Abstract
Stimulation of the opioid receptor-like1 (ORL-1) receptor by nociceptin (NC) produces hyperalgesia and reverses the antinociceptive effects induced by opioids. Most studies concerning the central effects of NC were conducted using acute pain models. The role NC may play in chronic inflammation remains unelucidated.
The present study was undertaken to assess the action of NC in the Freund's adjuvant-induced monoarthritic rat model. The effects of drugs known to act as analgesics in this model were evaluated. The effects of NC, NCNH2, and the ORL-1 ligand, [Phe1ψ(CH2-NH)Gly2]NC(1-13)NH2 ([F/G]NC(1-13)NH2), were also studied alone or in association with morphine.
NC (1–30 nmol, i.c.v.) was inactive, whilst NCNH2 (10 nmol, i.c.v.) exerted hyperalgesic effects (−4.5±0.9 vs −0.7±0.8 s of vehicle-treated animals). [F/G]NC(1-13)NH2 (0.01–10 nmol, i.c.v.) induced hyperalgesia in the arthritic paw (−3.3±0.6 vs −0.3±0.5 s of vehicle-treated animals; 10 nmol).
Both NC (0.01–10 nmol, i.c.v.) and [F/G]NC(1-13)NH2 (0.01–1 nmol, i.c.v), 30 min after morphine (3 mg kg−1, s.c.) induced an immediate and short-lived reversal of morphine effects (2.6±0.3 vs 10.4±1.0 and 1.2±1.5 vs 9.3±1.1 s of morphine alone, respectively), therefore displaying anti-opioid activity.
In the Freund's adjuvant-induced rat model of arthritis, both NC and [F/G]NC(1-13)NH2 act as anti-opioid peptides. Furthermore, NCNH2 and [F/G]NC(1-13)NH2 induce hyperalgesia when given alone. Further investigations and the identification of a centrally acting ORL-1 antagonist are necessary to better understand the role of NC in pain mechanisms.
Keywords: Nociceptin, [Phe1ψ(CH2-NH)Gly2]NC(1-13)NH2, Freund's adjuvant arthritis, hyperalgesia, chronic pain
Introduction
Nociceptin (NC) has been identified as the endogenous agonist for the opioid receptor-like 1 (ORL-1) receptor (Meunier et al., 1995; Reinscheid et al., 1995). Although structurally related to opioid peptides, NC selectively binds to the ORL-1 receptor but not to any subtype of the known opioid receptors. The ORL-1 receptor is widely expressed in the nervous system, and the peptide is likely to participate in a broad range of physiological and behavioural functions, such as pain modulation, food intake stimulation, regulation of anxiety responses and spatial memory (see Meunier, 1997; Henderson & McKnight, 1997; Darland et al., 1998 for reviews). In particular, conflicting results have been reported concerning the effects of NC in acute pain models. After intracerebroventricular (i.c.v.) administration, opposing results have been found using different pain models in both rats and mice, including hyperalgesia, reversal of opioid-analgesia, no effect, or even analgesia (see Meunier, 1997; Henderson & McKnight, 1997; Darland et al., 1998 for reviews). As with i.c.v. administration, at the spinal level NC does not induce a clear-cut activity, as shown by reports of either antinociceptive effects after intrathecal administration (Xu et al., 1996; Hao et al., 1997; Yamamoto & Nozaki Taguchi, 1997; Yamamoto et al., 1997a,1997b,1997c), or lack of activity (Reinscheid et al., 1995; Grisel et al., 1996). It has also been observed that NC induces allodynia following intrathecal administration in mice (Dawson-Basoa & Gintzler, 1997; Hara et al., 1997). Moreover, no measurable difference in nociceptive thresholds was observed between mice bearing ORL-1 genetic deletion and their wild-type counterparts, using both tail flick and acetic acid-induced writhing tests (Nishi et al., 1997).
In spite of some conflicting data, the overall trend, in the rat, is for NC to act supraspinally where it inhibits opioid-mediated antinociception, while convergent electrophysiological (Faber et al., 1996; Liebel et al., 1997) and behavioural data (Xu et al., 1996; Hao et al., 1997; Yamamoto & Nozaki Taguchi, 1997; Yamamoto et al., 1997a,1997b,1997c) indicate that this peptide has spinal antinociceptive effects.
Up to now, however, most of the investigations concerning the effects of i.c.v. administration of NC on pain modulation were conducted in experimental models of acute pain, whereas the role that NC might have in pain induced by chronic inflammation remains to be clarified. In man, chronic hyperalgesia can result from persistent inflammation of peripheral tissue, nerve damage or neuropathy. Adjuvant arthritis in rats is a widely used experimental model because in many respects it mimics arthritis in humans (Butler et al., 1992). Arthritic pain is a common and often debilitating chronic pain condition characteristic of a variety of arthritic diseases, such as osteoarthritis, juvenile or rheumatoid arthritis and gout.
The present study was undertaken to assess the effects of NC on pain response and morphine analgesia in Freund's adjuvant-induced arthritis in the rat, after supraspinal administration. For comparison, the effects of some reference analgesic compounds acting through different mechanisms of action were also evaluated. Furthermore, we have studied the effects of more stable analogues of NC, such as NC amide (NCNH2) and the ORL-1 ligand, [Phe1ψ(CH2-NH)Gly2]NC(1-13)NH2([F/G]NC(1-13)NH2; Guerrini et al., 1998) in the same chronic pain model.
