Abstract
Poly (ADP-ribose) synthetase (PARS) is a nuclear enzyme activated by strand breaks in DNA, which are caused by reactive oxygen species (ROS). Here we investigate the effects of the PARS inhibitors 3-aminobenzamide (3-AB), nicotinamide and 1,5-dihydroxyisoquinoline (ISO) on the circulatory failure and the organ injury/dysfunction caused by haemorrhage and resuscitation in the anaesthetized rat.
Haemorrhage (sufficient to lower mean arterial blood pressure to 50 mmHg for 90 min) and subsequent resuscitation with shed blood resulted (within 4 h after resuscitation) in a delayed fall in blood pressure to 66±4 mmHg (control, n=13). This circulatory failure was not affected by administration (5 min prior to resuscitation) of 3-AB (10 mg kg−1 i.v., n=7), nicotinamide (10 mg kg−1 i.v., n=6) or ISO (3 mg kg−1 i.v., n=6).
Haemorrhage and resuscitation also resulted in rises in the serum levels of urea and creatinine. This renal dysfunction was attenuated by 3-AB and nicotinamide, but not by nicotinic acid (n=7), an inactive analogue of nicotinamide. Although ISO (n=6) also attenuated the renal dysfunction caused by haemorrhage and resuscitation, its vehicle (10% DMSO, n=4) had the same effect.
Haemorrhagic shock resulted in enhanced serum levels of aspartate aminotransferase (AST), alanine aminotransferase (ALT) and lipase, indicating the development of hepatocellular and pancreatic injury, respectively. Similarly, haemorrhagic shock also resulted in an increase in the serum levels of creatine kinase (CK) indicating the development of neuromuscular injury. This was attenuated by 3-AB and nicotinamide, but not by nicotinic acid. Although ISO also attenuated the liver, pancreatic and neuromuscular injury caused by haemorrhagic shock, its vehicle had the same effect.
Thus, activation of PARS contributes to the organ injury and dysfunction caused by haemorrhage and resuscitation in the rat.
Keywords: Haemorrhagic shock, oxygen radicals, poly (ADP-ribose) polymerase, multiple organ failure, trauma
Introduction
Poly (adenosine 5′-diphosphate ribose) synthetase (PARS, E.C. 2.4.2.30) is a chromatin-bound enzyme which is abundantly present in the nuclei of numerous cell types (Ikai & Ueda, 1983). Activation of PARS is triggered by single strand breaks in DNA and subsequently catalyzes the transfer of ADP-ribose moeities from NAD to various nuclear proteins including histones and PARS (automodification domain) itself (Ueda & Hayaishi, 1985; Lautier et al., 1993). This reaction leads to the generation of nicotinamide, which is an inhibitor (negative feedback) of PARS activity. Continuous or excessive activation of PARS produces extended chains of ADP-ribose on nuclear proteins and results in a substantial depletion of intracellular NAD. As NAD functions as an electron carrier in the mitochondrial respiratory chain, NAD depletion rapidly leads to a fall in intracellular ATP levels. Moreover, nicotinamide can be recycled to NAD in a reaction that consumes ATP. Thus, activation of PARS leads to a fall in ATP (by two different mechanisms) which can ultimately cause cell death (Berger, 1985; Schraufstatter et al., 1986a, 1986b; Hyslop et al., 1988; Thies & Autor, 1991). Radicals including superoxide anions, hydrogen peroxide or hydroxyl radicals cause strand breaks in DNA, activation of PARS and depletion of NAD and ATP in cultured cells (Schraufstatter et al., 1986a, 1986b; Hyslop et al., 1988; Thies & Autor, 1991). Although nitric oxide (NO) has been shown to generate strand breaks in DNA (Zhang et al., 1994; Heller et al., 1995), this effect appears to be mediated by peroxynitrite rather than NO itself (Szabo et al., 1996). Inhibitors of PARS activity attenuate the fall in NAD and ATP and improve survival of cultured cells (e.g. fibroblasts, endothelial cells, smooth muscle cells, human cardiomyoblasts; human proximal tubule cells) exposed to oxygen-derived free radicals (Schraufstatter et al., 1986a, 1986b; Hyslop et al., 1988; Thies & Autor, 1991; Aalto & Raivo, 1993; Bowes et al., 1998; Chatterjee et al., 1999) or NO/peroxynitrite (Zhang et al., 1994; Heller et al., 1995; Szabo et al., 1996).
There is good evidence that haemorrhagic shock is associated with the generation of reactive oxygen species (ROS) including superoxide anions and hydrogen peroxide (see McCord, 1985; Redl et al., 1993). An enhanced formation of peroxynitrite may contribute to the circulatory dysfunction in rats with haemorrhagic shock (Szabo et al., 1995). The overproduction of ROS in haemorrhagic shock leads to a considerable oxidant stress as indicated by lipid peroxidation (Fleckenstein et al., 1991; Hamano et al., 1993) as well as the consumption of the endogenous antioxidant, vitamin E (Fleckenstein et al., 1991). Interventions which reduce the generation or the effects of ROS exert beneficial effects in a variety of models of haemorrhagic shock (Allan et al., 1986; Sanan et al., 1989; Mannion et al., 1994; Daughters et al., 1996; Simon et al., 1996; Fan et al., 1998; Mota-Filipe et al., 1999).
It has been suggested that the activation of PARS plays a role in the cardiovascular failure associated with haemorrhagic shock (Szabo et al., 1998; Szabo, 1998), but there is little information regarding the role of PARS in the pathophysiology of the organ dysfunction/injury associated with haemorrhagic shock. Here we investigate the effects of the PARS inhibitors 3-aminobenzamide and nicotinamide on the circulatory failure and the organ injury and dysfunction (renal dysfunction, liver injury and dysfunction, pancreatic injury) caused by severe haemorrhage and resuscitation in the anaesthetized rat.
