Abstract
The hyperpolarization-activated cation current (If) was recorded in single pacemaker cells of the porcine sino-atrial node, and the effects of genistein, an isoflavone inhibitor of tyrosine-specific protein kinases was investigated by the whole-cell patch clamp technique.
Genistein (20–500 μM) decreased If in a dose-dependent manner with an IC50 value of 62.3 μM and a maximum inhibition of 45.3%.
The effect on If appeared without altering the half-activation potential (control, −88.3±2.8 mV; genistein, −87.0±1.8 mV) and the slope factor (control, 8.0±0.3 mV; genistein, 8.6±0.7 mV) of the steady-state activation curve. No significant voltage-dependency was detected in the fully-activated current-voltage relation measured by the double-pulse protocols.
The inactive form of genistein analogue, daidzein (500 μM) or genistin (200 μM), were without effect. If was not affected by another tyrosine kinase inhibitor, tryphostin-47 (100 μM), but tyrphostin-25 (100–200 μM) suppressed If in an irreversible manner.
Neither bath nor intracellular application of the tyrosine phosphatase inhibitor, orthovanadate, affected If, and subsequent application of genistein inhibited If significantly.
These data indicate that the inhibition of If by genistein is not mediated through tyrosine kinase inhibition but through nonselective block of the If channels.
Keywords: Porcine sino-atrial node, hyperpolarization-activated inward current, tyrosine kinase, genistein, tyrphostins, orthovanadate
Introduction
Although it is well established that protein tyrosine kinase (PTK) plays a primary role in regulating cellular growth and differentiation, recent studies have demonstrated that PTK can also modulate various ionic channels such as the cloned K+ channel (Huang et al., 1993), the NMDA receptor channel (Wang & Salter, 1994) and the cystic fibrosis trasmembrane regulator Cl− channel (Shuba et al., 1996). In the heart, the hyperpolarization-activated cation current (If) is normally recorded in sino-atrial node (SAN) cells, atrio-ventricular node cells and Purkinje fibres. The current plays a role of maintaining the less negative potential during diastole, thereby facilitating spontaneous activity of these cells (Irisawa et al., 1993). Recently, If of rabbit SAN cells was shown to be inhibited by genistein, a PTK inhibitor (Wu & Cohen, 1997), indicating that If channels might be regulated by tyrosine phosphorylation.
On the other hand, genistein by itself has been shown to block various ionic channels including the voltage-gated K+ current of smooth muscle cells (Smirnov & Aaronson, 1995), L-type Ca2+ current in cardiac myocytes (Chiang et al., 1996) and cyclic AMP-dependent Cl− channel (Wang et al., 1998). Thus it is not yet clear whether or not If is regulated by tyrosine phosphorylation. To clarify the physiological importance of PTK in regulating If, it might be useful to examine the effects of tyrosine kinase inhibitors in different animal species. Since If plays a functional role in determining the heart rate, the contribution of If to the pacemaker potential and/or the extent of tyrosine phosphorylation of If channels might differ in various species. In the present study, therefore, we have isolated SAN cells of porcine hearts, which show spontaneous beating at a rate of approximately 80 min−1. We have shown that genistein inhibits If probably by nonselective block of the channels rather than through inhibiting PTK in porcine SAN cells.
Methods
Preparation
Porcine hearts were obtained from a slaughterhouse near the laboratory. Six-month pigs weighing 90–100 kg were killed by electrical shock and exsanguination, and their intra-thoracic and abdominal organs were quickly removed. The heart was also quickly removed, and the right coronary artery was cannulated, and ventricular branches of the coronary artery were clamped in order to facilitate perfusion toward the right atrium including the SAN region. The heart was first perfused with 150 ml Ca2+-free Tyrode solution (approximately 30°C) using a disposable syringe and was cooled down by subsequent perfusion with 250–300 ml cardioplegic solution (see below) at 4°C. Thereafter, the right atrial region enclosed laterally by the cristaterminalis and the interatrial septum was dissected out and stored in the cardioplegia at 4°C for transfer to the laboratory. Approximately 1 h elapsed before the following isolation procedure was commenced in the laboratory.
A piece of SAN tissue measuring approximately 20×5 mm was cut perpendicular to the crista terminalis into about 20 strips. The pieces of nodal tissue were then incubated in an oxygenated Ca2+-free Tyrode solution for 5 min at 34°C, and then in oxygenated Ca2+-free Tyrode solution containing 480 U ml−1 collagenase (Wako, Osaka, Japan), 0.1 mg ml−1 elastase (Wako, Osaka, Japan) and 3.5 U ml−1 hyaluronidase (Wako, Osaka, Japan) for 45 min at 34°C. After enzyme treatment, strips of SAN were placed into high-K+, low-Cl− medium (modified KB medium) and gently teased using forceps in a Petri dish. During this procedure, cells were released from the tissue and became suspended in the solution. The cell suspension was stored in the modified KB medium at 4°C for later use.
Solutions
The normal Tyrode solution contained (in mM): NaCl, 136.9; KCl, 5.4; MgCl2, 0.53; CaCl2, 1.8; NaH2PO4, 0.33; glucose 5.5; HEPES, 5.0 (pH=7.4 with NaOH). Ca2+-free Tyrode solution was made by simply omitting CaCl2 from the above normal Tyrode solution. The standard external solution contained (in mM): NaCl, 136.9; KCl, 5.4; MgCl2, 0.53; BaCl2, 1.0; NiCl2, 2.0; NaH2PO4, 0.33; glucose 5.5; HEPES, 5.0 (pH=7.4 with NaOH). The cardioplegia contained (in mM): NaCl, 82; KCl, 20; MgCl2, 0.5; glucose, 111.1; taurine, 20; HEPES, 10 (pH=7.4 with NaOH), and was supplemented with 20 U/l bovine insulin (Wako, Osaka, Japan). The modified KB medium contained (in mM): K-glutamate, 70; KCl, 20; KH2PO4, 10; MgCl2, 1.0; taurine, 20; glucose, 10; ethyleneglycol-bis-(2-aminoethyl ether)-N,N,N′,N′-tetraacetic acid (EGTA), 0.3 (pH=7.2 with KOH).
