Abstract
The heart normally acts as an electrical syncytium coupled via gap junctional channels. Since closure of these channels has been considered arrhythmogenic, we wanted to elucidate, how activation and repolarization wavefronts are altered during progressive pharmacological gap junctional uncoupling.
We used the well known gap junction uncoupler palmitoleic acid (PA). The specificity of PA was tested in rabbit papillary muscles, which exhibited slowed conduction without affecting action potential morphology. We submitted isolated rabbit hearts (Langendorff-technique) to increasing concentrations of palmitoleic acid (0.2, 1, 2, 5, 10, 20 μM), while 256 channel epicardial potential mapping was carried out.
In presence of PA activation recovery intervals (ARI) at the 256 electrodes became highly inhomogeneous with a dramatic increase in the dispersion of activation recovery intervals (from 6 to 35 ms, P>0.01; EC50=7 μM), while the mean ARI-duration at 256 sites remained stable. PA led to marked alterations of the activation pattern, expressed as percentage of unchanged activation vectors (reduction from 32 to 10%, P<0.01, EC50=3.3 μM), to prolongation of atrioventricular conduction time (from 58 to 107 ms, P<0.01; EC50=8 μM) of total activation time (from 7 to 14 ms, P<0.05, EC50=11 μM) and of QRS-complex-duration.
In additional experiments the ventricle was paced via a bipolar electrode during the mapping procedure. From the isochrones longitudinal and transversal velocities were assessed showing that PA reduced transversal conduction velocity more distinctly than longitudinal.
With regard to maximum effects and EC50 values we conclude that gap junction uncoupling by PA mainly affects atrioventricular conduction, ARI-dispersion and ventricular activation pattern. As important arrhythmogenic effects of uncoupling enhancement of dispersion with concomitant disturbation of the normal activation pattern and slowing of conduction might be considered.
Keywords: cardiac gap junction, palmitoleic acid, ventricular activation pattern, epicardial mapping, arrhythmia
Introduction
Coordinated activation and contraction are prerequisites for a normal and effective heart action. This is ensured by intercellular communication and coupling of the cells. This intercellular coupling in the heart is provided by gap junctional channels located close to the fascia adherens (Severs, 1990; Hoyt et al., 1989). The gap junction channels consist of two hexameric hemichannels (connexon) provided by each cell (Makowsky et al., 1977). Each cell provides one hemichannel of the intact gap junction. The connexins in a hexamer are arranged in a lettuce-like fashion with six lobes protruding from the extracellular surface which can interdigitate with the protrusions of the other connexon following a ‘lock and key' mechanism (Perkins et al., 1997). In the mammalian heart several isforms of connexins have been found: connexin 43, the most abundant connexin in the heart (Beyer et al., 1987); connexin 40, which is mainly found in atrium and specific conduction system as well as in endothelium (Kanter et al., 1992; Bastide et al., 1993); and connexin 45, which is found in the ventricle especially in embryonic tissue (Beyer, 1990; Veenstra et al., 1993). In the ventricle of adult mammalian heart coupling is provided predominantly by connexin 43 channels and to a lower extent connexin 45 (Kanter et al., 1994; Verheule et al., 1997). The gap junction channels are located mainly at the cell poles and only small amounts are found at the lateral cell borders (Oesthoeck et al., 1993) with a ventricular cardiomyocytes being connected to approximately 11 other myocytes (Saffitz et al., 1994). Thus, this specific type of distribution has been suggested to contribute to the anisotropy of the cardiac tissue with higher electrical resistance transverse to the fibre axis. Length to width ratio in the ventricle has been determined 6 : 1, but is reduced to 3.4 : 1 by the expression of gap junction channels at the lateral border as side-to-side connectors (Saffitz et al., 1995), which is in good accordance with an anisotropic ratio in the ventricle of 2 : 1–3 : 1. As a consequence conduction of electrical activation is faster along the fibres. The cardiac gap junction channels represent low resistance pathways, which also allow the passage of small molecules (<1000 Da). They are regulated by a large number of mechanisms, as for example phosphorylation processes via protein kinases such as PKC, PKA and PKG. Moreover, other factors like intracellular pH, [Na+], [Ca2+] and [ATP] regulate the channels (for a detailed overview see Dhein, 1998). The channels provide the electrical synchronization of neighbouring cells and directed conduction of the activation wavefront. The heart, thus, acts as a syncytium coupled via gap junctions. It has often been discussed that disturbance of the intercellular coupling may be an important factor for arrhythmogenesis (Quan & Rudy, 1990; Joyner, 1982; Dekker et al., 