Abstract
Flavonoids display a wide range of pharmacological properties including anti-inflammatory. Anti-mutagenic, anti-carcinogenic and anti-cancer effects. Here, we evaluated the effects of eight flavonoids on the tumour cell proliferation, cellular protein phosphorylation, and matrix metalloproteinase (MMPs) secretion.
Of the flavonoids examined, luteolin (Lu) and quercetin (Qu) were the two most potent agents, and significantly inhibited A431 cell proliferation with IC50 values of 19 and 21 μM, respectively.
The epidermal growth factor (EGF) (10 nM) promoted growth of A431 cells (+25±4.6%) and mediated epidermal growth factor receptor (EGFR) tyrosine kinase activity and autophosphorylation of EGFR were inhibited by Lu and Qu. At concentration of 20 μM, both Lu and Qu markedly decreased the levels of phosphorylation of A431 cellular proteins, including EGFR.
A431 cells treated with Lu or Qu exhibited protuberant cytoplasmic blebs and progressive shrinkage morphology. Lu and Qu also time-dependently induced the appearance of a ladder pattern of DNA fragmentation, and this effect was abolished by EGF treatment.
The addition of EGF only marginally diminished the inhibitory effect of luteolin and quercetin on the growth rate of A431 cells, treatment of cellular proteins with EGF and luteolin or quercetin greatly reduced protein phosphorylation, indicating Lu and Qu may act effectively to inhibit a wide range of protein kinases, including EGFR tyrosine kinase.
EGF increased the levels of matrix metalloproteinase-2 (MMP-2) and matrix metalloproteinase-9 (MMP-9), while Lu and Qu appeared to suppress the secretion of these two MMPs in A431 cells.
Examination of the relationship between the chemical structure and inhibitory effects of eight flavonoids reveal that the double bond between C2 and C3 in ring C and the OH groups on C3′ and C4′ in ring B are critical for the biological activities.
This study demonstrates that the inhibitory effects of Lu and Qu, and the stimulatory effects of EGF, on tumour cell proliferation, cellular protein phosphorylation, and MMP secretion may be mediated at least partly through EGFR. This study supports the idea that Lu and Qu may have potential as anti-cancer and anti-metastasis agents.
Keywords: Epidermal growth factor receptor, protein tyrosine kinase, matrix metalloproteinase, metastasis, luteolin, quercetin
Introduction
The flavonoids, which are benzo-γ-pyrone (phenylchromone) derivatives, comprise a very large class of naturally-occurring, low molecular weight polyphenol plant compounds. The flavonoids are classified into five categories: monomeric flavanols, flavanones, anthocyanidins, flavones, and flavonols. On average, the normal human diet contains approximately 1 g or more per day (Kuhnau, 1976), a quantity that provides pharmacologically significant concentrations in body fluids. Accumulating evidence shows that certain flavonoids not only inhibit growth of tumour cells but also induce cell differentiation. The inhibitory effects of flavonoids on the growth of malignant cells may partly be due to their suppressive effects on kinase activities involved in the regulation of cell proliferation. This has been shown in L1210 leukaemia cells (Soulinna et al., 1975), OVCA433 ovarian cancer cells (Scambia et al., 1990), gliosarcoma 9L cells (Kandaswami et al., 1991) and HTB 43 human squamous cells (Kandaswami et al., 1992). In addition, transformation of chick embryonic fibroblasts to malignant cells by the Rous sarcoma virus is dependent upon the expression of the src gene (pp60src) and can be inhibited by the flavonoids quercetin (Qu) and genistein (Gen) (Frank & Sartorelli, 1988). The pp60src gene product is a protein tyrosine kinase (PTK), the activity of which has been shown to be inhibited by quercetin (Constantinou et al., 1990). These findings raise the broad question of whether PTK may be involved in the transformation of normal cells to a malignant state. It is evident that quercetin was found to inhibit the activity of a tyrosine kinase which was thought to be responsible for the transformation of non-malignant fibroblast cells to sarcoma cells (Glossman et al., 1981). Also, quercetin inhibited the PTK activity of 9,10-dimethyl-1,2-benzanthracene (DMBA)-induced rat mammary tumour (Levi et al., 1984). Genistein, proposed to be a specific inhibitor of PTK (Akiyama et al., 1987), was found to inhibit growth and induce differentiation in human HL-60 and K-562 leukaemia cells (Constantinou et al., 1990). In addition, genistein inhibits EGF receptor tyrosine kinase activity in human epidermoid carcinoma A431 cells that constitutively express high level of epidermal growth factor receptor (EGFR) (Akiyama et al., 1987; Linassier et al., 1990). Merlino et al. (1984) found the caused of overproducing EGFRs in A431 cells is due to the gene amplification and translocation.
Protein phosphorylation/dephosphorylation is an important regulatory mechanism in the action of many hormones and growth factors, which transmit their messages via activation of cellular PTKs (Hunter & Cooper, 1985; Ullrich & Schlessinger, 1990; Cantley et al., 1991). The expression of PTKs, including several membrane-associated oncogene products, is elevated in tumour cells (Hunter & Cooper, 1985; Bishop, 1987; Linassier et al., 1990; Ullrich & Schlessinger, 1990; Cantley et al., 1991; Kolibaba & Druker, 1997). Moreover, this increase is associated with the development of various cancers (Pierce et al., 1988; Koch et al., 1991). Therefore, it is important to explore whether flavonoids that possess anti-proliferative activity have any significant effect on certain cellular protein phosphorylation. Moreover, it would be instructive to determine whether PTKs are the focal point for flavonoid action.
The lethality of malignant tumours is attributable in large part to metastasis of neoplastic cells (Taniguchi et al., 1992). Several agents, including flavonoids, have been reported to inhibit invasion and metastasis (Schneider & Schirner, 1993; Davies et al., 1993; Watson et al., 1995; Menon et al., 1995; Cha et al., 1996). Up to now, no established anti-metastatic agents are available for clinical use. Therefore, there is an urgent need for compounds capable of successfully interfering with one or more steps of the metastatic process (Yoneda et al., 1998). Metastasis involves matrix remoulding of organ tissues, which thus allow cells to migrate away from its origin and through circulation to a distant organ. Currently, matrix metalloproteinases (MMPs) are believed to play an important role in cancer metastasis. MMPs are Zinc-dependent endopeptidases that are secreted as proenzymes and then activated by the cleavage of the N-terminal peptide responsible for maintaining the latency of the enzyme (Matrisian, 1992; Liotta, 1992). Agents that regulate the secretion or activities of MMPs would be expected to limit the metastatic potential by preventing the degradation of basement membranes and the stromal connective tissue (Matrisian, 1990).
The prevention of neoplasia by agents that inhibit proliferation and stimulate differentiation appears to be an exciting prospect. The flavonoids, which are remarkable nontoxic (Rice-Evans et al., 1996) and could inhibit PTK activity, appear to have promise as anti-cancer agents and one worth further investigation. There were three objectives in this study. First purpose was to investigate the effects of flavonoids of different classes on the kinase activity and phosphorylation of cellular proteins in cancer cells. Also, the effect of flavonoids on EGF-induced tyrosine phosphorylation of cellular proteins including EGFR was determined. Second was to determine the effect of flavonoids on the basal and EGF-induced proliferation of cancer cells. Further, the molecular mechanism of the inhibitory effect of flavonoids on cell growth was determined. The third objective was to investigate the effect of flavonoids on the potential of cancer metastasis through determining their effects on the secretion of MMPs in cancer cell lines.
Methods
Quercetin and d-catechin, were purchased from Nacalai Tesque (Kyoto, Japan). Kaempferol, genistein, taxifolin, and naringenin were obtained from Sigma (St. Louis, MO, U.S.A.). Luteolin was from Extrasynthese (Genay, France). Wogonin was purchased from Kishida (Tokyo, Japan). The flavonoids were dissolved in dimethyl sulphoxide (DMSO), and stored in the dark at a concentration of 100 mM. RPMI-1640, Dulbecco's Modified Eagle Medium (DMEM) and foetal bovine serum were obtained from GIBCO (Grand Island, NY, U.S.A.). EGF, anti-phosphotyrosine, and anti-EGFR antibodies were purchased from UBI (Lake Placid, NY, U.S.A.). Purified human MMP-2, MMP-9, and anti-MMP-2 and MMP-9 antibodies were from Biogenesis (Sandown, NH, U.S.A.). Enhanced chemiluminescence reagent (ECL) and [γ-32P]-ATP were obtained from Amersham (Buckinghamshire, U.K.). Unless otherwise indicated, all other reagents were purchased from Sigma.
Growth experiments
The A431 (skin), MiaPaCa-2 (pancreas), Hep G2 (liver), and MCF-7 (breast) tumour cell lines were obtained from American Type Culture Collection (ATCC; Rockville, MD, U.S.A.). Cells were maintained at 37°C in a humidified atmosphere (95% air and 5% CO2) and grown as monolayers in plastic tissue flasks containing DMEM or RPMI-1640 supplement with 10% foetal bovine serum (GIBCO), 100 units ml−1 penicillin (GIBCO) and 100 μg ml−1 streptomycin (GIBCO) according to the recommendation of ATCC.
