Abstract
Cyclo-oxygenase is expressed in cells in two distinct isoforms. Cyclo-oxygenase-1 is present constitutively whilst cyclo-oxygenase-2 is expressed primarily after inflammatory insult. The activity of cyclo-oxygenase-1 and -2 results in the production of a variety of potent biological mediators (the prostaglandins) that regulate homeostatic and disease processes. Inhibitors of cyclo-oxygenase include the nonsteroidal anti-inflammatory drugs (NSAIDs) aspirin, ibuprofen and diclofenac. NSAIDs inhibit cyclo-oxygenase-2 at the site of inflammation, to produce their therapeutic benefits, as well as cyclo-oxygenase-1 in the gastric mucosa, which produces gastric damage. Most recently selective inhibitors of cyclo-oxygenase-2 have been developed and introduced to man for the treatment of arthritis. Moreover, recent epidemiological evidence suggests that cyclo-oxygenase inhibitors may have important therapeutic relevance in the prevention of some cancers or even Alzheimer's disease. This review will discuss how the new advancements in NSAIDs research has led to the development of a new class of NSAIDs that has far reaching implications for the treatment of disease.
Keywords: Cyclo-oxygenase-2, nonsteroidal anti-inflammatory drugs, rofecoxib, celecoxib, inflammation, prostaglandins
Introduction
Cyclo-oxygenase is the first enzyme in the formation of prostaglandins (PG) and thromboxane (TX) from arachidonic acid. Cyclo-oxygenase metabolites have a wide variety of physiological and pathophysiological effects and are involved in a number of homeostatic processes. However, it is the role of these metabolites in cardiovascular homeostasis and inflammatory oedema and pain which has made cyclo-oxygenase a therapeutic target that potentially affects the majority of individuals at some time in their lives.
The nonsteroidal anti-inflammatory drugs (NSAIDs), which include aspirin, owe their therapeutic and side effects to inhibition of cyclo-oxygenase. Up until the early 1990s the beneficial and the deleterious effects of NSAIDs were thought to be caused by inhibition of a single cyclo-oxygenase enzyme; inhibition of cyclo-oxygenase at inflammatory sites explaining their therapeutic actions and inhibition of cyclo-oxygenase in the gastric mucosa explaining their gastrotoxic effects. However, we now know that two forms of cyclo-oxygenase exist. A constitutive form, cyclo-oxygenase-1, and an inducible form, cyclo-oxygenase-2; the latter form being preferentially expressed at sites of inflammation. Thus, a new hypothesis has been suggested to explain the effects of NSAIDs; ‘inhibition of cyclo-oxygenase-1 accounts for the side effects whilst inhibition of cyclo-oxygenase-2 accounts for the therapeutic benefits of NSAIDs'. This review will discuss the respective similarities and differences in the biology of the two forms of cyclo-oxygenase. It will also pay particular attention to how recent discoveries relating to the functioning of cyclo-oxygenase-2 has led to the development of new and potentially better NSAIDs.
Cyclo-oxygenase isoforms
Cyclo-oxygenase-1; the constitutive isoform
Cyclo-oxygenase-1 is expressed in most mammalian cells under physiological conditions (Seibert et al., 1997). However endothelial cells, platelets and kidney tubule cells are notable in that they express particularly large amounts of cyclo-oxygenase-1. Cyclo-oxygenase-1 was initially identified and purified in the 1970s, using classical biochemical techniques, from bovine (Miyamoto et al., 1976) and sheep (Hemler & Lands, 1976) vesicular glands and found to be a membrane bound homo-dimer of 70 kDa. The protein contained both the cyclo-oxygenase and peroxidase activities required to form, respectively, prostaglandin PGG2 and PGH2. Either free or protein-bound heme was required for activity. The primary structure of cyclo-oxygenase-1 was later determined from the complementary DNA sequence of 2.7 kilobases (DeWitt & Smith, 1988).
Cyclo-oxygenase-2; the inducible isoform
Cyclo-oxygenase metabolites are released in high amounts locally at the site of inflammation or systemically after infection. Initially it was believed that this was due to an increase in supply of arachidonic acid. However, in 1990 it was demonstrated that the increase in prostaglandin formation following exposure of isolated cells in culture to inflammatory stimuli (see De Witt, 1991) was due to an increase in cyclo-oxygenase enzyme expression (Fu et al., 1990; Masferrer et al., 1990). We now know that this increased cyclo-oxygenase is not cyclo-oxygenase-1 but an inducible isoform, cyclo-oxygenase-2.
The identification of cyclo-oxygenase-2 was, in many respects, a triumph of molecular biology. Early experiments demonstrated that cultured epithelial cells contained two distinct mRNA species, recognized under low stringency conditions by cDNA probes designed for the known cyclo-oxygenase (cyclo-oxygenase-1) (Rosen et al., 1989). These probes hybridized to the predicted 2.8 kilobase mRNA but also to a novel 4.0 kilobase product. Importantly, these researchers also demonstrated that increases in the 4.0 kilobase product paralleled increases in enzymatic activity supporting the conclusion that this mRNA encoded for active protein. Later studies from the same group confirmed that these epithelial cells did indeed express two distinct forms of cyclo-oxygenase (Holtzman et al., 1992). In separate studies, Xie and co-workers (Xie et al., 1991) showed that mitogen stimulated chicken fibroblasts expressed a 4.1 kilobase mRNA which encoded a protein with 59% homology to the well characterized cyclo-oxygenase identified in sheep seminal vesicle (cyclo-oxygenase-1). Furthermore, phorbol esters were also shown to induce mouse fibroblasts to express an inducible cyclo-oxygenase (TIS10; Kujubu et al., 1991) with striking similarities to the protein identified in chicken cells (Xie et al., 1991). Finally, the inducible cyclo-oxygenase gene was directly demonstrated to encode a protein which had cyclo-oxygenase activities (Fletcher et al., 1992; O'Banion et al., 1992).
Differential induction of cyclo-oxygenase-1 and cyclo-oxygenase-2 transcription
The human cyclo-oxygenase-2 gene is 8.3 kilobases whereas the cyclo-oxygenase-1 gene is much larger at 22 kilobases (see Wu, 1995; Vane et al., 1998). As discussed above, the gene products also differ in size. Cyclo-oxygenase-1 mRNA is approximately 2.8 kilobases whilst cyclo-oxygenase-2 mRNA is approximately 4.0 kilobases. Analysis of the 5′-flanking untranslated regions of cyclo-oxygenase-1 and cyclo-oxygenase-2 show that the cyclo-oxygenase-1 gene exhibits the features of a housekeeping gene whereas the gene for cyclo-oxygenase-2 appears to be a primary response gene. For example, the 5′-flanking region of the cyclo-oxygenase-2 gene has a canonic TATA box 30 base pairs upstream from the transcription start site (Tazawa et al., 1994) whereas the same region in the cyclo-oxygenase-1 gene has no canonic TATA box (Wu, 1995). The cyclo-oxygenase-2 gene also contains a number of putative regulatory sites, including cyclic AMP response element, IL-6 response element, CCAAT/enhancer binding proteins, AP-2, nuclear factor-κB (NF-κB), Sp-1, PEA-3, GATA-1, and glucocorticoid response element (Wu, 1995). Interestingly, the cyclo-oxygenase-1 gene also has some putative regulatory sites, including Sp-1, PEA-3, AP2, NF-IL6, GATA-1 and shear stress response element (Wu, 1995). Characterization of the cyclo-oxygenase-2 gene as being a primary response gene has led to a larger number of studies investigating potential regulatory factors. These include in various cell types: cytokines, e.g. interleukin-1α (Ristimaki et al., 1994), tumour necrosis factor-α, interleukin-6, bacterial endotoxin and PMA (Geng et al., 1995); growth factors, e.g. epidermal growth factor (Hamasaki & Eling, 1995), platelet derived growth factor, serum (Xie & Herschman, 1996) and chorionic gonadotrophin (Han et al., 1996); locally acting mediators, e.g. 5-hydroxytryptamine (Stroebel & Goppelt-Struebe, 1994) and endothelin (Hughes et al., 1995); fatty acid related mediators, e.g. arachidonic acid (Barry et al., 1999), thromboxane A2 (Stroebel & Gopelt-Struebe, 1994) and platelet activating factor (Bazan et al., 1994); mechanical forces, e.g. pulsatile flow (Klein-Nulend et al., 1997) and cyclic stretch (Kato et al., 1998); and other stimuli such as circulating hormones, e.g. parathyroid hormone (Tetradis et al., 1996). In vivo local increases in cyclo-oxygenase-2 expression have been associated with inflammation (Vane et al., 1994), rheumatoid arthritis (Kang et al., 1996), seizures (Marcheselli & Bazan, 1996) and ischaemia (Planas et al., 1995). Cyclo-oxygenase-2 expression in the spinal cord is also elevated following peripheral inflammation (Beiche et al., 1996). The intracellular pathways regulating these events appear numerous and complicated, varying between cell types and cellular stimulus. These pathways include, but are not limited to, reactive oxygen intermediates (Feng et al., 1995), NF-κB and NF-IL6 (Yamamoto et al., 1995), Ras/Rac1/MEKK-1/JNK kinase/JNK signal transduction leading to phosphorylation of c-Jun (Xie & Herschman, 1996), ceramide (Hayakawa et al., 1996), mitogen-activated protein kinase (Hwang et al., 1997), AP-1 and CRE nuclear binding proteins (Miller et al., 1998), CCAAT/enhancer-binding proteins (Kim & Fischer, 1998) and protein kinase C/p42/p44 MAPK/p38 kinase (Barry et al., 1999). Despite the presence of many pathways regulating the expression of cyclo-oxygenase-2 it is widely found that cyclo-oxygenase-2 is down-regulated by glucocorticosteroids (Masferrer et al., 1994), and also by related agents such as 17β-estradiol (Morisset et al., 1998).
Enzyme homology
Interestingly, although the genes for cyclo-oxygenase-1 and cyclo-oxygenase-2 are clearly different (see above), the proteins share approximately 60% homology at the amino acid level (Xie et al., 1991). Cyclo-oxygenase-1 and cyclo-oxygenase-2 also both catalyse the formation of prostaglandin (PG) G2 followed by PGH2 from arachidonic acid, have a molecular weight of 70 kDa, and are identical in length. Studies of the tertiary structures of cyclo-oxygenase-1 and cyclo-oxygenase-2 have demonstrated that the amino acid conformation for the substrate binding sites and catalytic regions are almost identical (Kurumbail et al., 1996; Picot et al., 1994). However, there are important differences in these regions, particularly the exchange of Ile in cyclo-oxygenase-1 for Val in cyclo-oxygenase-2 at positions 434 and 523. These substitutions result in a larger and more flexible substrate channel in cyclo-oxygenase-2 than in cyclo-oxygenase-1 and also in the inhibitor binding site in cyclo-oxygenase-2 being 25% larger than that in cyclo-oxygenase-1 (Kurumbail et al., 1996). There are also differences in the amino acid sequences in the N and C terminus of these enzymes (Kurumbail et al., 1996; Picot et al., 1994). Cyclo-oxygenase-2 for instance, is lacking in a 17 amino acid sequence at the N terminus but has an extra 18 amino acid sequence at the C terminus. The functionality of these differences are yet not known.
Cyclo-oxygenase-1 and cyclo-oxygenase-2 are membrane bound proteins that reside, after synthesis and transport, primarily in the endoplasmic reticulum (Spencer et al., 1998). After the crystal structures of both isoforms of cyclo-oxygenase has been elucidated, it appeared likely that four amphipathic helices near the amino termini of these proteins would act as membrane binding domains (Krurumbail et al., 1996; Picot et al., 1994), a hypothesis that has been confirmed by experiments using chimeric proteins (Li et al., 1998).