Methods
Experimental animals
Male Lewis rats (Charles River, Calco, Italy) weighing 180–200 g were used. They were housed six per cage and kept under 12 h day/night cycle at a constant room temperature of 22°C. Food and water were available ad libitum. All behavioural tests were conducted in the morning.
Procedures involving animals and their care were conducted in conformity with the institutional guidelines, in compliance with the European Community Council Directive 86/609 (OJ L 358, 1, December 12, 1987).
Induction of arthritis
Under chloral hydrate anaesthesia (400 mg kg−1, i.p.) rats received a single subplantar injection of 100 μl of a 500 μg dose of heat-killed and dried Mycobacterium tuberculosis (H37 Ra, Difco Laboratories, Detroit, MI, U.S.A.) in a mixture of paraffin oil and an emulsifying agent, mannide monooleate (complete Freund's adjuvant, CFA). Control animals were injected with 0.1 ml mineral oil (incomplete Freund's adjuvant, IFA). Another group of animals was injected with saline to serve as naïve controls.
The plantar test
Thermal hyperalgesia to radiant heat was assessed by measuring the withdrawal latency as an index of nociception (Hargreaves et al., 1988). The plantar test (Basile, Comerio, Italy) was chosen because of its sensitivity to hyperalgesia. Briefly, the test consists of a movable infrared source placed below a glass plane onto which the rat is placed. A perspex enclosure defines the space within which the animal is unrestrained. It is divided into three compartments allowing three rats to be tested simultaneously. The infrared source was placed directly below the plantar surface of the hind-paw; both arthritic and contralateral paws were tested, and each animal served as its own control. The paw-withdrawal latency was defined as the time taken by the rat to remove its hind paw from the heat source. Three test trials, with an interval of 2 min were made and scores were averaged to yield a mean withdrawal latency. The radiant heat source was adjusted to result in baseline latencies of 10–12 s. Instrument cut-off was fixed at 21.5 s to prevent tissue damage.
Drugs
Morphine sulphate (SALARS, Como, Italy), R(+) baclofen hydrochloride (RBI, Natick, MA, U.S.A.) and naloxone hydrochloride (Sigma, St. Louis, MO, U.S.A.) were all dissolved in saline (0.16 M NaCl). Paracetamol (Sigma, St. Louis, MO, U.S.A.) was suspended in 0.4% methylcellulose (15 c.p.s.) dissolved in purified distilled water; indomethacin crystalline (Sigma, St. Louis, MO, U.S.A.) was dissolved in 0.2 M sodium phosphate solution. [F/G]NC(1-13)NH2 and NCNH2 were synthesized and purified as previously described (Guerrini et al., 1997; Calò et al., 1998a) and were stored as 1 mM solutions at −20°C, while NC (Phoenix Pharmaceuticals, Mountain View, CA, U.S.A.) was stored in the same condition using 5.5 mM concentrations. All peptides were dissolved in saline and diluted before use.
Intracerebroventricular injection
NC and other peptides were administered by i.c.v. (10 μl) injection. PE-10 tubing, 4 cm in length, was fixed on the skull at the following stereotaxic coordinates: AP=−0.8, L=1.5 and V=−4 according to Paxinos & Watson (1986). After completion of the experiments, to check the correct position of the cannula in the lateral ventricle, rats were treated i.c.v. with 3 μl of angiotensin II (1 mg ml−1) and returned to home cages. The angiotensin-induced water consumption was immediate and rats with a latency of more than 5 min were excluded from the study.
Data analysis
Statistical analysis for validation of the model was performed by the analysis of variance (ANOVA). The dose-response effects of standard compounds were examined by regression analysis. Raw latency data from each animal were converted to area under the curve (AUC), and values were calculated for the 120 min recording period. The dose-response effects of NC and related peptides as well as the interactions between NC or [F/G]NC(1-13)NH2 and morphine, were analysed using MANOVA. Interaction between morphine or [F/G]NC(1-13)NH2 with naloxone were analysed using ANOVA for 3×2 factorial design analysis.
Results
Monoarthritis model
In the first set of experiments, we examined the development of arthritis over 4 weeks. Animals injected with CFA showed a time-related redness and swelling in the injected paw and joint circumference. There were some alterations in spontaneous behaviour, including a reluctance to walk using the inflamed paw, and a persistent flexion of the knee joint on the affected side. Some animals did not use the arthritic paw for walking until 12–14 days post-treatment. Apart from these changes, rats behaved normally, they were neither aggressive nor hyperactive when stimulated by touch or sudden noise and appeared otherwise in good health. Occasionally, after 12–15 days, there were signs of inflammation in the contralateral paw.
When paw withdrawal latencies were measured before the injection of CFA, there was no significant difference between left and right hindpaws. Animals injected with CFA developed hyperalgesia quickly and latency times were lower on the inflamed paw than on the contralateral, IFA- or saline-treated paws (Figure 1). However, since after 12–14 days the animals appeared hypersensitive to manipulation of the affected limb, we carried out all the studies on day 10. At this time-point the inflamed-paw of CFA-treated rats exhibited a lower withdrawal latency to thermal stimuli (4.9±0.4 s) than the contralateral paw (9.4±0.7 s) and either of IFA- (10.9±0.9 s) or vehicle-treated rats (8.0±0.6 s).
Figure 1.