Cells at logarithmic growth phase were harvested using 0.25% trypsin-EDTA solution for 5 min, pelleted by centrifugation at 500×g for 5 min, washed once with medium and resuspended at a concentration of 1×104 cells ml−1 RPMI-1640 medium. Cells of 1×104 or 1×105 were inoculated in 24-well plates and 100×20 mm dish, respectively. The cells were then incubated at 37°C for 24 h to allow the attachment to plates after which the culture media were changed and flavonoids were added and provided final concentrations of 10, 20, 50 and 100 μM for varying time intervals. Cells were also treated with EGF at a concentration of 10 nM. Control wells received DMSO vehicle at a final concentration of 0.1%. This concentration was not found to affect cell growth. Cells were then incubated at 37°C in a 5% CO2−95% air atmosphere for various periods of time. At the end of incubation, cells (from triplicate wells representing each treatment condition) were harvested with 0.25% trypsin and 0.02% EDTA and counted using a Coulter Multisizer II counter (Coulter Electronics, Luton, U.K.). Cell viability was also determined using trypan blue dye exclusion method. Cell numbers was also determined as previously described (Mosmann, 1983) using a colourimetric assay with the reduction of 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide (MTT) as the assessable end-point.
Preparation of cell lysates
Cell lines were grown as described above. EGF- and flavonoid-treated cells were collected by trypsization and washed three times with PBS. The cells were then lysed in gold lysis buffer (GLB) containing 20 mM Tris, pH 7.9, 137 mM sodium chloride, 10% (v v−1) glycerol, 1% (v v−1) Triton X-100, 1 mM sodium orthovanadate, 1 mM EGTA, 10 mM sodium fluoride, 1 mM sodium pyrophosphate, 100 μM β-glycerophosphate, 10 μg ml−1 leupetin, 10 μg ml−1 aprotinin and 2 mM phenylmethylsulphonyl fluoride (PMSF) (Samuels et al., 1993). Cell lysates were cleared by centrifugation at 12,000×g for 10 min at 4°C. The protein concentration of cell lysate was determined according to the method of Bradford (Bradford, 1976) and adjusted to 5 μg μl−1. The samples were then divided into 50-μl aliquots and stored at −70°C for further study.
Analysis of DNA fragmentation
Tumour cells at logarithmic growth phase were treated with various flavonoids at a concentration of 20 μM for 24, 48, or 72 h. Cells were then collected by cell scraper and centrifuged at 500×g for 5 min. The cell pellet was then washed twice with PBS, and DNA was isolated and quantified by the method of Armstrong et al. (1992). Ten micrograms of DNA was loaded into each well of 1.8% agarose gel and electrophoresed in a buffer containing 0.04 M Tris, 0.04 M sodium acetate and 1 mM EDTA, pH 8.0, at 100 V for 3 h at room temperature. Gels were stained with ethidium bromide, visualized by u.v. fluorescence, and photographed.
Total kinase activity assay
The total kinase assay procedures were performed as originally described by Cohen et al. (1982) with modifications (Lee et al., 1991). Briefly, the reaction mixtures in a final volume of 80 μl contained 50 μg cellular proteins in 20 mM HEPES buffer, pH 7.4, 1.0 mM MnCl2, and 20 μM flavonoids or 100 nM EGF, respectively. The reaction mixtures were preincubated at room temperature for 2 h and then chilled at 4°C for 10 min. The reaction was initiated by the addition of [γ-32P]-ATP (6–12×106 c.p.m.) and unlabelled ATP (60 μM). After 5 min, the reaction was stopped by pipetting 50 μl aliquots of the reaction mixtures onto 2.3-cm-diameter Whatman 3MM filter papers, which were immediately dropped into a solution of 10% trichloroacetic acid containing 0.01 M pyrophosphate. The filter papers were then washed sequentially with 10% trichloroacetic acid, 50% and 100% ethyl alcohol (10 ml each), and dropped into a beaker with 50 ml diethyl ether for 2 min and then air dried in a fume hood. The radioactivity was measured in a Beckman scintillation counter.
Immunoprecipitation
Cell lysates with equivalent amounts of proteins were incubated with anti-EGFR polyclonal antibodies (1 μg ml−1) for 4 h at 4°C with gentle shaking according to the manufacturer's recommendations. EGFR/anti-EGFR antibodies immuno-complexes were removed by centrifugation at 14,000×g at 4°C for 20 min. The supernatants were collected and subjected to total kinase assay as described above.
Gel electrophoresis, Western blotting and autoradiography
The same kinase assay reaction mixtures described above were also subjected to SDS-polyacrylamide gel electrophoresis (SDS–PAGE) to examine further changes of cellular protein phosphorylation in response to EGF and flavonoids. The kinase reactions were terminated by the addition of 50 μl sample treatment solution (0.5 M Tris-HCl, pH 6.9; 2% SDS; 20% glycerol; .01% bromophenol) followed by boiling for 3 min. The reaction products then were electrophoresed on 3–18% linear gradient SDS-polyacrylamide gels according to the method of Laemmli (Laemmli, 1970). Proteins were then electrophoretically transferred to nitrocellulose membranes by the method of Towbin (Towbin et al., 1979) and then autoradiographed with Kodak X-Omat AR film (Kodak, Rochester, NY, U.S.A.) between two intensifying screens at −70°C for 24 h. The intensity of 32P-labelled proteins was determined using a densitometer (Vilber Lourmat, France).
Detection and characterization of MMPs by gelatin zymography
The amounts of MMPs secreted from tumour cells treated with 10 nM EGF and 20 μM flavonoids were measured using gelatin zymography (Heussen & Dowdle 1980; Herron et al., 1986). In brief, samples of conditioned media and cell lysates were subjected to electrophoresis on 3–18% linear gradient SDS-polyacrylamide gels copolymerized with 0.1% porcine skin gelatin. The volume of each medium sample analysed was normalized according to the cell number. Electrophoresis was performed under non-reducing conditions in 25 mM Tris, 192 mM glycine, and 0.1% SDS at 15 mA gel−1 during stacking and at 12 mA gel−1 during separation. After electrophoresis, gels were first washed twice for 30 min in 2.5% Triton X-100 to remove SDS, and then twice in reaction buffer (50 mM Tris-HCl, pH 8.0, containing 5 mM CaCl2, 0.02% NaN3) for an additional 30 min. The gels were then incubated in reaction buffer at 37°C for 16 h, and then stained with 0.25% Coomassie brilliant blue R-250 in 10% acetic acid/ 30% methanol for 30 min and destained for 6 h in the same solution without dye. A clear zone on the gel indicates the presence of gelatinase activity. To characterize the gelatinases produced by cancer cells according to their sensitivity to specific inhibitors, gels (following electrophoresis and Triton-buffer wash) were incubated in reaction buffer containing MMP inhibitors (10 mM EDTA, or 1 mM 1,10-phenanthroline) (Hibbs et al., 1985). Gels were then processed as described above.
Human tumour samples
Liver, ovarian, prostate, and breast cancer tissue samples were obtained with informed consent from patients (National Taiwan University Hospital) and collected after surgical resection. Human tumour tissues were homogenized in PBS buffer, pH 7.2, containing 0.05% NP-40 (3 ml g−1). The soluble proteins were separated from pellets by centrifugation at 5000×g for 30 min. Crude human tissue proteins were then precipitated with 40% ammonium sulphate. After dialysis against 0.02 N Tris buffer, PH 7.4, the protein concentrations were determined according to the method of Bradford (Bradford, 1976). The tumour extracts were then subjected to the total kinase assay as previously described (Lee et al., 1991; Liebow et al., 1994). All human subjects involved in this study were under the authorization of Human Subject and Utilization Committee of Institute of Biological Chemistry, Academia Sinica.
Western blot analysis of EGFR, phosphotyrosine protein, and MMPs
After proteins were electrophoresed on 3–18% linear gradient SDS-polyacrylamide gels, the proteins were then electrophoretically transferred to nitrocellulose membrane according to the method of Towbin (1979). The electrophoretic blots were blocked in PBS containing 5% BSA for 2 h at room temperature, and then were rinsed in PBS three times and incubated for 2 h at room temperature with primary antibody appropriately diluted in PBS/1% BSA. The primary antibodies used were mouse monoclonal IgG-anti human EGFR (Upstate Biotechnology, Lake Placid, NY, U.S.A.), mouse monoclonal IgG-anti phosphotyrosine (Upstate Biotechnology, Lake Placid, NY, U.S.A.), and rabbit IgG anti-human MMPs (Biogenesis, Sandown, NH, U.S.A.). The nitrocellulose membranes were then extensively washed three times for 10 min each in PBS containing 0.1% Tween-20 (PBST), and incubated with secondary antibody (goat anti-rabbit or rabbit anti-mouse IgG) conjugated with horseradish peroxidase (1 : 10,000 dilution) (Transduction Lab., Lexington, KY, U.S.A.). The membranes were subsequently washed three times with PBST and twice with PBS. Bands were detected with enhanced chemiluminescence reagents on Kodak BioMax film. To ascertain that whether the 170 kDa phosphotyrosyl protein band detected by anti-phosphotyrosine antibody is EGFR, the same membrane was stripped of bound primary anti-phosphotyrosine antibody and secondary antibodies, and reprobed by incubating with anti-EGFR antibody and secondary antibody as described above.
Statistics
Results are expressed as mean±s.e.mean of 3–6 independent experiments. Statistical significance between two groups was determined by mean of an unpaired Student's t-test. A probability of P⩽0.05 was considered significant.
Results
Effects of flavonoids on kinase activities of cellular proteins of cancer cell lines and solid tumours
To determine the relationship between chemical structure of flavonoids on the kinase activities of cellular proteins in A431 cells, eight different flavonoids of five classes were examined: luteolin and wogonin (flavones); quercetin and kaempherol (flavonols); taxifolin and naringenin (flavanones); d-catechin (catechins); and genistein (isoflavone). The structures of these flavonoids are shown in Figure 1.
Figure 1.