Cyclo-oxygenase substrate, products and receptors
Substrate
The first step in the formation of prostaglandins is the liberation of arachidonic acid from membrane bound phospholipids. This usually follows the action of phospholipase enzymes, primarily phospholipase A2. Like cyclo-oxygenase, phospholipase A2 is expressed in a number of different isoforms, some of which are present constitutively, and some of which appear after inflammatory insult (see Cirino, 1998). However, it is the cytosolic 85 kDa phospholipase A2 that most commonly supplies the arachidonic acid for prostaglandin production. Unlike cyclo-oxygenase, this form of phospholipase A2 requires calcium and calmodulin for activation. Thus, cells expressing cyclo-oxygenase-1 require only the calcium activation of phospholipase A2 for prostaglandin production to follow. Indeed, it has long been considered that phospholipase and not cyclo-oxygenase is the rate limiting step in prostaglandin production (see Cirino, 1998). More recently, however, with so much research attention being paid to cyclo-oxygenase-2, the role of phospholipases in the liberation of prostaglandins by inflamed cells and tissues expressing cyclo-oxygenase-2 has often been overlooked. However, as is the case for cyclo-oxygenase-1, the ability of cells and tissues expressing cyclo-oxygenase-2 in vitro (Saunders et al., 1999) and in vivo (Hamilton et al., 1999) to release prostanoids is greatly enhanced when substrate is provided or phospholipase activated. It is not yet clear exactly how phospholipase activity synchronises with cyclo-oxygenase in cells expressing both cyclo-oxygenase isoforms. However, it has been suggested that some level of compartmentalization is present in cells and that distinct pools of arachidonic acid are utilized by cyclo-oxygenase-1 and cyclo-oxygenase-2 (Reddy et al., 1992). Because of the relationship between substrate liberation and prostanoid release, we have hypothesized that the cyclo-oxygenase-1-dependent release of products from cells represents a one component system (Figure 1) whilst cyclo-oxygenase-2 dependent release represents a two component model (Hamilton et al., 1999; Saunders et al., 1999).
Figure 1.
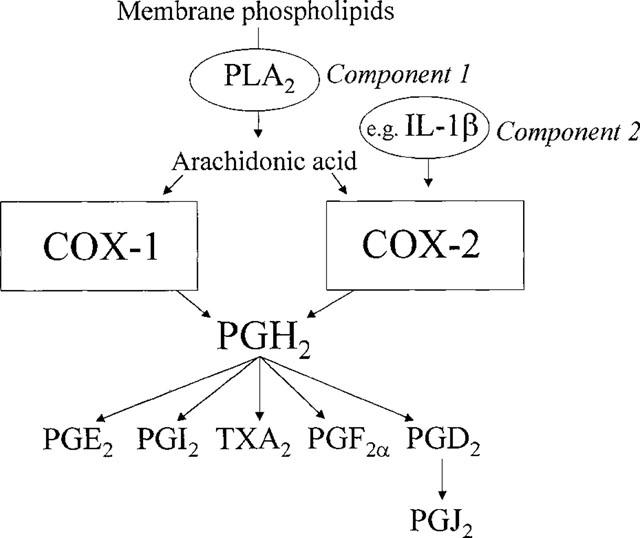
One and two component models of prostanoid production by, respectively, cyclo-oxygenase-1 (COX-1) and cyclo-oxygenase-2 (COX-2). PLA2, phospholipase A2; PG, prostaglandin.
Products
Once arachidonic acid has been supplied both isoforms of cyclo-oxygenase form PGG2 and PGH2 via identical enzymatic processes. Following these steps, PGH2 can be metabolized by different enzyme pathways to a range of products with potent biological effects (Figure 1). Prostacyclin (PGI2), the main cyclo-oxygenase product of endothelial cells, is formed by prostacyclin synthetase. Thromboxane (TXA2), the main product of platelets, is formed by thromboxane synthetase. PGD2 and PGF2 are formed from PGH2 by their respective synthase enzymes in a variety of cell types. PGE2 is formed both by enzymatic (PGE2 isomerase) and non-enzymatic pathways. The profile of products made by cells expressing cyclo-oxygenase-1 or cyclo-oxygenase 2 is therefore determined by the presence of different ‘down stream' enzymes. However, when cells express large amounts of cyclo-oxygenase-2 the PGH2 formed appears to be saturating for the PG synthase enzymes resulting in the formation of proportionately larger amounts of PGE2 (Bishop-Bailey et al., 1997a), possibly by non-enzymatic conversion. This is probably explained by the apparent lack of regulation of synthase enzymes at sites of inflammation.
The isoprostanes are a group of prostaglandin-like compounds formed under conditions of oxidative stress (see Morrow & Roberts, 1997). Although originally it was thought that these products were formed independently of cyclo-oxygenase, it is now clear that there are also cyclo-oxygenase-dependent pathways of formation. Thus, isoprostanes can be formed along with other arachidonic acid metabolites when cyclo-oxygenase-2 is induced (Jourdan et al., 1999). Moreover, there is also evidence that platelets form isoprostanes, presumably via the action of cyclo-oxygenase-1 (Pratico et al., 1995). Clearly, however, isoprostanes formed during oxidative stress are not dependent upon the activity of cyclo-oxygenase (Jourdan et al., 1999).
One interesting observation has been that the products formed by cyclo-oxygenase-1 and cyclo-oxygenase-2 diverge following treatment with aspirin. Aspirin acetylates serine 530 in cyclo-oxygenase, which has the effect of irreversibly inactivating cyclo-oxygenase-1. This explains the anti-platelet activity of aspirin. By contrast, aspirin-acetylated cyclo-oxygenase-2 produces R15HETE instead of PGH2 (Holtzman et al., 1992; Meade et al., 1993). The biological significance of this property of aspirin and cyclo-oxygenase-2 is not known. However, R15HETE is further metabolized by 5 lipoxygenase to form novel lipoxins (Clària & Sherhan, 1995), which may have effects in inflammation (Mitchell & Belvisi, 1997). These observations give rise to the intriguing possibility that part of the beneficial effects of aspirin could be attributed to the production of anti-inflammatory products.
Receptors
As outlined above the effects resulting from induction of cyclo-oxygenase-2 at a particular site will be partly dependent upon the distribution of synthetase enzymes and the oxidative state of the cell; i.e. these will regulate the mix of arachidonic acid metabolites released. Clearly, however, another factor regulating the overall biological consequence of cyclo-oxygenase-2 induction is the distribution of prostanoid receptors on local target tissues and cells. There are a number of different prostanoid receptor types, and yet more subtypes, linked to a range of signal transduction pathways. These prostanoid receptors are cell membrane spanning G-protein coupled receptors that mediate the physiological actions of the principal prostanoid metabolites (see Figure 1). Five major subdivisions of the prostanoid receptor family have been defined pharmacologically, and these correspond to each of the metabolites. The main endogenous receptor for PGI2 is the IP receptor. IP receptors are linked to activation of adenylate cyclase and so the action of PGI2 upon IP receptors is to elevate cyclic AMP, generally leading in many cells to an inhibition of active processes. For instance, in vascular smooth muscle activation of IP receptors promotes vasodilatation and in platelets leads to a reduction in aggregation and adhesion (Armstrong, 1996). TXA2 is the principle ligand for TP receptors. TP receptors are linked to activation of the inositolphosphate pathway with subsequent increases in intracellular calcium, generally leading to activation of target cells. For instance, in direct contrast to IP receptors, activation of TP receptors on vascular smooth muscle causes contraction and on platelets causes aggregation and adhesion (Negishi et al., 1995). There are four characterized receptors that PGE2 preferentially activates, EP1-4. Each EP receptor is linked to a different transduction pathway giving rise to activation or inhibition of cellular responses (Pierce & Regan, 1998). PGF2α and PGD2 activate FP and DP receptors, respectively. FP and DP receptors are linked to activation of inositolphosphates and generally result in activation of cellular processes including smooth muscle contraction.
Recently much interest has been paid to the role of the nuclear hormone receptor, peroxisome proliferator-activated receptor-γ (PPAR-γ), in the biological actions of PGJ2 and related metabolites (Forman et al., 1995; Kliewer et al., 1995). In particular PGJ2 has been found to activate PPAR-γ leading to changes in cell proliferation, such as promoting adipogenesis. Recently, it has been shown that PGF2α can negatively regulate PPAR-γ (Reginato et al., 1998), probably via an indirect action following activation of cell surface FP receptors. Interestingly, it appears that cyclo-oxygenase-2 and PPAR-γ are co-localized under some circumstances. This is because, cyclo-oxygenase-2 is located on the nuclear membrane during synthesis and transport to the endoplasmic reticulum. It is, therefore, possible that cyclo-oxygenase-2 products preferentially activate PPAR or even conventional prostanoid receptors located on the nuclear membrane.
Other substrates
As mentioned above, the main substrate for cyclo-oxygenase-1 or cyclo-oxygenase-2 is the n-6 polyunsaturated fatty acid, arachidonic acid (20 : 4). However, other fatty acids can be utilized by both cyclo-oxygenase-1 and cyclo-oxygenase-2 under certain conditions. For instance, the inhibitory effects of n-3 polyunsaturated fatty acids on platelet function are thought to be due to inhibition of thromboxane production following their competition with arachidonic acid for the active site of cyclo-oxygenase-1 in platelets. Indeed, the n-3 fatty acid, eicosapentaenoic acid (EPA), can be metabolized by plateletes, at the expense of arachidonic acid, to produce TXA3 (see Goodnight et al., 1992). TXA3 has considerably weaker biological actions than the arachidonic acid derivative TXA2. Thus, the anti-platelet effects of dietary EPA are probably exerted via effects on cyclo-oxygenase-1 metabolites. In addition to its effects on platelets, EPA also appears to substitute as a cyclo-oxygenase substrate in other cells as well. For example in isolated endothelial cells in culture (cyclo-oxygenase-1; Mitchell et al., 1993) treatment with EPA leads to the formation of PGI3 (Bordet et al., 1986; Takahata & Yamanaka, 1987). In vivo, however, a number of studies have shown that humans taking EPA experience decreases in TXA2 production without any change (von Shacky and Weber, 1985) or even in increase in PGI2 formation (DeCaterina et al., 1990; Honstra et al., 1990). Thus, in man, EPA further tips the PGI2 : TXA2 balance in the favour of cardio-protection. The ability of purified preparations of cyclo-oxygenase-2 and cyclo-oxygenase-1 to metabolize other substrates (Percival et al., 1994) has recently been compared. Both cyclo-oxygenase-1 and -2 catabolized substrates with the following rank order of efficiency, arachidonic acid>dihomo-gamma-linolenate>linoleate>alpha-linolenate. Gamma linolenate, alpha linolenate and EPA were very poor substrates for both forms, but in both cases were better metabolized by cyclo-oxygenase-2 than by cyclo-oxygenase-1 (Laneuville et al., 1995). The greater promiscuity of cyclo-oxygenase-2 is thought to be due to the larger substrate channel in this isoform (see Vane et al., 1998).
Cyclo-oxygenase-2 as a therapeutic target
Inflammation
Cyclo-oxygenase products, mainly PGE2, modulate the classical signs of inflammation. The two best studied inflammatory roles of cyclo-oxygenase products are induction of swelling and pain. In the case of ‘swelling' PGEs are thought to cause plasma exudation in a synergistic fashion with other mediators such as complement factor 5a (Williams & Peak, 1977). One of the most important inflammatory disease targets associated with cyclo-oxygenase-2 is arthritis. In animal models of arthritis cyclo-oxygenase-2 is induced and thought to be responsible for the associated increase in PG production (Anderson et al., 1996). Cyclo-oxygenase-2 expression has been identified in human osteoarthritis affected cartilage (Amin et al., 1997) as well as in synovial tissue taken from patients with rheumatoid arthritis (Kang et al., 1996).
In the case of pain, PGEs appear to ‘sensitize' peripheral sensory nerve endings located at the site of inflammation (Bley et al., 1998). In addition, cyclo-oxygenase products are thought to act in the spinal cord to facilitate the transmission of pain responses (Beiche et al., 1996; Yamamoto et al., 1996). However, despite a clear role for cyclo-oxygenase-2 in causing inflammatory swelling in animal models (see Chan & Rodger, 1997), the relative role of the two isoforms in pain is more complex. Clearly the perception of acute pain is more likely to be modulated by cyclo-oxygenase-1 as time for induction must elapse for cyclo-oxygenase-2. However, a number of studies have strongly implicated a role for cyclo-oxygenase-2 in inflammatory pain. For instance, highly selective cyclo-oxygenase-2 inhibitors (e.g. DFU) inhibit hyperalgesia in rats (Riendeau et al., 1997). Moreover, the process of sensing pain may actually lead to the induction of cyclo-oxygenase-2 induction in the spinal cord, as has been demonstrated in rats exposed to inflammatory stimuli in the paw (Beiche et al., 1996; Gardiner et al., 1997). Importantly, selective inhibitors of cyclo-oxygenase-2, e.g. rofecoxib, have been shown to be analgesic in humans when used for post-dental surgery pain (Morrison et al., 1999). The relative contributions of cyclo-oxygenase-1 and cyclo-oxygenase-2 in chronic pain (i.e. associated with rheumatoid or osteo-arthritis) remain to be established.