Methods
Surgical procedure
This study was carried out on 74 male Wistar rats (Tuck, Rayleigh, Essex, U.K.) weighing 250–320 g receiving a standard diet and water ad libitum. All animals were anaesthetized with thiopentone sodium (120 mg kg−1 i.p.) and anaesthesia was maintained by supplementary injections of thiopentone sodium as required. The trachea was cannulated to facilitate respiration and rectal temperature was maintained at 37°C with a homeothermic blanket. The right femoral artery was catheterized and connected to a pressure transducer (Senso-Nor 840, Senso-Nor, Horten, Norway) for the measurement of phasic and mean arterial blood pressure (MAP) and heart rate (HR) which were displayed on a data acquisition system (MacLab 8e, ADI Instruments, Hastings, U.K.) installed on an Apple Macintosh computer. The right carotid artery was cannulated to bleed the animals (see below). The jugular vein was cannulated for the administration of drugs. The bladder was also cannulated to facilitate urine flow and to prevent the possibility of the development of post-renal failure. Upon completion of the surgical procedure, cardiovascular parameters were allowed to stabilize for 15 min. Then, blood was withdrawn from the catheter placed in the carotid artery in order to achieve a fall in MAP to 50 mmHg within 10 min. Thereafter, MAP was maintained at 50 mmHg for a total period of 90 min by either withdrawal (during the compensation period) or re-injection of blood. At 90 min after initiation of haemorrhage, the shed blood was re-injected into the animal along with an equivalent volume of Ringers lactate solution.
Evaluation of the effects of various PARS inhibitors on the delayed circulatory failure and MODS: experimental design
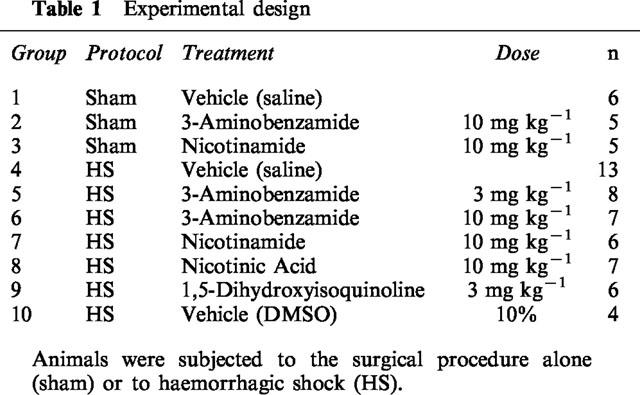
In the first study aimed at elucidating the effects of inhibitors of PARS activity in haemorrhagic shock, all animals were randomized into nine groups (see Table 1). Different groups of animals were subjected to 90 min of haemorrhage followed by resuscitation with shed blood and an equivalent volume of Ringer's Lactate solution for 4 h and treated with either saline (vehicle for 3-AB and nicotinamide), 10% DMSO (vehicle for ISO), 3-AB, nicotinamide, ISO (chemically distinct inhibitors of PARS activity) or nicotinic acid (negative control for nicotinamide). In addition, we have evaluated the effects of saline (vehicle), 3-AB and nicotinamide in rats subjected to the same surgical procedure, but which were not subjected to haemorrhagic shock (sham-operated rats) (see Table 1).
Table 1.
Experimental design
Quantification of organ function and injury
Four hours after resuscitation (end of the experiment), 1.5 ml of blood was collected into a serum gel S/1.3 tube (Sarstedt, Germany) from the catheter placed in the right carotid artery. The blood sample was centrifuged (1610×g for 3 min at room temperature) to separate plasma. All plasma samples were analysed within 24 h by a contract laboratory for veterinary clinical chemistry (Vetlab Services, Sussex, U.K.). The following marker enzymes were measured in the plasma as biochemical indicators of multiple organ injury/dysfunction: (1) Liver injury was assessed by measuring the rise in plasma levels of alanine aminotransferase (ALT, a specific marker for hepatic parenchymal injury) and aspartate aminotransferase (AST, a non-specific marker for hepatic injury) (Baue, 1993). (2) Renal dysfunction was assessed by measuring the rises in plasma levels of creatinine (an indicator of reduced glomerular filtration rate, and hence, renal failure) and urea (an indicator of impaired excretory function of the kidney and/or increased catabolism) (see Thiemermann et al., 1995). (3) In addition, we have evaluated the rises in the serum levels of lipase, a specific indicator for the development of pancreatic injury. (4) Finally, we determined the increase in the serum levels of creatine kinase, an indicator for the development of muscle (skeletal or cardiac) or brain injury.
In order to ensure that the PARS inhibitor 3-AB does not interfere with the determination of any of the above parameters of organ injury, we have carried out the following experiment. Serum (250 μl) was obtained from seven rats (four subjected to haemorrhagic shock and three subjected to the surgical procedure) and then spiked with either 250 μl of saline or with 250 μl containing 0.24 mg ml−1 of 3-AB. Given an estimated blood volume of ∼25 ml of blood per rat, we have estimated the maximal concentration of 3-AB in the blood to be 0.12 mg ml−1. The above assay has taken this estimation into account and, hence, the final concentration of 3-AB in the sample (comprising of 250 μl of serum plus 250 μl of saline containing 3-AB) was 0.12 mg ml−1. We have then subjected this sample to analysis by VetLab and determined the following parameters: urea, creatinine, AST, ALT, CK and lipase.
Materials
Unless otherwise stated, all compounds were obtained from Sigma-Aldrich Company Ltd. (Poole, Dorset, U.K.). Thiopentone sodium (Intraval Sodium®) was obtained from Rhône Mérieux Ltd. (Harlow, Essex, U.K.). All stock solutions were prepared in non-pyrogenic saline (0.9% NaCl; Baxter Healthcare Ltd., Thetford, Norfolk, U.K.).
Statistical evaluation
All data are presented as mean±s.e.mean of n observations, where n represents the number of animals or blood samples studied. For repeated measurements (haemodynamics) a 2-factorial analysis of variance (ANOVA) was performed. Data without repeated measurements (multiple organ injury/failure) was analysed by 1-factorial ANOVA, followed by a Dunnett's test for multiple comparisons. A P-value of less than 0.05 was considered to be statistically significant.