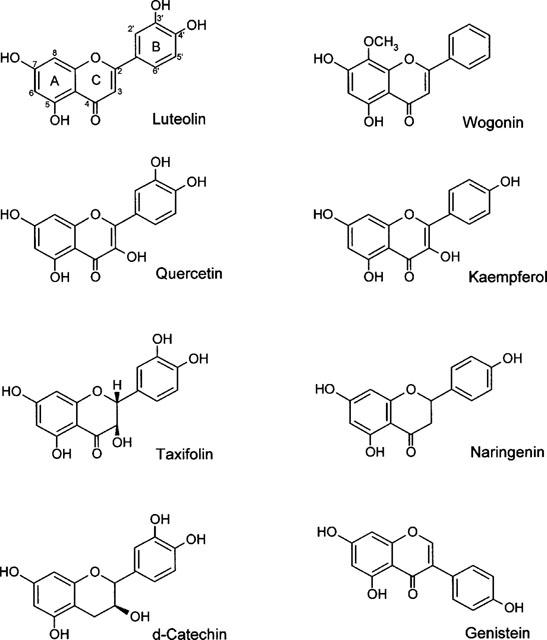
The chemical structures of flavonoids. Flavone: luteolin and wogonin; Flavonol: quercetin and kaempferol; Flavanones: taxifolin and naringenin; Catechin: d-catechin; Isoflavone: genistein.
The total kinase activities of the cellular proteins was measured as incorporation of 32P from [γ-32P]-ATP, in response to the various treatments. The addition of EGF to the reaction mixture resulted in a 25±4.6% increase in total phosphorylation, while luteolin, quercetin and genistein decreased the total phosphorylation by −47±9.9, −27±6.1 and −17±3.6%, respectively, as compared with the control value (Table 1). All other flavonoids produced no change or less than 10% decrease of the total phosphorylation. We further analysed the phosphorylation pattern of cellular proteins that were treated with EGF or each of the eight flavonoids using SDS-polyacrylamide gel electrophoresis. Among these flavonoids, only luteolin (Lu) and quercetin (Qu) at 20 μM greatly reduced the phosphorylation of most proteins including a 170-kDa protein, seen in the control sample with the exception of the 69-kDa band (Figure 2A). Also, genistein at 20 μM slightly reduced (about 12%) the phosphorylation of the 170-kDa protein. None of the other flavonoids produced detectable changes in protein phosphorylation. Administration of EGF to A431 cells, served as a positive control, stimulated the phosphorylation of most protein bands including the 170-kDa band (Figure 2A). In order to address whether the activity seen at 170-kDa represents EGFR, we performed immunoprecipitation using EGFR antibodies to remove EGFR prior to the determination of total kinase activity of A431 cellular proteins. This resulted in a great reduction of the phosphorylated 170-kDa band (Figure 2B).
Table 1.
Effect of flavonoids on the phosphorylation of A431 cellular protein
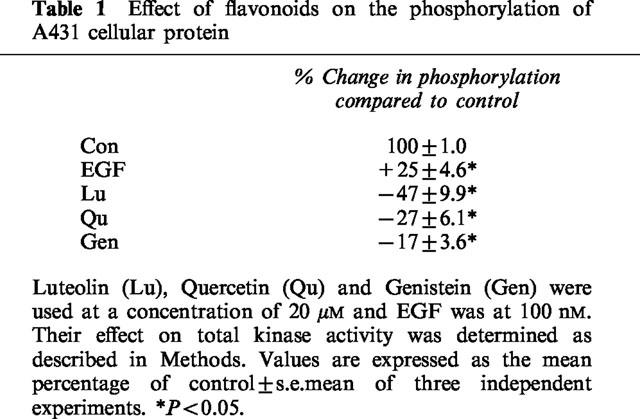
Figure 2.
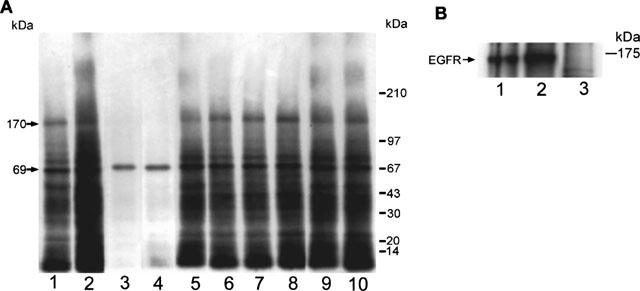
Effect of flavonoids and EGF on the kinase activity of A431 cellular proteins. (A) Cell lysates of 50 μg proteins were incubated with various flavonoids at 20 μM or with 100 nM EGF in the presence of [γ-32P]-ATP. Lanes: 1, control; 2, EGF; 3, luteolin; 4, quercetin; 5, genistein; 6, Taxifolin; 7, d-catechin; 8, naringenin; 9, kaempferol; 10, wogonin. Note that luteolin and quercetin both dramatically inhibited the phosphorylation of most protein bands, including the EGF receptor band (170-kDa), whereas genistein produced only a marginal (12%) decrease of the phosphorylation level of the 170-kDa band. (B) Cell lysates with equivalent amounts of protein (50 μg) were immunoprecipitated with anti-EGFR antibodies. After centrifugation, the supernatants were collected and then subjected to total kinase activity assay as described above. Lanes: 1, cell lysate; 2, EGF+cell lysate; 3. EGFR-removed cell lysate.
Owing to the dramatic inhibitory effect of luteolin and quercetin on kinase activity of cellular proteins of A431 cells, we also tested their effects on several other cancer cell lines. Both luteolin and quercetin significantly inhibited total kinase activities of cellular proteins in hepatic cancer cells Hep G2, breast cancer cells MCF-7 and pancreatic cancer cells MiaPaCa-2 (−30±4.5 and −23±2.7% for HepG2; −24±3.6 and −33±5.3% for MCF-7; −42±6.6 and −37±5.1% for MiaPaCa-2, respectively). In addition, we examined the effect of five flavonoids: luteolin, quercetin, d-catechin, genistein and taxifolin on the kinase activities of solid tumour proteins. Both luteolin and quercetin showed the inhibitory effect on the endogenous kinase activity of hepatic cancer homogenates, while d-catechin, genistein and taxifolin exhibited a lesser extent of inhibition (Figure 3). In response to luteolin and quercetin, similar effects of inhibition were seen in the ovarian, breast and prostate tumour samples.
Figure 3.
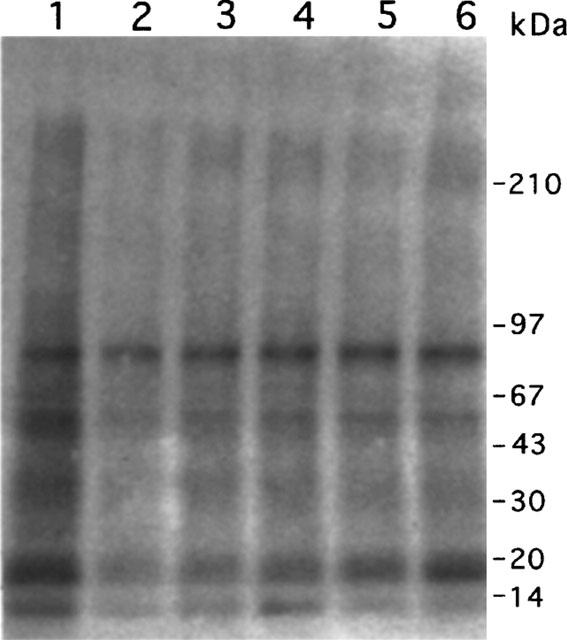
Effects of flavonoids on the kinase activity of liver tumour proteins. Liver tumour extracts of 50 μg proteins were incubated with various flavonoids (20 μM) in the presence of [γ-32P]-ATP. Lanes: 1, control; 2, luteolin; 3, quercetin; 4, d-catechin; 5, genistein; 6, taxifolin.
Inhibitory effect of luteolin and quercetin on EGF-induced tyrosine phosphorylation
In order to determine whether flavonoids might inhibit the EGF-induced tyrosine phosphorylation, A431 cell lysates were treated with EGF alone or in combination with various concentrations of luteolin or quercetin. Samples were analysed for the presence of phosphotyrosine proteins by immunoblotting. Incubation of cellular proteins with 100 nM EGF for 1 h consistently increased tyrosine phosphorylation of several proteins including the 170-kDa band as compared to the control (Figure 4A, lanes 1 and 2). Luteolin (Lu) greatly inhibited the EGF-stimulated tyrosine phosphorylation of most proteins in a dose-dependent manner from 10–80 μM (Figure 4A, lanes 3–6). Quercetin (Qu) exhibited similar effect as luteolin did. This 170-kDa phosphotyrosyl protein was identified as EGFR by reprobing the same blot with EGFR antibody (Figure 4B). Based on Figure 4A and B, it is noted that luteolin and quercetin suppressed the EGF-induced tyrosine phosphorylation of EGFR while both flavonoids had no effect on the EGFR protein levels under this condition.
Figure 4.