Cancer
One of the exciting observations associated with the use of NSAIDs is the association with a reduction in the incidence of colon cancer. Indeed, a retro-prospective study revealed the startling findings that patients taking relatively low doses of aspirin, a maximum effect being seen at four to six tablets per week, for long periods of time had substantially reduced risks of developing colon cancers (Giovannucci et al., 1995). It is not entirely clear how this protective effect of NSAIDs is exerted. However, adenocarcinomas in human subjects appear associated with marked increases in cyclo-oxygenase-2 expression (Smalley & DuBois, 1997) and evidence from studies with isolated cells in culture (DuBois et al., 1998) or animal models (Williams et al., 1997) similarly points to cyclo-oxygenase-2 being the level at which the beneficial effects of NSAIDs are exerted. The process underlying these effects is thought to be the ability of prostaglandins produced by cyclo-oxygenase-2 to slow down the rate of apoptosis in cancerous cells. This response is reduced by exposure to NSAIDs of the colon cancer cells (See DuBois et al., 1998). The effect of cyclo-oxygenase-2 selective NSAIDs on colon cancer in man is currently being investigated.
Cyclo-oxygenase-2 has now been identified as present in a number of other cancers including, oesophageal (Zimmerman et al., 1999), gastric (Murata et al., 1999), and pancreatic (Tucker et al., 1999) cancer. Whether or not cyclo-oxygenase-2 inhibitors can provide some level of protection in these forms of cancer remains to be established.
Alzheimer's disease
In addition to colon cancer, epidemiological evidence has suggested that in patients taking NSAIDs there is a reduced risk of developing Alzheimer's disease (see Pasinetti, 1998). Indeed, in 1997 an inverse correlation was drawn between onset and severity of Alzheimer's and the intake of NSAIDs, usually ibuprofen. The protective effects of NSAIDs in this setting are most likely related to reduction of inflammation as paracetamol is without effect (Stewart et al., 1997). Several groups have published supporting evidence for a role of cyclo-oxygenase-2 in this disease. Pasinetti & Aisen (1998) have shown that cyclo-oxygenase-2 expression is increased in the frontal cortex of brains from patients with Alzheimer's disease. Moreover, both isoforms of cyclo-oxygenase, as well as PPAR-γ receptor, expression are increased in the temporal cortex of brains from these patients (Kitamura et al., 1999). The link between the inflammatory properties of prostaglandins and Alzheimer's is not completely understood. However, animal studies have shown that after kainic acid induced seizures, cyclo-oxygenase-2 is induced in neurones that are susceptible to apoptosis (Tocco et al., 1997). Nevertheless, it is possible that the epidemiological data showing that NSAIDs reduce Alzheimer's may have nothing to do with cyclo-oxygenase-2 inhibition. Indeed, the anti-platelet properties of NSAIDs may also relieve or prevent Alzheimer's. For example, de la Torre and co-workers (de la Torre et al., 1997) hypothesised that Alzheimer's disease could be caused by the inappropriate development and invasion of capillaries in and around the brain tissue. The possibility of coagulation leading to ischaemic damage via blockade of these capillaries would be greatly reduced by aspirin and related drugs via an action on platelet cyclo-oxygenase-1.
The introduction of cyclo-oxygenase-2 selective NSAIDs into the general population for the treatment of arthritis and pain will allow, in time, epidemiological comparisons to be made with traditional drugs for their effects on the development of Alzheimer's disease.
Nonsteroidal anti-inflammatory drugs (NSAIDs) and cyclo-oxygenase isoforms
NSAID is a ‘catch all' name for a large number of chemically distinct drugs. Together they represent the single most important group of self-prescribed pharmaceuticals and the most widely used drug class. The therapeutic benefits of all NSAIDs include inhibition of swelling and/or pain at the site of inflammation. In addition, aspirin also offers protection against stroke and thrombosis, Alzheimer's disease and cancer (see above). There are, however, side effects of NSAIDs that limit their use in some patients. Most common among these side effects is irritation and damage to the gastro-intestinal mucous, particularly the gastric mucosa. Each member of the NSAID family has some level of individual effects, however, the unifying common mechanism of action of all is inhibition of cyclo-oxygenase (Vane, 1971). With identification of two distinct forms of cyclo-oxygenase came a new hypothesis to explain the effects of NSAIDs; ‘inhibition of cyclo-oxygenase-2 accounts for the therapeutic benefits and inhibition of cyclo-oxygenase-1 for the side-effects of NSAIDs' (Mitchell et al., 1994). The following explains why this hypothesis was presented and how its validity was proven.
Relationship between the selectivity of NSAIDs for cyclo-oxygenase-1 and gastric side effects
As a class the NSAIDs represent a major risk for morbidity and mortality from gastro-intestinal damage, perforation, ulcers and bleeding. In the U.S.A. the number of deaths per year due to NSAIDs is approximately 16,500 with 107,000 hospitalizations in the same period (Fries et al., 1998). In the U.K., it is estimated that 12,000 ulcer complications and 1200 deaths per year are directly linked to NSAID intake (Hawkey, 1996). However, some NSAIDs cause more gastro-intestinal side effects than others (Henry et al., 1996). A number of different experimental assays have been used to compare the potencies of NSAIDs on cyclo-oxygenase−1 and cyclo-oxygenase−2. On the whole, studies have shown that the ability of a given NSAID to inhibit cyclo-oxygenase-1 correlates with the degree of side effects it causes.
Comparison of the selectivity's of NSAIDs for cyclo-oxygenase-1 versus cyclo-oxygenase-2
In 1993, two studies showed that a range of contemporary NSAIDs preferentially inhibited cyclo-oxygenase-1 (Meade et al., 1993; Mitchell et al., 1993). Meade and co-workers used semi-purified preparations of murine cyclo-oxygenase-1 and -2 and monitored activity by an oxygen electrode. In our study (Mitchell et al., 1993), the production of PGs from intact cells expressing cyclo-oxygenase-1 (bovine endothelial cells) or cyclo-oxygenase-2 (LPS-activated J774 murine macrophages) was used as an index of enzyme activity. In addition, we compared NSAIDs potencies in whole cells with those of broken cell membranes and purified protein (Mitchell et al., 1994). In both of the early reports, the rank orders of selectively of NSAIDs for cyclo-oxygenase-1 were found to be associated with the risk of these agents producing gastro-intestinal side effects. Thus, the hypothesis that cyclo-oxygenase-1 inhibition was linked to NSAID side effects was suggested. Since these studies were made a number of variations, and improvements have been made to in vitro assays used to screen novel NSAIDs, with the intention of identifying cyclo-oxygenase-2 selective compounds (see Vane et al., 1998). A number of important criteria have been identified for optimum reliability of cyclo-oxygenase screens that are; (1), the use of both isoforms from a common species (preferably human); (2), common incubation times of drugs with both cyclo-oxygenase systems; (3), presence of realistic levels of plasma; (4), equivalent levels of substrate being available to both isoforms. To these ends, we have recently developed an improved cyclo-oxygenase-1–cyclo-oxygenase-2 screen that takes all of the points into consideration (Warner et al., 1999).
The classical NSAIDs currently used fall into three categories for the induction of gastro-intestinal side effects (see Figure 2). Ketorolac, ketoprofen, indomethacin, tolmetin and piroxicam constitute the first and most dangerous group of drugs. Fenoprofen, aspirin, naproxen and sulindac represent a mid-range gastro-intestinal risk. By contrast, ibuprofen, diclofenac and diflunisal appear to produce relatively fewer gastro-intestinal events (Garcia Rodriguez et al., 1998; Henry et al., 1998). Comparison of the relative activities of all these NSAIDs as inhibitors of human cyclo-oxygenase-1 versus cyclo-oxygenase-2 supports the idea that it is inhibition of cyclo-oxygenase-1 rather than inhibition of cyclo-oxygenase-2 that underlies the gastrotoxic effects of the NSAIDs (Figure 2).
Figure 2.
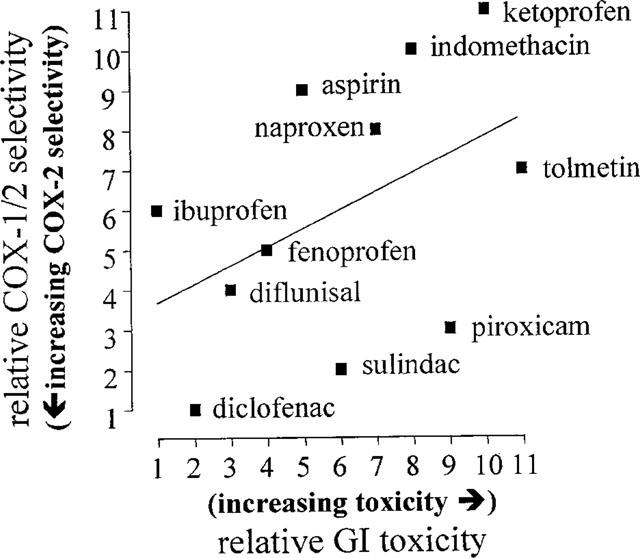
Relationship for NSAIDs between relative activities against cyclo-oxygenase-1 versus cyclo-oxygenase-2 (IC80 ratios, Warner et al., 1999) and risk of producing gastrointestinal toxicity (Henry et al., 1996).
Development of cyclo-oxygenase-2 selective compounds
The use of cyclo-oxygenase screens in vitro has been remarkably successful and has led to the development of a number of experimental cyclo-oxygenase-2 selective compounds. These include from Merck Frosst (Canada), L-745,337 (Chan et al., 1995), DFU (5,5-dimethyl-3-(3-fluorophenyl)-4 (4-methylsulphonyl)phenyl-2 (5H)-furanone; Riendeau et al., 1997) and DFP (3-(2-propyloxy)-4-(4-methylsulphonylphenyl)-5,5-dimethylfuranone; Black et al., 1999), and from Monsanto-Searle (U.S.A.), SC58125 (Guo et al., 1996). Furthermore this approach has led to the development of two cyclo-oxygenase-2 selective NSAIDs with FDA approval that are currently or about to be available in the U.S.A. These are rofecoxib (Vioxx™: Chan et al., 1999) from Merck-Frosst and celecoxib (Celebrex™: Geiss, 1999) from Monsanto (Figure 3). In addition to the development of new compounds by these screens, it has been possible to identify currently prescribed NSAIDs that are cyclo-oxygenase-2 selective. For example, using whole blood assays compounds displaying more than 5 fold selectivity for cyclo-oxygenase-2 would include etodolac, meloxicam and nimesulide (Warner et al., 1999) (Figure 4).
Figure 3.
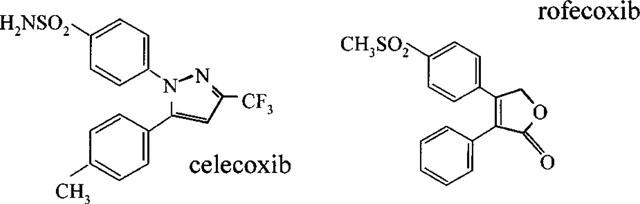
Chemical structures of celecoxib and rofecoxib.
Figure 4.
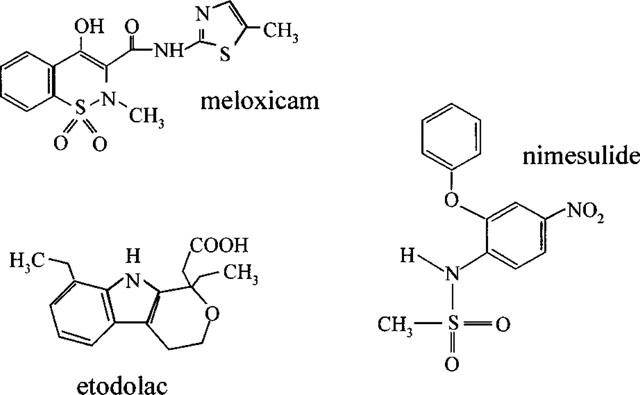
Chemical structures of etodolac, meloxicam and nimesulide.
