Abstract
The effect of reboxetine, a novel antidepressant drug that potently and selectively inhibits neuronal noradrenaline (NA) uptake, on brain extracellular monoamines was studied by microdialysis.
Fifteen mg kg−1 i.p. reboxetine raised extracellular NA in the frontal cortex (by 242%) and dorsal hippocampus (by 240%).
Idazoxan (1 mg kg−1 s.c.), given 60 min after 15 mg kg−1 reboxetine, markedly potentiated the effect on extracellular NA in the frontal cortex (by 1580%) and dorsal hippocampus (by 1360%), but had no effect by itself.
Twenty-four hours after the last injection of a chronic schedule (15 mg kg−1 i.p. once daily for 14 days) reboxetine had no effect on basal extracellular concentrations of NA in the dorsal hippocampus and a challenge dose of reboxetine (15 mg kg−1) raised extracellular NA similarly in rats treated chronically with reboxetine (by 353%) and saline (by 425%).
Ten and 20 μg kg−1 i.p. clonidine dose-dependently reduced hippocampal extracellular NA similarly in rats given chronic reboxetine (by 32% and 57%) and saline (by 42% and 56%).
Extracellular concentrations of dopamine and 5-HT in the striatum were similar in rats treated chronically with reboxetine and saline. A challenge dose of reboxetine (15 mg kg−1) had no effect on striatal extracellular dopamine and slightly increased striatal extracellular 5-HT to a similar extent in rats treated chronically with reboxetine (by 137%) and saline (by 142%).
The results suggest that combining reboxetine with an α2-adrenoceptor antagonist may facilitate its antidepressant activity. Repeated treatment confirmed that reboxetine is fairly selective for the noradrenergic system but provided no evidence of adaptive changes in that system that could facilitate its effect on extracellular NA.
Keywords: α2-Adrenoceptors, dopamine, dorsal hippocampus, frontal cortex, 5-HT, idazoxan, microdialysis, noradrenaline, reboxetine, striatum
Introduction
Most antidepressant drugs alleviate depression only after a few weeks, presumably because adaptive neuronal changes are necessary for their beneficial effects. Recent studies with selective 5-HT reuptake inhibitors (SSRIs) have shown that their effect on extracellular 5-HT in terminal regions is attenuated by their simultaneous ability to activate somatodendritic 5-HT1A receptors by increasing endogenous 5-HT in the raphe region (Adell & Artigas, 1991; Invernizzi et al., 1991; 1992; Hjorth & Auerbach, 1994; Arborelius et al., 1996). Somatodendritic 5-HT1A receptors are desensitized after chronic treatment with SSRIs and this facilitates their effects on extracellular 5-HT in terminal regions (Invernizzi et al., 1994; 1996; Rutter et al., 1994). The slow increase in serotonergic transmission in these regions may help explain why the beneficial effects of SSRIs take so long to appear.
Microdialysis studies with cocaine (Thomas et al., 1994) and desipramine (Mateo et al., 1998) suggested that cortical noradrenaline (NA) release was modulated by α2-adrenoceptors in the locus coeruleus. Electrophysiological studies suggested that presynaptic α2-adrenoceptors become subsensitive during long-term treatment with desipramine (McMillen et al., 1980; Lacroix et al., 1991), but there is no evidence that chronic treatment with NA uptake inhibitors facilitates their effect on extracellular NA in terminal regions in the brain by desensitizing inhibitory α2-autoreceptors.
Reboxetine is a novel antidepressant drug that potently and selectively inhibits NA uptake (Ki values in nM are eight for NA, 1070 for 5-HT and >10,000 for DA; Montgomery, 1999); unlike tricyclic antidepressant drugs, it has no appreciable action on muscarinic cholinergic and α1-adrenergic receptors (Riva et al., 1989). The main objectives of the present study were: (1) to prove that blockade of α2-adrenoceptors by idazoxan increases the effect of reboxetine on extracellular NA in some terminal regions of the rat brain; (2) to examine whether chronic treatment with reboxetine has more effect on extracellular concentrations of NA in a brain terminal region than a single injection, in analogy with the effects of long-term treatment with SSRIs on extracellular 5-HT; (3) to assess the sensitivity of α2-adrenoceptors regulating NA release by studying the ability of clonidine, an α2-adrenoceptor agonist, to reduce hippocampal extracellular NA in rats treated chronically with reboxetine and saline (4) to asses the selectivity of reboxetine's effects on the noradrenergic system after chronic treatment by examining how it affects extracellular dopamine (DA) and 5-HT in the striatum.
