Abstract
The D3 dopamine receptor presumably activates Gi/Go subtypes of G-proteins, like the structurally analogous D2 receptor, but its signalling targets have not been clearly established due to weak functional signals from cloned receptors as heterologously expressed in mostly non-neuronal cell lines.
In this study, recombinant human D3 receptors expressed in a human neuroblastoma cell line, SH-SY5Y, produced much greater signals than those expressed in a human embryonic kidney cell line, HEK293. Quinpirole, a prototypic agonist, markedly inhibited forskolin-stimulated cyclic AMP production and Ca2+-channel (N-type) currents in SH-SY5Y cells, and enhanced GTPγ35S binding in isolated membranes, nearly ten times greater than that observed in HEK293 cell membranes.
GTPγ35S-bound Gα subunits from quinpirole-activated and solubilized membranes were monitored upon immobilization with various Gα-specific antibodies. Gαo subunits (not Gαi) were highly labelled with GTPγ35S in SH-SY5Y, but not in HEK293 cell membranes, despite their abundance in the both cell types, as shown with reverse transcription-polymerase chain reaction and Western blots. N-type Ca2+ channels and adenylyl cyclase V (D3-specific effector), on the other hand, exist only in SH-SY5Y cells.
More efficient coupling of the D3 receptor to Go subtypes in SH-SY5Y than HEK293 cells may be attributed, at least in part, to the two D3 neuronal effectors only present in SH-SY5Y cells (N-type Ca2+-channels and adenylyl cyclase V). The abundance of Go subtypes in the both cell lines seems to indicate their availability not a limiting factor.
Keywords: Human D3 dopamine receptor, Go subtypes of G-proteins, SH-SY5Y cells, signalling of D3 dopamine receptor, GTP35S bound Gα subunits
Introduction
The D3 dopamine receptor, a G protein-coupled receptor (GPCR) with seven transmembrane segments, belongs to a family of D2-like dopamine receptors (D2–D4) (Kebabian & Calne, 1979; Civelli et al., 1993; Seeman & Van Tol, 1994; Missale et al., 1998), and has been the target of potential drugs for psychotic disorders and Parkinson's disease (Sokoloff et al., 1990; Sokoloff & Schwarz, 1995). Although the human D3 receptor has been heterologously expressed and examined in various mammalian cells, its signalling pathways have not been clearly established. The D3 receptor is known to inhibit adenylyl cyclases, but its signal was considerably weaker than that of the D2 and D4 receptor in Chinese hamster ovary (CHO) cells, a putatively responsive cell line, (Chio et al., 1994; Potenza et al., 1994; McAllister et al., 1995; Missale et al., 1998), or hardly detectable in many other cell lines, such as C6 glioma, LTK-, a rat fibroblast, SK-N-MC human epithelioma, CCL1.3 fibroblast, and GH4C1 pituitary cells (Sokoloff et al., 1990; Seabrook et al., 1992; Tang et al., 1994; Freedman et al., 1994; Pilon et al., 1994; MacKenzie et al., 1994; Cox et al., 1995). Also absent were other signals frequently observed with some GPCRs, like the release of arachidonate or inositol-1,4,5-trisphophate (IP3), and the modulation of K+- or Ca2+-channels (Seabrook et al., 1992; Freedman et al, 1994). Even in the first step of G-protein activation, agonist-induced GTPγ35S binding, the D3 response was much smaller than that of the D2 or D4 receptors in CHO cells (Gardner et al., 1996). Further understanding of D3 signalling pathways, therefore, seems to depend on the discovery of a cell line containing appropriate cellular machinery for D3 signals. In this study we report greater agonist-induced responses for the human D3 receptor heterologously expressed in human neuroblastoma cells (SH-SY5Y) as compared to those in human embryonic kidney cells (HEK293), and its efficient coupling to the Go subtype of G proteins.
Methods
A [3H]-IP3 radioreceptor assay kit, a FlashPlate [125I]-cyclic AMP assay kit, [3H]-raclopride and GTPγ35S were purchased from DuPont NEN. A PRISM Ready Reaction DyeDeoxy Terminal Cycle Sequencing Kit was purchased from Perkin-Elmer/Applied Biosystems Division, and a mammalian expression vector, PCI-Neo, from Promega. The antibodies selective for Gαi-Gαo, Gαs, Gαq/11 or Gα13 were purchased from Calbiochem, and the antibodies selective for Gαi3 or Gαo (K-20) from Santa Cruz Biotechnology. The mouse monoclonal antibody against bovine Go protein was obtained from Chemicon. Hybond-P (polyvinylidene difluoride membrane) and ECL Plus Western blotting detection system were purchased from Amersham Pharmacia Biotech.
The human cDNA for the D3 dopamine receptor was cloned from a human cDNA library and inserted into a PCI-Neo mammalian expression vector. This was expressed in HEK293 or SH-SY5Y cells, using Ca2+ phosphate precipitation techniques, and subsequent G-418 selection. Membranes from transfected cells were prepared by standard procedures including cell homogenization and differential centrifugation as described elsewhere (Pregenzer et al., 1993). Binding of radioactive ligands was measured in membranes expressing the recombinant receptor, using filtration techniques as described elsewhere (Pregenzer et al., 1993). Briefly, [3H]-raclopride binding was measured in medium containing (mM): NaCl 100, MgCl2 2, EDTA 1, 4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid (HEPES) 20 (pH 7.4) and 5–15 μg membrane protein, using the radioactive ligand at varying concentrations (0.1–20 nM for typical binding profiles). Incubations were performed in a total volume of 500 μl at 23°C for 60 min. Reaction mixtures were filtered over Whatman GF/B filters under vacuum, and filters were washed three times with 4 ml of ice cold 50 mM Tris/HCl buffer (pH 7.4). Non-specific binding was estimated in the presence of excess unlabelled raclopride (10 μM). All the stock solutions for ligands were prepared in 0.1 % ascorbic acid (w v−1). Displacements of [3H]-raclopride by test compounds (competition assays) were carried out in the same assay buffer with the radioactive ligand at 1 nM.