Confirmation of the ‘cyclo-oxygenase-2 hypothesis' in animal models
The effects of experimental cyclo-oxygenase-2 selective inhibitors have been studied in a number of animal models of inflammation and hyperalgesia. As a general rule inhibitors with more than 10 fold selectivity for cyclo-oxygenase-2, have proven to inhibit inflammatory responses without causing gastro-intestinal side effects. For example in 1995, Chan and co-workers showed that the L-745,337, inhibited cyclo-oxygenase-2 with more than a 500 fold selectivity. This compound was compared with indomethacin and found to be equally effective at inhibiting carageenin-induced paw oedema. However, unlike indomethacin, L745-337 did not cause any gastric lesions (Chan et al., 1995). Similarly, clinical trials with cyclo-oxygenase-2 selective agents such as celecoxib and rofecoxib have indicated that when used at therapeutic doses these produce significantly less gastrointestinal damage than traditional NSAIDs (Geiss, 1999; Lanza et al., 1999). Other cyclo-oxygenase-2 selective inhibitors have been used to implicate a role for this isoform in hyperalgesia associated with thermal trauma (Dirig et al., 1998) or intraplantar Freund's complete adjuvant (Hay et al., 1997). In addition, cyclo-oxygenase-2 activity may contribute to hyperexcitability of sensory nerves in the spinal chord (Willingale et al., 1997) and also to the genesis of fever (Schwartz et al., 1999). At the same time, however, it must also be noted that other studies have demonstrated pro-inflammatory roles for prostanoids generated by cyclo-oxygenase-1 (Wallace et al., 1998b).
Homeostatic/beneficial roles of cyclo-oxygenase-2
Constitutive expression of cyclo-oxygenase-2
Kidney
In addition to its expression at sites of inflammation cyclo-oxygenase-2 is also expressed constitutively in a number of tissues including the kidney and the brain. At these sites cyclo-oxygenase-2 activity is likely to be beneficial to the body forming prostanoids important to homeostatic processes. For example, in animal kidneys cyclo-oxygenase-1 and cyclo-oxygenase-2 are co-localized in the macula densa (Harris et al., 1994), although in man cyclo-oxygenase-2 appears more associated with podocytes. In addition, cyclo-oxygenase-2 has been identified in a subset of thick ascending limb cells, where it has been suggested to be involved in the handling of ions (Vio et al., 1997). People taking NSAIDs are at an increased risk of side effects in the kidney, but the incidence of these are much lower than those in the gastrointestinal tract (see Vane et al., 1998). Indeed, it seems that formation of PGs generally only become important when the kidney is ‘stressed', following for instance volume depletion, and PGI2 in particular becomes essential to sustaining renal vasodilatation and blood flow. Despite the kidney expressing cyclo-oxygenase-2 constitutively the clinical trials to date have not identified any deleterious effects of cyclo-oxygenase-2-selective inhibitors. However, in salt-depleted individuals the cyclo-oxygenase-2-selective inhibitor, celecoxib, has been reported to cause sodium and potassium retention, leading to the conclusion that cyclo-oxygenase-2 selectivity does not spare the kidney under all circumstances (Rossat et al., 1999).
Central Nervous System (CNS)
Cyclo-oxygenase-1 and cyclo-oxygenase-2 are both localized in the brain and spinal cord. However, there appears to be specific regions of these structures where either isoform is expressed predominately. For instance cyclo-oxygenase-2 is mainly expressed in the cortex, hypothalamus and hippocampus (Breder et al., 1995; Breder & Soper, 1996). By contrast, cyclo-oxygenase-1 is expressed in almost all structures of the central nervous system and is particularly highly localized in the forebrain. It is not yet clear what the specific roles of the two isoforms are in the central nervous system. However, it has been known for some time that PGs modulate activities such as thermoregulation and sleep by actions on or within nerves.
Vas deferens
Recently, using immunohistochemical techniques, McKanna and co-workers (1998) have reported that cyclo-oxygenase-2 is the predominant isoform present in the vas deferens of the rat. In particular, cyclo-oxygenase-2 appears highly localized to the epithelium lining this structure. As may be expected, cyclo-oxygenase-1 and not cyclo-oxygenase-2 was predominately expressed in the seminal vesicles. Cyclo-oxygenase-2 expression was regulated by androgen and suggested to be involved in penile erection (McKanna et al., 1998).
Pancreas
It has been known for some time that PGE2 inhibits the glucose-dependent release of insulin (see Robertson, 1998). However, it has recently been suggested that cyclo-oxygenase-2 is expressed constitutively in the pancreatic islets and that this isoform mediates PG production by these cells. In fact, this isoform predominates in islet cells isolated from hamster and human pancreas (Sorli et al., 1998). These observations suggest that specific cyclo-oxygenase-2 inhibitors may have effects on glucose regulation in some patients.
Gastrointestinal tract
As discussed above, gastric damage is the main side effect associated with inhibition of cyclo-oxygenase-1. Thus, cyclo-oxygenase-2 inhibitors are expected to cause fewer gastric side effects. Recent studies conducted by Zimmerman and co-workers (1998) suggests that cyclo-oxygenase-2, along with cyclo-oxygenase-1, is expressed in human gastric mucosa. Furthermore cyclo-oxygenase-2 selective inhibitors suppress the formation of PGs from samples of human gastric (Zimmerman et al., 1998) and colonic tissue (McCartney et al., 1999). Thus, a number of lines of evidence suggest that cyclo-oxygenase-2 is expressed throughout the human gastrointestinal tract. This ‘constitutive' expression may be a result of low level trauma and/or infection that often occurs without obvious macroscopic symptoms. Whether cyclo-oxygenase-2 activity in the gut acts in the same way was as cyclo-oxygenase-1, to protect it from damage, remains to be established. Nevertheless, these observations suggest that some patients may experience gastric side effects when taking cyclo-oxygenase-2 selective inhibitors (see Wallace et al., 1998a). Indeed, use of selective inhibitors of cyclo-oxygenase-2 in animal models of ulceration and gastrointestinal damage have suggested that cyclo-oxygenase-2 products promote gastrointestinal healing (Reuter et al., 1996; Mizuno et al., 1997; Ukawa et al., 1998).
Protective/beneficial effects of induced cyclo-oxygenase-2
When discussing the role of cyclo-oxygenase-2, it should not be forgotten that the ‘inflammatory response' is in itself a defence mechanism to protect the body from noxious stimuli. In this way, when cyclo-oxygenase-2 is induced at sites of inflammation and the response is appropriately limited, it can be considered a beneficial process. It is only when the response continues unnecessarily that cyclo-oxygenase-2 activity becomes deleterious. There are now emerging a number of other areas in which cyclo-oxygenase-2 induction has beneficial or protective properties including in the cardiovascular and respiratory systems as well as in the reproductive tract.
Cyclo-oxygenase-2 in the cardiovascular system
The activity of cyclo-oxygenase-1 in endothelial cells is thought to be beneficial, contributing to the normal functioning of the cardiovascular system, via the release of PGI2. PGI2 is a vasodilator, with potent inhibitory actions on platelet function (Moncada et al., 1976; Radomski et al., 1976; Sneddon & Vane, 1988), and is an endogenous anti-lipidemic agent (Willis et al., 1986). Indeed, it has been suggested that increasing PGI2 production or applying exogenous PGI2-mimetics could be therapeutically beneficial in cardiovascular diseases such as atherosclerosis (Willis et al., 1986). Recently it has been shown that cyclo-oxygenase-2 becomes induced in animal arterial vessels after physical damage or exposure to pro-inflammatory cytokines (Rimarachin et al., 1994). Similarly, we have shown that in human vessels in vitro inflammatory agents including interleukin (IL)-1β, tumour necrosis factor (TNF)α, interferon (INF)γ or endotoxin induce cyclo-oxygenase-2 within the vascular smooth muscle component. Moreover, this induction is able to rectify the loss of PG production that follows endothelial damage (Bishop-Bailey et al., 1997b; 1998; Jourdan et al., 1997). Induction of cyclo-oxygenase-2 in human vascular smooth muscle cells appears to have protective functions. Indeed, our studies show that cyclo-oxygenase-2 expression in the smooth muscle of damaged vessels suppresses inflammatory events such as cell proliferation (Bishop-Bailey et al., 1997b), cytokine release (see Mitchell and Evans, 1998) and adhesion receptor expression (Bishop-Bailey et al., 1998). These observations strongly suggest that cyclo-oxygenase-2 is induced as a protective defence enzyme in human cardiovascular disease (see Mitchell & Evans, 1998). Most recently, the group of Fitzgerald have shown that two cyclo-oxygenase-2 selective inhibitors, celecoxib (McAdam et al., 1999) and rofecoxib (Catella-Lawson et al., 1999) reduce circulating levels of prostacyclin in healthy human volunteers. This data suggests that cyclo-oxygenase-2 is a feature of the human cardiovascular system under physiological conditions. However, despite its selectivity in broken cell systems (Gierse et al., 1999) it should be noted that celecoxib has also been shown to inhibit cyclo-oxygenase-1 in intact human cells (Warner et al., 1999).
Cyclo-oxygenase-2 in the respiratory tract
Cyclo-oxygenase-2 is induced by cytokines in a number of airway cells including the epithelium (Mitchell et al., 1994) and underlying smooth muscle (Belvisi et al., 1998). Asthma and related diseases are characterized by excessive proliferation of airway cells, which contributes to airway narrowing in some patients. Cyclo-oxygenase-2 induction inhibits proliferation of human airway smooth muscle cells, suggesting a protective role of this enzyme in diseases such as asthma (Belvisi et al., 1998).
A subset of asthmatic patients experience symptoms after taking aspirin and related drugs (Kowalski, 1995). This form of asthma is termed ‘aspirin-sensitive asthma'. Although the biochemical mechanisms underlying aspirin-sensitive asthma are still being debated, there is a general level of acceptance that cyclo-oxygenase activity suppresses leukotriene production (Kowalski, 1995). Thus when cyclo-oxygenase is blocked by aspirin in sensitive patients, leukotriene production increases and asthma symptoms ensue. Aspirin sensitive asthmatics express cyclo-oxygenase-2 in their airways (Sousa et al., 1997; Cowburn et al., 1998). It is not yet clear whether cyclo-oxygenase-2 activity contributes to the ‘leukotriene brake' associated with PG production in these patients. However, it is tempting to speculate that cyclo-oxygenase-2 induction in airway cells leads to beneficial products that limit the production of leukotrienes.
Cyclo-oxygenase-2 in reproduction
There are several lines of evidence that suggest that cyclo-oxygenase-2 is important in positive feed-back mechanisms associated with reproduction. Both cyclo-oxygenase-1 and cyclo-oxygenase-2 are expressed in the uterine epithelium at different times in early pregnancy. For example, cyclo-oxygenase-1 is expressed preferentially in the uterine epithelium prior to implantation. After the embryo attaches, cyclo-oxygenase-1 is then down regulated (Chakraborty et al., 1996). Cyclo-oxygenase-2 is expressed in the luminal epithelium and subepithelial stromal cells at the time of attachment of the blastocyst. Thus, expression of cyclo-oxygenase-2 may be important for localized increased uterine permeability and the attachment reaction (Chakraborty et al., 1996).
Summary and conclusions
This year is the centenary of aspirin. It remains one of the most popular self-prescribed preparations world wide. There have been a number of important scientific observations made during the first 100 years of aspirin that have led us to the identification of better drugs and identified new therapeutic targets. We now know that aspirin and related drugs inhibit the two identified forms of cyclo-oxygenase. Inhibition of the constitutively expressed cyclo-oxygenase-1 contributes significantly to the side effects of these drugs. Inhibition of the inducible cyclo-oxygenase-2 accounts for the beneficial anti-inflammatory and perhaps the analgesic, effects of these drugs. Using this principle as an incentive, assays have been developed and used to screen for new and better aspirin-like drugs. Indeed, several selective inhibitors of cyclo-oxygenase-2 have been identified. As predicted, the selective cyclo-oxygenase-2 inhibitors are anti-inflammatory without being ulcerogenic.
In addition to arthritis, there now seems to be two important diseases where cyclo-oxygenase-2 activity predominates and is deleterious, these are cancer (colon) and Alzheimer's. Thus, new selective inhibitors of cyclo-oxygenase-2 may have far reaching therapeutic potential.
There are clearly identified areas where cyclo-oxygenase-2 induction may be of benefit to the body. These are in major blood vessels and airways as well as in the gastro-intestinal and urogenital tract. Thus, we could anticipate potential unexpected side effects of cyclo-oxygenase-2 selective drugs in some patients.
Acknowledgments
J.A. Mitchell is a Wellcome Career Development Fellow; T.D. Warner is a British Heart Foundation Lecturer (FS/95003). The authors would also like to acknowledge generous research support provided by Boehringer Ingelheim Pharma KG and NicOx S.A.