The pipette solution for the conventional whole-cell recording contained (in mM): KOH, 150; HCl, 30; NaCl, 10; CaCl2, 2; EGTA, 5; Na2ATP, 5; Na2GTP, 0.1; MgCl2, 5; HEPES 5.0 (pH=7.4 with aspartic acid). The free concentrations of Ca2+ and Mg2+ were calculated to be 0.1 μM and 0.55 mM, respectively (Fabiato & Fabiato, 1979). The pipette solution for the nystatin-perforated patch recording contained (in mM) NaCl, 10; KCl, 30; KOH, 120; HEPES 5.0 (pH=7.2 with aspartic acid). Nystatin was dissolved in methanol as a 10 mg ml−1 stock solution and added to the pipette solution to obtain a final concentration of 200 μg ml−1.
Drugs
Genistein (Wako, Osaka, Japan), daidzein (Fujicco, Kobe, Japan), genistin (Sigma, St Louis, U.S.A.), tyrphostin-47 (Sigma, St Louis, U.S.A.) and tyrphostin-25 (Sigma, St Louis, U.S.A.) were dissolved in dimethyl sulphoxide as 100 mM stock solutions, and diluted in the standard external solution to give final concentrations as described in the text.
An aqueous stock (100 mM) solution of orthovanadate (Wako, Osaka, Japan) was freshly prepared and the pH was adjusted to 10 (Gordon, 1991). The stock solution was boiled for 15–20 min before dilution into the superfusate before use, and the pH was readjusted to 7.4 with HCl. Orthovanadate has been used to prevent the effect of genistein on various ionic currents. Since orthovanadate provides an indication of the mechanism by which genistein affected If in the present study, we considered it was important to confirm that our preparation of orthovanadate was indeed active. Consequently, we therefore repeated the preliminary experiments that the activation by genistein of the cyclic AMP-dependent Cl− current was antagonized by orthovanadate (Shuba et al., 1996). In four cells examined, 0.1–1 mM orthovanadate inhibited the Cl− current activated by 50–100 μM genistein almost completely (data not shown).
Electrophysiological experiments
Whole-cell recordings were performed using the conventional whole-cell patch clamp techniques (Hamill et al., 1981) and the nystatin-perforated patch clamp method (Horn & Marty, 1988). Patch electrodes were made of glass capillaries having 1.5 mm o.d. and their resistance was in the range 3–5 Mω when filled with the internal solution. The currents were recorded with a patch clamp amplifier (List EPC-7, Darmstadt, FRG). The reference electrode was Tyrode-agar with an integral Ag-AgCl wire. Current and voltage signals were digitized on line at a sampling frequency of 200 Hz using pClamp software (Axon, U.S.A.). All experiments were carried out at 35– 37°C.
Results are expressed as the mean±s.e.mean. Comparisons between two groups were performed by paired or unpaired Student's t-test and values of P<0.05 were considered statistically significant.
Results
Inhibition of If by genistein
Porcine SAN cells were visually identified as long, spindle-shaped cells with faint striations and centrally located nuclei, i.e. similar to rabbit SAN cells (Denyer & Brown, 1990). In addition, the membrane currents typical for cardiac pacemaker cells were confirmed as illustrated in Figure 1. In control Tyrode solution, square pulses of 3 s duration were applied from a holding potential of −40 mV to various potentials. Upon depolarization activation of the inward Ca2+ current was followed by slower activation of the delayed rectifier K+ current. On the other hand, the activation of If was evident on hyperpolarization negative to −70 mV. At potentials around −50 mV, only few time-dependent current changes were observed and the current-voltage curves showed minimal slope conductance (Figure 1B). These properties are qualitatively in agreement with those described in pacemaker cells of rabbit (for review see Irisawa et al., 1993) and guinea-pig (Guo et al., 1997).
Figure 1.
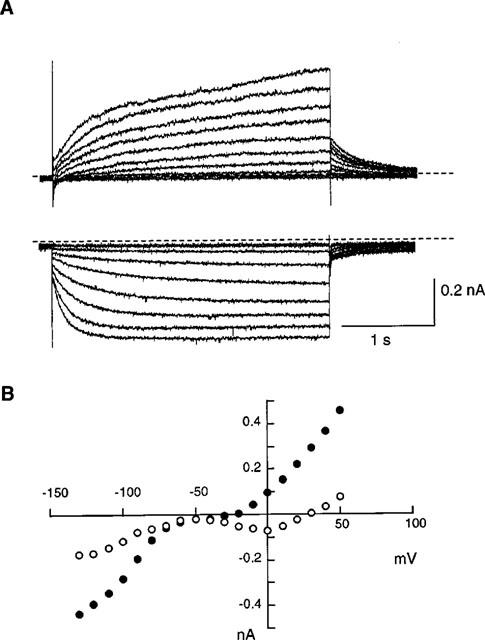
Whole-cell currents of porcine sinoatrial node cells in the normal Tyrode solution. (A) Current traces recorded by applying 3 s depolarizing (upper traces) or hyperpolarizing (lower traces) pulses from a holding potential of −40 mV in 10 mV increments. Dotted lines indicate the zero current level. (B) Current-voltage relations for the initial currents (open circles) and the currents at the end of the 3 s pulses (solid circles).