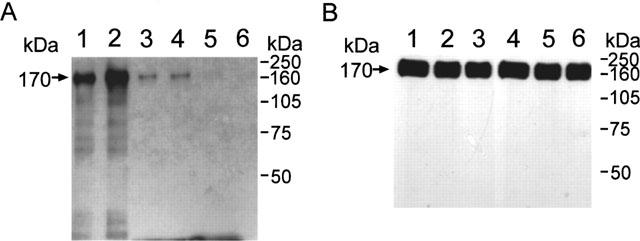
Effect of luteolin (Lu) and EGF on the tyrosine kinase activity of A431 cellular proteins. Cell lysates of 50 μg proteins, in the presence of ATP, were incubated with or without various concentrations of luteolin and EGF. The reaction mixtures were subjected to immunoblotting analysis. Lanes: 1, Control; 2, 100 nM EGF; 3–6, luteolin of 10, 20, 40 and 80 μM in the presence of 100 nM EGF, respectively. Anti-phosphotyrosine protein antibody was used to detect phosphotyrosyl protein (A). The same blot was then stripped off primary and secondary antibodies, and reprobed with anti-EGFR antibody (B). Note that similar amounts of the 170-kDa EGFR band were observed in all six lanes (B), while the phosphorylation levels were varied (A).
Effects of flavonoids on the growth of cancer cell lines
It has been suggested that the extent of tyrosine phosphorylation is an important indicator for cell growth, thus we examined the effects of flavonoids on the growth of A431 cells in culture. Treatment of A431 cells with flavonoids at 20 μM for 24 h revealed that luteolin and quercetin were the two most effective growth inhibitors (approximately −23±3.1 and −21±2.4%, respectively) amongst the eight flavonoids investigated (Figure 5A). Genistein, taxifolin and d-catechin exhibited slight inhibitory effect on cell growth, while naringenin, kaempferol and wogonin had no effect. EGF at 10 nM stimulated the growth of A431 cells by about 25±4.8%. Luteolin and quercetin also suppressed the EGF-stimulated growth of A431 cells (Figure 5B).
Figure 5.
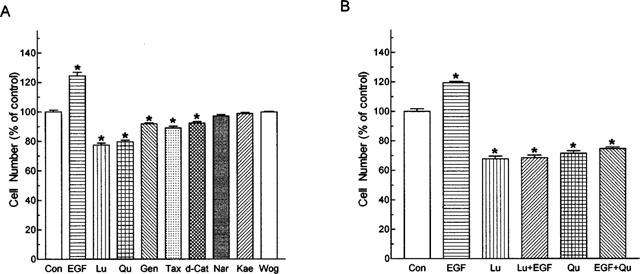
Effects of flavonoids on the growth (A) unstimulated and (B) EGF-stimulated A431 cells. Cells were treated with flavonoids (20 μM) and/or EGF (10 nM) for 24 h. Cell numbers of the culture were then determined. Each bar represents the mean (±s.e.mean) of six replicates from a representative experiment. Three separate experiments were performed and all showed similar results. The mean cell number of control groups was 6.08×105. Con, control; Lu, luteolin; Qu, quercetin; Gen, genistein; Tax, taxifolin; d-Cat. d-catechin; Nar, naringenin; Kae, kaempferol; Wog. wogonin.
Luteolin and quercetin were further demonstrated to inhibit the growth of A431 cells in a dose-dependent (10–50 μM) and a time-dependent manner (12–72 h) (Figure 6). This inhibitory effect was already evident after 24-h treatment. The estimated dose of growth inhibition (IC50) for luteolin and quercetin were 19 and 21 μM, respectively.
Figure 6.
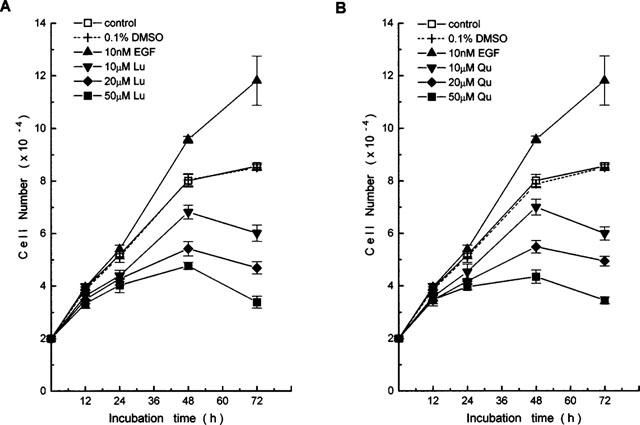
Effects of (A) luteolin and (B) quercetin on the growth of A431 cells. Cells were treated with various concentrations of flavonoids for 12–72 h. At the indicated time, cell numbers of the cultures were determined. Each point represents the mean (±s.e.mean) of triplicate wells from one of three separate representative experiments, all of which gave similar results, Lu, luteolin; Qu, quercetin.
The potential of luteolin and quercetin to inhibit cell proliferation was further supported by studies of HepG2, MCF-7, and MiaPaCa-2 cell lines. The percentages of growth inhibition by luteolin and quercetin at concentration of 20 μM were: −14.4±1.1 and −13.0±2.1% for HepG2; −13.5±0.9 and −13.0±1.5% for MCF-7; −24.1±1.8 and −25.3±1.6% for MiaPaCa-2, respectively (Figure 7).
Figure 7.
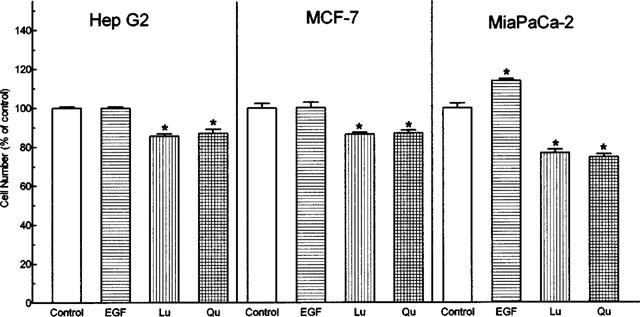
Effects of EGF, luteolin (Lu) and quercetin (Qu) on the growth of HepG2, MCF-7 and MiaPaCa-2 tumour cell lines. Ten nM EGF, 20 μM Lu or Qu were added to the cultures at time zero. After 48 h, the cells were harvested and the numbers were determined. The cell numbers of control groups in HepG2, MCF-7, and MiaPaCa 2 were 6.23×105, 6.18×105, and 6.90×105, respectively. Each bar represent the mean (±s.e.mean) of triplicate wells from one of three separate experiments, all of which gave similar results.
Mechanism of luteolin- and quercetin-induced inhibition of cell proliferation
A431 and MiaPaCa-2 cell lines are known to possess EGFR (Cohen et al., 1982; Lee et al., 1991), and EGF promotes growth of both cell lines. The dephosphorylation of EGFR can also be responsible for tumour growth inhibition (Lee et al., 1991; 1997). Therefore, A431 and MiaPaCa-2 are ideal for investigating the involvement of tyrosine kinases in signal transduction in regulation of cell growth.
The cell morphology and the molecular integrity of DNA were examined in A431 cells after incubation with luteolin and quercetin to reveal how these two agents affect cell homeostasis. A431 cells treated with 20 μM luteolin or quercetin for 24 h exhibited protuberant cytoplasmic blebs and progressive shrinkage, as shown in Figure 8. The cells then shrank to a round configuration, and detached from the flasks after a 48–72 h incubation period. In addition, A431 cells treated with either luteolin or quercetin exhibited a loss of DNA integrity, showing a ladder pattern of multiples of 180–200 base pairs, characteristic of apoptosis of A431 cells (Figure 9).
Figure 8.
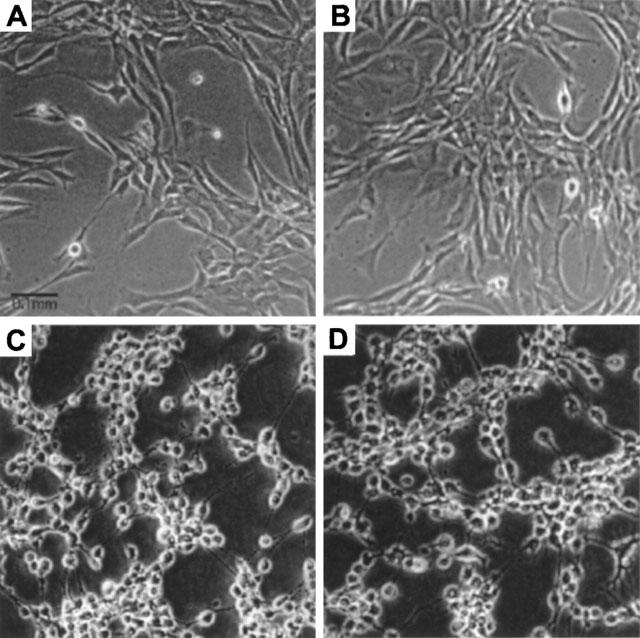
Effects of EGF, luteolin and quercetin on the morphology of A431 cells. Cells treated with 10 nM EGF, 20 μM luteolin, or 20 μM quercetin for 48 h. Cells were photographed under phase contrast microscopy. (A) control; (B) EGF; (C) luteolin and (D) quercetin. Magnification 200×.
Figure 9.
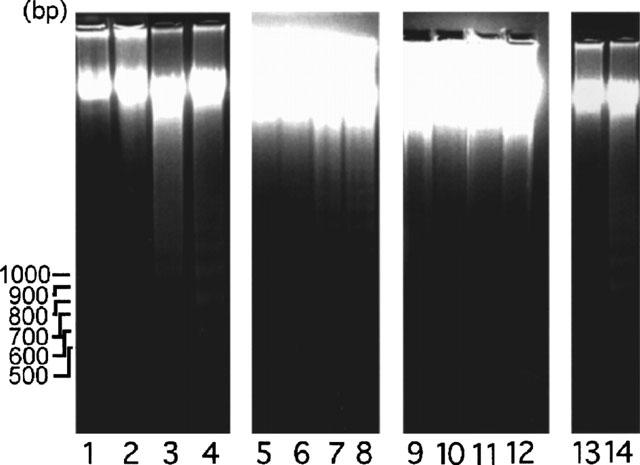
Effects of luteolin, quercetin and genistein on DNA integrity of A431 cells. Cells were treated with 0, 20, 50 and 100 μM of luteolin (lanes 1–4), quercetin (lanes 5–8) or genistein (lanes 9–12) for 24 h. Lane 13, A431 cells treated with 50 μM luteolin for 24 h and then treated with 10 nM EGF for 24 h. Lane 14, A431 cells treated with 50 μM for 48 h. At the end of the culture, approximately 2×106 cells were harvested. DNA was isolated from each sample and subjected to electrophoresis in a 1.8% agarose gel. EGF was shown to abrogate DNA degradation caused by luteolin (lane 13) and quercetin (data not shown). This ladder pattern of DNA fragments is a characteristic of apoptosis.