Results
Effects of PARS inhibitors on the delayed vascular decompensation (circulatory failure) caused haemorrhage
Baseline values of MAP in all groups of animals ranged from 123±8 to 140±6 mmHg, and were not significantly different between groups (Table 2). In sham-operated rats (no haemorrhage), neither administration of saline nor administration (at 90 min) of any of the PARS inhibitors had any effect on MAP (Table 2). In rats subjected to haemorrhage, resuscitation with shed blood led to an immediate increase in blood pressure from ∼50 mmHg to 104±3 mmHg. Thereafter, there was a progressive decline in MAP to approximately 65 mmHg at the end of the experiment (Table 2). None of the PARS inhibitors used attenuated the delayed fall in MAP associated with haemorrhage (Table 2).
Table 2.
Alterations in mean arterial pressure (MAP) and heart rate (HR)
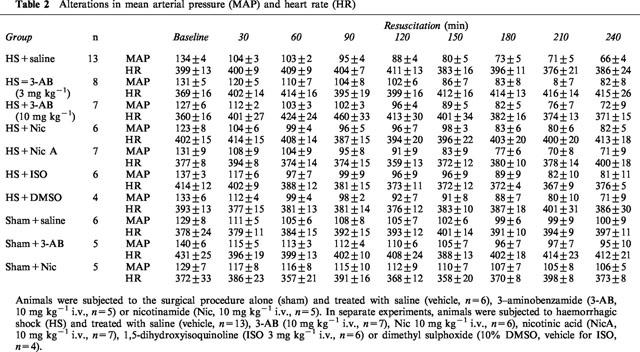
Baseline values of heart rate (HR) in all groups of animals ranged from 360±16 to 431±12 beats per minute (b.p.m.), and were not significantly different between groups (Table 2). In sham-operated rats, neither administration of saline nor administration of any of the PARS inhibitors had any effect on MAP (Table 2). Haemorrhagic shock did also not cause a significant alteration in heart rate (Table 2, P>0.05).
Effects of PARS inhibitors on the multiple organ dysfunction syndrome caused by haemorrhage in the rat
Effects on the renal injury/dysfunction
In sham-operated rats, administration of saline, 3-AB (10 mg kg−1), nicotinamide, nicotinic acid or ISO did not result in any significant alterations in the plasma levels of urea or creatinine (Table 4). When compared with sham-operated rats, haemorrhage/resuscitation resulted in significant rises in the plasma levels of urea (Figure 1a) and creatinine (Figure 1b), demonstrating the development of renal dysfunction. Treatment of rats subjected to haemorrhage and resuscitation with the PARS inhibitors 3-AB (10 mg kg−1) and nicotinamide attenuated the renal dysfunction caused by haemorrhage and resuscitation. In contrast, the lower dose of 3-AB (3 mg kg−1; Table 3) or nicotinic acid (negative control for nicotinamide, Figure 1) had no significant effect. Although ISO also attenuated the rise in the serum levels of urea and creatinine, its vehicle (10% DMSO) had a similar effect (Figure 1).
Table 4.
Alterations in the serum levels or urea, creatinine, aspartate amino transferasae (AST), alanine aminotrans-ferase (ALT), lipase and creatine kinase (CK)
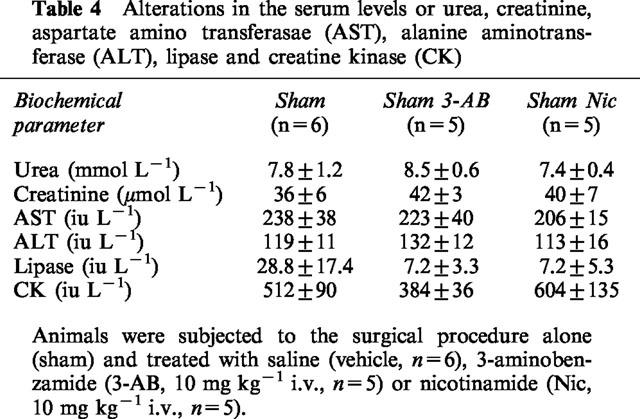
Figure 1.
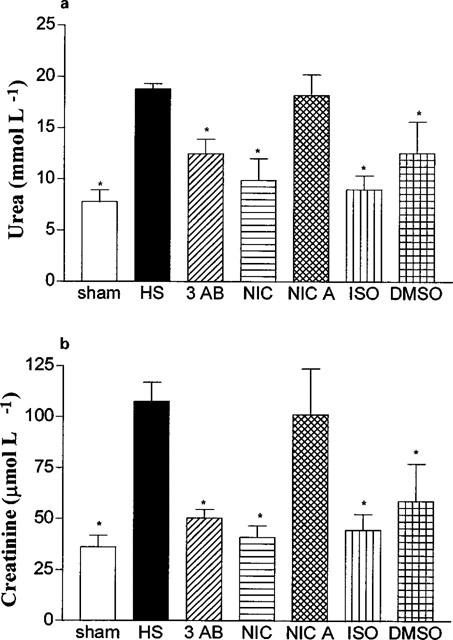
Alterations in the serum levels of urea (a) and creatinine (b). Animals were subjected to the surgical procedure alone and treated with saline (open column, n=6) or to haemorrhagic shock and treated with vehicle (HS, solid column, n=13), 3-AB (10 mg kg−1 i.v., n=7), Nic (10 mg kg−1 i.v., n=6), nicotinic acid (NicA, 10 mg kg−1 i.v., n=7), 1,5-dihydroxyisoquinoline (ISO, 3 mg kg−1 i.v., n=6) or dimethyl sulphoxide (10% DMSO, vehicle for ISO, n=4). *P<0.05 when compared to HS-control.
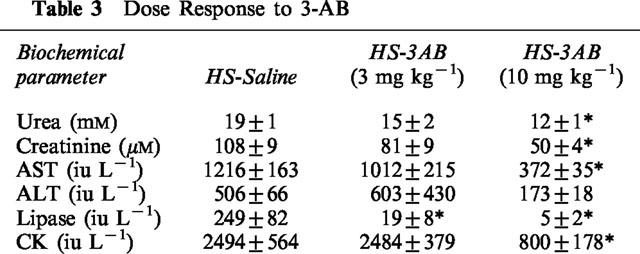
Table 3.