GTPγ35S binding was measured following the procedures reported earlier (Chabert et al, 1994; Pregenzer et al., 1997) in medium containing (mM) HEPES 25,: NaCl 100, EDTA 1, MgCl2 3, dithiothreitol, 0.003% digitonin (w v−1) 0.5, GTPγ35S (5–3×105 cpm/assay) 2, and about 20 μg membrane protein in a volume of 120 μl. The use of digitonin at trace amounts here was solely to make the membranes permeable to GTPγ35S. Test ligands were included at 10 μM, unless indicated otherwise. Membranes were preincubated with 100 μM 5′-adenylylimidodiphosphate for 30 min, and then with 10 μM GDP for 10 min on ice. The reaction was initiated by adding treated membranes to reaction mixtures and continued at 30°C for 30 min. Reaction mixtures were filtered over Whatman GF/B filters under vacuum, and filters were washed three times with 4 ml of an ice-cold buffer containing (mM): NaCl 100, Tris/HCl, pH 8.0 20, MgCl2 25. Agonist-induced GTPγ35S binding was obtained by subtracting that observed without agonists. The binding data were analysed using a nonlinear regression method (Sigma Plot), and presented as mean±s.e.mean from three or more experiments.
Agonist-induced IP3 release in intact cells was measured using the [3H]-IP3 radioreceptor assay. Briefly the cells were seeded on a 24-well plate and grown to about 80% confluency. The cells were treated with 10 μM quinpirole or 1 mM carbachol at 37°C for 0, 30, 45 or 60 s, and the reaction was stopped with trichloroacetic acid (TCA, 20% final concentration). After removal of TCA with 1,1,2-trichloro-1,2,2-trifluoroethane and trioctyl amine, an aliquot was analysed for IP3, using [3H]-IP3/IP3 receptor preparations from calf cerebellum, following the protocols provided by the vendor (DuPont NEN). For each experiment, a dose-response profile for IP3 was constructed by adding known amounts of exogenous IP3 to TCA extracts of untreated cells.
Cellular changes in cyclic AMP were measured using a FlashPlate [125I]-cyclic AMP assay. Briefly, cells were grown to about 80% confluency in a 96-well plate, and then treated with forskolin at a submaximal concentration (typically 10 μM) with or without quinpirole or test ligands for 30 min in the presence of a phosphodiesterase inhibitor. Cyclic AMP in cell lysates was measured, through competition between [125I]-cyclic AMP and non-radioactive antigen for a fixed number of antibody binding sites in microplates coated with solid scintillant.
The whole-cell configuration of the patch clamp technique (Hamill et al., 1981) was used to record Ca2+-channel currents, as described elsewhere (Ito et al., 1994). Briefly, currents were recorded by a current-voltage converter consisting of an Axopatch-1D amplifier and a CV-4 headstage (Axon Instrument Co., U.S.A.), and stored on diskettes in an IBM-PC compatible computer using the ‘pClamp' acquisition software (Axon Instrument Co.). The Pipette solution contained (mM): N-methylglucamine 140, EGTA 10, glucose 5 and HEPES 10, pH 7.2. External solution contained (mM): BaCl2 50, CsCl 50, tetraethylammonium chloride 25, glucose 25, HEPES 10, (pH 7.3), GTP 0.3 and tetrodotoxin 0.5. All experiments were performed at room temperature (23°C).
GTPγ35S-bound Gα subunits of G proteins were identified following the method described elsewhere (Okamoto et al., 1992) with some modifications. The key change was that receptor-activation by agonists in the presence of GTPγ35S preceded membrane solubilization with detergents while the sequence was reversed in the original procedure. This change is beneficial in that G proteins are activated prior to general structural disruptions of GPCRs, G proteins and surrounding membranes by detergents. Briefly, membranes (∼50 μg or less membrane proteins) were incubated in the presence of GTPγ35S (4 nM) and quinpirole (10 μM) under the conditions identical to those for GTPγ35S binding as described above. Such treated membranes were solubilized with an equal volume of a buffer containing (mM): Tris/HCl, 100, pH 8.0, MgCl2 10, NaCl 100 and 3-[(3-cholamidopropyl)dimethylammonio]-1-propane-sulfonate (CHAPS, w v−1) 0.6% for 30 min on ice, and then diluted to a final CHAPS concentration to 0.125% (w v−1). Aliquots of the mixtures (typically 300 μl) were transferred to wells which had been coated successively with goat anti-rabbit (or anti-mouse) antibodies (1 : 100 dilution), bovine serum albumin (5 mg ml−1) and one of the affinity-purified antibodies specific for various Gα subunits (1 : 200 dilution). Amounts of membranes were chosen to be in the range where quinpirole-induced GTPγ35S association with the antibody for Gαi–Gαo linearly increased. Here we used the antibody selective for Gαi–Gαo (against the C-terminal sequence of Gαi3, KNNLKECGLY (345–354) which shares the same last four residues (CGLY) with Gαo), the antibodies selective for Gαs (against the C-terminal sequence, 385–394), Gαq/11 (against the common C-terminal sequence, QLNLKEYNLV), or Gα13 (against the sequence, 367-377) (Calbiochem). We also used the antibody cross-reacting with Gαi1, Gαi2 and Gαi3 due to their common residues (KNNLK), but not with Gαo (Santa Cruz), and the mouse monoclonal antibody raised against bovine Go protein (Chemicon). The plates were incubated for 1 h at room temperature. Individual wells were washed free of unbound GTPγ35S, and counted using a standard scintillation cocktail and a β-counter. Ligand specific-GTPγ35S binding was computed by subtracting that observed without test ligands.
Transcripts for Gαo and adenylyl cyclase V were estimated using semi-quantitative reverse transcription with polymerase chain reaction (RT–PCR). RT reactions were carried out with an aliquot of RNA fractions (2 μg) extracted from SH-SY5Y or HEK293 cells, RNase inhibitor, oligo dT, random hexamers, dNTP (1.5 mM) and reverse transcriptase (Life Technologies) in a vendor-supplied buffer, for 1 h at 37°C. PCR was performed in a 50 μl reaction mixture containing 2 μl of RT reaction mixtures, 1 U Amplitaq DNA polymerase in the vendor supplied buffer, 200 μM each dNTP, 10 pmol primers. The cycle parameters were at 94° for 30 s, 54° for 30 s and 72° for 90 s with a final extension for 10 min after 28 cycles. For Gαo, the primers were designed from the published sequence of the human Gαo1 (bp 726–746 and bp 1029–1049) and Gαo2 (bp 729–748 and bp 1025–1047) (Tsukamoto et al., 1991), and for adenylyl cyclase V (bp 1192–1212 and bp 1484–1502) from the partial sequence of human adenylyl cyclase V in Genbank (Accession number M83533). RT–PCR yielded fragments of the expected size. Additionally, all PCR products from the two cell lines were purified through agarose gel electrophoresis, and were directly sequenced using the PRISM Ready Reaction DyeDeoxy Terminal Cycle Sequencing Kit.