Methods
Animals
Male Sprague-Dawley rats (CD-COBS,Charles River, Italy) weighing between 170 and 350 g were used. They were housed at constant room temperature (22±2°C) and relative humidity (60±5%), with a regular 12/12 h light/dark cycle. Food and water were available ad libitum. Procedures involving animals and their care were conducted in conformity with the institutional guidelines that are in compliance with national (D.L. n. 116, G.U., suppl. 40, 18 Febbraio 1992, Circolare N° 8, G.U., 14 Luglio, 1994) and international laws and policies (EEC Council Directive 86/609, OJ L 358,1, Dec. 12, 1987; Guide for the Care and Use of Laboratory Animals, U.S. National Research Council, 1996). All efforts were made to minimize suffering and to limit the number of animals used in the experiments.
Treatments
Extracellular NA was measured in the dorsal hippocampus and frontal cortex of freely-moving rats given 15 mg kg−1 i.p. reboxetine 60 min before 1 mg kg−1 s.c. idazoxan. The effect of chronic treatment on extracellular NA in the dorsal hippocampus was studied in a group of rats given reboxetine (15 mg kg−1) or saline (2 ml kg−1) i.p. once daily for 14 days. A challenge dose of 15 mg kg−1 i.p. reboxetine or 10 and 20 μg kg−1 i.p. clonidine was given 24 h after the last injection of the chronic schedule.
Finally, the effects of reboxetine was studied on the extracellular concentrations of DA and 5-HT in the striatum of rats given reboxetine (15 mg kg−1) or saline (2 ml kg−1) i.p. once daily for 14 days. A challenge dose of 15 mg kg−1 i.p. reboxetine was given 24 h after the last injection.
The reboxetine dose was selected on the basis of a previous study showing an ID50 of 7.51 mg kg−1 for inhibiting NA uptake (Riva et al., 1989). Preliminary experiments were done to select the dose of idazoxan with little or no effect on extracellular NA to facilitate investigating the interaction with reboxetine.
Drugs
Reboxetine methansulphonate (Pharmacia & Upjohn, Milano, Italy) and clonidine hydrochloride (Boehringer Ingelheim, Italy) were dissolved in saline and injected i.p. Idazoxan hydrochloride (RBI, Natick, MA, U.S.A.) was dissolved in saline and injected s.c. Doses are referred to the salt.
Microdialysis procedures
The day before the microdialysis experiment rats were deeply anaesthetized with an injection of 3 ml kg−1 i.p. equithesin (1.2 g pentobarbital, 5.3 g chloral hydrate, 2.7 g MgSO4, 49.5 ml propylene glycol, 12.5 ml ethanol and 58 ml distilled water). They were placed on a stereotaxic apparatus (David Kopf Instruments, Tujunga, CA, U.S.A.) and a vertical dialysis probe made of Cuprophan (Sorin Biomedica, Italy; 200 μm inner diameter with 3000 Da molecular weight cutoff) essentially as described by Robinson & Wishaw, (1988) was implanted in the dorsal hippocampus (2 mm long) and frontal cortex (4 mm long) at the following stereotaxic coordinates (from bregma and skull surface): dorsal hippocampus, AP=−3.3; V=−4.2; L=±1.9; frontal cortex, AP=±4.2; V=−5.5; L=±0.7 (Paxinos & Watson, 1982). In rats given chronic reboxetine a horizontal probe made of Cuprophan was used to increase the sensitivity of NA assay in experiments in which a marked reduction of extracellular NA was expected. In fact, hippocampal basal levels of NA (fmol/40 μl) are approximately 23 and 3 respectively using the horizontal and vertical probes. The probes were positioned at the following stereotaxic coordinates: dorsal hippocampi AP=5.7, V=6.7 and striata AP=9.7, V=5.6 from the interaural line according to the atlas of Paxinos & Watson (1982). A single horizontal dialysis probe was implanted in each rat whereas two vertical probes were implated in the frontal cortex and dorsal hippocampus of the same rat. The horizontal dialysis probe had a 7-mm long glue-free zone for the dorsal hippocampi, and two 4-mm zones with a 2-mm glued area in between for the two striata. Bilateral marks were made on the probes to help position them.
The rat's skull was exposed and two holes were made on the lateral surfaces at the level of the target area. Dialysis fibres, held straight by tungsten wires inside, were inserted transversely into the brain so that the glue-free zones were exactly in the target areas. The tungsten wires were removed and stainless steel cannulas (22 gauge, about 12 mm long) were glued to the ends of the probe. To prevent clogging, a thin fishing line (0.08 mm outer diameter) was inserted into the lumen immediately before the probe was cemented to the skull with dental cement. Finally, the skin was sutured and the rats were allowed to recover from surgery overnight, one per cage, with food and water ad libitum.
The day after the surgical procedure the dialysis probes were perfused with aCSF containing (mM): NaCl 145, CaCl2 1.26, KCl 3, MgCl2 1 at pH 7.4 with sodium phosphate buffer at a constant flow rate of 2 μl min−1, using a microperfusion pump (CMA 100, CMA Microdialysis, Sweden). Samples were collected at 20-min intervals and directly injected into the high-performance liquid chromatography (HPLC) system with electrochemical detector for the assay. Rats were treated with drugs when a stable basal concentration of monoamines was obtained (usually 3–4 h from the beginning of the experiment).
