and also at carbachol- or ionomycin-stimulated conditions, resulting in an impairment of endothelium-dependent relaxation. This dissociation may be explained by the abnormality in intracellular Ca2+ metabolism linked to the receptor activation and apparent decrease in Ca2+- sensitivity of eNOS. However, MCT-treatment does not seem to down-regulate the guanylate cyclase/cyclic GMP pathway.
Acknowledgments
This work was supported by the Grant-in-Aid for Scientific research from the Ministry of Education, Science, Sports and Culture, Japan.
Abbreviations
- eNOS
endothelial NO synthase
- GAPDH
glyceraldehyde 3-phosphate dehydrogenase
- iNOS
inducible NO synthase
- LV+S
left ventricle plus septum
- MCT
monocrotaline
- PSS
physiological salt solution
- RV
right ventricle
- SNP
sodium nitroprusside
- [Ca2+]i intracellular Ca2+ level; [Ca2+]o
Ca2+ concentration in PSS
References
- ADNOT S., RAFFESTIN B., EDDAHIBI S., BRAQUET P., CHABRIER P.-E. Loss of endothelium-dependent relaxant activity in the pulmonary circulation of rats exposed to chronic hypoxia. J. Clin. Invest. 1991;87:155–162. doi: 10.1172/JCI114965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ALTIERE R.J., OLSON J.W., GILLESPIE M.N. Altered pulmonary vascular smooth muscle responsiveness in monocrotaline-induced pulmonary hypertension. J. Pharmacol. Exp. Ther. 1985;236:390–395. [PubMed] [Google Scholar]
- BRADFORD M. A rapid and sensitive method for the quantitation of microgram quantities of protein. Anal. Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- BUSSE R., MULSCHE A. Calcium-dependent nitric oxide synthesis in endothelial cytosol is mediated by calmodulin. FEBS Lett. 1990;265:133–136. doi: 10.1016/0014-5793(90)80902-u. [DOI] [PubMed] [Google Scholar]
- CHOMCZYNSKI P., SACCI N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chliriform extraction. Anal. Biochem. 1987;162:156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- COLDEN-STANFIELD M., SCHILLING W.P., RITCHIE A.K., ESKIN S.G., NAVARO L.T., KUNZE D.L. Bradykinin-induced increases in cytosolic calcium and ionic currents in cultured bovine aortic endothelial cells. Circ. Res. 1987;61:632–640. doi: 10.1161/01.res.61.5.632. [DOI] [PubMed] [Google Scholar]
- DINH-XUAN A.T. Endothelial modulation of pulmonary vascular tone. Eur. Respir. J. 1992;5:757–762. [PubMed] [Google Scholar]
- DINH-XUAN A.T., HIGENBOTTAM T.W., CLELLAND C.A., PEPKE-ZABA J., CREMONA G., BUTT A.Y., LARGE S.R., WELLS F.C., WALLWORK J. Impairement of endothelium-dependent pulmonary-artery relaxation in chronic obstructive lung disease. N. Engl. J. Med. 1991;324:1539–1547. doi: 10.1056/NEJM199105303242203. [DOI] [PubMed] [Google Scholar]
- DINH-XUAN A.T., PEPKE-ZABA J., BUTT A.Y., CREMONA G., HIGENBOTTAM T.W. Impairment of pulmonary-artery endothelium-dependent relaxation in chronic obstructive lung disease is not due to dysfunction of endothelial cell membrane receptors not to L-arginine deficiency. Br. J. Pharmacol. 1993;109:587–591. doi: 10.1111/j.1476-5381.1993.tb13611.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FRASCH H.F., MARSHALL C., MARSHALL B.E. Endothelin-1 is elevated in monocrotaline pulmonary hypertension. Am. J. Physiol. 1999;276:L304–L310. doi: 10.1152/ajplung.1999.276.2.L304. [DOI] [PubMed] [Google Scholar]
- GHODSI F., WILL J.A. Changes in pulmonary structure and function induced by monocrotaline intoxication. Am. J. Physiol. 1981;240:H149–H155. doi: 10.1152/ajpheart.1981.240.2.H149. [DOI] [PubMed] [Google Scholar]