Abbreviations
- DFP
(3-(2-propyloxy)-4-(4-methylsulphonylphenyl)-5,5-dimethylfuranone
- DFU
dimethyl-3-(3-fluorophenyl)-4-(4-methylsulphonyl)phenyl-2(5H)-furanone
- EPA
eicosapentaenoic acid
- NSAID
nonsteroidal anti-inflammatory drugs
- PPAR
peroxisome proliferator-activated receptor
- PG
prostaglandin
- TX
thromboxane
References
- AMIN A.R., ATTUR M., PATEL R.N., THAKKER G.D., MARSHALL P.J., REDISKE J., STUCHIN S.A., PATEL I.R., ABRAMSON S.B. Superinduction of cyclo-oxygenase-2 activity in human osteoarthritis-affected cartilage: influence of nitric oxide. J. Clin. Invest. 1997;99:1231–1237. doi: 10.1172/JCI119280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ANDERSON G.D., HAUSER S.D., MCGARITY K.L., BREMER M.E., ISAKSON P.C., GREGORY S.A. Selective inhibition of cyclo-oxygenase (COX)-2 reverses inflammation and expression of COX-2 and interleukin-6 in rat adjuvant arthritis. J. Clin. Invest. 1996;97:2672–2679. doi: 10.1172/JCI118717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ARMSTRONG R.A. Platelet prostanoid receptors. Pharmacol. Ther. 1996;72:171–191. doi: 10.1016/s0163-7258(96)00103-9. [DOI] [PubMed] [Google Scholar]
- BARRY O.P., KAZANIETZ M.G., PRACTICO D., FITZGERALD G.A. Arachidonic acid in platelet microparticles up-regulates cyclo-oxygenase-2 dependent prostaglandin formation via a protein kinase C/mitogen-activated protein kinase-dependent pathway. J. Biol. Chem. 1999;274:7545–7556. doi: 10.1074/jbc.274.11.7545. [DOI] [PubMed] [Google Scholar]
- BAZAN N.G., FLETCHER B.S., HERSCHMAN H.R., MUKHERJEE P.K. Platelet-activating factor and retinoic acid synergistically activate the inducible prostaglandin synthase gene. Proc. Natl. Acad. Sci. U.S.A. 1994;91:5252–5256. doi: 10.1073/pnas.91.12.5252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BEICHE F., SCHEURER S., BRUNE K., GEISSLINGER G., GOPPELT-STRUEBE M. Up-regulation of cyclo-oxygenase-2 mRNA in the rat spinal cord following peripheral inflammation. FEBS Lett. 1996;390:165–169. doi: 10.1016/0014-5793(96)00604-7. [DOI] [PubMed] [Google Scholar]
- BELVISI M.G., SAUNDERS M., YACOUB M., MITCHELL J.A. Cyclo-oxygenase-2 induction has a profound inhibitory effect on human airway smooth muscle cell proliferation. Br. J. Pharmacol. 1998;125:1102–1108. doi: 10.1038/sj.bjp.0702104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BISHOP-BAILEY D., LARKIN S.W., WARNER T.D., CHEN G., MITCHELL J.A. Characterization of the induction of nitric oxide synthase and cyclo-oxygenase in rat aorta in organ culture. Br. J. Pharmacol. 1997a;121:125–133. doi: 10.1038/sj.bjp.0701100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BISHOP-BAILEY D., PEPPER J.R., HADDAD E.B., NEWTON R., LARKIN S.W., MITCHELL J.A. Induction of cyclo-oxygenase-2 in human saphenous vein and internal mammary artery. Arteriosclerosis Thromb. Vasc. Biol. 1997b;17:1644–1648. doi: 10.1161/01.atv.17.9.1644. [DOI] [PubMed] [Google Scholar]
- BISHOP-BAILEY D., PEPPER J.R., LARKIN S.W., MITCHELL J.A. Differential induction of cyclo-oxygenase-2 in human arterial and venous smooth muscle: role of endogenous prostanoids. Arteriosclerosis Thromb. Vas. Biol. 1998;18:1655–1661. doi: 10.1161/01.atv.18.10.1655. [DOI] [PubMed] [Google Scholar]
- BLACK W.C., BRIDEAU C., CHAN C.C., CHARLESON S., CHAURET N., CLAVEAU D., ETHIER D., GORDON R., GREIG G., GUAY J., HUGHES G., JOLICOEUR P., LEBLANC Y., NICOLL-GRIFFITH D., OUIMET N., RIENDEAU D., VISCO D., WANG Z., XU L., PRASIT P. 2,3-Diarylcyclopentenones as orally active, highly selective cyclo-oxygenase-2 inhibitors. J. Med. Chem. 1999;42:1274–1281. doi: 10.1021/jm980642l. [DOI] [PubMed] [Google Scholar]
- BLEY K.R., HUNTER J.C., EGLEN R.M., SMITH J.A.M. The role of IP prostanoid receptors in inflammatory pain. TIPS. 1998;19:141–147. doi: 10.1016/s0165-6147(98)01185-7. [DOI] [PubMed] [Google Scholar]
- BORDET J.C., GUICHARDANT M., LAGARDE M. Arachidonic acid strongly stimulated prostaglandin I3 (PGI3) production from eicosapentaenoic acid in human endothelial cells. Biochem. Biophys. Res. Commum. 1986;135:403–410. doi: 10.1016/0006-291x(86)90009-4. [DOI] [PubMed] [Google Scholar]
- BREDER C.D., SAPER C.B. Expression of inducible cyclo-oxygenase mRNA in the mouse brain after systemic administration of bacterial lipopolysaccharide. Brain Res. 1996;713:64–69. doi: 10.1016/0006-8993(95)01474-8. [DOI] [PubMed] [Google Scholar]
- BREDER C.D., DEWITT D., KRAIG R.P. Characterisation of inducible cyclo-oxygenase in rat brain. J. Comp. Neurol. 1995;355:296–315. doi: 10.1002/cne.903550208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CATELLA-LAWSON F., MCADAM B., MORRISON B.W., KAPOOR S., KUJUBU D., ANTES L., LASSETER K.C., QUAN H., GERTZ B.J., FITZGERALD G.A. Effects of specific inhibition of cyclo-oxygenase-2 on sodium balance, hemodynamics, and vasoactive eicosanoids. J. Pharmacol. Exp. Ther. 1999;289:735–741. [PubMed] [Google Scholar]
- CHAKRABORTY I., DAS S.K., WANG J., DEY S.K. Developmental expression of the cyclo-oxygenase-1 and cyclo-oxygenase-2 genes in the peri-implantation mouse uterus and their differential regulation by the blastocyst and ovarian steroids. J. Mol. Endocrinol. 1996;6:107–122. doi: 10.1677/jme.0.0160107. [DOI] [PubMed] [Google Scholar]
- CHAN C.C., BOYCE S., BRIDEAU C., FORD-HUTCHINSON A.W., GORDON R., GUAY D., HILL R.G. , LI C.S., MANCINI J., PENNETON M., PRASIT P., RASORI R., RIENDEAU D., ROY P., TAGARI P., VICKERS P., WONG E., RODGER I.W. Pharmacology of a selective cyclooxygenase-2 inhibitor, L-745,337: a novel nonsteroidal anti-inflammatory agent with an ulcerogenic sparing effect in rat and nonhuman primate stomach. J. Pharmacol. Exp. Ther. 1995;274:1531–1537. [PubMed] [Google Scholar]
- CHAN C.C., RODGER I.W. Selective cyclo-oxygenase-2 inhibitors as potential therapeutic agents for inflammatory diseases. Adv. Exp. Med. Biol. 1997;407:157–161. doi: 10.1007/978-1-4899-1813-0_24. [DOI] [PubMed] [Google Scholar]
- CHAN C., BOYCE S., BRIDEAU C., CHARLESON C., CROMLISH W., ETHIER D., EVANS J., FORD-HUTCHINSON A.W., FORREST M.J., GAUTHIER J.Y., GORDON R., GRESSER M., GUAY J., KARGMAN S., KENNEDY B., LEBLANC Y., LEGER S., MANCINI J., O'NEILL G.P., OUELLET M., PATRICK D., PERCIVAL M.D., PERRIER H., PRASIT P., RODGER I. Rofecoxib [Vioxx, MK-0966; 4-(4′-Methylsulfonylphenyl)-3-pheyl-2-(5H)-furanone]: a potent and orally active cyclo-oxygenase-2 inhibitor. Pharmacological and biochemical profiles. J. Pharmacol. Exp. Ther. 1999;290:551–560. [PubMed] [Google Scholar]
- CIRINO G. Multiple controls in inflammation. Extracellular and intracellular phospholipase A2, inducible and constitutive cyclo-oxygenase, and inducible nitric oxide synthase. Biochem. Pharmacol. 1998;55:105–111. doi: 10.1016/s0006-2952(97)00215-3. [DOI] [PubMed] [Google Scholar]
- CLÀRIA D., SHERHAN C.N. Aspirin triggers previously undescribed bioactive eicosanoids by human endothelial cell-leukocyte interactions. Proc. Natl. Acad. Sci. U.S.A. 1995;92:9475–9479. doi: 10.1073/pnas.92.21.9475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- COWBURN A.S., SLADEK K., SOJA J., ADAMEK L., NIZANKOWSKA E., SZCZEKLIK A., LAM B.K., PENROSE J.F., AUSTEN F.K., HOLGATE S.T., SAMPSON A.P. Overexpression of leukotriene C4 synthase in bronchial biopsies from patients with aspirin-intolerant asthma. J. Clin. Invest. 1998;101:834–846. doi: 10.1172/JCI620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DECATERINA R., GIANNESSI D., MAZZONE A., BERNINI W., LAZZERINI G., MAFFEI S., CERRI M., SALVATORE L., WEKSLER B. Vascular prostacyclin is increased in patients ingesting omega-3 poly-unsaturated fatty acids before coronary artery bypass graft surgery. Circulation. 1990;82:428–436. doi: 10.1161/01.cir.82.2.428. [DOI] [PubMed] [Google Scholar]
- DE LA TORRE J.C. Cerebromicrovascular pathology in Alzheimer's disease compared to normal aging. Gerontology. 1997;43:26–43. doi: 10.1159/000213834. [DOI] [PubMed] [Google Scholar]
- DEWITT D.L. Prostaglandin endoperoxide synthase: regulation of enzyme expression. Biochim. Biophys. Acta. 1991;1083:121–134. doi: 10.1016/0005-2760(91)90032-d. [DOI] [PubMed] [Google Scholar]
- DEWITT D.L., SMITH W.L. Primary structure of prostaglandin G/H synthase from sheep vesicular gland determined from the complementary DNA sequence. Proc. Natl. Acad. Sci. U.S.A. 1988;85:1412–1416. doi: 10.1073/pnas.85.5.1412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DIRIG D.M., ISAKSON P.C., YAKSH T.L. Effect of COX-1 and COX-2 inhibition on induction and maintenance of carrageenan-evoked thermal hyperalgesia in rats. J. Pharmacol. Exp. Ther. 1998;285:1031–1038. [PubMed] [Google Scholar]
- DUBOIS R.N., ABRAMSON S.B., CROFFORD L., GUPTA R.A., SIMON L.S., VAN DE PUTTE L.B.A., LIPSKY P.E. Cyclo-oxygenase in biology and disease. FASEB J. 1998;12:1063–1073. [PubMed] [Google Scholar]
- FENG L., XIA Y., GARCIA G.E., HWANG D., WILSON C.B. Involvement of reactive oxygen intermediates in cyclo-oxygenase-2 expression induced by interleukin-1, tumor necrosis factor-α, and lipopolysaccharide. J. Clin. Invest. 1995;95:1669–1675. doi: 10.1172/JCI117842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FLETCHER B.S., KUJUBU D.A., PERRIN D.M., HERSCHMAN H.R. Structure of the mitogen-inducible TIS10 gene and demonstration that the TIS10-encoded protein is a functional prostaglandin G/H synthase. J. Biol. Chem. 1992;267:4338–4344. [PubMed] [Google Scholar]
- FORMAN B.M., TONTONOZ P., CHEN J., BRUN R.P., SPIEGELMAN B.M., EVANS R.M. 15-Deoxy-Δ 12, 14-prostaglandin J2 is a ligand for the adipocyte determination factor PPAR gamma. Cell. 1995;83:803–812. doi: 10.1016/0092-8674(95)90193-0. [DOI] [PubMed] [Google Scholar]
- FRIES J.F., SINGH G., RAMEY D.R.The clinical epidemiology of non-steroidal anti-inflammatory drug gastropathy Clinical Significance and Potential of Selective COX-2 Inhibitors 1998London, U.K.: The William Harvey Press; 57–65.In: Vane, J.R., Botting, R.M. (eds) [Google Scholar]