After establishment of the conventional whole-cell clamp condition in the normal Tyrode solution, the external solution was changed to the standard external solution. If was elicited with double pulses of 2 s hyperpolarization to −120 mV followed by 1 s depolarization to +10 mV, which were applied from a holding potential of −40 mV every 20 s. Using this pulse, If was recognized as a slowly activating inward current upon hyperpolarization and an outward tail current on depolarization to +10 mV. Genistein (100 μM) decreased the amplitude of both If and the outward tail current within 1 min. The effect was reversed upon wash-out (Figure 2A). It was recently shown that in feline atrial cells, genistein showed biphasic effects on the L-type Ca2+ current via an inhibition of PTK and that these effects were observed under the perforated-patch recording condition (Wang & Lipsius, 1998). In order to explore the possibility that such effects might also exist for If, the same protocol was tested with the nystatin-perforated patch clamp condition (Figure 2B). The obtained time course was essentially similar to that with the ruptured patch clamp method (Figure 2A). It was also noted that the delayed rectifier K+ current, recorded upon depolarization, was also decreased by genistein in a reversible manner. This finding is in good accordance with a previous report that the delayed rectifier K+ current in guinea-pig ventricular cells is inhibited by genistein (Washizuka et al., 1998).
Figure 2.
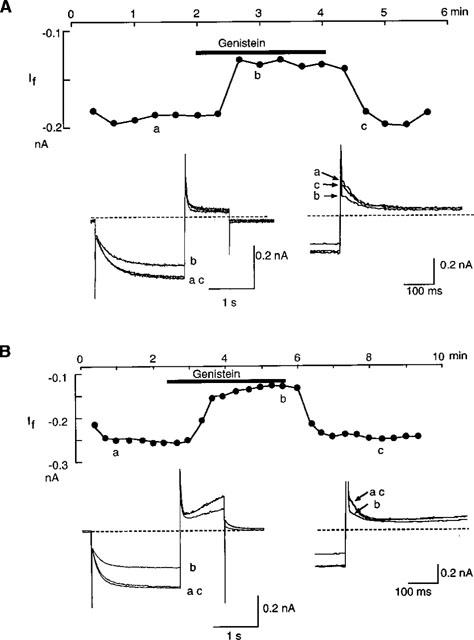
Inhibition by genistein of If. If was recorded by the ruptured (A) and nystatin-perforated patch clamp method (B). If was elicited by 2 s hyperpolarizing pulses from −40 mV to −120 mV every 20 s. Upper panels show changes of If amplitude during the genistein application. The bar above the graph indicates an application of 100 μM genistein. The current amplitude was measured as the time dependent inward current which was activated during the hyperpolarizing pulse. The lower left panels show superimposed original current traces obtained at the points indicated in the left graphs. The dotted line indicates the zero current level. In the lower right panels, tail currents are shown in an expanded time scale.
When the concentration of genistein was cumulatively increased to 20, 100 and 200 μM, the amplitude of If at −120 mV was decreased in a concentration-dependent manner (Figure 3A). The amplitude of If was measured as the time-dependent inward current at −120 mV, normalized in reference to the control. The results are summarized in Figure 3B (open circles, ruptured patch method; solid circles, nystatin-perforated patch method). No significant difference was detected in the If amplitudes obtained by ruptured- or perforated-patch clamp methods. The relationship between the concentration of genistein ([genistein]) and the relative amplitude (I) was fitted by the following equation,
where Idrug-ins is drug-insensitive component, IC50 is the half-maximal dose of genistein and n is the Hill's coefficient. The values of Idrug-ins, IC50 and n were 45.3%, 62.3 μM and 2.1, respectively. Since there was no significant difference between the ruptured- and perforated-patch recordings, the following experiments were carried out by the ruptured patch clamp method.
Figure 3.
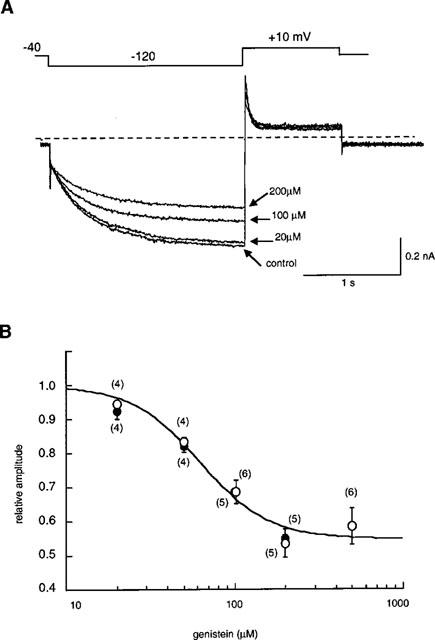
Concentration-dependent inhibition of If by genistein. (A) Superimposed current traces obtained at 20, 100, 200 μM genistein in the same cell. The If was recorded by the ruptured patch clamp method. The dotted line indicates the zero current level. The pulse protocol is shown above the records. (B) The relationship between the concentration of genistein and the If amplitude (open circles, ruptured patch clamp method; solid circles, nystatin-perforated patch clamp method). The smooth curve was obtained by the least squares fit with the Hill's equation, 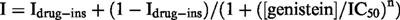 , where Idrug-ins is drug-insensitive component, IC50 is the half-maximal dose of genistein and n is the Hill's coefficient. The values of Idrug-ins, IC50 and n were 45.3%, 62.3 μM and 2.1, respectively. Numbers of data are indicated in parentheses.