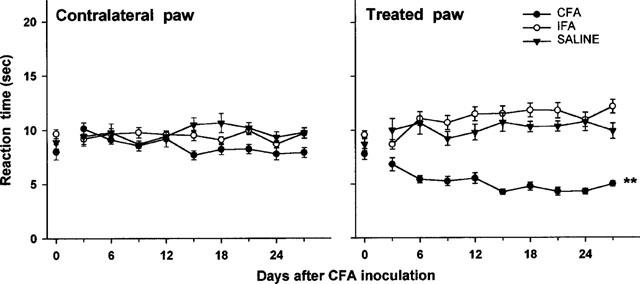
Development of thermal hyperalgesia following subplantar injection of 100 μl of saline, incomplete Freund's adjuvant (IFA) or complete Freund's adjuvant (CFA) in the paw of rats. Withdrawal latency (s) at radiant heat was measured in both the treated and the contralateral paw. Each point represents the mean±s.e.mean of nine animals per group. **P<0.01 vs saline or IFA-treated group (ANOVA).
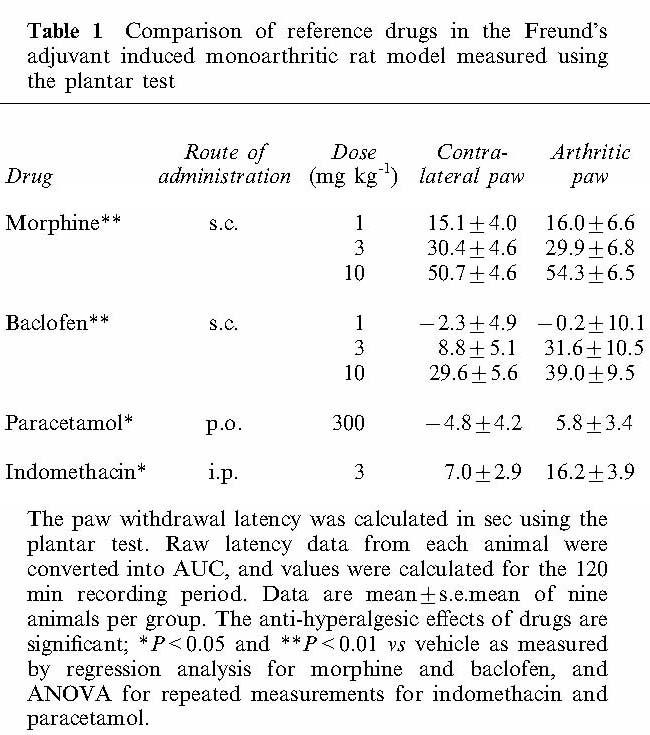
Results obtained using reference compounds are summarized in Table 1. Both morphine and baclofen administered at the doses of 1, 3 and 10 mg kg−1, s.c., produced clear dose-response effects. However, higher doses of baclofen induced other effects, such as muscle relaxation. Non steroidal anti-inflammatory drugs, such as indomethacin and paracetamol were also examined. These drugs were able to reduce inflammatory pain, but to a lesser extent compared with either morphine or baclofen.
Table 1.
Comparison of reference drugs in the Freund's adjuvant induced monoarthritic rat model measured using the plantar test
Effects of NC and NC-related peptides
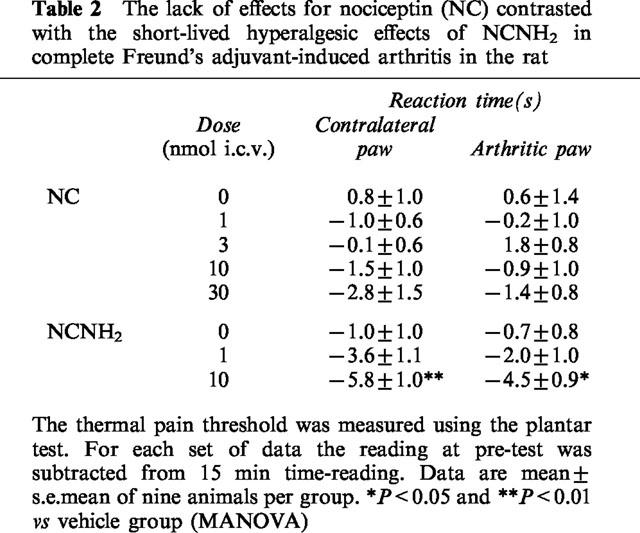
NC was tested at the doses of 1, 3, 10 and 30 nmol, i.c.v. Rats treated with 10 and 30 nmol of NC showed disruption of balance and motor control, had flaccid muscle tone and lost their righting reflex, exhibited increased diuresis, but could react to stimuli. However, NC was unable to modify paw withdrawal latency (Table 2). Instead, NCNH2 at 10 nmol, i.c.v. significantly reduced the withdrawal latency in both treated or untreated paws, with a peak effect 15 min after its administration (Table 2). NCNH2 produced behavioural changes somewhat similar to those of NC given at 10 and 30 nmol. At 1 nmol, i.c.v. NCNH2 did not induce any changes of either paw response or behaviour.
Table 2.