At the end of the experiments rats were deeply anaesthetized with chloral hydrate and killed by decapitation. The brains were immediately removed, frozen and sliced in 40-μm sections for examination of the probe track. Only rats with correct probe placement were considered in the results.
Analytical procedure
Extracellular concentrations of NA were measured using a high-performance liquid chromatograph (Coulochem 5100 A, ESA, Bedford, MA, U.S.A.) equipped with an analytical cell consisting of two in-series electrodes (mod. 5011). The first electrode was set at −0.2 V and the second at +0.25 V. NA was read as the second electrode output signal. Separation was obtained using a reverse-phase column (Hypersil-ODS 5 μm, 125×3.1 mm, Bischoff). The mobile phase, consisting of (mM): citric acid 25, sodium acetate 24, sodium octyl sulphate 1.55, Na2EDTA 0.1 and 80 ml l−1 CH3OH, was pumped at a constant flow rate of 1 ml min−1 with a Constametric III pump (LDC/Milton Roy, Riviera Beach, FL, U.S.A.).
DA was separated through a 150×4.6 mm column (Supelcosil LC18-DB, 5 μm, Supelco, Bellefonte, PA, U.S.A.) using a mobile phase containing 0.1 M sodium acetate, 0.34 mM sodium octyl sulphate, 0.1 mM Na2EDTA, 60 ml l−1 CH3OH, pH 4.2 with acetic acid. A constant flow rate of 1 ml min−1 was maintained by a Constametric 3200 pump (LDC/Milton Roy, Riviera Beach, FL, U.S.A.). DA was measured by a Coulochem II electrochemical detector equipped with a 5011 analytical cell. The first electrode was set at +350 mV and the second at −190 mV. DA was read as the second electrode output signal. 5-HT was measured as described by Invernizzi et al. (1992).
Reagents were of analytical grade and were purchased from Merck (Bracco, Milan, Italy) or Sigma-Aldrich (Milan, Italy).
Data calculation and statistics
Basal values of extracellular neurotransmitters were defined as the means of three consecutive stable samples before drug treatment and were expressed as fmol 40 μl−1 (not corrected for recovery).
The overall effects of reboxetine, idazoxan and their combination on extracellular NA were estimated from the area under the curve (AUC) calculated for each rat by subtracting the basal extracellular NA values from each concentration after drug administration over the interval 0–180 min. The resulting data were compared by two-way ANOVA (factorial design) followed by Tukey's test for post-hoc comparison of the single means. Basal values of rats treated chronically with saline or drugs were compared by Student's t-test.
The effects of a challenge i.p. dose of reboxetine or clonidine in rats chronically treated with saline or reboxetine was compared by analysis of variance for repeated measures (split-plot), with treatment (tr) as between-subject factor and time (t) as within-subject factor. When significant effects were found, post-hoc comparisons were made by Tukey's test.
Results
Effect of idazoxan on reboxetine-induced rise of extracellular NA in the dorsal hippocampus and frontal cortex
Reboxetine (15 mg kg−1) cause a similar increase in extracellular NA in the dorsal hippocampus and frontal cortex by 240% (Figure 1) and 242% (Figure 2) respectively. The effect persisted until the end of the observation period (3 h after injection). The overall effect, estimated from the AUC, was significant in both brain regions (P<0.05, Tukey's test). Idazoxan (1 mg kg−1) by itself had no significant effect in either region, but significantly potentiated the increase of extracellular NA induced by reboxetine in the dorsal hippocampus (Fint(1,22)=19.2, P<0.001, two-way ANOVA) and frontal cortex (Fint(1,16)=9.0, P<0.01, two-way ANOVA). No significant difference was found between the effects of the idazoxan-reboxetine combination in the dorsal hippocampus and frontal cortex (Fint(1,24)=1.6, P>0.05; two-way ANOVA). In rats given reboxetine+idazoxan, extracellular NA was maximally increased at 20 min after idazoxan by 1360% and 1580% respectively in the dorsal hippocampus and frontal cortex. The effect of idazoxan was relatively short-lasting since no differences in extracellular NA were observed in rats given reboxetine+saline and reboxetine+idazoxan 100 min after injection of the α2-adrenoceptor antagonist.
Figure 1.