1996; Spach & Heidlage, 1995). In small slices (5*5*6 mm) of sheep ventricular tissue Delmar and coworkers (1987) showed that reducing gap junctional coupling by heptanol resulted in inhibition of transversal rather than longitudinal conduction. Recently knock out mice have been produced in which Cx43 was knocked out. While the homozygous mice died shortly after birth due to malformations of the pulmonary outflow tract of the right ventricle (Reaume et al., 1995), the heterozygous mice survived. Hearts from (Cx43 +/−)-mice showed a significant slowing of conduction when Cx43 content is reduced by 50%, while atrial conduction was not affected (Thomas et al., 1998). As could be expected the ventricular QRS-complex was broadened in Cx43 (+/−)-mice, whereas the transmembrane action potential shape was unaltered (Guerrero et al., 1997). In contrast, in Cx40 knock out mice no changes in P-wave, QRS-complex or atrioventricular transmission were observed (Simon et al., 1998). However, Willecke et al. (1998) found significantly prolonged atrioventricular conduction intervals as well as prolonged QS and QTmax intervals in Cx40(−/−)-mice.
In order to determine the consequences of a pharmacological inhibition of gap junctional uncoupling in whole heart preparations, heptanol has been used in several studies (Delmar et al., 1987; Brugada et al., 1991; Boersma et al., 1994). These investigators showed that transverse conduction was more sensitive to heptanol than longitudinal conduction. However, in these studies the dispersion of action potential duration as an indicator of synchronization and as a parameter indicating possible arrhythmogenic effects has not been determined. In addition, heptanol has to be used in rather high concentrations, i.e. in a millimolar range, and in this concentration range affects other ion channels as well, so that the transmembrane action potential is affected.
Thus, it would be interesting to use substances which more or less specifically inhibit gap junctions. Palmitoleic acid has been shown to uncouple cells with an EC50 of about 5 μM (Burt et al., 1991). Thus, we decided to measure the influence of palmitoleic acid (1) on papillary muscle transmembrane action potentials and conduction velocity in papillary muscles, in order to find out in which concentration range conduction velocity is affected without changes in transmembrane action potentials, and (2) on whole heart electrophysiology assessed by 256 epicardial potential mapping (Dhein et al., 1993). Since theory would predict that conduction as well as synchronization should be affected (Lesh et al., 1989), we defined the total activation time, i.e. the time which is needed for activation of the whole hearts surface, the peak-to-peak amplitude of the ventricular QRS complex (as an indicator of the velocity of the local activation velocity (Spach & Dolber, 1986)) and the dispersion of epicardial action potential duration as lead parameters of this study. In addition, the influence on the geometry of the epicardial activation process should be analysed.
Methods
Heart-preparation and epicardial mapping
All experiments were performed in accordance with the ethical rules of the Council for International Organization of Medical Science and the German laws for animal welfare. The method of heart preparation and epicardial potential mapping has been described in more detail previously (Dhein et al., 1993) and will be explained only briefly in the following paragraph.
Male white New Zealand rabbits (conventional, normally fed ad libitum, 1500–1800 g, Rollie, Langenhagen, Germany) were treated with 1000 IU/kg heparin i.v. 5 min before they were stunned by a sharp blow on the neck and killed rapidly by subsequent exsanguination as approved by the local committee for animal care. The heart was excised, prepared and perfused according to the Langendorff-technique at constant pressure (70 cm H2O) with Tyrode's solution of the following composition: (mM) Na+ 161.02, K+ 5.36, Ca2+ 1.8, Mg2+ 1.05, Cl− 147.86, HCO3− 23.8, PO42− 0.42 and glucose 11.1 equilibrated with 95% O2 and 5% CO2 (pH=7.4). The surface temperature of the heart was 37°C. The hearts were connected to a 256 channel mapping system HAL3 (ELSA, Aachen, Germany, temporal resolution: 4 kHz per channel; amplitude resolution: 0.04 mV, interchannel coupling <−60 dB; bandwidth of the system: 0.5–20 kHz, data were not filtered) as described previously (Dhein et al., 1988). 256 AgCl electrodes (diameter: 70 μm) were cast in four polyester plates (in 8*8 orthogonal matrices with 1 mm interelectrodes distance), which were attached to the hearts surface in an elastic manner, so that they could follow the hearts movements easily without dislocation. The hearts were beating at their spontaneous rate. The four plates were located at (1) the right wall (64 channels); (2) the front wall (64 channels); (3) the left wall (64 channels); and (4) the back wall (64 channels), so that both ventricles were mapped with a total of 256 electrodes.