- GRYNKIEWICZ G., POENIE M., TSIEN R. A new generation of Ca2+ indicators with greatly improved fluorescence properties. J. Biol. Chem. 1985;260:3440–3450. [PubMed] [Google Scholar]
- HALLAM T.J., PEARSON J.D. Exogenous ATP raises cytoplasmic free calcium in fura-2 loaded piglet aortic endothelial cells. FEBS Lett. 1986;207:95–99. doi: 10.1016/0014-5793(86)80019-9. [DOI] [PubMed] [Google Scholar]
- HAYASHI Y., HUSSA J.F., LALICH J.J. Cor pulmonale in rats. Lab. Invest. 1967;16:875–876. [PubMed] [Google Scholar]
- ITO K., NAKASHIMA T., MURAKAMI K., MURAKAMI T. Altered function of pulmonary endothelium following monocrotaline-induced lung vascular injury in rats. Br. J. Pharmacol. 1988;94:1175–1183. doi: 10.1111/j.1476-5381.1988.tb11636.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- JOHNS A., TATEGEN T.W., LODGE N.J., RYAN U.S., VAN BREEMEN C., ADAMS D.J. Calcium entry through receptor-operated channels in bovine pulmonary artery endothelial cells. Tissue Cell. 1987;19:733–745. doi: 10.1016/0040-8166(87)90015-2. [DOI] [PubMed] [Google Scholar]
- KARAKI H., OZAKI H., HORI M., MITSUI-SAITO M., AMANO K.-I., HARADA K.-I., MIYAMOTO S., NAKAZAWA H., WON K.-J., SATO K. Calcium movements, distribution, and functions in smooth muscle. Pharmacol. Rev. 1997;49:157–230. [PubMed] [Google Scholar]
- LE CRAS T.D., XUE C., RENGASAMY A., JOHNS R.A. Chronic hypoxia upregulates endothelial and inducible NO synthase gene and protein expression in rat lung. Am. J. Physiol. 1996;270:L164–L170. doi: 10.1152/ajplung.1996.270.1.L164. [DOI] [PubMed] [Google Scholar]
- LIU Y., JONES A.W., STUREK M. Attenuated Ca2+ response to acetylcholine in endothelial cells from aorta of aldosterone-salt hypertensive rats. Am. J. Hypertens. 1995;8:404–408. doi: 10.1016/0895-7061(94)00205-p. [DOI] [PubMed] [Google Scholar]
- LUCKHOFF A., BUSSE R. Calcium influx into endothelial cells and formation of endothelium-derived relaxing factor is controlled by membrane potential. Pflügers Arch. 1990;416:305–311. doi: 10.1007/BF00392067. [DOI] [PubMed] [Google Scholar]
- MATHEW R., ZEBALLOS G.A., TUN H., GEWITZ M.H. Role of nitric oxide and endothelin-1 in monocrotaline-induced pulmonary hypertension in rats. Cardiovasc. Res. 1995;30:739–746. [PubMed] [Google Scholar]
- MEYRICK B., GAMBLE W., REID L. Development of Cratalaria pulmonary hypertension: hemodynamic and structural study. Am. J. Physiol. 1980;239:H692–H702. doi: 10.1152/ajpheart.1980.239.5.H692. [DOI] [PubMed] [Google Scholar]
- MICHEL J.B., FERON O., SACKS D., MICHEL T. Reciprocal regulation of endothelial nitric-oxide synthase by Ca2+-calmodulin and caveolin. J. Biol. Chem. 1997a;272:15583–15586. doi: 10.1074/jbc.272.25.15583. [DOI] [PubMed] [Google Scholar]
- MICHEL J.B., FERON O., SASE K., PRABHAKAR P., MICHEL T. Caveolin versus Calmodulin-counterbalancing allosteric modulators of endothelial nitric oxide synthase. J. Biol. Chem. 1997b;272:25907–25912. doi: 10.1074/jbc.272.41.25907. [DOI] [PubMed] [Google Scholar]
- MONCADA S., PALMER R.M.J., HIGGS E.A. Nitric oxide: Physiology, pathophysiology, and pharmacology. Pharmacol. Rev. 1991;43:109–142. [PubMed] [Google Scholar]
- NIWA M., TSUTSUMISHITA Y., KAWAI Y., TAKAHARA H., NAKAMURA N., FUTAKI S., TAKAISHI Y., KONDOH W., MORITOKI H. Suppression of inducible nitric oxide synthase mRNA expression by tryptoquinone A. Biochem. Biophys. Res. Commun. 1996;224:579–585. doi: 10.1006/bbrc.1996.1067. [DOI] [PubMed] [Google Scholar]