For Western blots, aliquots of cell lysates (20 μg protein) in the presence of protease inhibitors (leupeptin, 2 μg/ml and phenylmethylsulfonylfluoride, 1 mM) were separated on sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS–PAGE), using reducing gels and transferred to Hybond-P™ (polyvinylidene difluoride membrane). Membranes were probed with the Gαo-specific polyclonal rabbit antibody K-20 (1 : 500 dilution) (Santa Cruz), which was raised against a highly divergent Gαo domain, KMVCDVVSRMEDTEPFSAEL (105–124), and was not cross-reactive with other Gα subunits. Detection was performed using the ECL Plus Western blotting detection system.
Results
We examined ligand binding properties of the human D3 dopamine receptor expressed in HEK293 or SH-SY5Y cells. Scatchard analysis of binding data for [3H]-raclopride at various concentrations, fit to the binding equation with a single class of binding sites (linearity) and yielded dissociation constant (KD) of 1.5±0.1 and 2.1±0.3 nM, and maximal binding of 1.9±0.2 and 2.3±0.2 pmoles/mg protein in membranes from HEK293 and SH-SY5Y cells, respectively. Competition binding experiments using [3H]-raclopride, were carried out with standard dopaminergic ligands at various concentrations. The inhibition constants (KI) for spiperone, sulpiride, raclopride, quinpirole, dopamine, pramipexole and terguride (Table 1), as obtained using Cheng and Prusoff equation (Cheng & Prusoff, 1973) were in good agreement between the two cell lines, and were also comparable with those reported in the literature (Sokoloff et al, 1990). For the D3 receptor, agonists are known to display small affinity differences (less than 10 fold) between their high and low affinity sites (G-protein coupled and uncoupled phenotype, respectively) (Sokoloff et al., 1990), which could be further obscured by high receptor densities in cloned cells (Boundy et al, 1996; Butkerait et al., 1995). Consistent with this expectation, data from the displacement of [3H]-raclopride by quinpirole were fitted to the one-site binding model with its KI of 27±2 nM (Figure 1) and were not altered upon addition of GTPγS at 10 μM (data not shown).
Table 1.
Comparison of binding properties of the human D3 dopamine receptor expressed in SH-SY5Y and HEK293 cells
Figure 1.
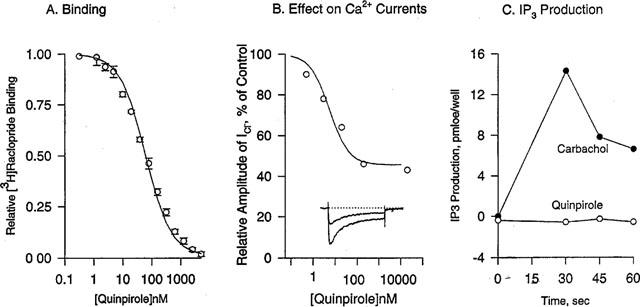
Plots showing the displacement of [3H]-raclopride binding to D3 by quinpirole, effects of quinpirole on N-type Ca2+-channel currents and on IP3 production in SH-SY5Y cells expressing the human D3 dopamine receptor. (A) Binding of [3H]-raclopride was measured in the presence of quinpirole at various concentrations in isolated membranes from SH-SY5Y cells. The solid line represents the data fitting with the binding equation for a single class of binding sites with the KI of 27±2 nM. (B) Quinpirole concentration-dependently blocked Ca2+-channel currents (Ba2+ as the charge carrier) in SH-SY5Y cells expressing the human D3 dopamine receptor. The inset shows representative traces for Ca2+-channel currents in the absence or presence of quinpirole at 1 μM, obtained using a pulse potential of 25 mV for 400 ms from a holding potential of −100 mV. The amplitude of the peak current without the drug was 120 pA. The dotted line represents the current baseline obtained upon application of La3+ (100 μM) at the end of the recording. (C) Quinpirole at 10 μM had no effect on intracellular level of IP3 in SH-SY5Y cells while carbachol at 1 mM transiently enhanced IP3 in the same cell line.
High threshold-activated Ca2+ channel currents in SH-SY5Y cells are largely blocked by ω-conotoxin (Friederich et al., 1993; Reeve et al., 1995), as N-type channels. Here, currents (Ba2+ ions as charge carriers) were evoked by pulses of 25 mV and 400 ms-duration from a holding potential of −100 mV. Quinpirole concentration-dependently blocked Ca2+-channel currents in SH-SY5Y cells heterologously expressing the human D3 receptor, with an IC50 value of 5.6±1.8 nM and maximal inhibition of 55±3 % (Figure 1). In untransfected cells, no effect on Ca2+ channel currents was observed with quinpirole at 1 μM. Certain GPCRs activate phospholipase C, via Gαq/11 or Gβγ subunits, and increase intracellular IP3. Quinpirole failed to enhance IP3 release in SH-SY5Y cells expressing D3 while carbachol markedly increased IP3 release in the same cell line by activation of endogenous m3 muscarinic receptors (Figure 1).
Agonist-bound GPCRs catalyze the exchange of GDP with GTP on G-protein α subunits as the first step of G-protein activation. This step was monitored with GTPγ35S, a slowly hydrolyzing GTP analogue. Quinpirole concentration-dependently enhanced GTPγ35S binding in membranes from the SH-SY5Y cells, with a half maximal concentration (EC50) of 25±4 nM, and maximal binding of 149±21 fmoles/mg protein, but by only 14±6 fmoles/mg protein in membranes from HEK293 cells (Figure 2). Quinpirole-induced GTPγ35S binding in SH-SY5Y cell membranes was blocked by haloperidol (10 μM), an antagonist (Figure 2). Haloperidol alone reduced the basal GTPγ35S binding by 17%, as normalized to that observed with quinpirole, probably due to some population of receptors in constitutively active states. Several known agonists for D3 were also examined for their effects on GTPγ35S binding in SH-SY5Y cell membranes. Dopamine, pramipexole and terguride concentration-dependently enhanced GTPγ35S binding with an EC50 value of 42±4, 18±2, and 1.4±0.2 nM, respectively, and maximal stimulation of 96±4, 97±9 and 47±5%, respectively, as normalized to that of quinpirole (Table 2).