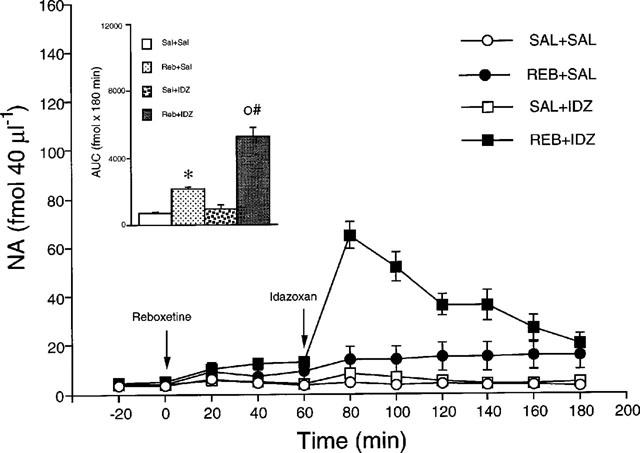
Extracellular concentrations of NA in the dorsal hippocampus of rats given saline (Sal+Sal), 15 mg kg−1 reboxetine (Reb+Sal), saline+1 mg kg−1 idazoxan (Sal+IDZ) and reboxetine+idazoxan (Reb+IDZ). Inset: AUC calculated from 0–180 min. Each group is the mean±s.e.mean of 6–7 rats. *P<0.05 vs Sal+Sal; °P<0.05 vs Reb+Sal; #Fint. P<0.05 (two-way ANOVA). The arrows indicate drug injection.
Figure 2.
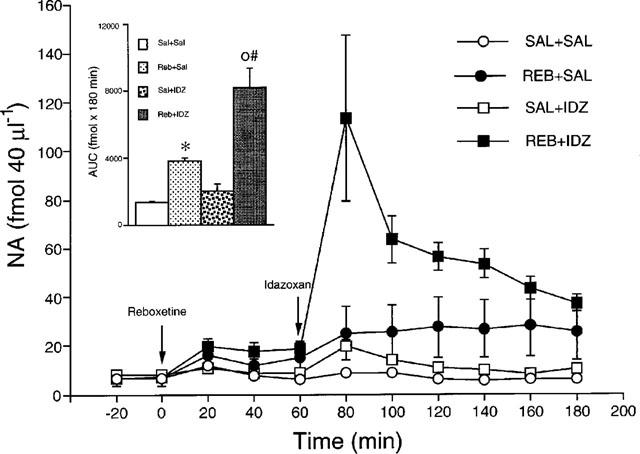
Extracellular concentrations of NA in the frontal cortex of rats given saline (Sal+Sal), 15 mg kg−1 reboxetine (Reb+Sal), saline+1 mg kg−1 idazoxan (Sal+IDZ) and reboxetine+idazoxan (Reb+IDZ). Inset: AUC calculated from 0–180 min. Each group is the mean±s.e.mean of 6–7 rats. *P<0.05 vs Sal+Sal; °P<0.05 vs Reb+Sal; #Fint. P<0.05 (two-way ANOVA). The arrows indicate drug injection.
Effect of reboxetine on extracellular concentrations of NA in the dorsal hippocampus of rats chronically treated with saline or reboxetine
As shown in Figure 3, 24 h after the last injection of a 14-day schedule with saline or 15 mg kg−1 reboxetine once daily the basal extracellular concentrations of NA in the dorsal hippocampus were no different in the two groups. A challenge dose of 15 mg kg−1 reboxetine significantly raised extracellular NA in rats chronically treated with saline. The effect was significant throughout the experiment (180 min). Reboxetine raised extracellular NA similarly in the dorsal hippocampus of animals treated chronically with the drug (Ftr(1,10)=0.8, P>0.05; Ft(9,90)=14.7, P<0.01; Ftrxt(9,90)=0.4, P>0.05).
Figure 3.
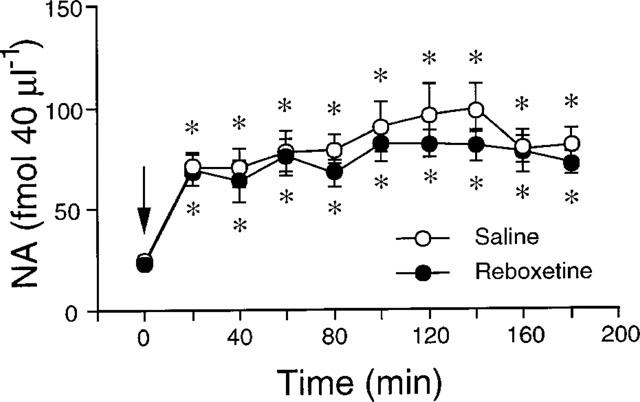
Effect of 15 mg kg−1 i.p. reboxetine on extracellular NA in the dorsal hippocampus of rats given saline or 15 mg kg−1 reboxetine once daily for 14 days. Each group is the mean±s.e.mean of 6–7 rats. *P<0.05 vs respective basal values (time 0). The arrow indicates drug injection.