Dose Response to 3-AB
Effects on the liver injury/dysfunction
Neither saline nor any of the PARS inhibitors used caused any significant alterations in the plasma levels of AST or ALT in sham-operated rats (Table 4). In contrast, haemorrhage/resuscitation caused a significant increase in the plasma levels of AST (Figure 2a) and ALT (Figure 2b) and, hence, hepatocellular injury. Treatment of rats subjected to haemorrhage and resuscitation with the PARS inhibitors 3-AB and nicotinamide reduced the liver injury caused by haemorrhage and resuscitation. In contrast, the lower dose of 3-AB (3 mg kg−1; Table 3) or nicotinic acid (negative control for nicotinamide, Figure 2) had no significant effect on the hepatocellular dysfunction caused by haemorrhagic shock. Although ISO also attenuated the rise in the serum levels of AST and ALT, its vehicle (10% DMSO) had a similar effect (Figure 2).
Figure 2.
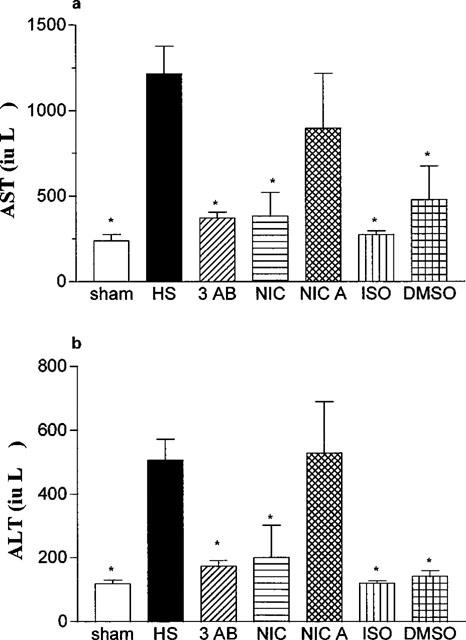
Alterations in the serum levels of aspartate aminotransferase (AST, a) and alanine aminotransferase (ALT, b). Animals were subjected to the surgical procedure alone and treated with saline (open column, n=6) or to haemorrhagic shock and treated with vehicle (HS, solid column, n=13), 3-AB (10 mg kg−1 i.v., n=7), Nic (10 mg kg−1 i.v., n=6), nicotinic acid (NicA, 10 mg kg−1 i.v., n=7), 1,5-dihydroxyisoquinoline (ISO, 3 mg kg−1 i.v., n=6) or dimethyl sulphoxide (10% DMSO, vehicle for ISO, n=4). *P<0.05 when compared to HS-control.
Effects on the pancreatic injury
In sham-operated rats, neither administration of saline nor of any of the PARS inhibitors used had any significant effect on the plasma levels of lipase (Table 4). Haemorrhagic shock on the other hand resulted in significant rises in the plasma levels of lipase (Figure 3), demonstrating the development of pancreatic injury. Treatment of rats subjected to haemorrhage and resuscitation with the PARS inhibitors 3-AB (3 or 10 mg kg−1; Table 3; Figure 3) or nicotinamide (Figure 3) reduced the pancreatic injury caused by haemorrhagic shock. In contrast, nicotinic acid (negative control for nicotinamide) was without effect (Figure 3). Although ISO also reduced the rise in the serum levels of lipase, its vehicle had a similar effect (Figure 3).
Figure 3.
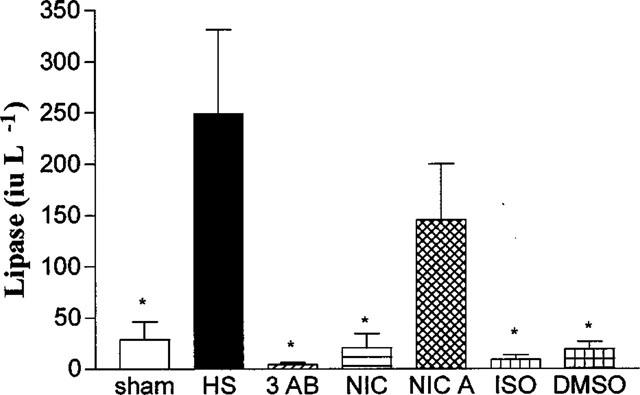
Alterations in the serum levels of lipase. Animals were subjected to the surgical procedure alone and treated with saline (open column, n=6) or to haemorrhagic shock and treated with vehicle (HS, solid column, n=13), 3-AB (10 mg kg−1 i.v., n=7), Nic (10 mg kg−1 i.v., n=6), nicotinic acid (NicA, 10 mg kg−1 i.v., n=7), 1,5-dihydroxyisoquinoline (ISO, 3 mg kg−1 i.v., n=6) or dimethyl sulphoxide (10% DMSO, vehicle for ISO, n=4). *P<0.05 when compared to HS-control.
Effects on the increase in the serum levels of creatine kinase
Neither saline nor any of the PARS inhibitors used had any significant effect on the plasma levels of creatine kinase (Table 4). When compared with sham-operated rats, haemorrhage and resuscitation resulted in significant rises in the plasma levels of creatine kinase (Figure 4), indicating that an injury to either myocytes (skeletal or cardiac) or brain has occurred. Treatment of rats subjected to haemorrhage and resuscitation with the PARS inhibitors 3-AB and nicotinamide attenuated the rise in creatine kinase caused by haemorrhage and resuscitation. In contrast, the lower dose of 3-AB (3 mg kg−1; Table 3) or nicotinic acid (negative control for nicotinamide, Figure 4) had no significant effect on the rise in the serum levels of CK. Although ISO also attenuated the rise in the serum levels of creatine kinase, its vehicle had a similar effect (Figure 4).
Figure 4.