, where Idrug-ins is drug-insensitive component, IC50 is the half-maximal dose of genistein and n is the Hill's coefficient. The values of Idrug-ins, IC50 and n were 45.3%, 62.3 μM and 2.1, respectively. Numbers of data are indicated in parentheses.
Kinetic properties of If inhibition by genistein
Figure 4 shows families of current traces recorded in the absence and presence of 200 μM genistein. Currents were obtained by 2 s hyperpolarizing pulses from −40 mV to various potentials in 10 mV steps followed by depolarization to +10 mV (Figure 4A inset). The amplitude of the tail current was measured and normalized relative to maximum tail current in the control. The relation between the test potentials (Vm) and the current amplitude was fitted by the Boltzmann equation,
where V1/2 is the half-activated potential and S indicates the slope factor. The values of S and V1/2, determined by a least-squares fit, were 6.8 mV and −89.4 mV in the control and 7.8 mV and −90.5 mV in the presence of 200 μM genistein, respectively (Figure 4A). Over an average of five cells, V1/2 was −88.3±2.8 mV and −87.0±1.8 mV, and S was 8.0±0.3 mV and 8.6±0.7 mV in the absence and presence of 200 μM genistein, respectively. The maximum activation was reduced to 45.6±3.6% (Figure 4B).
Figure 4.
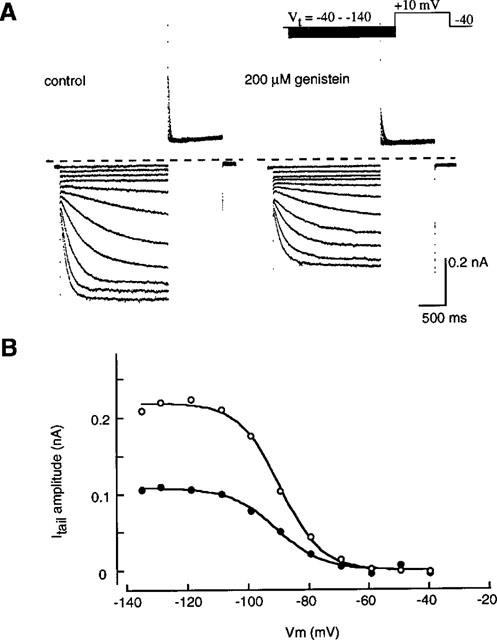
Voltage-dependent activation of If. (A) The original current recordings obtained in the absence (left panel) and presence (right panel) of 200 μM genistein. The pulse protocol is shown in the upper part. (B) Relationships of voltage and activation of If obtained from (A). The tail current amplitude was normalized in reference to that of the maximal amplitude in the absence of genistein and plotted against the membrane potential. The smooth curves were drawn by the least squares fit with the Boltzmann equation, 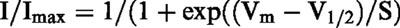 the values of S and V1/2 were 6.8 mV and −89.4 mV in the control, and 7.8 mV and −90.5 mV in the presence of 200 μM genistein, respectively.
the values of S and V1/2 were 6.8 mV and −89.4 mV in the control, and 7.8 mV and −90.5 mV in the presence of 200 μM genistein, respectively.
The fully-activated I-V relation was determined by the double-pulse protocol. As shown in Figure 5, the membrane was depolarized to various potentials after activating If at −120 mV (inserted current traces) and the current amplitude was measured at the peak (open circle) and near the end (solid circle) of the tail current, except at potentials more positive than −10 mV where the time-dependent outward current was activated after the decay of If. In these cases, the baseline level was measured immediately after outward tail current. Genistein decreased the amplitude of If at all potentials. Over an average of four cells, the reversal potential of If was −33.2±4.3 mV and −35.3±4.3 mV in the absence and presence of 200 μM genistein, respectively. These results, together with the effect of genistein on the voltage dependent activation (Figure 4), indicate that genistein decreases the If conductance without altering its kinetics.
Figure 5.
Fully-activated current-voltage relation of If. Original current recordings obtained in the absence and presence of 200 μM genistein are shown in upper panels. Instantaneous (open circles) and late (filled circles) current-voltage relationships are shown in lower panel. The late current was measured near the end of the repolarizing pulse, which was preceded by hyperpolarization to −120 mV.
Effects of daidzein and genistin on If
To address whether genistein decreased If amplitude through inhibiting PTK, we examined the effect on If of daidzein and genistin, inactive analogues of genistein on PTK (Akiyama et al., 1987). As shown in Figure 6, 500 μM daidzein hardly affected If, which is in sharp contrast to marked If inhibition observed at the subsequent application of 500 μM genistein. Essentially similar results were obtained in five other cells with daidzein and also with 200 μM genistin, in four cells (data not shown).
Figure 6.
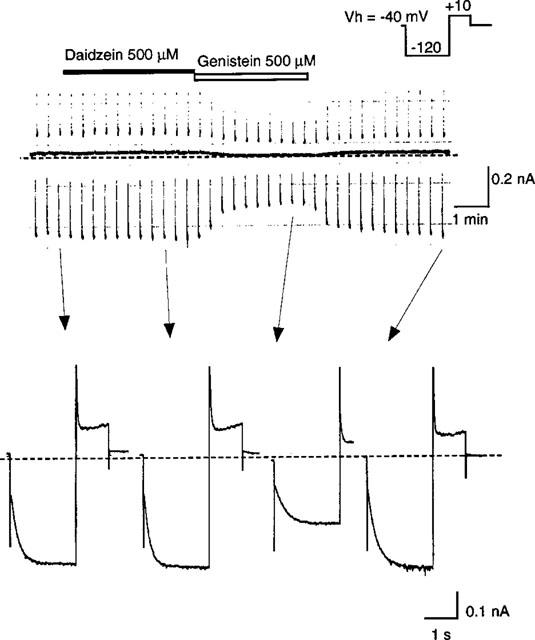
Effects of daidzein and genistin on If. Upper panel shows a current trace on the chart recorder. The double pulse protocol shown in the inset was repeated every 20 s. The filled and open bar above the trace indicate an application of 500 μM daidzein and 500 μM genistein, respectively. The lower panel shows original current recordings obtained at the points indicated in the upper panel. The dotted line indicates the zero current level.