Effect of clonidine on extracellular concentrations of NA in the dorsal hippocampus of rats treated chronically with saline or reboxetine
Administered 24 h after the last injection of the chronic schedule with reboxetine, 10 μg kg−1 clonidine significantly reduced by about 40% extracellular NA in the dorsal hippocampus of rats given saline or reboxetine for 2 weeks (Figure 4a). No significant difference in the effects of clonidine was found between the two experimental groups (Ftr(1,10)=0.0, P>0.05; Ft(6,60)=6.5, P<0.01; Ftr x t(6,60)=1.02, P>0.05). A higher dose of clonidine (20 μg kg−1) reduced by about 60% extracellular NA in rats treated chronically with saline or reboxetine (Figure 4b). The effect of clonidine in the two experimental groups was not significantly different (Ftr(1,8)=0.2, P>0.05; Ft(6,48)=8.8, P<0.01; Ftrxt(6,48)= 0.6, P>0.05).
Figure 4.
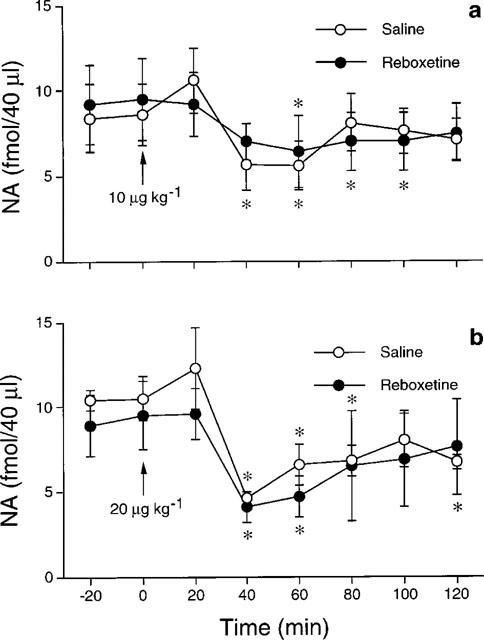
Effect of 10 (a) and 20 (b) μg kg−1 i.p. clonidine on extracellular NA in the dorsal hippocampus of rats given saline or 15 mg kg−1 reboxetine once daily for 14 days. Each group is the mean±s.e.mean of 5–6 rats. *P<.05 vs respective basal values (time 0). The arrow indicates drug injection.
Effect of reboxetine on extracellular concentrations of DA and 5-HT in the striatum of rats chronically treated with saline or reboxetine
Twenty-four hours after the last of the chronic injections with saline or 15 mg kg−1 reboxetine, basal extracellular concentrations of DA (Figure 5a) or 5-HT (Figure 5b) were not changed in the striatum in either group. The challenge dose of 15 mg kg−1 reboxetine did not affect extracellular DA (Ftr(1,11)=0.0, P>0.05; Ft(9,99)=1.8, P>0.05; Ftrxt(9,99)=0.1, P>0.05). The challenge dose raised extracellular 5-HT in the striatum of rats chronically treated with saline or reboxetine, but with no significant differences between the two treatments (Ftr(1,14)=0.1, P>0.05; Ft(9,126)=7.1 P<0.01; Ftrxt(9,126)=0.8, P>0.05). The effect was significant respectively 20 and 40 min after injection (P<0.05, Tukey's test) in rats given chronic reboxetine and saline.
Figure 5.

Effect of 15 mg kg−1 i.p. reboxetine on extracellular DA (a) and 5-HT (b) in the striatum of rats given saline or 15 mg kg−1 reboxetine once daily for 14 days. Each group is the mean±s.e.mean of 6–8 rats. *P<0.05 vs respective basal values (time 0). The arrow indicates drug injection.
Discussion
It has been proposed that the therapeutic activity of SSRIs or selective inhibitors of NA reuptake may be improved by combining them with presynaptic receptor antagonists, to enhance the effects on the extracellular concentrations of their respective neurotransmitters in the brain (Artigas et al., 1996; Mateo et al., 1998). Blockade of 5-HT1A receptors does not enhance the effects of SSRIs on extracellular 5-HT to the same extent in all brain regions, the dorsal hippocampus being particularly insensitive (Invernizzi et al., 1997; Romero & Artigas, 1997). In view of the proposed importance of the dorsal hippocampus for antidepressant activity (de Montigny et al., 1993), we studied whether idazoxan modified the effect of reboxetine on extracellular NA in the dorsal hippocampus in comparison with the effect in the frontal cortex. Microdialysis studies using local drug application have found that α2-adrenoceptor antagonists and desipramine caused greater increases in extracellular NA in hippocampus than in frontal cortex (Thomas & Holman, 1991; van Veldhuizen et al., 1994). The dose of idazoxan used in the present study (1 mg kg−1) did not significantly modify extracellular NA concentrations in hippocampus and frontal cortex whereas reboxetine caused a similar increase of extracellular NA in both regions. The present results with reboxetine are therefore at variance with those on desipramine showing a preferential effect on hippocampal NA release (Thomas & Holman, 1991). Idazoxan enhanced the effect of reboxetine on extracellular NA to a similar extent in the frontal cortex and dorsal hippocampus. It seems therefore that blockade of α2-adrenoceptors increases the effect of a selective NA uptake inhibitor on brain extracellular NA more uniformly than previously obtained by combining presynaptic receptor antagonists with SSRIs.