To determine if cell growth inhibition by luteolin (or quercetin) could be reversed by the addition of EGF, we repeated the culture experiments as described above, except that after 48 h luteolin (20 μM) was replaced with fresh medium containing 10 nM EGF. For the cells continuously treated with luteolin or quercetin for an additional 24 h, the cell growth remained inhibited. However, when flavonoids were removed and cells were incubated with EGF alone, the cells started growing and became viable. No nuclear digestion was observed (Figure 9, lane 13). This finding suggests that limited exposure to luteolin and quercetin does not condemn these cells to apoptosis.
Luteolin and quercetin suppress and EGF stimulates metastasis-associated properties
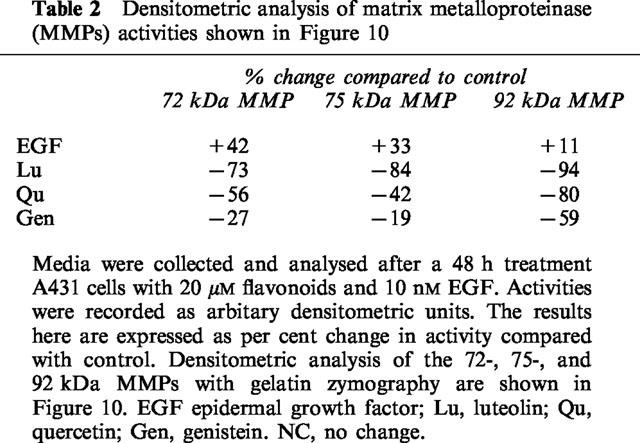
Our preliminary data indicated that EGF not only promotes growth of A431 cells and stimulates phosphorylation of membrane proteins, but also stimulates the secretion of MMPs (Lee et al., 1997). This prompted us to investigate whether flavonoids would oppose the action of EGF on the secretion of gelatinases. To determine whether or not luteolin and quercetin exert inhibitory effects on gelatinase activity, we first examined conditioned media, and cell lysates for gelatinase activity. Control cells secreted significant quantities of two gelatinases (92-kDa and 72-kDa), as well as smaller quantities of 75-kDa band. Secretion of the 92-, 75- and 72-kDa gelatinases was stimulated by EGF and inhibited by luteolin and quercetin (Figure 10A). Genistein also exerted a strong inhibitory effect on secretion of these gelatinases. The inhibitory effects of luteolin, quercetin, and genistein on the secretion of these gelatinases are summarized in Table 2.
Figure 10.
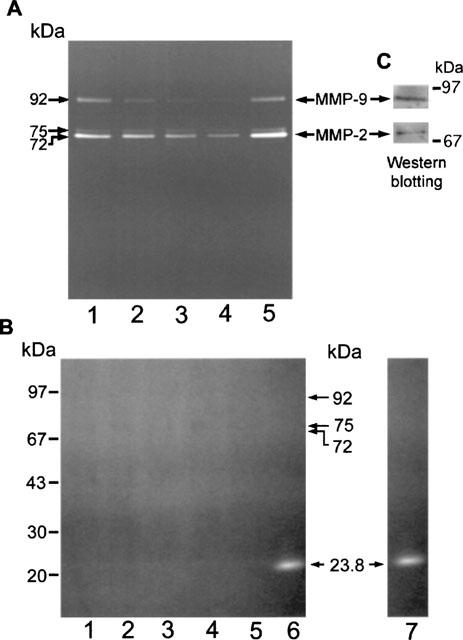
Effects of flavonoids and EGF on the secretion of gelatinases in A431 cells. Cells at 40% confluence were treated with vehicle (lane 1), 20 μM genistein, luteolin, or quercetin (lanes: 2–4, respectively), 10 nM EGF (lane 5) in serum-free medium for 48 h. The conditioned or without flavonoids and EGF (lane 1) in serum-free medium for 48 h. The conditioned media were collected and subjected to gelatin zymography analysis. Representative zymograms of gels in the absence (A) or the presence (B) of a metalloproteinase inhibitor 5 mM 1,10-phenanthroline (or EDTA) during substrate buffer incubation. Trypsin (lane 6), a serine protease, was not inhibited by metalloproteinase inhibitor. The conditioned media were collected and normalized by cell numbers prior to gelatin zymography analysis. (C), Immunoblotting analysis using anti-MMP-9 and MMP-2 indicated that the identity of 92- and 72-kDa gelatinases were MMP-9 and MMP-2, respectively.
Table 2.
Densitometric analysis of matrix metalloproteinase (MMPs) activities shown in Figure 10
The secreted gelatinases were further characterized according to the biochemical dependence of the enzyme activity and their immunoreactivity. Addition of 5 mM 1,10-phenanthroline (Figure 10B) or 10 mM EDTA (data not shown), abolished the gelatinase activity of all three gelatinases except that trypsin (Figure 10B, lane 6) was observed to retain proteolytic activity. Trypsin was also observed showing the proteolytic activity in the absence of 1,10-phenanthroline (Figure 10B, lane 7). Immunoblotting analysis demonstrated that the 92-kDa gelatinase is MMP-9 and the 72-kDa gelatinase is MMP-2 (Figure 10C); both are type IV collagenases. The identity of the 75-kDa gelatinase remains to be determined.
The cell gelatinase content was examined to determine if flavonoid treatment primarily inhibits the secretion of gelatinases. A431 cells cultured in the presence of luteolin, quercetin, DMSO, or EGF were harvested and cell lysates were assayed for gelatinase activity. The activities of most of the gelatinases in the cell lysates were too weak for any changes to be detected, with the exception of the 92-kDa gelatinase (Figure 11). Treatment of cells with luteolin caused a notable increase in the cellular content of the 92-kDa gelatinase, whereas EGF treatment caused the opposite effect. This result, together with the data shown in Figure 10, indicates that EGF stimulates the secretion of the 92-kDa gelatinase and thereby reduces the cellular content, whereas luteolin exerts the opposite effect, inhibiting secretion of this gelatinase. Similar results were observed using quercetin. This result suggests that suppression of EGFR tyrosine kinase activity by luteolin and quercetin could lead to down-regulation MMP secretion.
Figure 11.
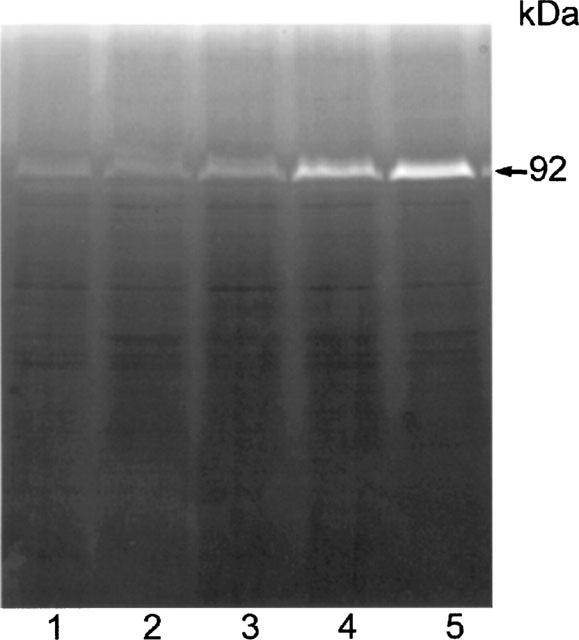
Effect of luteolin on gelatinase activity of A431 cell lysates. Cells were treated with EGF or luteolin for 24 h. Cell lysates containing 50 μg proteins were analysed by gelatin zymography. Lanes: 1, control; 2, EGF; lanes 3–5; 10, 20 and 50 μM luteolin, respectively. Similar results were observed in A431 cells using the same concentration of quercetin in the same experiments.
Discussion
Inhibition of cellular protein kinase activity and/or activation of protein phosphatase activity correlate with growth inhibition in Du145 (prostate) (Peterson & Barnes, 1993), A431 (skin) and MiaPaca-2 (pancreas) (Liebow et al., 1989; Lee et al., 1991) tumour cells. Flavonoids have been found to inhibit the activity of protein tyrosine kinases, with the same potency as they inhibit growth of human gliosarcoma and squamous cell carcinoma (Middleton & Kandaswami, 1993; Kandaswami et al., 1991; 1992); ovarian (Scambia et al., 1990) and NIH3T3 cells (Linassier et al., 1990). This correlation between the efficacy of kinase activity inhibition and growth inhibition suggests that net phosphorylation of one or more signal molecules may well be the determining factor for growth control. This phenomenon was also observed in parallel studies using a tyrosine phosphatase stimulator (Lee et al., 1991; Liebow et al., 1989). It is therefore of great interest to examine and select the more potent flavonoids capable of inhibiting both kinase activity and growth of tumour cells.