Figure 2.
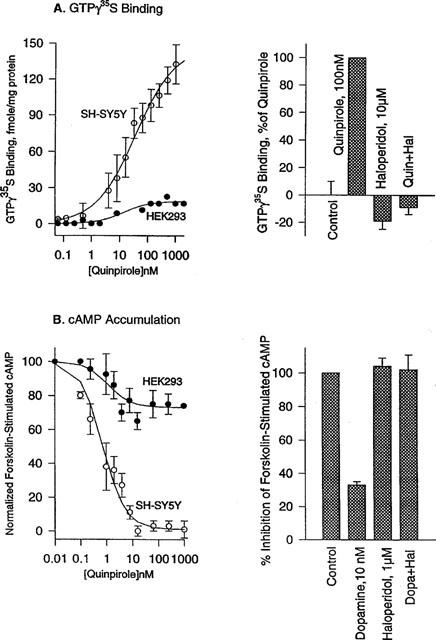
Comparison of quinpirole-induced GTPγ35S binding in isolated membranes and inhibition of forskolin (10 μM)-stimulated cyclic AMP production in HEK293 cells and SH-SY5Y cells expressing the human D3 dopamine receptor. (A) Quinpirole dose-dependently increased GTPγ35S binding in SH-SY5Y cell membranes, nearly 10 times more than that in HEK293 cell membranes. The quinpirole-induced GTPγ35S binding was blocked by haloperidol, which by itself reduced the basal GTPγ35S binding by 17%. (B) Quinpirole dose-dependently blocked forskolin-stimulated cyclic AMP production in SH-SY5Y cells much more robustly than in HEK293 cells. The degrees of inhibition at various concentrations were normalized to the maximal inhibition observed with quinpirole in SH-SY5Y cells in parallel assays. Dopamine similarly inhibited the cyclic AMP production in SH-SY5Y cells and its action was blocked by haloperidol.
Table 2.
Intrinsic efficacy of standard agonists for the human D3 dopamine receptor as measured with GTPγ35S binding and inhibition of cyclic AMP production
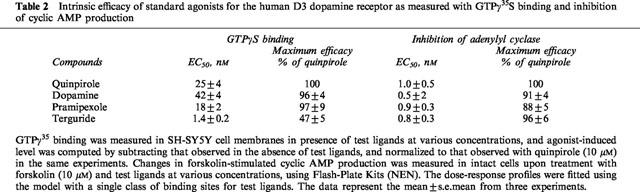
We examined the effect of quinpirole on forskolin-stimulated cyclic AMP production in SH-SY5Y and HEK293 cells, where forskolin at 10 μM (a submaximal concentration) typically increased cyclic AMP by 4–5 pmoles per well (a 96-well plate). Quinpirole concentration-dependently reduced the cyclic AMP increase in SH-SY5Y cells, and its maximal inhibition amounted to 63±10%. Composite dose-response profiles (Figure 2), when normalized to maximal inhibition observed with individual experiments, showed an IC50 value of 0.95±0.5 nM for quinpirole. Parallel assays in HEK293 cells showed the maximal inhibition of adenylyl cyclases by quinpirole amounting to 27±3% of that observed in SH-SY5Y cells, and with an IC50 value of 1.1±0.4 nM (Figure 2). We also examined the effects of several other agonists on adenylyl cyclases in SH-SY5Y cells. Dopamine, pramipexole and terguride concentration-dependently reduced forskolin-stimulated cyclic AMP with EC50 values of 0.8±0.2, 0.5±0.3 and 0.8±0.3 nM, respectively, and maximal inhibition of 91±4, 92±5 and 96±6%, respectively, as normalized to that of quinpirole (Table 2). Haloperidol by itself had no appreciable effect on forskolin-stimulated cyclic AMP level, but blocked the dopamine action, as expected for an antagonist (Figure 2). Note that the agonist EC50 values in this cyclic AMP assay were 30–50-fold less than those in the GTPγ35S assay, except for terguride with only a 2 fold difference. Moreover, terguride behaved like a full agonist with the cyclic AMP assay (96% of quinpirole), but like a partial agonist with the GTPγ35S assay (45% of quinpirole), as noted above. It appears that the maximal inhibition of adenylyl cyclase by agonists may occur at a receptor occupancy considerably lower than that observed with GTPγ35S binding. Moreover, terguride, a seemingly partial agonist in the GTPγ35S assay, may achieve maximal inhibition of adenylyl cyclase like full agonists, at its receptor occupancy much greater than that for full agonists.