The lack of effects for nociceptin (NC) contrasted with the short-lived hyperalgesic effects of NCNH2 in complete Freund's adjuvant-induced arthritis in the rat
The ORL-1 ligand [F/G]NC(1-13)NH2 (0.01, 0.1, 1 and 10 nmol, i.c.v.) reduced withdrawal latency in both paws at the concentrations of 1 and 10 nmol in the arthritic paw and 10 nmol in the contralateral paw, as shown in Figure 2. This peptide produced longer lasting effects, being effective from 15 min up to 60 min. The behaviour of the animals was similar to that observed with NC, although less prominent.
Figure 2.
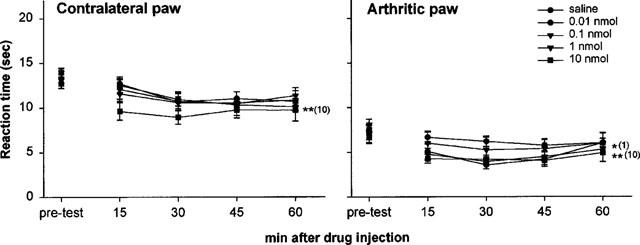
[F/G]NC(1-13)NH2 caused thermal hyperalgesia in the Freund's adjuvant-induced monoarthritic rat model. Animals were given the peptide at 0.01, 0.1, 1 and 10 nmol, i.c.v. Each point represents the mean±s.e.mean of nine animals per group. *P<0.05 and **P<0.01 vs vehicle group (MANOVA).
Naloxone, at a dose (10 mg kg−1, i.p.) which blocked completely the effects of morphine (3 mg kg−1, s.c.) did not change the pronociceptive effects of [F/G]NC(1-13)NH2 at 1 nmol, i.c.v. (Figure 3). When [F/G]NC(1-13)NH2 (0.1 and 1 nmol, i.c.v.) was injected in association with NCNH2 it did not modify the pronociceptive effects elicited by the effective dose of NCNH2 (10 nmol, i.c.v.). In particular, NCNH2 15 min after injection decreased the threshold latency in the arthritic paw by about 30%, and the same effects were seen with [F/G]NC(1-13)NH2 coinjection. The reaction time in the three groups was: −3.4±0.6 (NCNH2), −4.6±0.7 (NCNH2+0.1 nmol [F/G]NC(1-13)NH2) and −3.2±0.5 s (NCNH2+1 nmol [F/G]NC(1-13)NH2).
Figure 3.
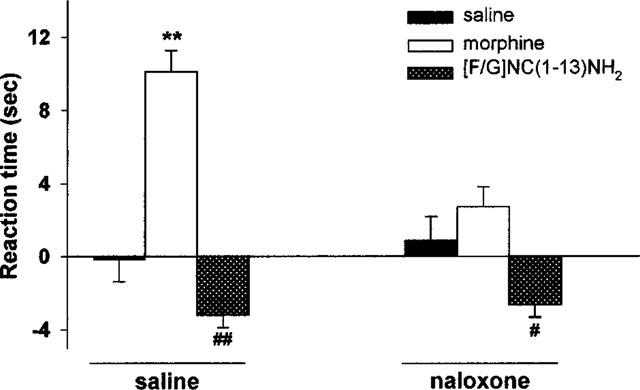
Interaction between naloxone and morphine or [F/G]NC(1-13)NH2 in the Freund's adjuvant-induced monoarthritic rat model. Naloxone (10 mg kg−1, i.p.) was injected 15 min prior to the administration of either the synthetic peptide (1 nmol, i.c.v.) or morphine (3 mg kg−1, s.c.). Bars represent the mean±s.e.mean of nine animals per group. The pre-test reading (s) was subtracted from the results taken at 15 or 30 min following administration of the peptide or morphine, respectively. Naloxone significantly reduced morphine-induced analgesia but did not reverse [F/G]NC(1-13)NH2-induced hyperalgesia. **P<0.01 vs saline group (ANOVA for 3×2 factorial design analysis). #P<0.05 and ##P<0.01 vs saline group (MANOVA).
Interaction with morphine
Morphine, when administered at the dose of 3 mg kg−1, s.c., induced clear antihyperalgesic effects, which lasted at least 90 min. The peak effect occurred at 30 min and was 60% above baseline values. Given i.c.v. 30 min after morphine, NC (0.01–10 nmol, i.c.v.) induced an immediate and short-lasting reversal of morphine-induced analgesia at the doses of 1 and 10 nmol (Figure 4A). However, NC was not effective when injected 5 min before morphine injection (data not shown).
Figure 4.
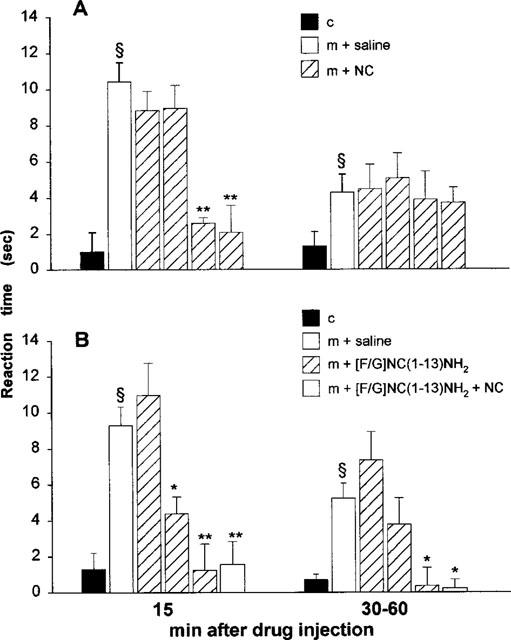
Anti-morphine effects of NC (A) and [F/G]NC(1-13)NH2 (B) in the Freund's adjuvant-induced monoarthritic rat model. Control group (c) was treated with both i.c.v. and s.c. saline injections. NC (0.01, 0.1, 1 and 10 nmol, i.c.v.), [F/G]NC(1-13)NH2 (0.01, 0.1 and 1 nmol, i.c.v.) or the two peptides together (1 nmol, i.c.v.) were given 30 min after morphine (m; 3 mg kg−1, s.c.). Bars represent the mean±s.e.mean of nine animals per group. For each set of data the pre-test reading (s) was subtracted from either the 15 min reading, or the combined average of the 30, 45 and 60 min readings post-peptide administration. ≈rcub;P<0.01 vs saline group, and *P<0.05 and **P<0.01 vs morphine group (MANOVA).