We administered palmitoleic acid in cumulative concentrations of 0.2, 1, 2, 5, 10 and 20 μM, each concentration being applied for a period of 15 min. For comparison a time control series was carried out without any treatment. Each experimental series was carried out with n=7 experiments.
Epicardial potential mapping was performed in each experimental phase during periods of constant cycle length of at least 4 min, in order to make it possible to compare the activation patterns (of single heart beats) or their alterations.
In addition, the functional parameters maximum systolic left ventricular pressure (LVP), basic cycle length (BCL) and coronary flow (CF) were assessed continuously as described (Dhein et al., 1993).
The delay between the end of the pacing stimulus and the first normal ventricular activation was assessed as PQ-time as a measure for the atrioventricular conduction time.
For evaluation of the mapping data the activation time points at each electrode were determined as t(dU/dtmin) (Dhein et al., 1993; Durrer & Van der Tweel, 1954). Next, the repolarization time points were determined as t(dU/dtmax) during the T-wave as described (Dhein et al., 1993; Millar et al., 1985). After automatic determination activation and repolarization timepoints were verified (or corrected if necessary) manually by the experimentor. From these data for each electrode an activation-recovery-interval (ARI, corresponding to the epicardial potential duration) was calculated. The corresonding distribution of ARI was analysed for each area of the heart (i.e. front, left, right or back wall) calculating the standard deviation of ARI at 64 electrodes and expressed as ARI-dispersion. From the activation time points an activation sequence was determined. We determined those electrodes which were activated before any of the neighbouring ones and defined them as breakthrough-points, which can be considered as the origins of epicardial activation (Arisi et al., 1983). These breakthrough-points were determined for heart beats under control conditions and for heart beats under treatment. Heart beats in the various phases of the experiment were compared to those under control conditions by calculating the percentage of breakthrough-points with identical location as compared to their location under control conditions (identical=deviating not more than 1 mm from their location under control conditions, this parameter was named BTP). That means, that two identical heart beats should reveal a breakthrough-point-similarity of 100%. It is, however known from previous studies, that identical heart beats do occur only rarely and that arrhythmogenic stimuli can reduce breakthrough-point-similarity (Dhein et al., 1988, 1989, 1990, 1993). In the above studies we defined a lower limit of 50% breakthrough-point-similarity beneath which it was only a matter of time until arrhythmia would occur.
In a similar way the spread of epicardial excitation was analysed. In order to allow a quantitative and comparative description of the activation process for each electrode an activation vector was calculated from the activation times and the locations of the surrounding electrodes which were activated after the central electrode (i.e. a maximum number of eight), as described by Müller et al. (1991). These vectors give direction and apparent velocity of local activation. The percentage of similar vectors (VEC) between heart beats in the various experimental phases compared with those under control conditions was determined (vectors deviating not more than 5° from their original direction were considered to be similar). The critical value beneath which arrhythmia often occurs (see above) for VEC-similarity is 10% as determined in previous studies (Dhein et al., 1988, 1989, 1990, 1993).
Taken together, the parameters BTP and VEC characterize the geometry of the epicardial activation process, and represent the beat similarity of the cardiac impulse as compared to heart beats under control conditions. Thus, decreasing values for BTP or VEC indicate progressive deviation from the initial (control) activation pattern.