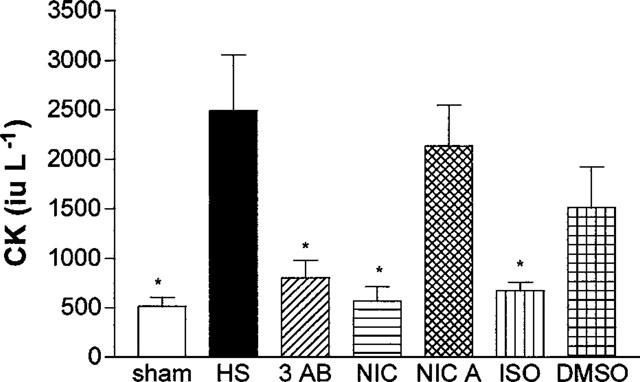
Alterations in the serum levels of creatine kinase (CK). Animals were subjected to the surgical procedure alone and treated with saline (open column, n=6) or to haemorrhagic shock and treated with vehicle (HS, solid column, n=13), 3-AB (10 mg kg−1 i.v., n=7), Nic (10 mg kg−1 i.v., n=6), nicotinic acid (NicA, 10 mg kg−1 i.v., n=7), 1,5-dihydroxyisoquinoline (ISO, 3 mg kg−1 i.v., n=6) or dimethyl sulphoxide (10% DMSO, vehicle for ISO, n=4). *P<0.05 when compared to HS-control.
Effects of 3-AB on the determination of creatinine, urea, AST, ALT, CK and lipase
In a separate set of experiments, plasma samples obtained from rats subjected to haemorrhagic shock or sham-operation were spiked with 3-AB in order to evaluate whether 3-AB affects the determination of any of the above parameters of organ injury (see Methods for details). We found that 3-AB had no significant effect on the determination of creatinine (without 3-AB: 16±1 μM; with 3-AB 14±2 μM; P>0.05, n=7), urea (without 3-AB: 6±1 mM; with 3-AB 6±1 mM; P>0.05, n=7), AST (without 3-AB: 112±21 iu L−1; with 3-AB 112±20 iu L−1; P>0.05, n=7), ALT (without 3-AB: 64±8 iu L−1; with 3-AB 64±8 iu L−1; P>0.05, n=7), CK (without 3-AB: 190±38 iu L−1; with 3-AB 229±57 iu L−1; P>0.05, n=7) and lipase (without 3-AB: 19±3 iu L−1; with 3-AB 14±4 iu L−1; P>0.05, n=7).
Discussion
Haemorrhage for 90 min followed by resuscitation with shed blood (for 4 h) resulted in a substantial increase in the plasma levels of urea and creatinine indicating the development of acute renal dysfunction. Haemorrhage and resuscitation also caused an increase in the plasma levels of the transaminases AST and ALT, indicating the development of hepatocellular injury. Indeed, we have previously confirmed (by using light microscopy) that the model of haemorrhagic shock used here results in a substantial degree of tissue injury to the lung, kidney, intestine and liver (Mota-Filipe et al., 1999). In addition, haemorrhage and resuscitation resulted in a substantial increase in the serum levels of lipase indicating the development of pancreatic injury. Haemorrhage and resuscitation also caused a significant increase in the serum levels of creatine kinase, which is a cytosolic enzyme contained in large amounts in skeletal or cardiac muscle and in the brain. We report here that the administration (at 5 min prior to resuscitation) of three, chemically distinct PARS inhibitors (3-AB, nicotinamide and ISO) attenuated (i) the renal dysfunction, (ii) the liver injury, (iii) the pancreatic injury and (iv) the muscle and/or brain injury caused by haemorrhage and resuscitation.
We propose that the beneficial effects of 3-AB and nicotinamide are due to the ability of these agents to inhibit PARS activity. Although not very potent in vitro, 3-AB is a well-documented, water-soluble PARS inhibitor. The dose of 3-AB used in our study has been shown to inhibit PARS activity in the rat in vivo. As nicotinamide is a vitamin, it could be argued that the beneficial effects of nicotinamide observed in our study are due to non-specific effects of nicotinamide. This is, however, unlikely, as nicotinic acid, a structural analogue of nicotinamide, which does not inhibit PARS activity (Thiemermann et al., 1997; Bowes et al., 1998; 1999), failed to reduce the organ injury and dysfunction caused by haemorrhage and resuscitation. It could also be argued that the beneficial effects of nicotinamide could be secondary to the ability of nicotinamide to replenish intracellular NAD levels via the re-synthesis of NAD from nicotinamide (Carson et al., 1986). This is also very unlikely, as nicotinic acid, which can also be re-synthesized to NAD, did not reduce the organ injury and dysfunction caused by haemorrhage and resuscitation in the rat.
We have recently documented that tempol, a small molecule which crosses biological membranes and functions as an intracellular radical scavenger, reduces the multiple organ failure caused by haemorrhage and resuscitation (Mota Filipe et al., 1999). The PARS inhibitors used here however, do not scavenge reactive oxygen-radicals (Bowes et al., 1998). Thus, we propose that the beneficial effects of PARS inhibitors are not due to their ability to scavenge radicals. This notion is supported by the finding that these agents are unable to attenuate the development of single strand breaks in DNA caused by hydrogen peroxide in proximal tubule cells of the rat in vitro (Chatterjee et al., 1999). In contrast, desferrioxamine and catalase attenuate the DNA strand breaks caused by H2O2 in the same cells (positive control) (Chatterjee et al., 1999).
ISO is a more potent inhibitor of PARS activity than analogues of benzamide, such as 3-AB (Bowes et al., 1999). Although ISO abolished the renal dysfunction, the hepatocellular injury and the pancreatic injury associated with haemorrhage and resuscitation, a similar beneficial effect was also observed with its vehicle DMSO. As DMSO is a scavenger of hydroxyl radicals, it is possible that the observed beneficial effects of ISO are, at least in part, due to the ability of DMSO to scavenge hydroxyl radicals in vivo. Indeed, agents which either reduce the generation of hydroxyl radicals in vivo (e.g. deferroxamine) or agents which scavenge hydroxyl radicals (Mota Filipe et al., 1999) reduce the organ injury/dysfunction associated with haemorrhagic shock.