Taking advantage of robust agonist-induced GTPγ35S binding in SH-SY5Y cell membranes, we attempted to trap quinpirole-dependent GTPγ35S-labelled Gα subunits from solubilized membranes, with Gα-specific antibodies, following the procedure previously described (Okamoto et al., 1992), with some modifications (see Methods). After receptor activation with quinpirole at 10 μM in the presence of GTPγ35S (4 nM), G proteins were solubilized with 0.3% CHAPS (w v−1), and trapped with antibodies specific for various Gα subunits; Gαi, Gαo, Gαi–Gαo (cross-reacting with both), Gαs, Gαq/11, G13. The highest level of GTPγ35S was observed with the antibody for Gαi–Gαo (Figure 3). Typically, GTPγ35S associated with the antibody was about three times greater than that observed in the control well without quinpirole activation; e.g., 6182±325 vs 1610±240 c.p.m. per well. The next highest level of GTPγ35S was associated with Gαo, 75±3% of that for Gαi–Gαo, followed by Gαi, only 8±5%. Relative degrees of GTPγ35S-association with the other antibodies, as normalized to that observed with the antibody against Gαi–Gαo in SH-SY5Y cell membranes, were 7±3, −1±2 and −2±3% for Gαs, Gαq/11, G13, respectively (Figure 3). These results point to Gαo subtypes of G proteins as the primary targets of D3 (Figure 3). Moreover, quinpirole (200 nM)-induced GTPγ35S association with the Gαi–Gαo antibody was blocked by coapplication of haloperidol (10 μM) (data not shown). Similar experiments with membranes from HEK293 cells expressing D3 showed no marked association of GTPγ35S with any antibodies we tested, including those specific for Gαi–Gαo or Gαo (Figure 3). Two isotypes of Gαo (Gαo1 and Gαo2) are known (Tsukamoto et al., 1991), and their presence in the two cell lines was examined, using RT–PCR with isotype-specific primers, in a semi-quantitative manner (see Methods). Gαo1 transcripts were well amplified in samples from HEK293 and SH-SY5Y cells, and Gαo2 transcripts were detectably amplified, but its product was somewhat less for HEK293 than SH-SY5Y cells (Figure 4). Direct sequencing of the PCR products from the both cell lines confirmed their identities as Gαo1 and Gαo2 (Tsukamoto et al., 1991). The protein levels of Gαo subtypes in the two cell lines were estimated with Western blots using the antibody specific for Gαo, albeit not isotype-specific. The Western blot (Figure 4) displayed the primary band at the molecular weight of 39 k.d.a., which is identical to that of Gαo subunits as resolved with SDS–PAGE. Intensities of the 39 k.d.a. band indicate comparable abundance of Gαo subunits in the two cell lines. A minor band at the molecular weight of 31 k.d.a. was detected only in SH-SY5Y cells (Figure 4), probably partially digested Gαo.
Figure 3.
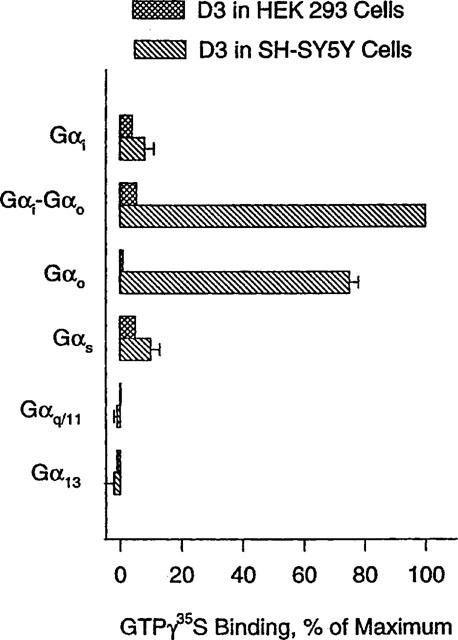
Plot showing relative levels of quinpirole-induced GTPγ35S association with the antibodies specific for various Gα subunits. Membranes from SH-SY5Y and HEK293 cells expressing the human D3 dopamine receptor were treated with quinpirole at 10 μM in the presence of GTPγ35S at 4 nM, and then solubilized with 0.3% CHAPS (w v−1). The mixtures were diluted to final CHAPS concentration of 0.125% (w v−1) and then added to the wells coated with indicated antibodies for Gα isotypes. After washing out unbound GTPγ35S, the wells were counted for 35S radioactivity. The GTPγ35S binding level for each antibody was normalized to that observed with the Gαi–Gαo antibody. The data represent the mean±s.e.mean from four experiments.
Figure 4.
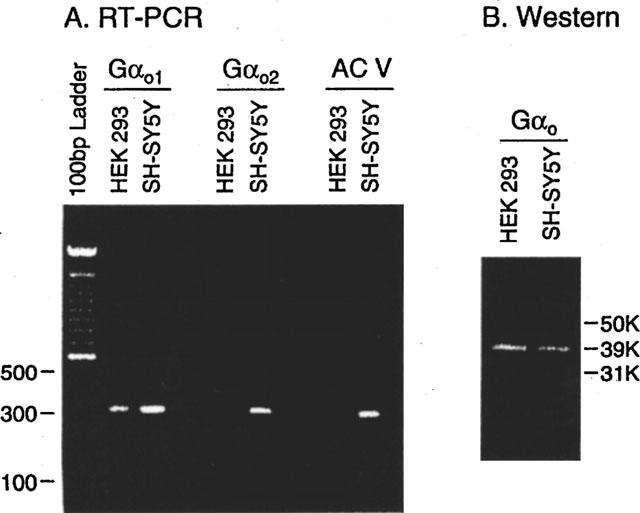
Detecting transcripts for Gαo1, Gαo2 and adenylyl cyclase V (ACV) via RT–PCR, and expression levels of Gαo subunits via Western blots with cell lysates from HEK293 and SH-SY5Y cells. (A) RT–PCR were carried with RNA extracts from HEK293 and SH-SY5Y cells and primers specific for Gαo1, Gαo2 and ACV as described in detail in Methods. (B) Cell lysates from HEK293 and SH-SY5Y cells were resolved on reducing SDS–PAGE gels, transferred to PVDF membranes, and blotted with the antibody specific for Gαo subunits.
Among nine isotypes of adenylyl cyclases in mammalian cells, adenylyl cyclase V (ACV) has been recently proposed as a selective D3 target, because its heterologous coexpression with the D3 dopamine receptor in HEK293 cells resulted in robustly enhanced quinpirole-induced inhibition of forskolin-stimulated cyclic AMP production (Robinson & Caron, 1997). We examined here the presence of ACV transcripts in the two cell lines, using RT–PCR with ACV-specific primers (see Methods). Transcripts for ACV were well amplified in samples from SH-SY5Y cells, but were not detectably amplified in those from HEK293 cells. The identity of the PCR product from SH-SY5Y cells was confirmed with direct sequencing.
Discussion
In this study we have shown that the human D3 dopamine receptor, when heterologously expressed in SH-SY5Y cells, produced robust functional signals, which included agonist-induced stimulation of GTPγ35S binding, inhibition of adenylyl cyclases and reduction of N-type Ca2+-channel currents. These responses were much greater than those obtained with HEK 293 cells, where the receptor was expressed as abundantly as in SH-SY5Y cells. Agonist-induced GTPγ35S binding, representing the first step of G protein activation, was nearly 10 fold greater in SH-SY5Y than HEK293 cell membranes. Immobilization studies with antibodies specific for various Gα subunits revealed that quinpirole induced exclusive associations of GTPγ35S with Gαo subtypes of G-proteins in SH-SY5Y cells, but not with Gαi. It should be pointed out that the specificity of the Gαo-and Gαi-selective antibodies has been well documented by their vendors (see Methods), and antibody efficiencies to bind target subunits appear to be comparable. For example, similar studies with 5-HT1B receptors which are known to couple to Gi but not Go subtypes showed the expected pattern, robust associations of GTPγ35S with Gαi and Gαi–Gαo, but not Gαo (unpublished results). Immunoprecipitation studies with the antibodies specific for other α subunits, Gαs–Gαq/11 and Gα13, revealed no appreciable association with GTPγ35S in SH-SY5Y cell membranes.