In the present study, we examined the effects of eight flavonoids of different classes on the growth of tumour cell lines. Our data indicate that among the flavonoids investigated, luteolin and quercetin were the most potent inhibitors of the proliferation of A431 and other cell lines, such as MiaPaCa-2, MCF-7, and Hep G2. Luteolin and quercetin inhibited A431 proliferation in a dose dependent manner. This is in agreement with other reports that flavonoids have anti-proliferative effects upon human cancer cells in vitro (Scambia et al., 1990; Fotsis et al., 1997). In this study we intended to determine whether the anti-proliferative mechanism might correlate with decreases in cellular protein kinase activities. The EGFR tyrosine kinase activity was reported to be suppressed by genistein (Akiyama et al., 1987; Linassier et al., 1990) and quercetin (Agullo et al., 1997). Relating a particular cellular response, such as growth rate, to changes in cellular protein phosphorylation in response to a particular tyrosine kinase stimulator like EGF or a tyrosine kinase inhibitor like luteolin is a rather crude measure of intracellular signalling. However, our observations indicated that both luteolin and quercetin inhibit EGFR tyrosine kinase activity, suggest the hypothesis that these two agents may contribute to inhibiting growth in A431 cells by down-regulating EGFR. The effects of EGFRs on cell growth are known to be mediated through tyrosine phosphorylation. Therefore, the inhibitory effect of luteolin and quercetin on growth of A431 cells might be the result of changes of phosphorylation of certain regulatory proteins including EGFR (Figures 2 and 4).
The response of solid tumour proteins to luteolin, in terms of phosphorylation, was also examined. As shown in Figure 3, liver carcinoma homogenates showed a marked decrease in the levels of protein phosphorylation in response to luteolin and quercetin in vitro. Similar phosphorylation patterns were observed in breast, prostate, and ovarian carcinoma proteins (data not shown). However, these results could not indicate which of those proteins are particularly affected by the addition of flavonoids. Also, the failure to detect a clearly phosphorylated 170-kDa band under these conditions could be explained by the fact that the amount of 170-kDa protein present in tumour extract is not sufficient to yield measurable phosphorylation (Lee et al., 1991). The results of this work demonstrated the existence of endogenous kinases in tumours, and that these kinases can be inhibited by flavonoids in vitro. These data suggest that luteolin and quercetin might reduce the growth rates of cancers in vivo. The growth inhibition of individual cancers in response to luteolin and quercetin might be predicted by testing the reduction in the levels of phosphorylation produced by flavonoids in tissue samples of individual cancers.
Having established the inhibitory effects of luteolin and quercetin on kinase activity (Figures 234) and cell proliferation (Figures 567), we considered it important to determine whether these two agents exhibit a structure-activity relationship in terms of biological function. Flavonoids are competitive inhibitors for the ATP binding site on a variety of enzymes such as PKC (Graziani et al., 1983), a region of considerable homology among kinases. Of the eight flavonoids tested, luteolin and quercetin exerted the strongest inhibitory effects on cell growth, kinase activity, and MMP secretion. Luteolin and quercetin have similar structures, with the same double bond between C2 and C3 in ring C, and the same OH groups on C3′ and C4′ in ring B (Figure 1). From a structural point of view, we suggest that the double bond between C2 and C3 results in ring B and ring C being on the same plane, which might be critical for access to the kinase substrate binding site. Based on our data and others (Agullo et al., 1997), we suggest that the two adjacent polar OH groups on C3′ and C4′ of ring B are required for suppression kinase activity. Di-hydrogenation of the double bond at C2 and C3 on ring C (taxifolin) or the absence of one or two OH groups on ring B (kaempferol, wogonin), or both (naringenin), results in abolishing the biological activity. It is noteworthy that the position, number and substitution of the hydroxyl group of the B ring, and saturation of the C2–C3 bond are important factors affecting flavonoids inhibition of phosphatidylinositol 3-kinase reported by Agullo et al. (1999). However, a more systematic study, employing X-ray crystallography of luteolin (or quercetin) and known kinase data, is required to elucidate the detailed structure-function relationship involved in the interaction of flavonoids and ATP binding sites. This data may provide a basis for the further design of specific inhibitors of protein kinases.
Studies using leukaemia cell lines suggest a role of tyrosine phosphorylation in the regulation of apoptosis (Yousefi et al., 1994). Tyrosine kinase inhibitors, such as genistein and herbimycin A, can block the effect of growth factors on growth factor-dependent cell lines (Akiyama et al., 1987; Otani et al., 1993). Apoptosis is often associated with a characteristic pattern of oligonucleosomal DNA fragmentation, and such DNA fragmentation in A431 and MiaPaCa-2 cells became evident after 24 h treatment with luteolin and quercetin (Figure 9) in culture. As shown in Figure 9, lane 13, we found that the addition of EGF to the Lu-treated cells abrogates the induction of apoptosis. The mechanism responsible for which is not understood yet, however, our result and previous reports (Yousefi et al., 1994; Savill et al., 1993) support the hypothesis that tyrosine phosphorylation is a ‘balancing act' between activation and inhibition of apoptosis in tumour cells. More evidence is required before a comprehensive model can be formulated to explain the control of apoptosis; however, the evidence presented here suggests that changes in the phosphorylation levels of certain proteins may well be intimately linked to this process.
MMPs have been implicated in processes leading to cancer invasion and metastasis (Liotta et al., 1991; 1992), and may also play a major role in tumour angiogenesis (Liotta, 1992). These contentions are supported by the fact that the levels of some MMPs are elevated in certain cancer types. These enzymes, therefore, appear to be the appropriate targets for the development of anti-cancer and anti-metastasis agents. Of considerable interest in this study was the marked decrease in the secretion of the 92- and 72-kDa gelatinases from A431 cells in response to luteolin and quercetin. These two gelatinases appear to be type IV collagenases, i.e. MMP-9 and MMP-2, as demonstrated by Western blotting analysis. MMP-9 and MMP-2 are expressed in various human epithelial cancers, and their levels seem to be related to the metastatic potential and malignancy (McDonnel et al., 1990; Davis et al., 1993; Naylor et al., 1994; Hamdy et al., 1994; Liabakk et al., 1996). Synthetic peptides which inhibit MMP activity can block malignant tumour growth in animals (Watson et al., 1995; Cha et al., 1996). The expression and activities of MMPs are regulated at several levels (Matrisian, 1990). Studies over the past decade have shown that MMPs are regulated by a variety of growth factors (Graziani et al., 1983; Kerr et al., 1988; 1990; McDonnel et al., 1993). EGF, the typical example, was found to induce the secretion of MMPs (Kerr et al., 1990). Our study also demonstrated that EGF stimulates the secretion of MMP-9 and MMP-2 from A431 cells (Figure 10). These results, therefore, raise the possibility that EGF not only enhances the secretion of MMPs, but might also in turn enhance the metastatic potential of tumours. Conversely, agents that inhibit EGFR tyrosine kinase activity may have potential in the prevention of metastasis. Scholar & Toews (1994) reported that the tyrosine kinase inhibitor genistein could inhibit invasion by tumour cells, possibly via repression of tyrosine phosphorylation. The association of Her-2/neu overexpression with metastatic potential in cancer cells provides further role of MMPs in metastasis (Zhang et al., 1998). Moreover, suppression of Her-2/neu tyrosine kinase activity can also repress the metastatic potential of tumour cells bearing this gene (Zhang et al., 1998).
In the present study, we investigated the possible interactions of luteolin and quercetin with the secretion of MMPs. Our finding indicated that luteolin and quercetin could decrease the secretion MMP-9 and MMP-2 in A431 cells (Figure 10). To test the hypothesis that the secretion of MMPs is inhibited, cell lysates from EGF or flavonoid treated cells were also assayed for gelatinase activity. Our previous study demonstrated that EGF stimulates the secretion of the MMP-9 and MMP-2 and thereby reduces the cellular content (Lee et al., 1997). In this study, our cell lysate gelatinase activity assay data revealed that luteolin and quercetin (data not shown) could retain the intracellular concentration of the MMP-9 (Figure 11). However, we could not rule out the possibility that both luteolin and quercetin inhibit specially the synthesis of MMPs. Taken together, the cell lysate and media MMP activity assay data suggest that treatment with flavonoids could inhibit secretion of these lytic enzymes from tumour cells, and thereby reduce the potential for metastasis.
In summary, we provide evidence for a role of protein phosphorylation in the regulation of apoptosis and MMP secretion in tumour cells in response to luteolin and quercetin. Our findings suggest that these natural products may lead to growth inhibition of tumours and a reduced likelihood of cancer metastasis, and also encourages the clinical investigation of luteolin and quercetin in the prevention of cancer invasion. Targeting specific genes that regulate expression of MMP could offer an approach to the treatment of cancers.
Acknowledgments
Dr Middleton, who is now deceased, provided an inspiration and impetus for the accomplishment of this work. This manuscript, therefore, is dedicated to Dr Middleton for his intellectual and personal contribution to this work in particular and science in general. The study is supported by NSC grants 84-2311-B-001-056 and 85-2311-B-001-088 (M.-T. Lee).