In principle, severe haemorrhage followed by resuscitation leads to ischaemia and reperfusion (injury) of target organs including the heart, liver, brain and kidney (Flaherty & Wesfeldt, 1988). There is good evidence that various, chemically distinct inhibitors of PARS activity (including 3-AB, nicotinamide and ISO) reduce the degree of tissue injury associated with regional myocardial ischaemia and reperfusion of the heart (Thiemermann et al., 1997; Zingarelli et al., 1997; 1998; Bowes et al., 1999), the brain (Eliasson et al., 1997), the gut (Cuzzocrea et al., 1997) and the kidney (Chatterjee et al., 1999). Most notably, the degree of tissue injury caused by ischaemia and reperfusion of the heart (Zingarelli et al., 1998; Walles et al., 1998a, 1998b; Grupp et al., 1999) and brain (Eliasson et al., 1997) is attenuated in mice in which the gene for PARS has been disrupted by gene-targeting (PARS knock out or −/− mice). We, therefore, propose that severe haemorrhage and resuscitation leads to organ ischaemia (McCord, 1985; Flaherty & Wesfeldt, 1988), the generation of oxygen- or nitrogen-derived free radicals upon reperfusion (Zweier et al., 1987; Nunes et al., 1995), strand breaks in DNA (Carson et al., 1986) and ultimately PARS activation. The resultant excessive activation of PARS contributes to the organ injury and dysfunction associated with severe haemorrhage and resuscitation.
The PARS inhibitor 3-AB (15 mg kg−1) has been reported to attenuate the delayed circulatory failure (e.g. fall in blood pressure, cardiac output and stroke volume) associated with severe haemorrhage in the pig. Thus, it has been suggested (Szabo et al., 1998) that the beneficial effects of 3-AB in haemorrhagic shock are due to an improved cardiac performance. We have not measured the effects of any of the PARS inhibitors used in our study on cardiac performance. It should be noted that the mean arterial blood pressure of rats treated with 3-AB, nicotinamide or ISO were higher (at the end of the resuscitation period) than in the control group. Although consistent, the observed effect of the PARS inhibitors on blood pressure was small, but not significant. Thus, we provide no evidence that the PARS inhibitors used in this study attenuate the delayed fall in blood pressure caused by severe haemorrhage and resuscitation.
In conclusion, this study demonstrates that three chemically distinct inhibitors of PARS activity attenuate the renal dysfunction, the hepatocellular injury and the pancreatic injury associated with severe haemorrhage and resuscitation. As the beneficial effects of the potent and specific PARS inhibitor ISO were – in part – due to its vehicle, further studies with potent and specific inhibitors of PARS activity are warranted to ensure that the reduction by the agents of the multiple organ failure in haemorrhagic shock is indeed due to their ability to inhibit PARS activity.
Acknowledgments
HM Filipe was funded by a post-doctoral grant provided by the Portuguese Fundação para a Ciência e Tecnologia (Praxis XXI/BPD/16333/98). M.C McDonald is a recipient of a PhD studentship provided by the Joint Research Board of St. Bartholomew's Hospital Medical College (G7Z4). C. Thiemermann is a Senior Fellow of the British Heart Foundation (FS 96/018).
Abbreviations
- 3-AB
3-aminobenzamide
- ALT
alanine aminotransferase
- AST
aspartate aminotransferase
- ATP
adenosine triphosphate
- HR
heart rate
- ISO
1,5-dihydroxyisoquinoline
- MAP
mean arterial blood pressure
- NAD
nicotinamide adenine dinucleotide
- NO
nitric oxide
- PARS
poly (ADP-ribose) synthetase
- ROS
reactive oxygen species
References
- AALTO T.K., RAIVIO K.O. Nucleotide depletion due to reactive oxygen metabolites in endothelial cells: effects of antioxidants. Pediatric Res. 1993;34:572–576. doi: 10.1203/00006450-199311000-00004. [DOI] [PubMed] [Google Scholar]
- ALLAN G., CAMBRIDGE D., LEE-TSANG-TANG L., VAN WAY C.W., WHITING M.V. The protective action of allopurinol in an experimental model of haemorrhagic shock and reperfusion. Br. J. Pharmacol. 1986;89:149–155. doi: 10.1111/j.1476-5381.1986.tb11130.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BANASIK M., KOMURA H., SHIMOYAMA M., UEDA K. Specific inhibitors of poly (ADP-ribose) synthetase and mono (ADP-ribosyl) transferase. J. Biol. Chem. 1992;267:1569–1575. [PubMed] [Google Scholar]
- BAUE A.E.The multiple organ or system failure syndrome Pathophysiology of Shock, Sepsis and Organ Failure 1993Springer Verlag: Berlin; 1004–1018.In: Schlag, G. & Redl, H. (eds) [Google Scholar]
- BERGER N.A. Poly(ADP-ribose) in the cellular response to DNA damage. Rad. Res. 1985;101:4–15. [PubMed] [Google Scholar]
- BOWES J., PIPER J., THIEMERMANN C. Inhibitors of the activity of poly (ADP-ribose) synthetase reduce the cell death caused by hydrogen peroxide in human cardiac myoblasts. Br. J. Pharmacol. 1998;124:1760–1766. doi: 10.1038/sj.bjp.0702009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BOWES J., MCDONALD M.C., PIPER J., THIEMERMANN C. Inhibitors of poly (ADP-ribose) synthetase protect rat cardiomyocytes against oxidant stress. Cardiovasc. Res. 1999;41:126–134. doi: 10.1016/s0008-6363(98)00221-1. [DOI] [PubMed] [Google Scholar]