From the above considerations, it is reasonable to propose that Go subtypes are the major G proteins activated by D3 dopamine receptors in SH-SY5Y, but not in HEK293 cells. Gαo subunits are highly enriched in neurons, and also seem to be reasonably abundant in HEK293 cells, as shown in this study by RT–PCR with Gαo specific primers and by Western blots with the Gαo-specific antibody (K-20 from Santa Cruz). The K-20 antibody which was raised against the internal sequence unique for Gαo (Residue 105–124) has been previously documented for its binding specificity and efficiency for Gαo in Western blots (Schandar et al., 1998). This apparent presence of Gαo in HEK293 cells raises the question of why D3 receptors failed to produce robust G protein couplings (e.g., as measured with agonist-induced GTPγ35S binding) which seems to occur promiscuously between G proteins and various receptors in isolated/reconstituted states. One possibility has been recently proposed that such couplings would not occur in intact cells in the absence of target effectors. Such coupling specificities in cells may require formation of structurally restrained complexes among receptors, G-proteins and target effectors (Chidiac, 1998).
Numerous Gαo-coupled effectors would be expected to exist in neuronal cell lines, and here we examined only two effectors, adenylyl cyclases and N-type Ca2+ channels. Adenylyl cyclases consist of nine isotypes including the most recently discovered type IX (Premont et al., 1996). Among the multiple isotypes, ACV has been reported to be a selective target for D3 (Robinson & Caron, 1997). Only when the D3 receptor and ACV were coexpressed in HEK293 cells, quinpirole robustly blocked forskolin-stimulated cyclic AMP, despite the presence of other endogenous adenylyl cyclases such as ACII, III, IV and VII (Hellevuo et al., 1993). Using RT–PCR, we have shown here the presence of ACV transcripts in SH-SY5Y cells, but not in HEK293 cells. In the brain, ACV is highly expressed in selective regions, such as the striatum and nucleus accumbens which are the important regions for both dopaminergic and serotonergic neurotransmission (Glatt & Snyder, 1993; Premont et al., 1996). ACV thus appears to be a physiologically relevant target for the D3 receptor. In addition to ACV, SH-SY5Y cells contain high threshold N-type Ca2+ channels, selectively inhibited by ω-conotoxin and also by Go coupled receptors, such as neuropeptide Y receptors (Friederich et al., 1993; Reeve et al., 1995). Generally, N-type Ca2+-channel currents are inhibited by various Go coupled receptors, such as opiate, muscarinic, GABAB and neuropeptide Y receptors, but are much less so by Gi coupled receptors (Lledo et al., 1992). The potent inhibition of Ca2+-channel currents in SH-SY5Y cells by quinpirole is consistent with the coupling of D3 to Go subtypes of G proteins. As described above, Gαo subunits consists of the two splice variants, Gαo1 and Gαo2 (Tuskamoto et al, 1991), in HEK293 and SH-SY5Y cells, but their relative abundance will not be known, until the antibodies specific for the isotypes become available. So far, however, no isotype-specific functional differences have been established, e.g., both isotypes have been reported to be active for N-type Ca2+ channels (Lledo et al., 1992). Gαo subtypes seem not to be a limiting factor in D3 signallings in HEK293 cells.
In this study, we were able to point out two effectors, N-type Ca2+ channels and ACV which could contribute, at least in part, to the D3 signalling differences in HEK293 and SH-SY5Y cells, including robust agonist-induced GTPγ35S binding. Yet, a number of questions remain to be resolved. (1) To what extent do these effectors contribute to the GTPγ35S binding? (2) What other effectors are involved in D3 receptor signaling in SH-SY5Y cells? (3) Are there other molecular entities required for D3 signallings? Recently, caveolins, representing a family of scaffolding proteins for organizing preassembled signalling complexes at the plasma membranes, have been proposed to regulate the activation state of associated signalling molecules (Okamoto et al., 1998). Some of these questions could be resolved in the near future by removing ACV and/or N-type Ca2+ channels from SH-SY5Y cells, using various molecular biological techniques, and also by expressing these effectors in HEK293 cells.
In this study, intrinsic efficacy of several standard ligands was compared as measured with agonist-induced GTPγ35S binding and inhibition of forskolin-stimulated cyclic AMP production in SH-SY5Y cells. One noteworthy point is that the agonist EC50 values for the cyclic AMP assay were 30–50-fold less than those for the GTPγ35S assay (Table 2). This indicates that the receptor occupancy required for the maximal inhibition of adenylyl cyclases by agonists was considerably lower than that observed for the GTPγ35S assay. Moreover, terguride behaved like a partial agonist in the GTPγ35S assay (45% of quinpirole), but behaved like a full agonist in cyclic AMP assay (97% of quinpirole), when its receptor occupancy became much greater than that for full agonists. This points out difficulties in using the cyclic AMP assay or other low threshold assays for estimating intrinsic efficacy of test ligands, because of large receptor reserves. In this respect, the GTPγ35S assay seems to be advantageous for intrinsic efficacy measurements because the assay, representing the first step of G-protein activation, seems to be solely dependent on the number of functionally activated receptor complexes without downstream amplification or threshold steps.
In summary, human D3 dopamine receptors appear to activate Go subtypes of G proteins in SH-SY5Y cells, as evidenced by robust agonist-induced GTPγ35S binding, but not in HEK293 cells, due to the presence of D3-specific target effectors (e.g., ACV and N-type Ca2+ channels) only in SH-SY5Y cells.