Similarly, [F/G]NC(1-13)NH2 (0.01–1 nmol, i.c.v.) at the doses of 0.1 and 1 nmol, reversed morphine-induced antinociception, with an action more prolonged than that of NC (Figure 4B). Administered in combination with NC (1 nmol, i.c.v.), [F/G]NC(1-13)NH2 (1 nmol, i.c.v.) reduced the antinociceptive effects of morphine to a similar extent to that shown when given alone.
Discussion
Intraplantar injection of complete Freund's adjuvant in the rat paw leads, in a few days, to persistent hyperalgesia which is reduced or eliminated by a variety of analgesic anti-inflammatory agents, such as morphine, paracetamol, indomethacin and even baclofen, the GABAB receptor agonist.
Indeed, several endogenous systems have been shown to intervene in the inflammatory reaction, for instance cytokines (Woolf et al., 1997), neurokinins (Goff et al., 1998), prostaglandins (Hay et al., 1997), a series of factors that, at the same time, modify local vascular functions and neuronal reactivity to injury. As shown by Goff et al. (1998), induction of arthritis by Freund's adjuvant in the rat paw is accompanied by an increase of binding sites for μ receptors in the laminae I and II, sites that appear to be formed de novo, since their mRNA expression in the spinal cord is enhanced ipsilaterally to the injured paw (Maekawa et al., 1995). NC and ORL-1 may also intervene in the neuronal plasticity changes that occur after peripheral application of carrageenan or Freund's adjuvant in the rat. In fact, mRNA expression for the peptide and for ORL-1 binding sites are enhanced in the dorsal root ganglia ipsilaterally to the peripheral lesion (Andoh et al., 1997a,1997b; Jia et al., 1998) and this coincides with the attenuation of thermal hyperalgesia (Jia et al., 1998), a finding that is consistent with the antihyperalgesic activity of NC administered intrathecally in carrageenan (Yamamoto et al., 1997a; Hao et al., 1998) or formalin-induced inflammation (Erb et al., 1997; Yamamoto et al., 1997b), neuropathic pain (Yamamoto & Nozaki Taguchi 1997; Yamamoto et al., 1997c; Hao et al., 1998) and spinal cord injury (Hao et al., 1998).
In addition to its antinociceptive effects at the spinal cord level, NC has been shown to antagonize some central effects of morphine (Mogil et al., 1996) and this appears to be confirmed in this investigation. The present results show that when injected via i.c.v. NC acts as an anti-opioid peptide in the pain response associated with Freund's adjuvant-induced arthritis, a chronic rat model of inflammation. While NC did not produce noticeable effects of its own on pain threshold, it decreased the antinociceptive effects of morphine when injected at the higher doses of 1 and 10 nmol, i.c.v. These effects were observed both with NCNH2 and [F/G]NC(1-13)NH2, the latter being a new compound which has been shown to exert NC-like (agonistic) effects in the spinal cord (Carpenter & Dickenson, 1998; Xu et al., 1998) and brain (Calò et al., 1998b). NC was either much weaker or inactive, while NCNH2 and [F/G]NC(1-13)NH2 were found to be active also on the contralateral paw, suggesting that peptides might be involved not only in hyperalgesic responses after tissue injury, but could be also important in the response to an acute noxious stimulus. These findings point to the importance of NC degradation and suggest that peptides which are partially protected from proteolytic hydrolysis will be needed to evaluate the effects of exogenous NC. The present results confirm those of Xu et al. (1998), who showed that [F/G]NC(1-13)NH2, given intrathecally reduces the flexor reflex in the rat longer than NC; this effect is not modified by naloxone and is attributed to activation of ORL-1. In other studies, [F/G]NC(1-13)NH2 was shown to act as partial or full agonist in the tail withdrawal test in mice (Calò et al., 1998b), in in vivo electrophysiological recordings of rat dorsal horn neurones (Carpenter & Dickenson, 1998), in the flexor reflex in spinalized rats (Xu et al. 1998) and in recombinant chinese hamster ovary cells expressing the human receptor for NC (Butour et al., 1998). Conversely, it acts as an antagonist in the mouse vas deferens, guinea-pig ileum (Guerrini et al., 1998) and isolated bronchus (Shah et al., 1998; Rizzi et al., 1999), and in regulation of cardiovascular function in unanaesthetized mice (Madeddu et al., 1999). It has been postulated that partial or full agonistic activities of [F/G]NC(1-13)NH2 may depend on the receptor density (Toll et al., 1998). Thus, in the CNS, where the number of receptors is expected to be high, [F/G]NC(1-13)NH2 is a full agonist, mimicking the actions of NC. In the rat cardiovascular system, where functional sites contributing to blood pressure changes may be present in various structures, [F/G]NC(1-13)NH2 reduces the hypotension and bradycardia induced by NC but maintains some residual agonistic activity by itself (Bigoni et al., 1999), while in isolated organs, such as the mouse vas deferens, where the number of ORL-1 is relatively low, [F/G]NC(1-13)NH2 acts as an antagonist, that competitively blocks the effects of NC. The hypothesis of the existence of two different receptors for NC has also been proposed (Calò et al., 1998b; Butour et al., 1998) but neither biochemical nor genetic evidence has been found to support it. Furthermore, in mice bearing deletion of ORL-1 receptors there are no binding sites for [125I]-NC and NC does not produce noticeable effects in pain models (Nishi et al., 1997; internal data).