Effects of other PTK inhibitors on If
We then investigated effects of other PTK inhibitors, tyrphostins, on If. It is well known that tyrphostins bind to the substrate subsite of PTK domain (Gazit et al., 1989), whereas genistein competes with the ATP binding site of PTK (Akiyama et al., 1987; 1991). In Figure 7A, current traces obtained in the absence (left) and presence (right) of 100 μM tyrphostin-25 are shown. The If was elicited by 2 s hyperpolarization from −40 mV to various potentials followed by depolarization to +10 mV. It is clearly shown that tyrphostin-25 suppressed If at all potentials. Figure 7B shows the averaged time course of the effect produced by 100 (open circles) and 200 μM (filled circles) tyrphostin-25 (n=4). In contrast to the reversible inhibition by genistein, the amplitude of If continued to decrease while tyrphostin-25 was washed out. Thus, tyrphostin-25-induced inhibition of If was irreversible. On the other hand, tyrphostin-47 hardly affected If (n=4; Figure 6C). Tyrphostin-47 is known to be a more potent and broad range PTK inhibitor than tyrphostin-25 (Gazit et al., 1989; Levitzki & Gazit, 1995). The findings indicate that tyrphostin-25 and genistein could inhibit If by mechanisms independent of tyrosine phosphorylation.
Figure 7.
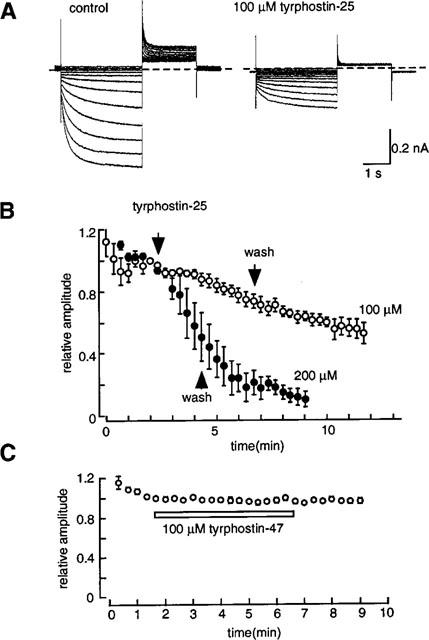
Effects of tyrphostin-25 and tyrphostin-47 on If. (A) The current traces recorded before (left) and during the application of 100 μM tyrphostin-25. (B) The time course of If inhibition by 100 (open circles) and 200 μM (filled circles) tyrphostin-25. The pulse protocol was the same as shown in Figure 6. The If amplitude was normalized to that recorded just before application of genistein and plotted against the experimental time. The times of application and removal of the drug are indicated by arrows. Each symbol indicates mean±s.e.mean of four cells. (C) The time course of changes in If amplitude during the application of 100 μM tyrphostin-47. The horizontal bar indicates the duration of application of tyrphostin-47. Each symbol indicates mean±s.e.mean of four cells.
Effects of protein tyrosine phosphatases inhibitor on If
The possibility that If is regulated by tyrosine phosphorylation was investigated by using a tyrosine phosphatase inhibitor, sodium orthovanadate (Swarup et al., 1982). In the experiment shown in Figure 8A, the pipette solution contained 0.2 mM orthovanadate and the external solution was changed to the standard external solution containing 2 mM orthovanadate after the rupture of the patch membrane at time zero. If was monitored by applying a 2 s hyperpolarizing pulse to −120 mV from −40 mV every 1 min. During the course of experiments over 25 min, no significant difference was detected in the time course of the If amplitude between the control currents (open circles) and those recorded in the presence of orthovanadate (filled circles). Furthermore, pretreatment of myocytes with both intra- and extra-cellular orthovanadate did not significantly affect the response to genistein (Figure 8B). In Figure 7C, results obtained from five cells are summarized. Orthovanadate failed to modify If (95.7±4.7%, NS), or the effects of genistein (64.2–5.3%) compared with those obtained using genistein alone (Figure 8B).
Figure 8.
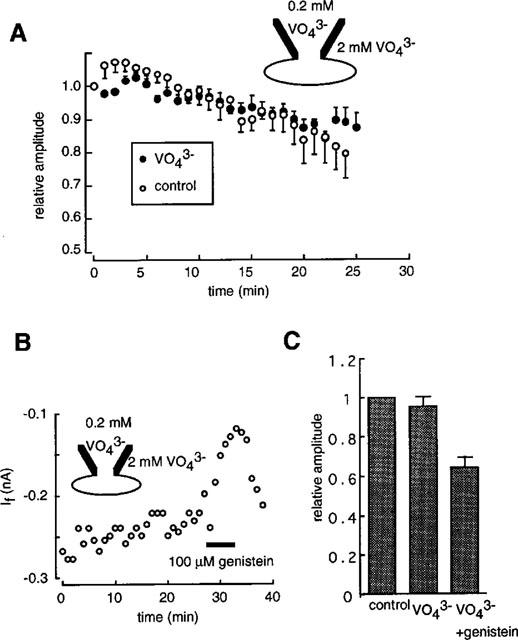
Effects of orthovanadate on If. (A) The time course of If in the absence (open circles) and in the presence of orthovanadate (filled circles). The pulse protocol was the same as shown in Figure 6. The If amplitude was normalized to that of the first record in each experiment. The data are mean±s.e.mean of five cells. (B) After the intra- (0.2 mM) and extra-cellular (2 mM) application of orthovanadate for 20 min, 100 μM genistein was applied during the period shown by the horizontal bar. The pulse protocol was the same as shown in Figure 6. The If amplitude was measured as the time dependent component of hyperpolarization activated inward current. (C) Summarized results obtained from five cells. The If amplitude was normalized to that recorded immediately after the start of the experiments. Each column indicates mean±s.e.mean.