- FU J.Y., MASFERRER J.L., SEIBERT K., RAZ A., NEEDLEMAN P. The induction and suppression of prostaglandin H2 synthase (cyclo-oxygenase) in human monocytes. J. Biol. Chem. 1990;265:16737–16740. [PubMed] [Google Scholar]
- GARCIA RODRIGUEZ L.A., CATTARUZZI C., GRAZIA TRONCON M., AGOSTINIS L. Risk of hospitalization for upper gastrointestinal tract bleeding associated with ketorolac, other nonsteroidal anti-inflammatory drugs, calcium antagonists and other antihypertensive drugs. Arch. Intern. Med. 1998;158:33–39. doi: 10.1001/archinte.158.1.33. [DOI] [PubMed] [Google Scholar]
- GARDINER N.J., GILETT S., GRUBB B.D. Cyclo-oxygenase in rat spinal cord: selective induction of COX-2 during peripheral inflammation. Br. J. Pharmacol. 1997;120:71P. doi: 10.1038/sj.bjp.0701548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GEISS S. Update on clinical developments with celecoxib, a new selective COX-2 inhibitor: what can we expect. Scand. J. Rheumatol. 1999;109 suppl.:31–37. [PubMed] [Google Scholar]
- GENG Y., BLANCO F.J., CORNELISSON M., LOTZ M. Regulation of cyclo-oxygenase-2 expression in normal articular chondrocytes. J. Immunol. 1995;155:796–801. [PubMed] [Google Scholar]
- GIERSE J.K., KOBOLDT C.M., WALKER M.C., SEIBERT K., ISAKSON P.C. Kinetic basis for selective inhibition of cyclo-oxygenases. Biochem. J. 1999;339:607–614. [PMC free article] [PubMed] [Google Scholar]
- GIOVANNUCCI E., EGAN K.M., HUNTER D.J., STAMPFER M.J., COLDITZ G.A., WILLET W.C., SPEIZER F.E. Aspirin and the risk of colorectal cancer in women. N. Engl. J. Med. 1995;333:609–614. doi: 10.1056/NEJM199509073331001. [DOI] [PubMed] [Google Scholar]
- GOODNIGHT S.H., CAIRNS J.A., FISHER M., FITZGERALD G.A. Assessment of the therapeutic use of n-3 fatty acids in vascular disease and thrombosis. Chest. 1992;102:S374–S384. doi: 10.1378/chest.102.4_supplement.374s. [DOI] [PubMed] [Google Scholar]
- GUO Q., WANG L.H., RUAN K.H., KULMACZ R.J. Role of Val509 in the time-dependent inhibition of human prostaglandin H synthase-s cyclo-oxygenase activity by isoform-selective agents. J. Biol. Chem. 1996;271:19134–19139. doi: 10.1074/jbc.271.32.19134. [DOI] [PubMed] [Google Scholar]
- HAMASAKI Y., ELING T.E. EGF and TPA stimulate de novo synthesis of PGHS-1 and PGHS-2 through different signal transduction pathways. Prostaglandins Leukot. Essent. Fatty Acids. 1995;53:225–229. doi: 10.1016/0952-3278(95)90121-3. [DOI] [PubMed] [Google Scholar]
- HAMILTON L.C., MITCHELL J.A, WARNER T.D. Synergy between cyclo-oxygenase-2 induction and arachidonic acid supply in vivo: consequences for nonsteroidal anti-inflammatory drug efficacy. FASEB J. 1999;2:245–251. doi: 10.1096/fasebj.13.2.245. [DOI] [PubMed] [Google Scholar]
- HAN S.W., LEI Z.M., RAO C.V. Up-regulation of cyclo-oxygenase-2 gene expression by chorionic gonadotrophin in mucosal cells from human fallopian tubes. Endocrinology. 1996;137:2929–2937. doi: 10.1210/endo.137.7.8770916. [DOI] [PubMed] [Google Scholar]
- HARRIS R.C., MCKANNA J.A., AKAI Y., JACOBSON H.R., DUBOIS R.N., BREYER M.D. Cyclo-oxygenase-2 is associated with the macula densa of rat kidney and increases with salt restriction. J. Clin. Invest. 1994;94:2504–2510. doi: 10.1172/JCI117620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HAWKEY C.J. Non-steroidal anti-inflammatory drug gastropathy: causes and treatment. Scand. J. Gastroenterol. 1996;220:124–127. doi: 10.3109/00365529609094763. [DOI] [PubMed] [Google Scholar]
- HAY C.H., TREVETHICK M.A., WHEELDON A., BOWERS J.S., DE BELLEROCHE J.S. The potential role of spinal cord cyclo-oxygenase-2 in the development of Freund's complete adjuvant-induced changes in hyperalgesia and allodynia. Neuroscience. 1997;78:843–150. doi: 10.1016/s0306-4522(96)00598-2. [DOI] [PubMed] [Google Scholar]
- HAYAKAWA M., JAYADEV S., TSUJIMOTO M., HANNUN Y.A., ITO F. Role of ceramide in stimulation of the transcription of cytosolic phospholipase A2 and cyclo-oxygenase-2. Biochem. Biophys. Res. Commun. 1996;220:681–686. doi: 10.1006/bbrc.1996.0464. [DOI] [PubMed] [Google Scholar]
- HEMLER M., LANDS W.E. Purification of the cyclo-oxygenase that forms prostaglandins. Demonstration of two forms of iron in the holoenzyme. J. Biol. Chem. 1976;251:5575–5579. [PubMed] [Google Scholar]
- HENRY D., DREW A., BEUZEVILLE S.Adverse drug reactions in the gastrointestinal system attributed to ibuprofen Safety and Efficacy of Non-prescription (OTC) Analgesics and NSAIDs 1998Dordrecht, The Netherlands: Kluwer Academic Publishers; 19–45.In: Rainsford, K.D., Powanda, M.C. (eds) [Google Scholar]
- HENRY D.H., LIM L.L.-Y., GARCIA-RODRIGUEZ L.A., PEREZ GUTTHAN S., CARSON J.L., GRIFFITH M., SAVAGE R., LOGAN R., MORIDE Y., HAWKEY C., HILLS S., FRIES J.T. Variability in risk of gastrointestinal complications with individual non-steroidal anti-inflammatory drugs: results of a collaborative meta-analysis. Brit. Med. J. 1996;312:1563–1566. doi: 10.1136/bmj.312.7046.1563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HOLTZMAN M.J., TURK J., SHORNICK L.P. Identification of a pharmacologically distinct prostaglandin H synthase in cultured epithelial cells. J. Biol. Chem. 1992;267:21438–21445. [PubMed] [Google Scholar]
- HONSTRA G., VAN HOUWELINGEN A.C., KIVITS G.A., FISHER S., UEDELHOVEN W. Influence of dietary fish on eicosanoid metabolism in man. Prostaglandins. 1990;40:311–329. doi: 10.1016/0090-6980(90)90018-q. [DOI] [PubMed] [Google Scholar]
- HUGHES A.K., PADILLA E., KUTCHERA W.A., MICHAEL J.R., KOHAN D.E. Endothelin-1 induction of cyclo-oxygenase-2 in rat mesangial cells. Kidney Int. 1995;47:53–61. doi: 10.1038/ki.1995.6. [DOI] [PubMed] [Google Scholar]
- HWANG D., JANG B.C., YU G., BOUDREAU M. Expression of mitogen-inducible cyclo-oxygenase induced by lipopolysaccharide: mediation through both mitogen-activated protein kinase and NK-κB signaling pathways in macrophages. Biochem. Pharmacol. 1997;54:87–96. doi: 10.1016/s0006-2952(97)00154-8. [DOI] [PubMed] [Google Scholar]
- JOURDAN K.B., EVANS T.W., GOLDSTRAW P., MITCHELL J.A. Isoprostane and PGE2 production human pulmonary artery smooth muscle cells: concomitant and differential release. FASEB J. 1999;113:1025–1030. doi: 10.1096/fasebj.13.9.1025. [DOI] [PubMed] [Google Scholar]
- JOURDAN K.B., MITCHELL J.A., EVANS T.W. Release of isoprostanes by human pulmonary artery in organ culture by a cyclo-oxygenase and nitric oxide synthase dependent mechanism. Biochem. Biophys. Res. Commun. 1997;120:1280–1285. doi: 10.1006/bbrc.1997.6523. [DOI] [PubMed] [Google Scholar]
- KANG R.Y., FREIRE-MOAR J., SIGAL E., CHU C.Q. Expression of cyclo-oxygenase-2 in human and an animal model of rheumatoid arthritis. Br. J. Rheumatol. 1996;35:711–718. doi: 10.1093/rheumatology/35.8.711. [DOI] [PubMed] [Google Scholar]
- KATO T., ISHIGURO N., IWATA H., KOJIMA T., ITO T., NARUSE K. Up-regulation of COX2 expression by uni-axial cyclic stretch in human lung fibroblast cells. Biochem. Biophys. Res. Commun. 1998;244:615–619. doi: 10.1006/bbrc.1998.8335. [DOI] [PubMed] [Google Scholar]
- KIM Y., FISCHER S.M. Transcriptional regulation of cyclo-oxygenase-2 in mouse skin carcinoma cells. Regulatory role of CCAAT/enhancer-binding proteins in the differential expression of cyclo-oxygenase-2 in normal and neoplastic tissues. J. Biol. Chem. 1998;273:27686–27694. doi: 10.1074/jbc.273.42.27686. [DOI] [PubMed] [Google Scholar]
- KITAMURA Y., SHIMOHAMA S., KOIKE H., KAKIMURA J.I., MATSUOKA Y., NOMURA Y., GEBICKE-HAERTER P.J., TANIGUCHI T. Increased expression of cyclo-oxygenase and peroxisome proliferator-activated receptor-gamma in Alzheimer's disease brains. Biochem. Biophys. Res. Commun. 1999;254:582–586. doi: 10.1006/bbrc.1998.9981. [DOI] [PubMed] [Google Scholar]
- KLEIN-NULEND J., BURGER E.H., SEMEINS C.M., RAISZ L.G., PILBEAM C.C. Pulsating fluid flow stimulates prostaglandin release and inducible prostaglandin G/H synthase mRNA expression in primary mouse bone cells. J. Bone Miner. Res. 1997;12:45–51. doi: 10.1359/jbmr.1997.12.1.45. [DOI] [PubMed] [Google Scholar]
- KLIEWER S.A., LENHARD J.M., WILLSON T.M., PATEL I., MORRIS D.C., LEHMANN J.M. A prostaglandin J2 metabolite binds peroxisome proliferator-activated receptor gamma and promotes adipocyte differentiation. Cell. 1995;83:813–819. doi: 10.1016/0092-8674(95)90194-9. [DOI] [PubMed] [Google Scholar]
- KOWALSKI M.L. Aspirin sensitive rhinosinusitis and asthma. Allergy Proc. 1995;16:77–80. doi: 10.2500/108854195778771417. [DOI] [PubMed] [Google Scholar]
- KUJUBU D.A., FLETCHER B.S., VARNUM B.C., LIM R.W., HERSCHMAN H.R. TIS10, a phorbol ester tumor-promoter inducible mRNA from Swiss 3T3 cells, encodes a novel prostaglandin synthase/cyclo-oxygenase homologue. J. Biol. Chem. 1991;266:12866–12672. [PubMed] [Google Scholar]
- KURUMBAIL R.G., STEVENS A.M., GIERSE J.K., MCDONALD J.J., STEGEMAN R.A., PAK J.Y., GILDEHAUS D., MIYASHIRO J.M., PENNING T.D., SEIBERT K., ISAKSON P.C., STALLINGS W.C. Structural basis for selective inhibition of cyclo-oxygenase-2 by anti-inflammatory agents. Nature. 1996;384:644–648. doi: 10.1038/384644a0. [DOI] [PubMed] [Google Scholar]
- LANEUVILLE O., BREUER D.K., XU N., HUAN Z.H., GAGE D.A., WATSON J.T., LAGARDE M., DEWITT D.L., SMITH W.L. Fatty acid substrate specificities of human prostaglandin-endoperoxide H synthase-1 and -2. Formation of 12-hydroxy-(9Z, 13E/Z, 15Z)-octadecatrienoic acids from alpha-linolenic acid. J. Biol. Chem. 1995;270:19330–19336. doi: 10.1074/jbc.270.33.19330. [DOI] [PubMed] [Google Scholar]
- LANZA F.L., RACK M.F., SIMON T.J., QUAN H., BOLOGNESE J.A., HOOVER M.E., WILSON F.R., HARPER S.E. Specific inhibition of cyclo-oxygenase-2 with MK-0966 is associated with less gastroduodenal damage than either aspirin or ibuprofen. Ailment. Pharmacol. Ther. 1999;13:761–767. doi: 10.1046/j.1365-2036.1999.00529.x. [DOI] [PubMed] [Google Scholar]