The regional selectivity of SSRIs on extracellular 5-HT may be due to different densities of presynaptic 5-HT1A receptors (Weissman-Nanoupolos et al., 1985) in the two serotonergic cell groups, the dorsal and median nuclei, that innervate the frontal cortex and dorsal hippocampus respectively (Invernizzi et al., 1997). Noradrenergic innnervation of the dorsal hippocampus and frontal cortex originates in the same cell group, the locus coeruleus (Lindvall & Bjorklund, 1974), and this may explain why reboxetine alone or in combination with idazoxan have similar effects on extracellular NA in both regions.
α2-Adrenoceptors in the noradrenergic terminals also exert an inhibitory control on NA release (Maura et al., 1992); thus it is not clear to what extent these receptors in the locus coeruleus or in nerve terminals contributed to the effect of systemically administered idazoxan on the reboxetine-induced increase of extracellular NA in the two terminal regions. That somatodendritic α2-adrenoreceptors modulate the effect of selective NA uptake inhibitors on extracellular concentrations of NA in terminal regions is strongly suggested by a recent report that an α2-adrenoceptor antagonist injected into the locus coeruleus markedly enhanced the effect of desipramine on dialysate NA in the cingulate cortex (Mateo et al., 1998).
Behavioural studies with clonidine have suggested that presynaptic α2-adrenoceptor function is attenuated by repeated treatment with various antidepressant drugs which increase synaptic levels of NA (Heal et al., 1991). There is also some electrophysiological evidence that presynaptic α2-adrenoceptors are desensitized after long-term treatment with desipramine (McMillen et al., 1980; Lacroix et al., 1991). We did in fact recently find that 10 mg kg−1 desipramine i.p. increased extracellular NA more in the dorsal hippocampus of rats after once daily doses of 10 mg kg−1 desipramine for 14 days (manuscript in preparation). To examine whether the same happened with reboxetine, we gave rats 15 mg kg−1 reboxetine once daily for 14 days, with a challenge dose 24 h after the last dose. The challenge dose raised extracellular NA similarly in rats treated chronically with reboxetine and vehicle. Moreover, 10 and 20 μg kg−1 clonidine reduced hippocampal extracellular concentrantions of NA similarly in rats treated chronically with reboxetine and saline. It seems therefore that these treatment conditions did not induce significant adaptive changes in noradrenergic transmission that could be reflected by changes in the drug's effect on extracellular NA after chronic treatment, probably due to the relatively rapid kinetics in rats (Strolin Benedetti et al., 1995). Adaptive changes may well occur during chronic treatment in depressed patients since reboxetine has a considerably longer plasma half-life in man than rats (Edwards et al., 1995). In view of this, experiments are in progress using osmotic minipumps to deliver continuous, adequate levels of reboxetine to rats.
In a final experiment, we studied the selective effect of reboxetine on the noradrenergic system by measuring extracellular concentrations of 5-HT and DA in the striatum under the same treatment conditions used for NA. Repeated doses of reboxetine did not affect the concentrations of 5-HT and DA in the rat striatum. A challenge dose of reboxetine had no effect on striatal DA but caused a slight, short-lasting increase of 5-HT at early times in vehicle and drug treated animals. An effect on 5-HT uptake mechanism is unlikely to have been involved since the selectivity ratio for inhibiting 5-HT and NA uptake is about 130 (Montgomery, 1999). We can also exclude that an artifact of the injection was involved since an i.p. injection of saline did not significantly modify striatal extracellular 5-HT (unpublished results). Perhaps, an increased noradrenergic transmission contributed to the effects on 5-HT, but further studies are needed to clarify whether reboxetine may cause significant and stable changes in extracellular 5-HT in certain brain regions.
In conclusion, the present study has shown that blockade of α2-adrenoceptors by idazoxan significantly increased the effect of reboxetine on extracellular NA in the frontal cortex and dorsal hippocampus of rats. Together with previous studies with desipramine (Mateo et al., 1998), these findings suggest that using reboxetine in combination with an α2-adrenoceptor antagonist may facilitate its antidepressant activity.
In our experimental conditions we obtained no evidence of adaptive changes in the noradrenergic system that could facilitate the effect of reboxetine on extracellular NA after long-term treatment. Experiments are in progress to investigate this further. Finally, for the first time, to our knowledge, it has been shown that reboxetine is fairly selective for the noradrenergic system in awake rats, at least judging from extracellular 5-HT and DA levels in the striatum.
Acknowledgments
This work was partly supported by a grant from Pharmacia & Upjohn, Milano, Italy.