- CARSON D.A., SET S., WASSON B., CARRERA C.J. DNA strand breaks, NAD metabolism and programmed cell death. Exp. Cell. Res. 1986;164:273–281. doi: 10.1016/0014-4827(86)90028-5. [DOI] [PubMed] [Google Scholar]
- CHATTERJEE P.K., CUZZOCREA S., THIEMERMANN C. Inhibitors of poly (ADP-ribose) synthetase protect rat proximal tubular cells against hydrogenperoxide-mediated oxidant stress. Kidney Int. 1999;56:973–984. doi: 10.1046/j.1523-1755.1999.00644.x. [DOI] [PubMed] [Google Scholar]
- CUZZOCREA S., ZINGARELLI B., COSTANTINO G., SZABO A., SALZMAN A.L., CAPUTI A.P., SZABO C. Beneficial effects of 3-aminobenzamide, an inhibitor of poly (ADP-ribose) synthetase in a rat model of splanchnic artery occlusion and reperfusion. Br. J. Pharmacol. 1997;121:1065–1074. doi: 10.1038/sj.bjp.0701234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DAUGHTERS K., WAXMAN K., GASSEL A., ZOMMER S. Anti-oxidant treatment for shock: vitamin E but not vitamin C improves survival. Am. Surg. 1996;62:789–792. [PubMed] [Google Scholar]
- ELIASSON M.J.L., SAMPEI K., MANDIR A.S., HURN P.D., TRAYSTMAN R.J., BAO J., PIEPER A., WANG Z., DAWSON T.M., SNYDER S.H., DAWSON V.L. Poly (ADP-ribose) polymerase gene disruption renders mice resistant to cerebral ischaemia. Nature Med. 1997;3:1089–1095. doi: 10.1038/nm1097-1089. [DOI] [PubMed] [Google Scholar]
- FAN J., MARSHALL J.C., JIMENEZ M., SHEK P.N., ZAGORSKI J., ROTSTEIN O.D. Hemorrhagic shock primes for increased expression of cytokine-induced neutrophil chemoattractant in the lung: role in pulmonary inflammation following lipopolysaccharide. J. Immunol. 1998;161:440–447. [PubMed] [Google Scholar]
- FLAHERTY J.L., WESFELDT M.L. Reperfusion injury. Free Radic. Biol. Med. 1988;5:409–419. doi: 10.1016/0891-5849(88)90115-3. [DOI] [PubMed] [Google Scholar]
- FLECKENSTEIN A.E., SMITH S.L., LINSEMAN K.L., BEUVING L.J., HALL E.D. Comparison of the efficacy of mechanistically different antioxidants in the rat hemorrhagic shock model. Circ. Shock. 1991;35:223–230. [PubMed] [Google Scholar]
- GRUPP I.L., JACKSON T.M., HAKE P., GRUPP G., SZABO C. Protection against hypoxia reoxygenation in the absence of poly (ADP-ribose) synthetase in isolated working hearts. J. Mol. Cell. Cardiol. 1999;31:297–303. doi: 10.1006/jmcc.1998.0864. [DOI] [PubMed] [Google Scholar]
- HAMANO K., TSUBOI H., SEYAMA A., ESATO K. Shock-reinfusion injury to the central organs and the effect of free radical scavengers in the rat. Surg. Today. 1993;23:891–896. doi: 10.1007/BF00311368. [DOI] [PubMed] [Google Scholar]
- HELLER B., WANG Z.Q., WAGNER E.F., RADONS J., BURKLE A., FEHSEL K., BURKART V., KOLB H. Inactivation of the poly(ADP-ribose)polymerase gene affects oxygen radical and nitric oxide toxicity in islet cells. J. Biol. Chem. 1995;270:11176–11180. doi: 10.1074/jbc.270.19.11176. [DOI] [PubMed] [Google Scholar]
- HYSLOP P.A., HINSHAW D.B., HALSEY W.A., SCHRAUFSTATTER I.U., SAUERHEBER R.D., SPRAGG R.G., JACKSON J.H., COCHRANE C.G. Mechanisms of oxidant-mediated cell injury: The glycolytic and mitochondrial pathways of ADP phosphorylation are major intracellular targets inactivated by hydrogen peroxide. J. Biol. Chem. 1988;263:1665–1675. [PubMed] [Google Scholar]
- IKAI K., UEDA K. Immunohistochemical demonstration of poly (adenosine diphosphate-ribose) synthetase in bovine tissues. J. Histochem. Cytochem. 1983;31:1261–1264. doi: 10.1177/31.11.6311893. [DOI] [PubMed] [Google Scholar]
- LAUTIER D., LAGVEUX J., THIBODEAU J., MENARD L., POIRER G.G. Molecular and biochemical features of poly (ADP-ribose) metabolism. Mol. Cell. Biochem. 1993;122:171–193. doi: 10.1007/BF01076101. [DOI] [PubMed] [Google Scholar]
- MANNION D., FITZPATRICK G.J., FEELEY M. Role of xanthine oxidase inhibition in survival from hemorrhagic shock. Circ. Shock. 1994;42:39–43. [PubMed] [Google Scholar]
- MCCORD J.M. Oxygen-derived free radicals in post-ischaemic tissue injury. N. Engl. J. Med. 1985;312:159–163. doi: 10.1056/NEJM198501173120305. [DOI] [PubMed] [Google Scholar]
- MOTA-FILIPE H., MCDONALD M., CUZZOCREA S., THIEMERMANN C. A membrane-permeable radical scavenger reduces the organ injury in hemorrhagic shock. Shock. 1999;12:255–261. doi: 10.1097/00024382-199910000-00002. [DOI] [PubMed] [Google Scholar]
- NUNES F.A., KUMAR C., CHANCE B., BRASS C.A. Chemiluminescent measurement of increased free radical formation after ischaemia-reperfusion. Dig. Dis. Sci. 1995;40:1045–1053. doi: 10.1007/BF02064197. [DOI] [PubMed] [Google Scholar]
- REDL H., GASSER H., SCHLAG G., MARZI I. Involvement of oxygen radicals in shock related cell injury. Br. Med. Bull. 1993;49:556–565. doi: 10.1093/oxfordjournals.bmb.a072630. [DOI] [PubMed] [Google Scholar]