Abbreviations
- DMSO
dimethyl sulphoxide
- ECL
enhanced chemiluminescence
- EDTA
ethylenediamine tetraacetic acid
- EGF
epidermal growth factor
- EGFR
epidermal growth factor receptor
- GLB
gold lysis buffer
- Lu
luteolin
- MMP
matrix metalloproteinase
- MTT
3-(4,5-dimethylthiazo-2-yl)-2,5-diphenyl tetrazolium bromide
- PKC
protein kinase C
- PMSF
phenylmethylsulphonyl fluoride
- PTK
protein tyrosine kinase
- Qu
quercetin
- SDS–PAGE
sodium dodecyl sulphate polyacrylamide gel electrophoresis
References
- AGULLO G., GAMET-PAYRASTRE L., MANENTI S., VIALA C., REMESY C., CHAP H., PAYRASTRE B. Relationship between flavonoid structure and inhibition of phosphatidylinositol 3-kinase: a comparison with tyrosine kinase and protein kinase C. Biochem. Pharmacol. 1997;53:1649–1657. doi: 10.1016/s0006-2952(97)82453-7. [DOI] [PubMed] [Google Scholar]
- AKIYAMA T., ISHIDA J., NAKAGAWA S., OGAWARA H., WATANABE S., ITOH N., SHIBUYA M., FUKAMI Y. Genistein, a specific inhibitor of tyrosine kinases. J. Biol. Chem. 1987;262:5592–5595. [PubMed] [Google Scholar]
- ARMSTRONG D.K., ISACCS J.T., OTTAVINO Y.L., DARIDSON N.E. Programmed cell death in an estrogen-independent human cancer cell line, MDA-MB-468. Cancer Res. 1992;52:3418–3424. [PubMed] [Google Scholar]
- BISHOP J.M. The molecular genetics of cancer. Science. 1987;235:305–311. doi: 10.1126/science.3541204. [DOI] [PubMed] [Google Scholar]
- BRADFORD M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the protein-dye binding. Anal. Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- CANTLEY L.C., AUGER K.R., CARPENTER C., DUCKWORTH B., GRAZIANI A., KAPELLER R., SOLTOFF S. Oncogenes and signal transduction. Cell. 1991;64:281–302. doi: 10.1016/0092-8674(91)90639-g. [DOI] [PubMed] [Google Scholar]
- CHA I.-J., BAE S.-K., LEE H.-Y., LEE O.-H., SATO H., SEIKI M., PARK B.-C., KIM K.-W. Anti-invasive activity of ursolic acid correlates with the reduced expression of matrix metalloproteinase-9 (MMP-9) in HT-1089 human fibrosacoma cells. Cancer Res. 1996;56:2281–2284. [PubMed] [Google Scholar]
- COHEN S., USHIRO H., STOSCHECK C., CHINKERS M. A native 170,000 epidermal growth factor receptor kinase complex from shed membrane vesicles. J. Biol. Chem. 1982;257:1523–1531. [PubMed] [Google Scholar]
- CONSTANTINOU A., KIGUCHI K., HUBERMAN E. Induction of differentiation and DNA strand breakage in human HL-60 and K-562 leukemia cells by genistein. Cancer Res. 1990;50:2618–2624. [PubMed] [Google Scholar]
- DAVIES B.D., WAXMAN J., WASAN H., ABEL P., WILLIAMS G., KRAUSZ T., NEZL D., THOMAS D., BALKWILL F.R. Levels of matrix metalloproteinases in bladder cancer correlate with tumor grade and invasion. Cancer Res. 1993;53:5365–5369. [PubMed] [Google Scholar]
- DAVIS B.D., BROWN P.D., EAST N., CRIMMIN M.J., BALKWILL F.R. A synthetic matrix metalloproteinase inhibitor decrease tumor burden and prolongs survival of mice bearing human ovarian carcinoma xenografts. Cancer Res. 1993;53:2087–2091. [PubMed] [Google Scholar]
- FOTSIS T., PEPPER M.S., AKTAS E., BREIT S., RASKU S., ADLERCREUTZ H., WAHALA K., MONTESANO R., SCHWEIGERER L. Flavonoids, dietary-derived inhibitors of cell proliferation and in vitro angiogenesis. Cancer Res. 1997;57:2916–2921. [PubMed] [Google Scholar]
- FRANK D.A., SARTORELLI A.C. Biochemical characterization of tyrosine kinase and phosphotyrosine phosphatase activities of HL-60 leukemia cells. Cancer Res. 1988;48:4299–4306. [PubMed] [Google Scholar]
- GLOSSMAN H.P., PRESEK P., EIGENBRODT E. Quercetin inhibits tyrosine phosphorylation by the cyclic nucleotide-independent transforming protein kinase, pp 60. Naunyn-Schmiedeberg's. Arch. Pharmacol. 1981;217:100–102. doi: 10.1007/BF00506266. [DOI] [PubMed] [Google Scholar]
- GRAZIANI Y., ERIKSON E., ERIKSON R.L. The effect of the phosphorylation activity of the ras sarcoma virus transforming gene product in vitro and in vivo. Eur. J. Biochem. 1983;135:583–589. doi: 10.1111/j.1432-1033.1983.tb07692.x. [DOI] [PubMed] [Google Scholar]
- HAMDY F.C., FADLON E.J., COTTAM D., LOWRY J., THURREL W., SILOCKS P.B., ANDERSON J.B., WILLIAMS J.L., REES R.C. Matrix metalloproteinase 9 expression in primary human prostatic adenocarcinoma and benign prostatic hyperplasia. Br. J. Cancer. 1994;69:177–182. doi: 10.1038/bjc.1994.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HERRON G.S., BANDA M.J., CLARK E.J., GAVRILOVIC J., WERB Z. Secretion of metalloproteinases bystimulated capillary endothelial cells. II. Expression of collagenase and stromelysin activities is regulated by endogenous inhibitors. J. Biol. Chem. 1986;261:2814–2818. [PubMed] [Google Scholar]
- HEUSSEN C., DOWDLE E.B. Electrophoretic analysis of plasminogen activators in polyacrylamide gels containing sodium dodecyl sulfate and copolymerized substrate. Anal. Biochem. 1980;102:196–202. doi: 10.1016/0003-2697(80)90338-3. [DOI] [PubMed] [Google Scholar]
- HIBBS M.S., HASTY K.A., SEYER J.M., KANG A.H., MAINARDI C.L. Biochemical and immunological characterization of the secreted forms of human neutrophil gelatinase. J. Biol. Chem. 1985;260:2493–2500. [PubMed] [Google Scholar]
- HUNTER T., COOPER J.A. Protein tyrosine kinase. Annul. Rev. Biochem. 1985;54:897–930. doi: 10.1146/annurev.bi.54.070185.004341. [DOI] [PubMed] [Google Scholar]
- KANDASWAMI C., PERKINS E., DRZEWIECKI G., SOLONIUK D.S., MIDDLETON Differential inhibition of proliferation of human squamous cell carcinoma, gliosarcoma and embryonic fibroblast-like lung cells in culture by plant flavonoids. Anti-Cancer Drugs. 1992;3:525–530. doi: 10.1097/00001813-199210000-00013. [DOI] [PubMed] [Google Scholar]
- KANDASWAMI C., PERKINS E., SOLONIUK D.S., DRZEWIECKI G., MIDDLETON E. Antiproliferative effects of citrus flavonoids on a human squamous cell carcinoma in vitro. Cancer Lett. 1991;56:147–152. doi: 10.1016/0304-3835(91)90089-z. [DOI] [PubMed] [Google Scholar]
- KERR L.D., HOLT J.T., MATRISIAN L.M. Growth factors regulate transin gene expression by c-fos and c-fos independent pathways. Science. 1988;242:1424–1427. doi: 10.1126/science.2462278. [DOI] [PubMed] [Google Scholar]
- KERR L.D., MILLER D.B., MATRISIAN L.M. TGF-β inhibition of transin/stromelysin gene expression is mediated through a c-fos binding sequence. Cell. 1990;61:267–278. doi: 10.1016/0092-8674(90)90807-q. [DOI] [PubMed] [Google Scholar]
- KOCH C.A., ANDERSON D., MORAN M.F., ELLIS C., PAWSON T. SH2 and SH3 domains: elements that control interactions of cytoplasmic signaling proteins. Science. 1991;252:668–674. doi: 10.1126/science.1708916. [DOI] [PubMed] [Google Scholar]
- KOLIBABA K.S., DRUKER B.J. Protein tyrosine kinases and cancer. Biochim Biophys Acta. 1997;1333:F217–F248. doi: 10.1016/s0304-419x(97)00022-x. [DOI] [PubMed] [Google Scholar]
- KUHNAU J. The flavonoids: a class of semi-essential food components: their role in human nutrition. World Rev. Nutr. Diet. 1976;24:117–191. [PubMed] [Google Scholar]
- LAEMMLI U.K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- LEE M.T., LEE P.P., KE F.C., LO T.B., LEE M.S., HUANG Y.T., LIEBOW C., SCHALLY A.V., HWANG J.J. Opposite effect of EGF and analog of LHRH and somatostatin on matrix metalloproteinases expression and cell growth of tumor cells. Proc. Am. Associ. Cancer. Res. 1997;38:405. [Google Scholar]
- LEE M.T., LIEBOW C., KAMER A., SCHALLY A.V. Effect of epidermal growth factor and analogue of luteinizing hormone releasing hormone and somatostatin on phosphorylation and dephosphorylation of tyrosine residues of specific protein substrates in various tumors. Proc. Natl. Acad. Sci. USA. 1991;88:1656–1660. doi: 10.1073/pnas.88.5.1656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LIABAKK N.B., TALBOT I., SMITH R.A., WILKINSON K., BALKWILL F. Matrix metalloproteinase 2 (MMP-2) and matrix metalloproteinase 9 (MMP-9) type IV collagenases in colorectal cancer. Cancer Res. 1996;56:190–196. [PubMed] [Google Scholar]