Discussion
In the present study we have isolated SAN cells from porcine hearts and recorded If, the properties of which were similar to those of rabbit SAN cells (Frace et al., 1992; Maruoka et al., 1994), i.e. the threshold for activation was around −70 mV with the half maximal activation of around −90 mV, and the reversal potential was approximately −35 mV with the high-K+ internal and standard external solutions. Using various inhibitors for PTK, the present study has focused on whether If channels were regulated by tyrosine phosphorylation. The results were as follows: (1) the amplitude of If was decreased by genistein, but not by daidzein and genistin which are inactive analogues of genistein; (2) the inhibition was observed irrespective of whether the current was recorded by either ruptured- or perforated-patch method (Figures 2 and 3); (3) the inhibition of If by genistein was characterized by a decrease of the maximal conductance without affecting the V½ of the activation curve (Figure 4B); (4) tyrphostin-47 did not affect If whereas tyrphostin-25 inhibited If in an irreversible manner, and (5) when SAN cells were pretreated with orthovanadate, a tyrosine phosphatase inhibitor, genistein still decreased the amplitude of If.
The finding that If inhibition by genistein was not accompanied by a shift of the activation curve is in sharp contrast to the reported finding that the decrease of intracellular cyclic AMP shifted the activation curve toward the negative direction (DiFrancesco & Tortora, 1991). Thus we conclude that If inhibition by genistein occurs independently from cyclic AMP-dependent pathways. On the other hand, it has been reported that calyculin A, a phosphatase inhibitor, increased the amplitude of If without affecting the activation curve in rabbit SAN cells (Accili et al., 1997), indicating that phosphorylation can modulate If. In the present study, however, orthovanadate failed to increase If. This might occur if the If channels were maximally phosphorylated by native PTK, assuming that tyrosine phosphorylation increases the amplitude of If. However, the finding that genistein inhibited If in myocytes pretreated with orthovanadate argues strongly against the phosphorylation hypothesis. In addition, the inhibition of If was not related to the potency of the PTK inhibitors used. Tyrphostin-47, which is known to be a more potent inhibitor than tyrphostin-25 (Gazit et al., 1989; Levitzki & Gazit, 1995), did not affect If whereas tyrphostin-25 inhibited If in an irreversible manner. Provided that If is regulated by PTKs, the finding might indicate that several different PTKs are present in sinoatrial node cells and that these PTKs are inhibited by tyrphostin-25 or -47 with different affinities. However, no biochemical data is available at present to support this idea, and the experiments with orthovanadate can not be explained entirely by the PTK mechanism. Thus, it appears that genistein and tyrphostin-25 are somewhat nonselective in their actions and that the inhibition of If occurs independently from tyrosine phosphorylation.
Nonselective effects of genistein on various ionic channels have been reported, including the voltage-gated Na+ current in cultured rat brain neurons (IC50=60 μM, Paillart et al., 1997), the Ca2+ current in vascular smooth muscle cells (IC50=36 μM, Wijetunge et al., 1992) and cardiac cells (Chiang et al., 1996), human cystic fibrosis transmembrane conductance regulator Cl− channels (Weinreich et al., 1997; Wang et al., 1998), and delayed rectifier K+ current in guinea-pig ventricular cells (IC50=30 μM, Washizuka et al., 1998). In the present study, genistein inhibited If with an IC50 value of 62.5 μM. It should be considered that genistein is poorly soluble in the test solution, and therefore the dose-response relation might have been distorted, particularly at concentrations higher than 200 μM. Nevertheless, the observed value seems in rough agreement with the non-selective inhibition of ionic currents reported in the previous studies (for references see above).
In vascular smooth muscle cells, tyrosine kinase inhibitors genistein and ST638 inhibited the voltage-gated K+ current (Smirnov & Aaronson, 1995). This effect was not mimicked by daidzein, which is often used with genistein as a control, since it is structurally similar but inactive as a PTK inhibitor (Akiyama et al., 1987, 1991). However, the inhibitory action of genistein was unaffected by orthovanadate (Smirnov & Aaronson, 1995), and the authors concluded that the effect of genistein was not due to PTK inhibition. Both daidzein and genistin may not be appropriate inactive control agents. In rabbit SAN cells, only the inhibition of If by genistein and the insensitivity to daidzein were described, but the effects of other PTK inhibitors and phosphatase inhibitors have not been extensively studied (Wu & Cohen, 1997). It might, therefore, be hard to conclude the inhibition of If in rabbit SAN cells is mediated through PTK suppression.
Recently it has been reported that genistein elicits biphasic actions on the L-type Ca2+ currents in feline atrial myocytes, i.e., the inhibition phase was followed by the stimulation of the current (Wang & Lipsius, 1998). The biphasic action was observed not with a ruptured patch method but with a nystatin-perforated patch method, and the stimulatory action of genistein was eliminated by cell dialysis. These findings might indicate that cytosolic PTKs are involved in the stimulatory action of genistein. In the present study, however, only the inhibition of If was observed both with the ruptured- and perforated-patch clamp methods. It is thus unlikely that genistein exerts stimulatory effects on If similar to that reported for the L-type Ca2+ current.