- SANAN S., SHARMA G., MALHOTRA R., SANAN D.P., JAIN P., VADHERA P. Protection by desferrioxamine against histopathological changes of the liver in the post-oligaemic phase of clinical haemorrhagic shock in dogs: correlation with improved survival rate and recovery. Free Radic. Res. Commun. 1989;6:29–38. doi: 10.3109/10715768909073425. [DOI] [PubMed] [Google Scholar]
- SCHRAUFSTATTER I.U., HINSHAW D.B., HYSLOP P.A., SPRAGG R.G., COCHRANE C.G. DNA strand breaks activate poly adenosine diphosphate -ribose polymerase and lead to depletion of nicotinamide adenine dinucleotide. J. Clin. Invest. 1986b;77:1312–1320. doi: 10.1172/JCI112436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SCHRAUFSTATTER I.U., HYSLOP P.A., HINSHAW D.B., SPRAGG R.G., SKLAR L.A., COCHRANE C.G. Hydrogen peroxide induced injury and its prevention by inhibitors of poly (ADP-ribose) polymerase. Proc. Natl. Acad. Sci. U.S.A. 1986a;83:4908–4912. doi: 10.1073/pnas.83.13.4908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SIMON H.M., SCALEA T., PASKANIK A., YANG B. Superoxide dismutase (SOD) prevents hypotension after hemorrhagic shock and aortic cross clamping. Am. J. Med. Sci. 1996;312:155–159. doi: 10.1097/00000441-199610000-00002. [DOI] [PubMed] [Google Scholar]
- SZABO A., HAKE P., SALZMAN A.L., SZABO C. 3-Aminobenzamide, an inhibitor of poly (ADP-ribose) synthetase, improves hemodynamics and prolongs survival in a porcine model of hemorrhagic shock. Shock. 1998;10:347–353. doi: 10.1097/00024382-199811000-00007. [DOI] [PubMed] [Google Scholar]
- SZABO C. Potential role of peroxynitrite-poly(ADP-ribose) synthetase pathway in a rat model of severe hemorrhagic shock. Shock. 1998;9:341–344. doi: 10.1097/00024382-199805000-00005. [DOI] [PubMed] [Google Scholar]
- SZABO C., SALZMAN A.L., ISCHIROPOULOS H. Peroxynitrite-mediated oxidation of dihydrorhodamine 123 occurs in early stages of endotoxic and hemorrhagic shock and ischemia-reperfusion injury. FEBS Lett. 1995;372:229–232. doi: 10.1016/0014-5793(95)00984-h. [DOI] [PubMed] [Google Scholar]
- SZABO C., ZINGARELLI B., SALZMAN A.L. Role of poly-ADP ribosyltransferase activation in the vascular contractile and energetic failure elicited by exogenous and endogenous nitric oxide and peroxynitrite. Circ. Res. 1996;78:1051–1063. doi: 10.1161/01.res.78.6.1051. [DOI] [PubMed] [Google Scholar]
- THIEMERMANN C., RUETTEN H., WU C.C., VANE J.R. The multiple organ dysfunction syndrome caused by endotoxin in the rat: attenuation of liver dysfunction by inhibitors of nitric oxide synthase. Br. J. Pharmacol. 1995;116:2845–2851. doi: 10.1111/j.1476-5381.1995.tb15935.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- THIEMERMANN C., BOWES J., MYINT F., VANE J.R. Inhibition of the activity of poly (ADP-ribose) synthetase reduces ischemia-reperfusion injury in the heart and skeletal muscle. Proc. Natl. Acad. Sci U.S.A. 1997;94:679–683. doi: 10.1073/pnas.94.2.679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- THIES R.L., AUTOR A.P. Reactive oxygen injury to cultured pulmonary artery endothelial cells: Mediation by poly (ADP-ribose) polymerase activation causing NAD depletion and altered energy balance. Arch. Biochem. Biophys. 1991;286:353–363. doi: 10.1016/0003-9861(91)90051-j. [DOI] [PubMed] [Google Scholar]
- UEDA K., HAYAISHI O. ADP-ribosylation. Ann. Rev. Biochem. 1985;54:73–100. doi: 10.1146/annurev.bi.54.070185.000445. [DOI] [PubMed] [Google Scholar]
- WALLES T., PIEPER A.A., ZHANG J.J., SNYDER S.H., ZWEIER J.L.Demonstration that poly (ADP-ribose) accumulation occurs in the post ischemic heart and is associated with myocardial necrosis Circulation 1998a98Suppl.I-260(Abstract) [Google Scholar]
- WALLES T., WANG P., PIEPER A., SNYDER S., ZWEIER J.L.Mice lacking poly (ADP-ribose) polymerase gene show attenuated cellular energy depletion and improved recovery of myocardial function following global ischemia Circulation 1998b98Suppl.I-260(Abstract) [Google Scholar]
- ZHANG J., DAWSON V.L., DAWSON T.M., SNYDER S.H. Nitric oxide activation of poly (ADP-ribose) synthetase in neurotoxicity. Science. 1994;263:687–689. doi: 10.1126/science.8080500. [DOI] [PubMed] [Google Scholar]
- ZINGARELLI B., CUZZOCREA S., ZSENGELLER Z., SALZMAN A.L., SZABO C. Protection against myocardial ischemia and reperfusion injury by 3 aminobenzamide, an inhibitor of poly (ADP-ribose) synthetase. Cardiovasc. Res. 1997;36:205–215. doi: 10.1016/s0008-6363(97)00137-5. [DOI] [PubMed] [Google Scholar]
- ZINGARELLI B., SALZMAN A.L., SZABO C. Genetic disruption of poly (ADP-ribose) synthetase inhibits the expression of P-selectin and intercellular adhesion molecule-1 in myocardial ischemia/reperfusion injury. Circ. Res. 1998;83:85–94. doi: 10.1161/01.res.83.1.85. [DOI] [PubMed] [Google Scholar]
- ZWEIER J.L., FLAHERTY J.T., WEISFELDT M.L. Direct measurement of free radical generation following reperfusion of ischemic myocardium. Proc. Natl. Acad. Sci. U.S.A. 1987;84:1404–1407. doi: 10.1073/pnas.84.5.1404. [DOI] [PMC free article] [PubMed] [Google Scholar]