Abbreviations
- DA
dopamine
- HPLC
high-performance liquid chromatography
- 5-HT
5-hydroxytryptamine
- NA
noradrenaline
- SSRIs
selective serotonin reuptake inhibitors
References
- ADELL A., ARTIGAS F. Differential effects of clomipramine given locally or systemically on extracellular 5-hydroxytryptamine in raphe nuclei and frontal cortex. N. S. Arch. Pharmacol. 1991;343:237–244. doi: 10.1007/BF00251121. [DOI] [PubMed] [Google Scholar]
- ARBORELIUS L., NOMIKOS G.G., HERTEL P., SALMI P., GRILLNER P., BACKLUND HK, B., HACKSELL U., SVENSSON T.H. The 5-HT1A receptor antagonist (S)-UH-301 augments the increase in the extracellular concentrations of 5-HT in the frontal cortex produced by both acute and chronic treatment with citalopram. N. S. Arch. Pharmacol. 1996;353:630–640. doi: 10.1007/BF00167182. [DOI] [PubMed] [Google Scholar]
- ARTIGAS F., ROMERO L., DE MONTIGNY C., BLIER P. Acceleration of the effect of selected antidepressant drugs in major depression by 5-HT1A antagonists. Trends Neurosci. 1996;19:378–383. doi: 10.1016/S0166-2236(96)10037-0. [DOI] [PubMed] [Google Scholar]
- DE MONTIGNY C., CHAPUT Y., BLIER P.Classical and novel targets for antidepressant drugs New Pharmacological Approaches to the Therapy of Depressive Disorders 1993Karger, Basel; 8–17.In: J. Mendlewicz, N. Brunello, S.Z. Langer and G. Racagni, eds [Google Scholar]
- EDWARDS D.M.F., PELLIZZONI C., BREUEL H.P., BERARDI A., CASTELLI M.G., FRIGERIO E., POGGESI I., ROCCHETTI M., DUBINI A., STROLIN BENEDETTI M. Pharmacokinetics of reboxetine in healthy volunteers. Single oral doses, linearity and plasma protein binding. Biopharm. Drug Disp. 1995;16:443–460. doi: 10.1002/bdd.2510160603. [DOI] [PubMed] [Google Scholar]
- HEAL D.J., PROW M.R., BUCKETT W.R. Effects of antidepressant drugs and electroconvulsive shock on pre- and postsynaptic α2-adrenoceptor function in the brain: rapid down-regulation by sibutramine hydrochloride. Psychopharmacology. 1991;103:251–257. doi: 10.1007/BF02244212. [DOI] [PubMed] [Google Scholar]
- HJORTH S., AUERBACH S.B. Further evidence for the importance of 5-HT1A autoreceptors in the action of selective 5-HT reuptake inhibitors. Eur. J. Pharmacol. 1994;260:251–255. doi: 10.1016/0014-2999(94)90346-8. [DOI] [PubMed] [Google Scholar]
- INVERNIZZI R., BELLI S., SAMANIN R.An increase of extracellular 5-HT in the dorsal raphe masks the effect of sertraline in the frontal cortex Monitoring Molecules in Neuroscience 1991University Centre for Pharmacy: Groningen; 253–255.In: H. Rollema, B.H.C. Westerink and W.J. Drijfhout, eds [Google Scholar]
- INVERNIZZI R., BELLI S., SAMANIN R. Citalopram's ability to increase the extracellular concentrations of 5-HT in the dorsal raphe prevents the drug's effect in the frontal cortex. Brain Res. 1992;584:322–324. doi: 10.1016/0006-8993(92)90914-u. [DOI] [PubMed] [Google Scholar]
- INVERNIZZI R., BRAMANTE M., SAMANIN R. Chronic treatment with citalopram facilitates the effect of a challenge dose on cortical 5-HT output: role of presynaptic 5-HT1A receptors. Eur. J. Pharmacol. 1994;260:243–246. doi: 10.1016/0014-2999(94)90344-1. [DOI] [PubMed] [Google Scholar]
- INVERNIZZI R., BRAMANTE M., SAMANIN R. Role of 5-HT1A receptors in the effects of acute and chronic fluoxetine on extracellular 5-HT in the frontal cortex. Pharmacol. Biochem. Behav. 1996;54:143–147. doi: 10.1016/0091-3057(95)02159-0. [DOI] [PubMed] [Google Scholar]
- INVERNIZZI R., VELASCO C., BRAMANTE M., LONGO A., SAMANIN R. Effect of 5-HT1A receptor antagonists on citalopram-induced increase in extracellular 5-HT in the frontal cortex, striatum and dorsal hippocampus. Neuropharmacology. 1997;36:467–473. doi: 10.1016/s0028-3908(97)00060-9. [DOI] [PubMed] [Google Scholar]
- LACROIX D., BLIER P., CURET O., DE MONTIGNY C. Effects of long-term desipramine administration on noradrenergic neurotransmission: electrophysiological studies in the rat brain. J. Pharmacol. Exp. Ther. 1991;257:1081–1090. [PubMed] [Google Scholar]