Moreover, the total activation time (TAT, [ms]) was assessed as the delay between activation of the first and activation of the last electrode. Furthermore, the amplitude of the ventricular activation complexes was assessed as the peak-to-peak amplitude (PTP, [mV]), which is known to be an indirect indicator of the velocity of the travelling activation wave in the tissue beneath the electrode (Spach & Dolber, 1986).
In order to assess the degree of anisotropy under the influence of palmitoleic acid, additional mapping experiments were carried out in which the ventricle was stimulated via a bipolar electrode (rectangular pulses of 1 ms duration and double threshold) and the activation pattern isochrones around this stimulation point were analysed. From the isochrones and the fibre orientation of the ventricle the longitudinal and transversal conduction velocity was calculated as described by Wit & Dillon (1993) and by Haverkamp et al. (1993). Briefly, longitudinal conduction velocity was evaluated from electrodes (distant from the stimuls site) on the long axis showing the shortest conduction time interval. Transverse conduction velocity was evaluated from electrodes on a line perpendicular to the long axis (90±5°) across the more closely spaced isochrones. Velocities were calculated by dividing the distance travelled by the wave front by the corresponding conduction time interval between the selected electrodes. We avoided such areas for electrode selection which exhibited sudden changes in the density of isochrones. VL and VT were determined at at least two sites in each map and the mean was taken for further analysis (for further details see Haverkamp et al., 1993).
Experiments on isolated papillary muscles
In addition, we performed experiments on isolated rabbit papillary muscles which were exposed to superfusion with increasing concentrations of palmitoleic-acid (0.2, 1, 2, 5, 10 μM). The following method has been already deseribed (Müller et al., 1997) and is described only briefly here. Right ventricular papillary muscles (diameter <1.5 mm, length ca. 3–5 mm) were prepared from excised hearts of male rabbits, placed in an organ bath, superfused with Tyrodes solution (37°C, composition as described above) and paced at 1 Hz near the base of the muscle with rectangular stimuli of double threshold. Intracellular action potentials were recorded using glass microelectrodes (filled with 3 M KCl, 10–20 MΩ) which were connected to a custom-built microelectrode amplifier (HSE-Mikroelektrodenverstärker, Hugo Sachs Elektronik, Hugstetten, Germany). Electrode capacitance was compensated before each experiment using the capacitance compensation circuit of the amplifier. Microelectrodes were impaled into the muscle near the tendineous end so that propagated action potentials could be registered. Only propagated action potentials (with a delay between stimulus end and start of the action potential of >3 ms) were admitted to data analysis. Only experiments with a stable recording over the entire experiment were included in the final analysis. From the digitized data we determined the delay between the end of the stimulus and the timepoints of the maximum upstroke velocity of the propagated action potential as the stimulus-response interval (SRI; [ms]), the maximum upstroke velocity (dV/dtmax; [V s−1]), the action potential duration at 90% repolarization and the resting membrane potential. In addition, we measured the distance [mm] between both electrodes and calculated the propagation velocity by dividing SRI by this distance. After 30 min of equilibration palmitoleic acid was administered in a cumulative manner (0.2, 1, 2, 5, 10 μM), each concentration for 12 min. For control the same experiments were carried out in absence of palmitoleic acid.
Experiments on isolated cardiomyocytes
Since in papillary muscles electrotonic influences on the action potential configuration can not be principally excluded, additional experiments were carried out on isolated ventricular myocytes. For that purpose the epicardium of adult male guinea-pig hearts was digested with collagenase according to standard protocols (Müller et al., 1997). Briefly, animals were killed by a sharp blow on the neck and exsanguination, hearts were removed, cannulated and perfused with Tyrode's solution (same composition as described above) for 10–15 min at room temperature at constant pressure of 60 cm H2O. Thereafter, the hearts were perfused with Ca2+-free Tyrode's solution for 2 min, followed by 2 min perfusion with a solution containing (mM) NaCl 20, potassium asparate 120, MgCl2 1, HEPES 10, glucose 10, pH 7.4. For digestion the same solution was supplemented with 6000 IU collagenase (type CLS II) 1 mg ml−1 bovine serum albumin (fraction IV) and 25 μM CaCl2, and perfused for 15 min in a recirculating manner (all solutions were gassed with 100% oxygen). Thereafter, the ventricles were cut off, the endocardium was gently removed and the remaining epicardium was minced and incubated in the collagenase solution for 5–10 min. After filtering through 250 μm mesh width nylon gauze the solution was centrifuged at 400 r.p.m. for 2 min. The resulting cells were stored in Ca2+-deprived Tyrode's solution (2.5×10−6 M), to which CaCl2 was added stepwise to a final concentration of 1.8 mM. Cells were transferred to the perfusion chamber and allowed to settle to the chambers bottom for 5 min. Cells were superfused with normal Tyrode's solution.