In conclusion, this study reports the anti-opioid effects of NC and NC related peptides in a model of chronic pain and inflammation. The discovery of potent and selective NC related compounds may be useful in developing clinically-relevant drugs. Further studies will be required to elucidate the complex role(s) that NC and its receptor play in pain and analgesia.
Acknowledgments
We thank Dr D. Regoli for his critical comments on the manuscript.
Abbreviations
- CFA
complete Freund's adjuvant
- [F/G]NC(1-13)NH2
[Phe1ψ(CH2-NH)Gly2]NC(1-13)NH2
- GABA
γ-aminobutyric acid
- IFA
incomplete Freund's adjuvant
- i.c.v
intracerebroventricular
- NC
nociceptin
- NCNH2
nociceptin amide
- ORL-1
opioid receptor-like 1 receptor
References
- ANDOH T., ITOH M., KURAISHI Y. Nociceptin gene expression in rat dorsal root ganglia induced by peripheral inflammation. Neuroreport. 1997a;8:2793–2796. doi: 10.1097/00001756-199708180-00028. [DOI] [PubMed] [Google Scholar]
- ANDOH T., ITOH M., KURAISHI Y.Expression and tissue distribution of prepronociceptin messenger RNA in rats with adjuvant-induced hyperalgesia Soc. Neurosci. 1997b704.10(Abstract) [Google Scholar]
- BIGONI R., GIULIANI S., CALO' G., RIZZI A., GUERRINI R., SALVADORI S., REGOLI D., MAGGI C.A. Characterization of nociceptin receptors in the periphery: in vitro and in vivo studies. Naunyn-Schmiedeberg's Arch. Pharmacol. 1999;359:160–167. doi: 10.1007/pl00005338. [DOI] [PubMed] [Google Scholar]
- BUTLER S.H., GODEFROY F., BESSON J.M., WEIL-FUGAZZA J. A limited arthritic model for chronic pain studies in the rat. Pain. 1992;48:73–81. doi: 10.1016/0304-3959(92)90133-V. [DOI] [PubMed] [Google Scholar]
- BUTOUR J.L., MOISAND C., MOLLEREAU C., MEUNIER J.C. [Phe1ψ(CH2-NH)Gly2] nociceptin-(1-13)-NH2 is an agonist of the nociceptin (ORL1) receptor. Eur. J. Pharmacol. 1998;349:R5–R6. doi: 10.1016/s0014-2999(98)00273-8. [DOI] [PubMed] [Google Scholar]
- CALO' G., GUERRINI R., BIGONI R., RIZZI A., BIANCHI C., REGOLI D., SALVADORI S. Structure-activity study of the NC(1-13)-NH2 N-terminal tetrapeptide and discovery of a nociceptin receptor antagonist. J. Med. Chem. 1998a;41:3360–3366. doi: 10.1021/jm970805q. [DOI] [PubMed] [Google Scholar]
- CALO' G., RIZZI A., MARZOLA G., GUERRINI R., SALVADORI S., BEANI L., REGOLI D., BIANCHI C. Pharmacological characterization of the nociceptin receptor mediating hyperalgesia in the mouse tail withdrawal assay. Br. J. Pharmacol. 1998b;125:373–378. doi: 10.1038/sj.bjp.0702087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CARPENTER K.J., DICKENSON A.H. Evidence that a peripheral ORL-1 receptor antagonist, acts as an agonist in the rat spinal cord. Br. J. Pharmacol. 1998;125:949–951. doi: 10.1038/sj.bjp.0702188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DARLAND T., HEINRICHER M.M., GRANDY D.K. Orphanin FQ/nociceptin: a role in pain and analgesia, but so much more. Trends Neurosci. 1998;21:215–221. doi: 10.1016/s0166-2236(97)01204-6. [DOI] [PubMed] [Google Scholar]
- DAWSON-BASOA M., GINTZLER A.R. Nociceptin (orphanin FQ) abolishes gestational and ovarian sex steroid-induced antinociception and induces hyperalgesia. Brain Res. 1997;750:48–52. doi: 10.1016/s0006-8993(96)01334-0. [DOI] [PubMed] [Google Scholar]
- ERB K., LIEBEL J.T., TEGEDER I., ZEILHOFER H.U., BRUNE K., GEISSLINGER G. Spinally delivered nociceptin/orphanin FQ reduces flinching behaviour in the rat formalin test. Neuroreport. 1997;8:1967–1970. doi: 10.1097/00001756-199705260-00034. [DOI] [PubMed] [Google Scholar]
- FABER E.S., CHAMBERS J.P., EVANS R.H., HENDERSON G. Depression of glutamatergic transmission by nociceptin in the neonatal rat hemisected spinal cord preparation in vitro. Br. J. Pharmacol. 1996;119:189–190. doi: 10.1111/j.1476-5381.1996.tb15969.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GOFF J.R., BURKEY A.R., GOFF D.J., JASMIN L. Reorganization of the spinal dorsal horn in models of chronic pain: correlation with behaviour. Neuroscience. 1998;82:559–574. doi: 10.1016/s0306-4522(97)00298-4. [DOI] [PubMed] [Google Scholar]