Abbreviations
- AC
adenylyl cyclase
- CHAPS
3-[(3-cholamidopropyl)dimethylammonio]-1-propane-sulfonate
- CHO
Chinese hamster ovary
- EC50
a half maximal concentration
- GPCR
G protein coupled receptor
- HEK
human embryonic kidney
- HEPES
4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid
- IC50
a half maximal inhibitory concentration
- IP3
inositol 1,4,5-triphosphate
- KD
dissociation constant
- KI
inhibition constant
- PCR
polymerase chain reaction
- RT
reverse transcription
- SDS–PAGE
sodium dodecyl sulphate-polyacrylamide gel electrophoresis
- TCA
trichloroacetic acid
References
- BOUNDY V.A., LU L., MOLINOFF P.B. Differential coupling of rat D2 dopamine receptor isotypes expressed in Spodoptera Frugiperda insect cells. J. Pharmacol. Exp. Therap. 1996;276:784–794. [PubMed] [Google Scholar]
- BUTKERAIT P., ZHENG Y., HALLAK H., GRAHAM T.E., MILLER H.A., BURRIS K.D., MOLINOFF P.B., MANNING D.R. Expression of the human 5-hydroxytryptamine1A receptor on Sf9 cells. J. Biol. Chem. 1995;270:18691–18699. doi: 10.1074/jbc.270.31.18691. [DOI] [PubMed] [Google Scholar]
- CHABERT C., CAVEGN C., BERNARD A., MILLS A. Characterization of the functional activity of dopamine ligands at human recombinant dopamine D4 receptors. J. Neurochem. 1994;63:62–65. doi: 10.1046/j.1471-4159.1994.63010062.x. [DOI] [PubMed] [Google Scholar]
- CHENG Y.-C., PRUSOFF W.H. Relationship between the inhibition constant (KI) and the concentration of inhibitor which causes 50 per cent inhibition (IC50) of an enzymatic reaction. Biochem. Pharmacol. 1973;22:3099–3108. doi: 10.1016/0006-2952(73)90196-2. [DOI] [PubMed] [Google Scholar]
- CHIDIAC P. Rethinking receptor-G protein-effector interactions. Biochem. Pharmacol. 1998;55:549–556. doi: 10.1016/s0006-2952(97)00361-4. [DOI] [PubMed] [Google Scholar]
- CHIO C.L., LAJINESS M.E., HUFF R.M. Activation of heterologously expressed D3 dopamine receptors: Comparison with D2 dopamine receptors. Mol. Pharmacol. 1994;45:51–60. [PubMed] [Google Scholar]
- CIVELLI O., BUNZOW J.R., GRANDY D.K. Molecular diversity of the dopamine receptors. Annu. Rev. Pharmacol. Toxicol. 1993;32:281–307. doi: 10.1146/annurev.pa.33.040193.001433. [DOI] [PubMed] [Google Scholar]
- COX B.A., ROSSER M.P., KOZLOWSKI M.R., DUWE K.M., EVE R.L., EVE K.A. Regulation and functional characterization of a rat recombinant dopamine D3 receptor. Synapse. 1995;21:1–9. doi: 10.1002/syn.890210102. [DOI] [PubMed] [Google Scholar]
- FREEDMAN S.B., PATEL S., MARWOOD R., EMMS F., SEABROOK G.R., KNOWLES M.R., MCALLISTER G. Expression and pharmacological characterization of the human D3 dopamine receptor. J. Pharmacol. Exp. Ther. 1994;268:417–426. [PubMed] [Google Scholar]
- FRIEDERICH P., NÜRNBERG B., SCHLUTZ G., HESCHELER J. Inversion of Ca2+ current modulation during recovery of neuroblastoma cells from pertussis toxin pretreatment. FEBS Lett. 1993;334:322–326. doi: 10.1016/0014-5793(93)80703-w. [DOI] [PubMed] [Google Scholar]
- GARDNER B., HALL D.A., STRANGE P.G. Pharmacological analysis of dopamine stimulation of [35S]-GTPγS binding via human D2short and D2long dopamine receptors expressed in recombinant cells. Br. J. Pharmacol. 1996;118:1544–1550. doi: 10.1111/j.1476-5381.1996.tb15572.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GLATT C.E., SNYDER S.H. Cloning and expression of an adenylyl cyclase localized to the corpus striatum. Nature (Lond.) 1993;361:536–538. doi: 10.1038/361536a0. [DOI] [PubMed] [Google Scholar]
- HAMILL O.P., MARTY A., NEHRE E., SAKMANN B., SIGWORTH F.J. Improved patch-clamp techniques for high resolution current recording from cells and cell free membrane patches. Pflüg. Arch. 1981;393:85–100. doi: 10.1007/BF00656997. [DOI] [PubMed] [Google Scholar]
- HELLUEVO K., YOSHIMURA M., KAO M., HOFFMAN P.L., COOPER D.M.F., TABAKOFF B. A novel adenylyl cyclase sequence cloned from the human erythroleukemia cell line. Biochem. Biophys. Res. Comm. 1993;192:311–318. doi: 10.1006/bbrc.1993.1415. [DOI] [PubMed] [Google Scholar]
- ITO C., IM W.B., TAGAGI H., TAKAHASHI M., TSUZUKI K., LIOU S.-Y., KNUIHARA M. U-92032, a T-type Ca2+ channel blocker and antioxidant, reduces neuronal ischemic injuries. Eur. J. Pharmacol. 1994;257:203–210. doi: 10.1016/0014-2999(94)90130-9. [DOI] [PubMed] [Google Scholar]
- KEBABIAN J.W., CALNE D.B. Multiple receptors for dopamine. Nature. 1979;277:93–96. doi: 10.1038/277093a0. [DOI] [PubMed] [Google Scholar]
- LLEDO P.M., HOMBURGER V., BOCKAERT J., VINCENT J.-D. Differential G protein-mediated coupling of D2 dopamine receptors to K+ and Ca2+ currents in rat anterior pituitary cell. Neuron. 1992;8:455–463. doi: 10.1016/0896-6273(92)90273-g. [DOI] [PubMed] [Google Scholar]
- MACKENZIE R.G., VANLEEUWEN D., PUGSLEY T.A., SHIH Y.H., DEMATTOS S., TANG L., TODD R.D., O'MALLEY K.L. Characterization of the human dopamine D3 receptor expressed in transfected cell lines. Eur. J. Pharmacol. 1994;266:79–85. doi: 10.1016/0922-4106(94)90212-7. [DOI] [PubMed] [Google Scholar]