Using 4–6 MΩ glass pipettes (filled with (mM) KCl 140, MgCl2 2, CaCl2 1, HEPES 10, pH adjusted to 7.2 with KOH) the cells were clamped in whole cell patch mode. Experiments were performed only when seal resistance exceeded 5 GΩ. For details of the amplifier and recording system please refer to Müller et al. (1997). In the current clamp mode brief currents (1 nA for 5 ms) were administered in order to elicit action potentials at a rate of 1 Hz. We recorded action potentials from these isolated ventricular myocytes before and after extracellular administration of palmitoleic acid (1, 10 and 20 μM) at 1 kHz sampling rate using the SEC-05 amplifier (npi-electronic, Tamm, Germany) equipped with the eggworks data acquisition system and software (npi-electronic, Tamm, Germany).
Statistics
All values are given as means±s.e.m of n=7 experiments in each series (whole hearts as well as papillary muscles or isolated cell experiments). Experimental data were analysed by computer-supported fitting to sigmoid curves and analysing the EC50 values using GraphPad-prism-software (GraphPad Software, San Diego, CA, U.S.A.). Significance was analysed using analysis of variance for comparison of multiple groups. If ANOVA indicated significant differences Wilcoxon rank test for paired observations or Mann-U-test for unpaired observations were performed. The level of significance was P<0.05%.
Chemicals
All chemicals used in this study were of analytical grade. All chemicals were purchased from Sigma (St. Louis, U.S.A.), except heparin which was from Serva (Heidelberg, Germany). Solutions of palmitoleic acid were prepared with 0.01% DMSO. Collagenase (type CLS II) was purchased from Biochrom (Berlin, Germany).
Results
Experiments on whole heart preparations
In the time control series, the stability of the parameters was assessed. As shown in Table 1, the electrophysiological parameters remained stable throughout the entire experimental phase except the similarity of the activation pattern which exhibited a progressive reduction in VEC, whereas >70% of the breakthrough-points remained stable. There was a decrease in the functional parameters LVP and CF as usual for this kind of experiment.
Table 1.
Data for the time control series
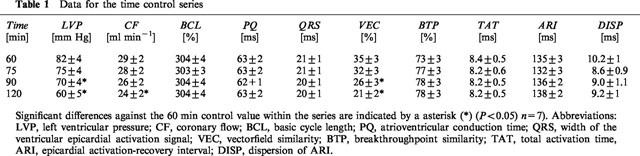
Under the influence of palmitoleic acid left ventricular pressue was reduced to 42±10% (10 μM) and 39±17% (20 μM) from initially 70±3 mm Hg. In parallel, coronary flow was reduced by the highest concentrations of palmitoleic acid to 41±6% (10 μM) and 24±10% (20 μM) from initially 29±2 ml min−1. In concentrations ⩽5 μM there was no effect of palmitoleic acid on both parameters and the data for left ventricular pressure and coronary flow did not differ significantly from the corresponding time control values.
With regard to the electrophysiological measurements it was found that palmitoleic acid led to dramatic increase (nearly 6 fold) in the dispersion of activation recovery intervals (from 6±1 to 35±4 ms at 20 μM, P>0.01; EC50=7±0.4 μM). At the lowest concentration (0.2 μM) an increase by +14±10% was observed (Figure 1).
Figure 1.