- LI Y., SMITH T., GRABSKI S., DEWITT D.L. The membrane association sequences of the prostaglandin endoperoxide synthases-1 and -2 isozymes. J. Biol. Chem. 1998;273:29830–29837. doi: 10.1074/jbc.273.45.29830. [DOI] [PubMed] [Google Scholar]
- MARCHESELLI V.L., BAZAN N.G. Sustained induction of prostaglandin endoperoxide synthase-2 by seizures in hippocampus. Inhibition by a platelet-activating factor antagonist. J. Biol. Chem. 1996;271:24794–24799. doi: 10.1074/jbc.271.40.24794. [DOI] [PubMed] [Google Scholar]
- MASFERRER J.L., REDDY S.T., ZWEIFEL B.S., SEIBERT K., NEEDLEMAN P., GILBERT R.S., HERSCHMAN H.R. In vivo glucorticoids regulate cyclo-oxygenase-2 but not cyclo-oxygenase-1 in peritoneal macrophages. J. Pharmacol. Exp. Ther. 1994;270:1340–1344. [PubMed] [Google Scholar]
- MASFERRER J.L., ZWEIFEL B.S., SEIBERT K., NEEDLEMAN P. Selective regulation of cellular cyclo-oxygenase by dexamethasone and endotoxin in mice. J. Clin. Invest. 1990;86:1375–1379. doi: 10.1172/JCI114850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MCADAM B.F., CATELLA-LAWSON F., MARDINI I.A., KAPOOR S., LAWSON J.A., FITZGERALD G.A. Systemic biosynthesis of prostacyclin by cyclo-oxygenase (COX)-2: the human pharmacology of a selective inhibitor of COX-2. Proc. Natl. Acad. Sci. U.S.A. 1999;96:272–277. doi: 10.1073/pnas.96.1.272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MCCARTNEY S.A., MITCHELL J.A., FAIRCLOUGH P.D., FARTHING M.J.G., WARNER T.D. Selective COX-2 inhibitors and human inflammatory bowel disease. Aliment. Pharmacol. Ther. 1999;13:1115–1117. doi: 10.1046/j.1365-2036.1999.00585.x. [DOI] [PubMed] [Google Scholar]
- MCKANNA J.A., ZHANG M.Z., WANG J.L., CHENG H., HARRIS R.C. Constitutive expression of cyclo-oxygenase-2 in rat vas deferens. Am. J. Physiol. 1998;275:R227–R233. doi: 10.1152/ajpregu.1998.275.1.R227. [DOI] [PubMed] [Google Scholar]
- MEAD E.A., SMITH W.L., DEWITT D.L. Differential inhibition of prostaglandin endoperoxide synthase (cyclo-oxygenase) isozymes by aspriin and other non-steroidal anti-inflammatory drugs. J. Biol. Chem. 1993;268:6610–6614. [PubMed] [Google Scholar]
- MILLER C., ZHANG M., HE Y., ZHAO J., PELLETIER J.P., MARTEL-PELLETIER J., DI BATTISTA J.A. Transcriptional induction of cyclo-oxygenase-2 gene by okadaic acid inhibition of phosphatase activity in human chondrocytes: co-stimulation of AP-1 and CRE nuclear binding proteins. J. Cell. Biochem. 1998;69:392–413. [PubMed] [Google Scholar]
- MITCHELL J.A., BELVISI M.G. Too many COX (cyclo-oxygenase) spoil the broth: aspirin-sensitive asthma and 5-lypoxygenase. Thorax. 1997;52:933–935. doi: 10.1136/thx.52.11.933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MITCHELL J.A., AKARASEREENONT P., THIEMERMANN C., FLOWER R.J., VANE J.R. Selectivity of nonsteroid anti-inflammatory drugs as inhibitors of constitutive and inducible cyclo-oxygenase. Proc. Natl. Acad. Sci. U.S.A. 1993;90:11693–11697. doi: 10.1073/pnas.90.24.11693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MITCHELL J.A., BELVISI M., AKARASEREENONT P., ROBBINS R., KWON O.J., CROXTALL J., BARNES P.J., VANE J.R. Induction of cyclo-oxygenase-2 by cytokines in human pulmonary epithelial cells: regulation by dexamethasone. Br. J. Pharmacol. 1994;113:1008–1014. doi: 10.1111/j.1476-5381.1994.tb17093.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MITCHELL J.A., EVANS T.W. Cyclooxygenase-2 as a therapeutic target. Inflam. Res. 1998;47:S88–S92. doi: 10.1007/s000110050287. [DOI] [PubMed] [Google Scholar]
- MIYAMOTO T., OGINO N., YAMAMOTO S., HAYAISHIM O. Purification of prostaglandin endoperoxide synthetase from bovine vesicular gland microsomes. J. Biol. Chem. 1976;251:2629–2636. [PubMed] [Google Scholar]
- MIZUNO H., SAKAMOTO C., MATSUDA K., WADA K., UCHIDA T., NOGUCHI H., AKAMATSU T., KASUGA M. Induction of cyclo-oxygenase-2 in gastric mucosal lesions and its inhibition by the specific antagonist delays healing in mice. Gastroenterology. 1997;112:387–397. doi: 10.1053/gast.1997.v112.pm9024292. [DOI] [PubMed] [Google Scholar]
- MONCADA S., GRYGLEWSKI R., BUNTING S., VANE J.R. An enzyme isolated from arteries transforms PG endoperoxides to an unstable substance that inhibits platelet aggregation. Nature. 1976;263:663–665. doi: 10.1038/263663a0. [DOI] [PubMed] [Google Scholar]
- MORISSET S., PATRY C., LORA M., DE BRUM-FERNANDES A.J. Regulation of cyclo-oxygenase-2 expression in culture by interleukin-1α, tumor necrosis factor-α, glucocorticoids, and 17β-estradiol. J. Rheumatol. 1998;25:1146–1153. [PubMed] [Google Scholar]
- MORRISON B.W., CHRISTENSEN S., YUAN W., BROWN J., AMLANI S., SEIDENBERG B. Analgesic efficacy of the cyclo-oxygenase-2-specific inhibitor rofecoxib in post-dental surgery pain: a randomized, controlled trial. Clin. Ther. 1999;21:943–953. doi: 10.1016/S0149-2918(99)80016-2. [DOI] [PubMed] [Google Scholar]
- MORROW J.D., ROBERTS L.J. The isoprostanes: unique bioactive products of lipid peroxidation. Prog. Lipid Res. 1997;36:1–21. doi: 10.1016/s0163-7827(97)00001-5. [DOI] [PubMed] [Google Scholar]
- MURATA H., KAWANO S., TSUJI S., TSUJI M., SAWAOKA H., KIMURA Y., SHIOZAKI H., HORI M. Cyclo-oxygenase-2 overexpression enhances lymphatic invasion and metastasis in human gastric carcinoma. Am. J. Gastroenterol. 1999;94:451–455. doi: 10.1111/j.1572-0241.1999.876_e.x. [DOI] [PubMed] [Google Scholar]
- NEGISHI M., SUGIMOTO Y., ICHIKAWA A. Molecular mechanisms of diverse actions of prostanoid receptors. Biochim. Biophys. Acta. 1995;259:109–119. doi: 10.1016/0005-2760(95)00146-4. [DOI] [PubMed] [Google Scholar]
- O'BANION M.K., WINN V.D., YOUNG D.A. CDAN cloning and functional activity of a glucocorticoid-regulated inflammatory cyclo-oxygenase. Proc. Natl. Acad. Sci. U.S.A. 1992;89:4888–4892. doi: 10.1073/pnas.89.11.4888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PASINETTI G.M. Cyclo-oxygenase and inflammation in Alzheimer's disease: experimental approaches and clinical interventions. J. Neurosci. Res. 1998;54:1–6. doi: 10.1002/(SICI)1097-4547(19981001)54:1<1::AID-JNR1>3.0.CO;2-M. [DOI] [PubMed] [Google Scholar]
- PASSINETTI G.M., AISEN P.S. Cyclo-oxygenase-2 expression is increased in frontal cortex of Alzheimer's disease brain. Neuroscience. 1998;87:319–324. doi: 10.1016/s0306-4522(98)00218-8. [DOI] [PubMed] [Google Scholar]
- PERCIVAL M.D., OUELLET M., VINCENT C.J., YERGEY J.A., KENNEDY B.P., O'NEILL G.P. Purification and characterisation of recombinant human cyclo-oxygenase-2. Arch. Biochem. Biophys. 1994;315:111–118. doi: 10.1006/abbi.1994.1478. [DOI] [PubMed] [Google Scholar]
- PICOT D., LOLL P.J., GARAVITO R.M. X-ray crystal structure of the membrane protein prostaglandin H2 synthase-1. Nature. 1994;367:243–249. doi: 10.1038/367243a0. [DOI] [PubMed] [Google Scholar]
- PIERCE K.L., REGAN J.W. Prostanoid receptor heterogeneity through alternative mRNA splicing. Life Sci. 1998;62:1479–1483. doi: 10.1016/s0024-3205(98)00093-9. [DOI] [PubMed] [Google Scholar]
- PLANAS A.M., SORIANO M.A., RODRIGUEZ-FARRE E., FERER I. Induction of cyclo-oxygenase-2 mRNA and protein following transient focal ischaemia in the rat brain. Neurosci. Lett. 1995;200:187–190. doi: 10.1016/0304-3940(95)12108-g. [DOI] [PubMed] [Google Scholar]
- PRATICO D., LAWSON J.A., FITZGERALD G.A. Cyclo-oxygenase-dependent formation of the isoprostane, 8-epi prostaglandin F2 alpha. J. Biol. Chem. 1995;270:9800–9808. doi: 10.1074/jbc.270.17.9800. [DOI] [PubMed] [Google Scholar]
- RADOMSKI M.W., PALMER R.M.J., MONCADA S. The anti-aggregating properties of vascular endothelium: interactions between prostacyclin and nitric oxide. Br. J. Pharmacol. 1976;92:181–187. doi: 10.1111/j.1476-5381.1987.tb11367.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- REDDY S.T., HERSCHMAN H.R. Ligand-induced prostaglandin synthesis requires expression of the TIS10/PGS-2 prostaglandin synthase gene in murine fibroblasts and macrophages. J. Biol. Chem. 1994;269:15473–15480. [PubMed] [Google Scholar]
- REGINATO M.J., KRAKOW S.L., BAILEY S.T., LAZAR M.A. Prostaglandins promote and block adipogenesis through opposing effects on peroxisome proliferator-activated receptor gamma. J. Biol. Chem. 1998;273:1855–1858. doi: 10.1074/jbc.273.4.1855. [DOI] [PubMed] [Google Scholar]
- REUTER B.K., ASFAHA S., BURET A., SHARKEY K.A., WALLACE J.L. Exacerbation of inflammation-associated colonic injury in rat through inhibition of cyclo-oxygenase-2. J. Clin. Invest. 1996;98:2076–2085. doi: 10.1172/JCI119013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RIENDEAU D., PERCIVAL M.D., BOYCE S., BRIDEAU C., CHARLESON S., CROMLISH W., ETHIER D., EVANS J., FALGUEYRET J.P., FORD-HUTCHINSON A.W., GORDON R., GREIG G., GRESSER M., GUAY J., KARGMAN S., LEGER S., MANCINI J.A., O'NEILL G., OUELLET M., RODGER I.W., THERIEN M., WANG Z., WEBB J.K., WONG E., CHAN C.C. Biochemical and pharmacological profile of a tetrasubstituted furanone as a highly selective COX-2 inhibitor. Br. J. Pharmacol. 1997;121:105–117. doi: 10.1038/sj.bjp.0701076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RIMARACHIN J.A., JACOBSON J.A., SZABO P., MACLOUF J., CREMINON C., WEKSLER B.B. Regulation of cyclo-oxygenase-2 expression in aortic smooth muscle cells. Arterioscler. Thromb. 1994;14:1021–1031. doi: 10.1161/01.atv.14.7.1021. [DOI] [PubMed] [Google Scholar]
- RISTIMAKI A., GARFINKEL S., WESSENDORF J., MACIAG T., HLA T. Induction of cyclo-oxygenase-2 by interleukin-1α. Evidence for post-transcriptional regulation. J. Biol. Chem. 1994;269:11769–11775. [PubMed] [Google Scholar]
- ROBERTSON R.P. Dominance of cyclo-oxygenase-2 in the regulation of pancreatic islet prostaglandin synthesis. Diabetes. 1998;47:1379–1383. doi: 10.2337/diabetes.47.9.1379. [DOI] [PubMed] [Google Scholar]