- GRISEL J.E., MOGIL J.S., BELKNAP J.K., GRANDY D.K. Orphanin FQ acts as a supraspinal, but not a spinal, anti-opioid peptide. Neuroreport. 1996;7:2125–2129. doi: 10.1097/00001756-199609020-00012. [DOI] [PubMed] [Google Scholar]
- GUERRINI R., CALO' G., RIZZI A., BIANCHI C., LAZARUS L.H., SALVADORI S., TEMUSSI P.A., REGOLI D. Address and message sequences for the NC receptor: a structure-activity study of NC-(1-13)-peptide amide. J. Med. Chem. 1997;40:1789–1793. doi: 10.1021/jm970011b. [DOI] [PubMed] [Google Scholar]
- GUERRINI R., CALO' G., RIZZI A., BIGONI R., BIANCHI C., SALVADORI S., REGOLI D. A new selective antagonist of the nociceptin receptor. Br. J. Pharmacol. 1998;123:163–165. doi: 10.1038/sj.bjp.0701640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HAO J.X., XU I.S., WIESENFELD-HALLIN Z., XU X.J. Anti-hyperalgesic and anti-allodynic effects of intrathecal nociceptin/orphanin FQ in rats after spinal cord injury, peripheral nerve injury and inflammation. Pain. 1998;76:385–393. doi: 10.1016/S0304-3959(98)00071-2. [DOI] [PubMed] [Google Scholar]
- HAO J.X., WIESENFELD HALLIN Z., XU X.J. Lack of cross-tolerance between the antinociceptive effect of intrathecal orphanin FQ and morphine in the rat. Neurosci. Lett. 1997;223:49–52. doi: 10.1016/s0304-3940(97)13401-2. [DOI] [PubMed] [Google Scholar]
- HARA N., MINAMI T., OKUDA ASHITAKA E., SUGIMOTO T., SAKAI M., ONAKA M., MORI H., IMANISHI T., SHINGU K., ITO S. Characterization of nociceptin hyperalgesia and allodynia in conscious mice. Br. J. Pharmacol. 1997;121:401–408. doi: 10.1038/sj.bjp.0701146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HARGREAVES K., DUBNER R., BROWN F., FLORES C., JORIS J. A new and sensitive method for measuring thermal nociception in cutaneous hyperalgesia. Pain. 1988;32:77–88. doi: 10.1016/0304-3959(88)90026-7. [DOI] [PubMed] [Google Scholar]
- HAY C.H., TREVETHICK M.A., WHEELDON A., BOWERS J.S., DE BELLEROCHE J.S. The potential role of spinal cord cyclooxygenase-2 in the development of Freund's complete adjuvant-induced changes in hyperalgesia and allodynia. Neuroscience. 1997;78:843–850. doi: 10.1016/s0306-4522(96)00598-2. [DOI] [PubMed] [Google Scholar]
- HENDERSON G., MCKNIGHT A.T. The orphan opioid receptor and its endogenous ligand-nociceptin/orphanin FQ. Trends Pharmacol. Sci. 1997;18:293–300. [PubMed] [Google Scholar]
- JIA Y.P., LINDEN D.R., SERIE J.R., SEYBOLD V.S.Peripheral inflammation increases nociceptin/orphanin FQ binding in the superficial laminae of the rat spinal cord Soc. Neurosci. 1998350.2(Abstract) [DOI] [PubMed] [Google Scholar]
- LIEBEL J.T., SWANDULLA D., ZEILHOFER H.U. Modulation of excitatory synaptic transmission by nociceptin in superficial dorsal horn neurones of the neonatal rat spinal cord. Br. J. Pharmacol. 1997;121:425–432. doi: 10.1038/sj.bjp.0701149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MADEDDU P., SALIS M.B., MILIA A.F., EMANUELI C., GUERRINI R., REGOLI D., CALO' G. Cardiovascular effects of nociceptin in unanesthetized mice. Hypertension. 1999;33:914–919. doi: 10.1161/01.hyp.33.3.914. [DOI] [PubMed] [Google Scholar]
- MAEKAWA K., MASABUMI M., TAKAHIRO M., SATOH M. Expression of mu and kappa, but not delta, opioid receptor mRNAs is enhanced in the spinal dorsal horn of the arthritic rats. Pain. 1995;64:365–371. doi: 10.1016/0304-3959(95)00132-8. [DOI] [PubMed] [Google Scholar]
- MEUNIER J.C. Nociceptin/orphanin FQ and the opioid receptor-like ORL1 receptor. Eur. J. Pharmacol. 1997;340:1–15. doi: 10.1016/s0014-2999(97)01411-8. [DOI] [PubMed] [Google Scholar]
- MEUNIER J.C., MOLLEREAU C., TOLL L., SUAUDEAU C., MOISAND C., ALVINERIE P., BUTOUR J.L., GUILLEMOT J.C., FERRARA P., MONSARRAT B. Isolation and structure of the endogenous agonist of opioid receptor-like ORL1 receptor. Nature. 1995;377:532–535. doi: 10.1038/377532a0. [DOI] [PubMed] [Google Scholar]