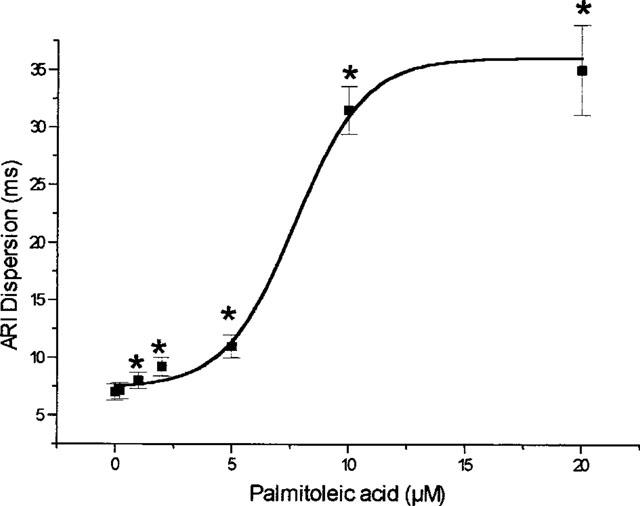
Concentration dependent influence of palmitoleic acid on the dispersion (DISP) of the activation recovery intervals (ARI) assessed as the standard deviation of ARI at 256 ventricular electrodes. Data from n=7 experiments are given as means±s.e.mean. Significant changes versus control conditions (60 min equilibration) are indicated by an * (P<0.05).
The mean activation recovery interval at the 256 sites, however, was nearly unaffected until a concentration of 10 μM was applied (control: 156±5 ms; 10 μM: 158±7 ms). Application of the highest concentration of 20 μM resulted in a prolongation of the mean activation recovery interval to 197±12 ms. Thus, while the mean activation recovery interval remained unchanged in concentrations ⩽10 μM, its distribution function becomes broadened, or in more mathematical terms platycurtotic, i.e. the standard deviation of this parameter, the dispersion of ARI at the 256 sites, was increased with an unchanged mean ARI.
Palmitoleic acid led to marked alterations of the activation pattern, expressed as the percentage of unchanged activation vectors (reduction from 32 to 10%, P<0.01, EC50=3.3±1.3 μM, Figure 2). In contrast, however, the location of the breakthrough-points remained unchanged and we found 75±5% of the breakthrough-points at the same localization as under control conditions, i.e. at the beginning of the experiment. The maximum similarity of two succeeding beats under control conditions was 75±4% in this series. Thus, in contrast to the activation vectors there was no influence of palmitoleic acid on BTP.
Figure 2.
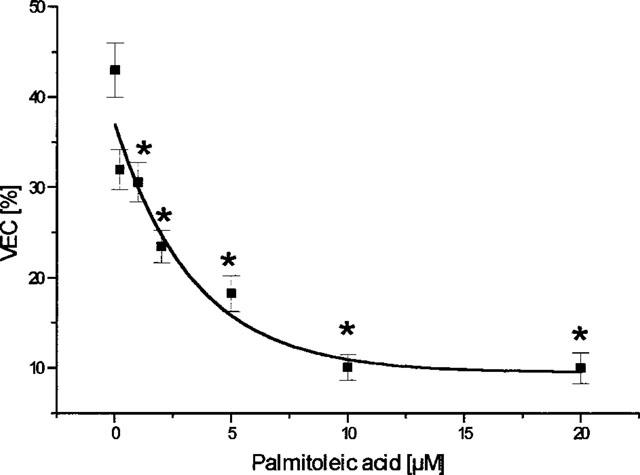
Influence of cumulative concentrations of palmitoleic acid on rabbit ventricular activation pattern. Data are given as the percentage of activation vectors being similar (deviating <5°) to the direction of the vector under control conditions, i.e. at 60 min equilibration. Data from n=7 experiments are given as means±s.e.mean. Significant changes versus control conditions (60 min equilibration) are indicated by an * (P<0.05).
The application of palmitoleic acid resulted in an increase in the total activation time (from 7 ms to 14 ms, P<0.05, EC50=10.8±2.2 μM, Figure 3a). In a similar manner the QRS-complex-duration was found to be prolonged with an EC50 of 14±3.3 μM and a maximum prolongation from 27.2±0.5 ms to 35.3±1.7 ms (20 μM) (Figure 3b). The peak-to-peak-voltage of the epicardial activation complex signals was found to be reduced dose-dependently by palmitoleic acid with an EC50 of 3.6±3 μM from 4.8±0.2 mV under control conditions to 3.28±0.21 mV (20 μM PA) (Figure 3c). In addition, palmitoleic acid led to a marked prolongation of the atrioventricular conduction time (from 58 to 107 ms, P<0.01; EC50=8.1±1.3 μM; Figure 3d). However, in concentrations up to 5 μM the effect was rather moderate and not significant.