- MCALLISTER G., KNOWLES M.R., WARD-BOOTH S.M., SINCLAIR H.A., PATEL S., MARWOOD R., EMMS F., PATEL S., SMITH A., SEABROOK G.R. Functional coupling of human D2, D3 and D4 dopamine receptors in HEK293 cells. J. Recept. Signal. Transduct. Res. 1995;15:267–281. doi: 10.3109/10799899509045220. [DOI] [PubMed] [Google Scholar]
- MISSALE C., RUSSEL S.W., JABER M., CARON M.G. Dopamine Receptors: From structure and function. Physiol. Rev. 1998;78:190–225. doi: 10.1152/physrev.1998.78.1.189. [DOI] [PubMed] [Google Scholar]
- OKAMOTO T., IKEZU T., MURAYAMA Y, OGATA E., NISHIMOTO I. Measurement of binding to specific G proteins in membranes using G-protein antibodies. FEBS Lett. 1992;305:125–128. doi: 10.1016/0014-5793(92)80878-k. [DOI] [PubMed] [Google Scholar]
- OKAMOTO T., SCHLEGEL A., SCHERER P.E., LISANTI M.P. Caveolins, a family of scaffolding proteins for organizing “preassembled signaling complexes” at the plasma membranes. J. Biol. Chem. 1998;273:5419–5422. doi: 10.1074/jbc.273.10.5419. [DOI] [PubMed] [Google Scholar]
- PILON C., LEVESQUE D., DIMITRIADOU V., GRIFFON N., MARTRES M.P., SCHWARZ J.C., SOKOLOFF P. Functional coupling of the human dopamine D3 receptor in a transfected NG 108-15 neuroblastoma-glioma hybrid cell line. Eur. J. Pharmacol. 1994;268:129–139. doi: 10.1016/0922-4106(94)90182-1. [DOI] [PubMed] [Google Scholar]
- POTENZA M.N., GRAMINSKI G.F., SCHMAUSS C., LEARNER M.R. Functional expression and characterization of human D2 and D3 dopamine receptors. J. Neurosci. 1994;14:1463–1476. doi: 10.1523/JNEUROSCI.14-03-01463.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PREGENZER J.F., ALBERTS G.L., BOCK J.H., SLIGHTOM J.L., IM W.B. Characterization of ligand binding properties of the 5-HT1D receptors cloned from chimpanzee, gorilla and rhesus monkey in comparison with those from the human and guinea pig receptors. Neurosci. Lett. 1997;235:117–120. doi: 10.1016/s0304-3940(97)00728-3. [DOI] [PubMed] [Google Scholar]
- PREGENZER J.F., IM W.B., CARTER D.B., THOMSEN D.R. Comparison of interaction of [3H]muscimol, t-butylbicyclophosphoro[35S]thionate, and [3H]flunitrazepam with cloned GABAA receptors of the α1β2 and α1β2γ2 subtypes. Mol. Pharamcol. 1993;43:801–806. [PubMed] [Google Scholar]
- PREMONT R.T., MATSUOKA I., MATTEI M.-G., POUILLE Y., DEFER N., HANOUNE J. Identification and characterization of a widely expressed form of adenylyl cyclase. J. Biol. Chem. 1996;271:13900–13907. doi: 10.1074/jbc.271.23.13900. [DOI] [PubMed] [Google Scholar]
- REEVE H.L., VAUGHAN P.F.T., PEERS C. Inhibition of N-type Ca2+ channel currents in human neuroblastoma (SH-SY5Y) cells by muscarine via stimulation of M3 receptors. Neuropharmacol. 1995;34:319–326. doi: 10.1016/0028-3908(94)00161-k. [DOI] [PubMed] [Google Scholar]
- ROBINSON S.W., CARON M.G. Selective inhibition of adenylyl cyclase type V by the dopamine D3 receptor. Mol. Pharmacol. 1997;52:508–514. doi: 10.1124/mol.52.3.508. [DOI] [PubMed] [Google Scholar]
- SCHANDAR M., LAUGWITZ K.-L., BOEKHOFF L., KRONER C., GUDERMAN T., SCHULTZ G., BREER H. Odorants selectively activate distinct G protein subtypes in olfactory cilia. J. Biol. Chem. 1998;273:16669–16677. doi: 10.1074/jbc.273.27.16669. [DOI] [PubMed] [Google Scholar]
- SEABROOK G.R., PATEL S., MARWOOD R., EMMS F., KNOWLES M.R., FREEDMAN S.B., MCALLISTER G. Stable expression of human D3 dopamine receptors in GH4C1 pituitary cells. FEBS Lett. 1992;312:123–126. doi: 10.1016/0014-5793(92)80918-7. [DOI] [PubMed] [Google Scholar]
- SEEMAN P., VAN TOL H.M. Dopamine receptor pharmacology. Trends in Pharmacol. Sci. 1994;15:264–270. doi: 10.1016/0165-6147(94)90323-9. [DOI] [PubMed] [Google Scholar]
- SOKOLOFF P., GIROS B., MARTRES M.-P., BOUTHENET M.L., SCHWARZ J.-C. Molecular cloning and characterization of a novel dopamine receptor (D3) as a target of neuroleptics. Nature. 1990;347:146–151. doi: 10.1038/347146a0. [DOI] [PubMed] [Google Scholar]
- SOKOLOFF P., SCHWARZ J.-C. Novel dopamine receptors half a decade later. Trends Pharmacol. Sci. 1995;16:270–275. doi: 10.1016/s0165-6147(00)89044-6. [DOI] [PubMed] [Google Scholar]
- TANG L., TODD R.D., HELLER A., O'MALLEY K.L. Pharmacological and functional characterization of D2, D3 and D4 dopamine receptors in fibroblast and dopaminergic cell lines. J. Pharmacol. Exp. Therap. 1994;270:475–479. [PubMed] [Google Scholar]
- TSUKAMOTO T., TOYAMA R., ITOH H., KOZASA T., MATSUOKA M., KAZIRO Y. Structure of the human gene and two rat cDNAs encoding the α chain of GTP-binding regulatory protein Go: Two different mRNAs are generated by alternative splicing. Proc. Natl. Acad. Sci., U.S.A. 1991;88:2974–2978. doi: 10.1073/pnas.88.8.2974. [DOI] [PMC free article] [PubMed] [Google Scholar]