- LIEBOW C., CREAN D.H., LEE M.T., KAMER A.R., MANG T.S., SCHALLY A.V. Synergistic effects of bombesin and epidermal growth factor. Proc. Natl. Acad. Sci. U.S.A. 1994;91:3804–3808. doi: 10.1073/pnas.91.9.3804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LIEBOW C., REILLY C., SERRNO M., SCHALLY A.V. Somatostatin analogue inhibit growth of pancreatic cancer by stimulating tyrosine phosphatase. Proc. Natl. Acad. Sci. U.S.A. 1989;86:2003–2007. doi: 10.1073/pnas.86.6.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LINASSIER C., PIERRE M., LE PECO J.-B., PIERRE J. Mechanism of action in NIH-3T3 cells of genistein, an inhibitor of EGF receptor tyrosine kinase activity. Biochem. Pharmacol. 1990;39:187–193. doi: 10.1016/0006-2952(90)90664-7. [DOI] [PubMed] [Google Scholar]
- LIOTTA L.A.Cancer cell invasion and metastasis Sci. Am. Feb. 199226654–59.62–63 [DOI] [PubMed] [Google Scholar]
- LIOTTA L.A., STEEG P.S., STETLER-STEVENSON Cancer metastasis and angiogenesis: an imbalance of positive and negative regulation. Cell. 1991;64:327–336. doi: 10.1016/0092-8674(91)90642-c. [DOI] [PubMed] [Google Scholar]
- LEVI J., TEUERSTEIN I., MARBACH M., RADIANS S., SHARONI Y. Tyrosine protein kinase activity in the DMBA-induced rat mammary tumor: inhibition by quercetin. Biochem. Biophys. Res. Commun. 1984;123:1227–1233. doi: 10.1016/s0006-291x(84)80264-8. [DOI] [PubMed] [Google Scholar]
- MATRISIAN L.M. Metalloproteinases and their inhibitors in matrix remolding. Trends Genet. 1990;6:121–125. doi: 10.1016/0168-9525(90)90126-q. [DOI] [PubMed] [Google Scholar]
- MATRISIAN L.M. The matrix-degrading metalloproteinases. BioEssays. 1992;14:455–462. doi: 10.1002/bies.950140705. [DOI] [PubMed] [Google Scholar]
- MCDONNEL S.E., FINGLETON B. Role of matrix metalloproteinase in invasion and metastasis: biology, diagnosis and inhibitors. Cytotechnology. 1993;12:367–384. doi: 10.1007/BF00744674. [DOI] [PubMed] [Google Scholar]
- MCDONNEL S.E., KERR L.D., MATRISIAN L.M. Epidermal growth factor stimulation of stromelysin mRNA in rat fibroblasts requires induction of proto-oncogene c-fos and c-jun and activation of protein kinase C. Mol. Cell. Biol. 1990;10:4284–4293. doi: 10.1128/mcb.10.8.4284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MENON L.G., KUTTAN R., KUTTAN G. Inhibition of lung metastasis in mice induced by B16F10 melanoma cells by polyphenolic compounds. Cancer Lett. 1995;95:221–225. doi: 10.1016/0304-3835(95)03887-3. [DOI] [PubMed] [Google Scholar]
- MERLINO G.T., XU Y.H., ISHII S., CLARK A.J., SEMBA K., YAMAMOTO T., PASTAN I. Amplification and enhanced expression of the epidermal growth factor receptor gene in A431 human carcinoma cells. Science. 1984;224:417–419. doi: 10.1126/science.6200934. [DOI] [PubMed] [Google Scholar]
- MIDDLETON E., KANDASWAMI C. ‘The Flavonoids: Advances in Research' 1993Chapman and Hall: London; 619–649.J.B. Harborne, (ed) [Google Scholar]
- MOSMANN T.J. Rapid colorimetric assay for cellular growth and survival: Application to proliferation and cytotoxicity. J. Immunol. Methods. 1983;65:55–63. doi: 10.1016/0022-1759(83)90303-4. [DOI] [PubMed] [Google Scholar]
- NAYLOR M.S., STAMP G.W., DAVIES B.D., BALKWILL F.R. Expression and activity of MMPs and their regulators in ovarian cancer. Intl. J. Cancer. 1994;58:50–56. doi: 10.1002/ijc.2910580110. [DOI] [PubMed] [Google Scholar]
- OTANI H., ERDOS M., LEONARD W.J. Tyrosine kinase(s) regulate apoptosis and bcl-2 expression in a growth factor-dependent cell line. J. Biol. Chem. 1993;268:22733–22736. [PubMed] [Google Scholar]
- PETERSON G., BARNES S. Genistein and biochanin A inhibit the growth of human prostate cancer cells but not epidermal growth factor receptor tyrosine autophosphorylation. Prostate. 1993;22:335–345. doi: 10.1002/pros.2990220408. [DOI] [PubMed] [Google Scholar]
- PIERCE J.H., RUGGIERO M., FLEMING T.P., DIFIORE P.P., GREENBERGER J.S., VARTICOVSKI L., SCHLESSINGER J., ROVERA G., AARONSON S.A. Signal transduction through the EGF receptor transfected in IL-3-dependent hematopoietic cells. Science. 1988;239:628–631. doi: 10.1126/science.3257584. [DOI] [PubMed] [Google Scholar]
- RICE-EVANS C.A., MILLER N.J., PAGANGA G. Structure-antioxidant activity relationships of flavonoids and phenolic acid. Free Radic. Biol. Med. 1996;20:933–956. doi: 10.1016/0891-5849(95)02227-9. [DOI] [PubMed] [Google Scholar]
- SAMUELS M.L., WEBER M.J., BISHOP J.M., MCMAHON M. Conditional transformation of cells and rapid activation of the mitogen-activated protein kinase cascade by an estradiol-dependent human raf-1 protein kinase. Mol. Cell. Biol. 1993;13:6241–6252. doi: 10.1128/mcb.13.10.6241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SAVILL J., FADOK V., HENSON P., HASLETT C. Phagocyte recognition of cells undergoing apoptosis. Immunol. Today. 1993;14:131–136. doi: 10.1016/0167-5699(93)90215-7. [DOI] [PubMed] [Google Scholar]
- SCAMBIA G., RANELLETTI F.O., BENEDETTI PANICI P., PIANTELLI M., BONANNO G., DE VINCENZO R., FERRANDINA G., RUMI C., LAROCCA L.M., MANCUSO S. Inhibitory effect of quercetin on OVCA 433 cells and presence of type II oestrogen binding sites in primary ovarian tumours and cultured cells. Br. J. Cancer. 1990;62:942–946. doi: 10.1038/bjc.1990.414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SCHNEIDER M.R., SCHIRNER M. Antimetastatic prostacyclin analogs. Drugs Future. 1993;18 1:29–48. [Google Scholar]
- SCHOLAR E.M., TOEWS M.L. Inhibition of invasion of murine mammary carcinoma cells by the tyrosine kinase inhibitor genistein. Cancer Lett. 1994;9 87:159–162. doi: 10.1016/0304-3835(94)90217-8. [DOI] [PubMed] [Google Scholar]
- SOULINNA E.M., BUCHSBAUM R.N., RACKER E. The effect of flavonoids on aerobic glycolysis and growth of tumor cells. Cancer Res. 1975;35:1865–1872. [PubMed] [Google Scholar]
- TANIGUCHI S., FUJIKI H., KOBAYASHI H., GO H., MIYADO K., SADANO H., SHIMOKAWA R. Effect of (−)-epigallocatechin gallate, the main constituent of green tea, on lung metastasis with mouse B16 melanoma cell lines. Cancer Lett. 1992;65:51–54. doi: 10.1016/0304-3835(92)90212-e. [DOI] [PubMed] [Google Scholar]
- TOWBIN H., STAEHELIN T., GORDON J. Electrophoretic transfer of proteins from poly-acrylamide gels to nitrocellular sheets: procedure and some application. Proc. Natl. Acad. Sci. U.S.A. 1979;76:4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ULLRICH A., SCHLESSINGER J. Signal transduction by receptors with tyrosine kinase activity. Cell. 1990;61:203–212. doi: 10.1016/0092-8674(90)90801-k. [DOI] [PubMed] [Google Scholar]
- WATSON S.A., MORRIS T.M., ROBINSON G., CRIMMIN M.J., BROWN P.D., HARDCASTLE J.D. Inhibition of organ invasion by the metalloproteinase inhibitor Batimastat (BB-94) in two human colon carcinoma metastasis models. Cancer Res. 1995;55:3629–3633. [PubMed] [Google Scholar]
- YONEDA J., KUNIYASU H., CRISPENS M.A., PRICE J.E., BUCANA C.D., FIDLER I.J. Expression of angiogenesis-related genes and progression of Human Ovarian carcinomas in nude mice. J. Natl Cancer. Inst. 1998;90 6:447–454. doi: 10.1093/jnci/90.6.447. [DOI] [PubMed] [Google Scholar]
- YOUSEFI S., GREEN D.R., BLASER K., SIMON H.-U. Protein tyrosine phosphorylation regulates apoptosis in human eosinophils and neutrophils. Proc. Natl. Acad. Sci. U.S.A. 1994;91:10868–10872. doi: 10.1073/pnas.91.23.10868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ZHANG L., LAU Y.-K., XI L., HONG R.-L., KIM D.S.H.L., CHEN C.-F., HORTOBAGYI C.C.-J., HUMG M.-C. Tyrosine kinase inhibitors, emodin and its derivate repress Her-2/neu-induced cellular transformation and metastasis-associated properties. Oncogene. 1998;16:2855–2863. doi: 10.1038/sj.onc.1201813. [DOI] [PubMed] [Google Scholar]