It has been shown that If exists in atrial and ventricular cells (Thuringer et al., 1992; Zhou & Lipsius, 1993; Yu et al., 1993), and the If occurrence became prominent during development of cardiac hypertrophy (Cerbai et al., 1994). Since PTK plays a role in the regulation of cellular growth and hypertrophy, functional linkage between If activation and tyrosine phosphorylation might be expected through the long term process. Recently, hyperpolarization-activated cation channels were cloned from the rat brain (Ludwig et al., 1998; Santoro et al., 1998), and the expressed current resembled those of If or Ih in neurons and cardiac cells, though it is not yet determined whether or not the members of the cloned channel family code for cardiac If channels. It would be interesting to know whether the expressed If channels are functionally phosphorylated by tyrosine kinase. Further studies are clearly necessary to elucidate this point.
In conclusion, the present study has demonstrated that genistein suppressed If of porcine SAN cells probably by the nonselective interaction with If channels rather than through inhibiting PTK.
Acknowledgments
We thank Dr A. Shinbo for the initial work of this study. This work was supported partly by grants from the Ministry of Education, Science, Sports and Culture of Japan, and from the Naitoh Memorial Foundation.
Abbreviations
- If
hyperpolarization-activated inward current
- PTK
protein tyrosine kinase
- SAN
sino-atrial node
References
- ACCILI E.A., REDAELLI G., DIFRANCESCO D. Differential control of the hyperpolarization-activated current (if) by cAMP gating and phosphatase inhibition in rabbit sino-atrial node myocytes. J. Physiol. 1997;500:643–651. doi: 10.1113/jphysiol.1997.sp022049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- AKIYAMA T., ISHIDA J., NAKAGAWA S., OGAWARA H., WATANABE S., ITOH N., SHIBUYA M., FUKAMI Y. Genistein, a specific inhibitor of tyrosine-specific protein kinases. J. Biol. Chem. 1987;262:5592–5595. [PubMed] [Google Scholar]
- AKIYAMA T., OGAWARA H. Use and specificity of genistein as inhibitor of protein-tyrosine kinases. Methods Enzymol. 1991;201:362–370. doi: 10.1016/0076-6879(91)01032-w. [DOI] [PubMed] [Google Scholar]
- CERBAI E., BARBIERI M., MUGELLI A. Characterization of the hyperpolarization-activated current, If, in ventricular myocytes isolated from hypertensive rats. J. Physiol. 1994;481:585–591. doi: 10.1113/jphysiol.1994.sp020465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CHIANG C.E., CHEN S.A., CHANG M.S., LIN H.N. Genistein directly inhibits L-type calcium currents but potentiates cAMP-dependent chloride currents in cardiac myocytes. Biochem. Biophys. Res. Commun. 1996;223:598–603. doi: 10.1006/bbrc.1996.0941. [DOI] [PubMed] [Google Scholar]
- DENYER J.C., BROWN H.F. Rabbit sino-atrial node cells: isolation and electrophysiological properties. J. Physiol. 1990;428:405–424. doi: 10.1113/jphysiol.1990.sp018219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DIFRANCESCO D., TORTORA P. Direct activation of cardiac pacemaker channels by intracellular cAMP. Nature. 1991;351:145–147. doi: 10.1038/351145a0. [DOI] [PubMed] [Google Scholar]
- FABIATO A., FABIATO F. Calculator programs for computing the composition of the solutions containing multiple metals and ligands used for experiments in skinned muscle cells. J. Physiol. 1979;75:463–505. [PubMed] [Google Scholar]
- FRACE A.M., MARUOKA F., NOMA A. Control of the hyperpolarization-activated cation current by external anions in rabbit sino-atrial node cells. J. Physiol. 1992;453:307–318. doi: 10.1113/jphysiol.1992.sp019230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GAZIT A., YAISH P., GILON C., LEVITZKI A. Tyrphostins I: synthesis and biological activity of protein tyrosine kinase inhibitors. J. Med. Chem. 1989;32:2344–2352. doi: 10.1021/jm00130a020. [DOI] [PubMed] [Google Scholar]
- GORDON J.A. Use of vanadate as protein-phosphotyrosine phosphatase inhibitor. Methods Enzymol. 1991;201:477–482. doi: 10.1016/0076-6879(91)01043-2. [DOI] [PubMed] [Google Scholar]
- GUO J., MITSUIYE T., NOMA A. The sustained inward current in sino-atrial node cells of guinea-pig heart. Pflügers Arch. 1997;433:390–396. doi: 10.1007/s004240050293. [DOI] [PubMed] [Google Scholar]
- HAMILL O.P., MARTY A., NEHER E., SAKMANN B., SIGWORTH F.J. Improved patch-clamp techniques for high-resolution current recording from cells and cell-free membrane patches. Pflügers Arch. 1981;391:85–100. doi: 10.1007/BF00656997. [DOI] [PubMed] [Google Scholar]