- PRIE S., STEWART D.J., DUPUIS J. EndothelinA receptor blockade improves nitric oxide-mediated vasodilation in monocrotaline-induced pulmonary hypertension. Circulation. 1998;97:2169–2174. doi: 10.1161/01.cir.97.21.2169. [DOI] [PubMed] [Google Scholar]
- RESTA T.C., GONZALES R.J., DAIL W.G., SANDERS T.C., WALKER B.R. Selective upregulation of arterial endothelial nitric oxide synthase in pulmonary hypertension. Am. J. Physiol. 1997;272:H806–H813. doi: 10.1152/ajpheart.1997.272.2.H806. [DOI] [PubMed] [Google Scholar]
- SATO K., OZAKI H., KARAKI H. Differential effects of carbachol in cytosolic calcium levels in vascular endothelium and smooth muscle. J. Pharmacol. Exp. Ther. 1990;255:114–119. [PubMed] [Google Scholar]
- SESSA W.C., HARRISON J.K., BARBER C.M., ZENG D., DURIEUX M.E., D'ANGELO D.D., LYNCH K.R., PEACH M.J. Molecular cloning and expression of a cDNA encoding endothelial cell nitric oxide synthase. J. Biol. Chem. 1992;267:15274–15276. [PubMed] [Google Scholar]
- SHAUL P.W., SMART E.J., ROBINSON L.J., GERMAN Z., YUHANNA I.S., YING Y., ANDERSON R.G., MICHEL T. Acylation targets endothelial nitric-oxide synthase to plasmalemmal caveolae. J. Biol. Chem. 1996;271:6518–6522. doi: 10.1074/jbc.271.11.6518. [DOI] [PubMed] [Google Scholar]
- TAKAHASHI T., KANDA T., INOUE M., SUMINO H., KOBAYASHI I., IWAMOTO A., NAGAI R.Endothelin converting enzyme inhibitor protects development of right ventricular overload and medial thickening of pulmonary arteries in rats with monocrotaline-induced pulmonary hypertension Life Sci. 199863137–143.PL [DOI] [PubMed] [Google Scholar]
- TODOROVICH-HUNTER L., JOHNSON D.J., RANGER P., KEELEY F.W., RABINOVITCH M. Altered elastin and collagen synthesis associated with progressive pulmonary hypertension induced by monocrotaline-A biochemical and ultrastructual study. Lab. Invest. 1988;58:79–91. [PubMed] [Google Scholar]
- TYLER R.C., MURAMATSU M., ADMAN S.H., STELZNER T.J., RODMAN D.M., BLICH K.D., MCMUTRY I.F. Variable expression of endothelial NO synthase in three forms of rat pulmonary hypertension. Am. J. Physiol. 1999;276:L297–L303. doi: 10.1152/ajplung.1999.276.2.L297. [DOI] [PubMed] [Google Scholar]
- URAKAWA N., KARAKI H., IKEDA M. Effects of ouabain and metabolic inhibiting factors on Ca distribution during K-induced contracture in guinea-pig taenia coli. Japan. J. Pharmacol. 1970;20:360–366. doi: 10.1254/jjp.20.360. [DOI] [PubMed] [Google Scholar]
- VORNDRAN C., MINTA A., POENIE M. New fluorescent calcium indicators designed for cytosolic retension or measuring calcium near membranes. Biophys. J. 1995;69:2112–2114. doi: 10.1016/S0006-3495(95)80082-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WANG R., SAUVE R., CHAMPLAIN D.J. Abnormal regulation of cytosolic free calcium in vascular endothelial cells from spontaneously hypertensive rats. J. Hypertens. 1995;13:993–1001. [PubMed] [Google Scholar]
- WANSTALL J.C., D'ONNELL S.R. Endothelin and 5-hydroxyrtyptamine on rat pulmonary artery in pulmonary hypertension. Eur. J. Pharmacol. 1990;176:159–168. doi: 10.1016/0014-2999(90)90524-a. [DOI] [PubMed] [Google Scholar]
- XUE C., JOHNS R.A. Upregulation of nitric oxide synthase correlates temporally with onset of pulmonary vascular remodeling in the hypoxic rat. Hypertension. 1996;28:743–753. doi: 10.1161/01.hyp.28.5.743. [DOI] [PubMed] [Google Scholar]