- ROSEN G.D., BIRKENMEIER T.M., RAZ A., HOLTZMAN M.J. Identification of a cyclo-oxygenate-related gene and its potential role in prostaglandin formation. Biochem. Biophys. Res. Common. 1989;164:1358–1365. doi: 10.1016/0006-291x(89)91819-6. [DOI] [PubMed] [Google Scholar]
- ROSSAT J., MAILLARD M., NUSSBERGER J., BRUNNER H.R., BURNIER M. Renal effects of selective cyclo-oxygenase-2 inhibition in normotensive salt-depleted subjects. Clin. Pharmacol. Ther. 1999;66:76–84. doi: 10.1016/S0009-9236(99)70056-1. [DOI] [PubMed] [Google Scholar]
- SAUNDERS M., BELVISI M.G., CIRINO G., BARNES P.J., MITCHELL J.A. Mechanisms of prostaglandin E2, release by intact cells expressing cyclo-oxygenase-2: evidence for a ‘two component' model. J. Pharmacol. Exp. Ther. 1999;288:1101–1108. [PubMed] [Google Scholar]
- SCHWARTZ J.I., CHAN C.C., MUKHOPADHYAY S., MCBRIDE K.J., JONES T.M., ADCOCK S., MORITZ C., HEDGES J., GRASING K., DOBRATZ D., COHEN R.A., DAVIDSON M.H., BACHMAN K.A., GERTZ B.J. Cyclo-oxygenase-2 inhibition by rofecoxib reverses naturally occurring fever in humans. Clin. Pharmacol. Ther. 1999;65:653–660. doi: 10.1016/S0009-9236(99)90087-5. [DOI] [PubMed] [Google Scholar]
- SEIBERT K., ZHANG Y., LEAHY K., HAUSER S., MASFERRER J., ISAKSON P. Distribution of COX-1 and COX-2 in normal and inflamed tissues. Adv. Exp.Med. Biol. 1997;400A:167–170. doi: 10.1007/978-1-4615-5325-0_24. [DOI] [PubMed] [Google Scholar]
- SMALLEY W.E., DUBOIS R.N. Colorectal cancer and nonsteroidal anti-inflammatory drugs. Adv. Pharmacol. 1997;39:1–20. doi: 10.1016/s1054-3589(08)60067-8. [DOI] [PubMed] [Google Scholar]
- SNEDDON J.M., VANE J.R. Endothelial derived relaxing factor reduces platelet adhesion to bovine endothelial cells. Proc. Natl. Acad. Sci. U.S.A. 1988;85:2800–2804. doi: 10.1073/pnas.85.8.2800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SORLI C.H., ZHANG H.J., ARMSTRONG M.B., RAJOTTE R.V., MACLOUF J., ROBERTSON R.P. Basal expression of cyclo-oxygenase-2 and nuclear factor-interleukin 6 are dominant and co-ordinately regulated by interleukin 1 in the pancreatic islet. Proc. Natl. Acad. Sci. U.S.A. 1998;95:1788–1793. doi: 10.1073/pnas.95.4.1788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SOUSA A.R., PFISTER R., CHRISTIE P.E., LANE S.J., NASSER S.M., SCHMITZ-SCHUMANN M., LEE T.H. Enhanced expression of cyclo-oxygenase isoenzyme 2 (COX-2) in asthmatic airways and its cellular distribution in aspirin-sensitive asthma. Thorax. 1997;52:940–945. doi: 10.1136/thx.52.11.940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SPENCER A.G., WOODS J.W., ARAKAWA T., SINGER I.I., SMITH W.L. Subcellular localization of prostaglandin endoperoxide H synthases-1 and -2 by immunoelectron microscopy. J. Biol. Chem. 1998;273:9886–9893. doi: 10.1074/jbc.273.16.9886. [DOI] [PubMed] [Google Scholar]
- STEWART W.F., KAWAS C., CORRADA M., METER E.J. Risk of Alzheimer's disease and duration of NSAID use. Neurology. 1997;489:626–632. doi: 10.1212/wnl.48.3.626. [DOI] [PubMed] [Google Scholar]
- STROEBEL M., GOPPELT-STRUEBE M. Signal transduction pathways responsible for serotonin-mediated prostaglandin G/H synthase expression in rat mesangial cells. J. Biol. Chem. 1994;269:22952–22957. [PubMed] [Google Scholar]
- TAKAHATA K., YAMANAKA M. PGI3 production from eicosapentaenoic acid in 3T3 fibroblast cells and cultured bovine pulmonary artery endothelial cells. Thromb. Res. 1987;45:581–589. doi: 10.1016/0049-3848(87)90321-5. [DOI] [PubMed] [Google Scholar]
- TAZAWA R., XU X.M., WU K.K., WANG L.H. Characterization of the genomic structure, chromosomal location and promoter of human prostaglandin H synthase-2 gene. Biochem. Biophys. Res. Commun. 1994;203:190–199. doi: 10.1006/bbrc.1994.2167. [DOI] [PubMed] [Google Scholar]
- TETRADIS S., PILBEAM C.C., LIU Y., KREAM B.E. Parathyroid hormone induces prostaglandin G/H synthase-2 expression by a cyclic adenosine 3′,5′-monophosphate-mediated pathway in the murine osteoblastic cell line MC3T3-E1. Endocrinology. 1996;137:5435–5440. doi: 10.1210/endo.137.12.8940368. [DOI] [PubMed] [Google Scholar]
- TOCCO G., FREIRE-MOAR J., SCHREIBER S., SAKHI S., AISEN P., PASINETTI G. Maturational regulation and regional induction of cyclo-oxygenase-2 in rat brain: implications for Alzeimer's disease. Exp. Neurol. 1997;144:339–349. doi: 10.1006/exnr.1997.6429. [DOI] [PubMed] [Google Scholar]
- TUCKER O.N., DANNENBERG A.J., YANG E.K., ZHANG F., TENG L., DALY J.M., SOSLOW R.A., MASFERRER J.L., WOERNER B.M., KOKI A.T., FAHEY T.J., III Cyclo-oxygenase-2 expression is up-regulated in human pancreatic cancer. Cancer Res. 1999;59:987–990. [PubMed] [Google Scholar]
- UKAWA H., YAMAKUNI H., KATO S., TAKEUCHI K. Effects of cyclo-oxygenase-2 selective and nitric oxide releasing nonsteroidal inflammatory drugs on mucosal ulcerogenic and healing responses of the stomach. Dig. Dis. Sci. 1998;43:2003–2011. doi: 10.1023/a:1018846912032. [DOI] [PubMed] [Google Scholar]
- VANE J.R. Inhibition of prostaglandin synthesis as a mechanism of action for aspirin-like-drugs. Nature. 1971;231:232–235. doi: 10.1038/newbio231232a0. [DOI] [PubMed] [Google Scholar]
- VANE J.R., BAKHLE Y.S., BOTTING R.M. Cyclo-oxygenase-1 and 2. Ann. Rev. Pharmacol. Toxicol. 1998;38:97–120. doi: 10.1146/annurev.pharmtox.38.1.97. [DOI] [PubMed] [Google Scholar]
- VANE J.R., MITCHELL J.A., APPLETON I., TOMLINSON A., BISHOP-BAILEY D., CROXTALL J., WILLOUGHBY D.A. Inducible isoforms of cyclo-oxygenase and nitric oxide synthase in inflammation. Proc. Natl. Acad. Sci. U.S.A. 1994;91:2046–2050. doi: 10.1073/pnas.91.6.2046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VIO C.P., CESPEDES C., GALLARDO P., MASFERRER J.L. The identification of cyclo-oxygenase-1 and cyclo-oxygenase-2. Hypertension. 1997;30:687–692. doi: 10.1161/01.hyp.30.3.687. [DOI] [PubMed] [Google Scholar]
- VON SCHACKY C., WEBER P.C. Metabolism and effects on platelet function of the purified eicosapentaenoic and docosahexaenoic acids in humans. J. Clin. Invest. 1985;76:2446–2450. doi: 10.1172/JCI112261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WALLACE J.L., BAK A., MCKNIGHT W., ASFAHA S., SHARKEY K.A., MACNAUGHTON W.K. Cyclo-oxygenase-1 contributes to inflammatory responses in rats and mice: implications for gastrointestinal toxicity. Gastroenterology. 1998b;115:101–109. doi: 10.1016/s0016-5085(98)70370-1. [DOI] [PubMed] [Google Scholar]
- WALLACE J.L., REUTER B.K., MCKNIGHT W., BAK A. Selective inhibitors of cyclo-oxygenase-2: are they really effective, selective, and GI-safe. J. Clin. Gastroenterol. 1998a;27:S28–S34. doi: 10.1097/00004836-199800001-00006. [DOI] [PubMed] [Google Scholar]
- WARNER T.D., GIULIANO F., VOJNOVIC I., BUKASA A., MITCHELL J.A., VANE J.R. Nonsteroid drug selectivities for cyclo-oxygenase-1 versus cyclo-oxygenase-2 correlate with human gastrointestinal toxicity: a full in vitro analysis. Proc. Natl. Acad. Sci. U.S.A. 1999;96:7563–7568. doi: 10.1073/pnas.96.13.7563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WILLIAMS C.S., SMALLY W., DUBOIS R.N. Aspirin use and potential mechanisms for colorectal cancer prevention. J. Clin. Invest. 1997;100:1–5. doi: 10.1172/JCI119651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WILLIAMS T.J., PECK M.J. Role of prostaglandin-mediated vasodilatation in inflammation. Nature. 1977;270:530–532. doi: 10.1038/270530a0. [DOI] [PubMed] [Google Scholar]
- WILLINGALE H.L., GARDINER N.J., MCLYMONT N., GIBLETT S., GRUB B.D. Prostanoids synthesized by cyclo-oxygenase isoforms in rat spinal cord and their contribution to the development of neuronal hyperexcitability. Br. J. Pharmacol. 1997;122:1593–1604. doi: 10.1038/sj.bjp.0701548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WILLIS A.L., SMITH D.L., VIGO C. Suppression of principal atherosclerotic mechanisms by prostacyclins and other eicosanoids. Prog. Lipid. Res. 1986;25:645–666. doi: 10.1016/0163-7827(86)90132-3. [DOI] [PubMed] [Google Scholar]
- WU K.K. Inducible cyclo-oxygenase and nitric oxide synthase. Adv. Pharmacol. 1995;33:179–207. doi: 10.1016/s1054-3589(08)60669-9. [DOI] [PubMed] [Google Scholar]
- XIE W., HERSCHMAN H.R. Transcriptional regulation of prostaglandin synthase 2 gene expression by platelet-derived growth factor and serum. J. Biol. Chem. 1996;271:31742–31748. doi: 10.1074/jbc.271.49.31742. [DOI] [PubMed] [Google Scholar]
- XIE W.L., CHIPMAN J.G., ROBERTSON D.L., ERIKSON R.L., SIMMONS D.L. Expression of a mitogen-responsive gene encoding prostaglandin synthase is regulated by mRNA splicing. Proc. Natl. Acad. Sci. U.S.A. 1991;88:2692–2696. doi: 10.1073/pnas.88.7.2692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- YAMAMOTO K., ARAKAWA T., UEDA N., YAMAMOTO S. Transcriptional roles of nuclear factor κB and nuclear factor-interleukin-6 in the tumour necrosis factor α-independent induction of cyclo-oxygenase-2 in MC3T3-E1 cells. J. Biol. Chem. 1995;270:31315–31320. doi: 10.1074/jbc.270.52.31315. [DOI] [PubMed] [Google Scholar]
- YAMAMOTO T., NOZAKI-TAGUCHI N. Analysis of the effects of cyclo-oxygenase (COX)-1 and COX-2 in spinal nociceptive transmission using indomethacin, a non-selective COX inhibitor, and NS398, a COX-2 selective inhibitor. Brain Res. 1996;739:104–110. doi: 10.1016/s0006-8993(96)00817-7. [DOI] [PubMed] [Google Scholar]
- ZIMMERMAN K.C., SARBIA M., SCHROR K., WEBER A.A. Constitutive cyclo-oxygenase-2 expression in healthy human and rabbit gastric mucosa. Mol. Pharmacol. 1998;54:536–540. doi: 10.1124/mol.54.3.536. [DOI] [PubMed] [Google Scholar]
- ZIMMERMAN K.C., SARBIA M., WEBER A.A., BORCHARD F., GABBERT H.E., SCHROR K. Cyclo-oxygenase-2 expression in human esophageal carcinoma. Cancer Res. 1999;59:198–204. [PubMed] [Google Scholar]