- HORN R., MARTY A. Muscarinic activation of ionic currents measured by a new whole-cell clamp method. J. Gen. Physiol. 1988;92:145–159. doi: 10.1085/jgp.92.2.145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HUANG X.Y., MORIELLI A.D., PERALTA E.G. Tyrosine kinase-dependent suppression of potassium channel by the G protein-coupled m1 muscarinic acetylcholine receptor. Cell. 1993;75:1145–1156. doi: 10.1016/0092-8674(93)90324-j. [DOI] [PubMed] [Google Scholar]
- IRISAWA H., BROWN H.F., GILLES W. Cardiac pacemaking in the sinoatrial node. Physiol. Rev. 1993;73:197–227. doi: 10.1152/physrev.1993.73.1.197. [DOI] [PubMed] [Google Scholar]
- LEVITZKI A., GAZIT A. Tyrosine kinase inhibition: an approach to drug development. Science. 1995;267:1782–1788. doi: 10.1126/science.7892601. [DOI] [PubMed] [Google Scholar]
- LUDWIG A., ZONG X., JEGLITSCH M., HOFMANN F., BIEL M. A family of hyperpolarization-activated mammalian cation channels. Nature. 1998;393:587–591. doi: 10.1038/31255. [DOI] [PubMed] [Google Scholar]
- MARUOKA F., NAKASHIMA Y., TAKANO M., ONO K., NOMA A. Cation-dependent gating of the hyperpolarization-activated cation current in the rabbit sino-atrial node cells. J. Physiol. 1994;477:423–435. doi: 10.1113/jphysiol.1994.sp020204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PAILLART C., CARLIER E., GUEDIN D., DARGENT B., COURAUD F. Direct block of voltage-sensitive sodium channels by genistein, a tyrosine kinase inhibitor. J. Pharmacol. Exp. Ther. 1997;280:521–526. [PubMed] [Google Scholar]
- SANTORO B., LIU D.T., YAO H., BARTSCH D., KANDEL E.R., SIEGELBAUM S.A., TIBBS G.R. Identification of a gene encoding a hyperpolarization-activated pacemaker channel of brain. Cell. 1998;93:717–729. doi: 10.1016/s0092-8674(00)81434-8. [DOI] [PubMed] [Google Scholar]
- SHUBA L.M., ASAI T., PELZER S, McDONALD T.F. Activation of cardiac chloride conductance by the tyrosine kinase inhibitor, genistein. Br. J. Pharmacol. 1996;119:335–345. doi: 10.1111/j.1476-5381.1996.tb15991.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SMIRONOV S.V., AARONSON P.I. Inhibition of vascular smooth muscle cell K+ currents by tyrosine kinase inhibitors genistein and ST 638. Circ. Res. 1995;76:310–316. doi: 10.1161/01.res.76.2.310. [DOI] [PubMed] [Google Scholar]
- SWARUP G., COHEN S., GARBERS D.L. Inhibition of membrane phosphotyrosyl-protein phosphatase activity by vanadate. Biochem. Biophys. Res. Commun. 1982;107:1104–1109. doi: 10.1016/0006-291x(82)90635-0. [DOI] [PubMed] [Google Scholar]
- THURINGER D., LAURIBE P., ESCANDE D. A hyperpolarization-activated inward current in human myocardial cells. J. Mol. Cell. Cardiol. 1992;24:451–455. doi: 10.1016/0022-2828(92)91833-q. [DOI] [PubMed] [Google Scholar]
- WANG F., ZEITWANGER S., YANG I.C., NAIRN A.C., HWANG T.C. Actions of genistein on cystic fibrosis transmembrane conductance regulator channel gating. Evidence for two binding sites with opposite effects. J. Gen. Physiol. 1998;111:477–490. doi: 10.1085/jgp.111.3.477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WANG Y.G., LIPSIUS S.L. Genistein elicits biphasic effects on L-type Ca2+ current in feline atrial myocytes. Am J. Physiol. 1998;275:H204–H212. doi: 10.1152/ajpheart.1998.275.1.H204. [DOI] [PubMed] [Google Scholar]
- WANG Y.T., SALTER M.W. Regulation of NMDA receptors by tyrosine kinases and phosphatases. Nature. 1994;369:233–235. doi: 10.1038/369233a0. [DOI] [PubMed] [Google Scholar]
- WASHIZUKA T., HORIE M., OBAYASHI K., SASAYAMA S. Genistein inhibits slow component delayed rectifier K currents via a tyrosine-kinase independent pathway. J. Mol. Cell. Cardiol. 1998;30:2577–2590. doi: 10.1006/jmcc.1998.0815. [DOI] [PubMed] [Google Scholar]
- WEINREICH F., WOOD P.G., RIORDAN J.R., NAGEL G. Direct action of genistein on CFTR. Pflügers Arch. 1997;434:484–491. doi: 10.1007/s004240050424. [DOI] [PubMed] [Google Scholar]
- WIJETUNGE S., AALKJAER C., SCHACHTER M., HUGHES A.D. Tyrosine kinase inhibitors block calcium channels currents in vascular smooth muscle cells. Biochem. Biophys. Res. Commun. 1992;189:1620–1623. doi: 10.1016/0006-291x(92)90262-j. [DOI] [PubMed] [Google Scholar]
- WU J.Y., COHEN I.S. Tyrosine kinase inhibition reduces if in rabbit sinoatrial node myocytes. Pflügers Arch. 1997;434:509–514. doi: 10.1007/s004240050430. [DOI] [PubMed] [Google Scholar]
- YU H., CHANG F., COHEN IS. Pacemaker current exists in ventricular myocytes. Circ. Res. 1993;72:232–236. doi: 10.1161/01.res.72.1.232. [DOI] [PubMed] [Google Scholar]
- ZHOU Z., LIPSIUS S.L. Effect of isoprenaline on If current in latent pacemaker cells isolated from cat right atrium: ruptured vs. perforated patch whole-cell recording methods. Pflügers Arch. 1993;423:442–447. doi: 10.1007/BF00374939. [DOI] [PubMed] [Google Scholar]