- MOGIL J.S., GRISEL J.E., REINSCHEID R.K., CIVELLI O., BELKNAP J.K., GRANDY D.K. Orphanin FQ is a functional anti-opioid peptide. Neuroscience. 1996;75:333–337. doi: 10.1016/0306-4522(96)00338-7. [DOI] [PubMed] [Google Scholar]
- NISHI M., HOUTANI T., NODA Y., MAMIYA T., SATO K., DOI T., KUNO J., TAKESHIMA H., NUKADA T., NABESHIMA T., YAMASHITA T., NODA T., SUGIMOTO T. Unrestrained nociceptive response and disregulation of hearing ability in mice lacking the nociceptin/orphanin FQ receptor. EMBO J. 1997;16:1858–1864. doi: 10.1093/emboj/16.8.1858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PAXINOS G., WATSON C. The rat brain in stereotaxic coordinates. Academic Press: New York; 1986. [DOI] [PubMed] [Google Scholar]
- REINSCHEID R.K., NOTHACKER H.P., BOURSON A., ARDATI A., HENNINGSEN R.A., BUNZOW J.R., GRANDY D.K., LANGEN H., MONSMA F.J., , JR, CIVELLI O. Orphanin FQ: a neuropeptide that activates an opioid like G protein-coupled receptor. Science. 1995;270:792–794. doi: 10.1126/science.270.5237.792. [DOI] [PubMed] [Google Scholar]
- RIZZI A., CALO' G., TREVISANI M., TOGNETTO M., FABBRI L., GUERRINI R., SALVADORI S., REGOLI D., GEPPETTI P. Nociceptin receptor activation inhibits tachykinergic non adrenergic non cholinergic contraction of guinea pig isolated bronchus. Life Sci. 1999;64:PL157–PL163. doi: 10.1016/s0024-3205(99)00045-4. [DOI] [PubMed] [Google Scholar]
- SHAH S., PAGE C.P., SPINA D. Nociceptin inhibits non-adrenergic non-cholinergic contraction in guinea-pig airway. Br. J. Pharmacol. 1998;125:510–516. doi: 10.1038/sj.bjp.0702068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TOLL L., BURNSIDE J., BERZETEI-GURSKE I.Agonist activity of the ORL1 antagonists is dependent upon receptor number International Narcotics Research Conference, 20–25 July 1998A89Garmisch-Partenkirchen, Germany; (Abstract) [Google Scholar]
- WOOLF C.J., ALLCHORNE A., SAFIEH-GARABEDIAN B., POOLE S. Cytokines, nerve growth factor and inflammatory hyperalgesia: the contribution of tumour necrosis factor alpha. Br. J. Pharmacol. 1997;121:417–424. doi: 10.1038/sj.bjp.0701148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- XU I.S., WIESENFELD-HALLIN Z., XU X.J. [Phe1 ψ (CH2-NH)Gly2]-nociceptin-(1-13)NH2, a proposed antagonist of the nociceptin receptor, is a potent and stable agonist in the rat spinal cord. Neurosci. Lett. 1998;249:127–130. doi: 10.1016/s0304-3940(98)00411-x. [DOI] [PubMed] [Google Scholar]
- XU X.J., HAO J.X., WIESENFELD HALLIN Z. Nociceptin or antinociceptin: potent spinal antinociceptive effect of orphanin FQ/nociceptin in the rat. Neuroreport. 1996;7:2092–2094. [PubMed] [Google Scholar]
- YAMAMOTO T., NOZAKI TAGUCHI N. Effects of intrathecally administered nociceptin, an opioid receptor-like1 receptor agonist, and N-methyl-D-aspartate receptor antagonists on the thermal hyperalgesia induced by partial sciatic nerve injury in the rat. Anesthesiology. 1997;87:1145–1152. doi: 10.1097/00000542-199711000-00019. [DOI] [PubMed] [Google Scholar]
- YAMAMOTO T., NOZAKI TAGUCHI N., KIMURA S. Effects of intrathecally administered nociceptin, an opioid receptor-like1 (ORL1) receptor agonist, on the thermal hyperalgesia induced by carrageenan injection into the rat paw. Brain Res. 1997a;754:329–332. doi: 10.1016/s0006-8993(97)00186-8. [DOI] [PubMed] [Google Scholar]
- YAMAMOTO T., NOZAKI TAGUCHI N., KIMURA S. Analgesic effect of intrathecally administered nociceptin, an opioid receptor-like receptor agonist, in the rat formalin test. Neuroscience. 1997b;81:249–254. doi: 10.1016/s0306-4522(97)00166-8. [DOI] [PubMed] [Google Scholar]
- YAMAMOTO T., NOZAKI TAGUCHI N., KIMURA S. Effects of intrathecally administered nociceptin, an opioid receptor-like1 (ORL1) receptor agonist, on the thermal hyperalgesia induced by unilateral constriction injury to the sciatic nerve in the rat. Neurosci. Lett. 1997c;224:107–110. doi: 10.1016/s0304-3940(97)13475-9. [DOI] [PubMed] [Google Scholar]