- LINDVALL O., BJORKLUND A. The organization of the ascending catecholamine neuron system in the rat brain as revealed by the glyoxylic acid fluorescence method. Acta Physiol. Scand. 1974;412 Suppl. I [PubMed] [Google Scholar]
- MATEO Y., PINEDA J., MEANA J. J. Somatodendritic α2-adrenoceptors in the locus coeruleus are involved in the in vivo modulation of cortical noradrenaline release by the antidepressant desipramine. J. Neurochem. 1998;71:790–798. doi: 10.1046/j.1471-4159.1998.71020790.x. [DOI] [PubMed] [Google Scholar]
- MAURA G., BONANNO G., RAITERI M. Presynaptic α2-adrenoceptors mediating inhibition of noradrenaline and 5-hydroxytryptamine release in rat cerebral cortex: further characterization as different α2-adrenoceptors subtypes. N. S. Arch. Pharmacol. 1992;345:410–416. doi: 10.1007/BF00176618. [DOI] [PubMed] [Google Scholar]
- MCMILLEN B.A., WARNACK W., GERMAN D.C., SHORE P.A. Effects of chronic desipramine treatment on rat brain noradrenergic responses to α-adrenergic drugs. Eur. J. Pharmacol. 1980;61:239–246. doi: 10.1016/0014-2999(80)90126-0. [DOI] [PubMed] [Google Scholar]
- MONTGOMERY S.A. Predicting response: noradrenaline reuptake inhibition. Int. Clin. Psychopharmacol. 1999;14 suppl 1:S21–S26. doi: 10.1097/00004850-199905001-00005. [DOI] [PubMed] [Google Scholar]
- PAXINOS G., WATSON C. Academic Press: Sydney; 1982. The rat brain in stereotaxic coordinates. [DOI] [PubMed] [Google Scholar]
- RIVA M., BRUNELLO N., ROVESCALLI A.C., GALIMBERTI R., CARFAGNA N., CARMINATI P., POZZI O., RICCIARDI S., RONCUCCI R., ROSSI A., RACAGNI G. Effect of reboxetine, a new antidepressant drug, on the central noradrenergic system: behavioural and biochemical studies. Journal Drug Development. 1989;1:243–253. [Google Scholar]
- ROBINSON T.E., WHISHAW I.Q. Normalization of extracellular dopamine in striatum following recovery from a partial unilateral 6-OHDA lesion of the substantia nigra: a microdialysis study in freely moving rats. Brain Res. 1988;450:209–224. doi: 10.1016/0006-8993(88)91560-0. [DOI] [PubMed] [Google Scholar]
- ROMERO L., ARTIGAS F. Preferential potentiation of the effect of 5-HT uptake inhibitors by 5-HT1A receptor antagonists in the dorsal raphe pathway: role of somatodendritic autoreceptors. J. Neurochem. 1997;68:2593–2603. doi: 10.1046/j.1471-4159.1997.68062593.x. [DOI] [PubMed] [Google Scholar]
- RUTTER J.J., GUNDLAH C., AUERBACH S.B. Increase in extracellular 5-HT produced by uptake inhibitors is enhanced after chronic treatment with fluoxetine. Neuroscience Letters. 1994;171:183–186. doi: 10.1016/0304-3940(94)90635-1. [DOI] [PubMed] [Google Scholar]
- STROLIN BENEDETTI M., FRIGERIO E., TOCCHETTI P., BRIANCESCHI G., CASTELLI M.G., PELLIZZONI C., DOSTERT P. Stereoselective and species-dependent kinetics of reboxetine in mouse and rat. Chirality. 1995;7:285–289. doi: 10.1002/chir.530070416. [DOI] [PubMed] [Google Scholar]
- THOMAS D.N., HOLMAN R.B. Regulation of endogenous noradrenaline release in rat hippocampus and frontal cortex: in vivo microdialysis. Br. J. Pharmacol. 1991;102:5P. [Google Scholar]
- THOMAS D.N., POST R.M., PERT A. Focal and systemic cocaine differentially affect extracellular norepinephrine in the locus coeruleus, frontal cortex and hippocampus of the anaesthetized rat. Brain Res. 1994;645:135–142. doi: 10.1016/0006-8993(94)91646-2. [DOI] [PubMed] [Google Scholar]
- VAN VELDHUIZEN M.J.A., FEENSTRA M.G.P., BOER G.J. Regional differences in the in vivo regulation of the extracellular levels of noradrenaline and its metabolites in rat brain. Brain Res. 1994;635:238–248. doi: 10.1016/0006-8993(94)91445-1. [DOI] [PubMed] [Google Scholar]
- WEISSMAN-NANOUPOLOS D., MACH E., MAGRE J., DEMASSEY Y., PUJOL J.-F. Evidence for the localization of 5-HT1A binding sites on 5-HT containing neurons in the raphe dorsalis and raphe centralis nuclei of the rat brain. Neurochem. Int. 1985;7:1061–1072. doi: 10.1016/0197-0186(85)90156-1. [DOI] [PubMed] [Google Scholar]