Figure 3.
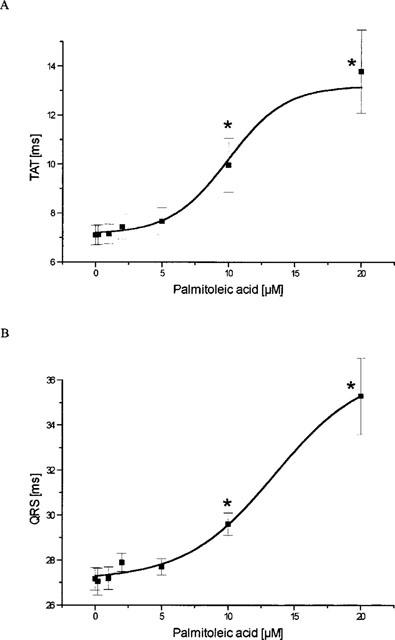
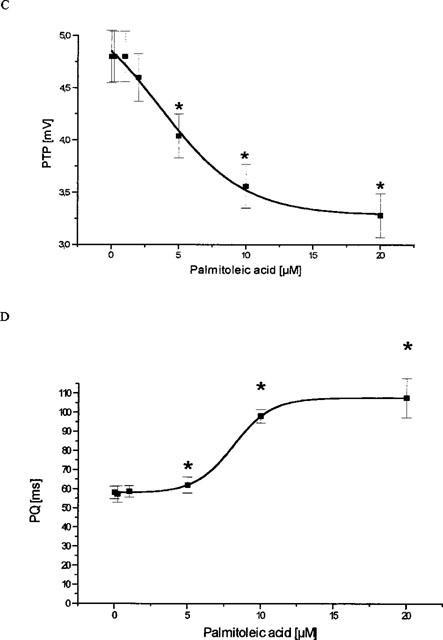
Influence of palmitoleic acid on cardiac ventricular and supraventricular conduction. (A) Effect of palmitoleic acid on ventricular total activation time (TAT), i.e. time necessary to activate the whole ventricular epicardial surface. (B) Action of palmitoleic acid on the width of the ventricular activation signal, i.e. the ventricular QRS complex. (C) Effect on the peak-to-peak-amplitude (PTP) of the ventricular QRS complex. (D) Influence of palmitoleic acid on the atrioventricular conduction time (PQ). In all four panels data are given from n=7 experiments as means±s.e.mean. Significant changes versus control conditions (60 min equilibration) are indicated by an * (P<0.05).
In additional experiments the ventricle was paced via a bipolar electrode during the mapping procedure. From the isochrones longitudinal and transversal velocities were assessed. Under control conditions longitudinal velocity was determined 0.56±0.1 m s−1 and transversal velocity 0.26±0.07 m s−1. As could be expected from the literature, we found that palmitoleic acid led to a reduction in transversal conduction velocity more distinctly than longitudinal velocity (to VL=0.36±0.06 (65±6% of the control value) vs VT=0.14±0.06 m s−1 (53±7% of the control) at 20 μM; see also Figure 4).
Figure 4.
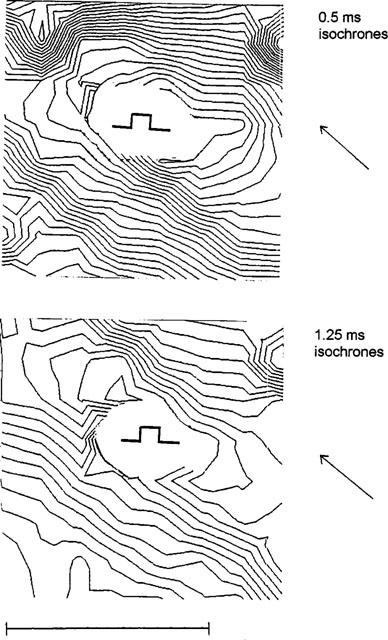
Influence of palmitoleic acid (10 μM) on the isochrone pattern of epicardial activation. The pattern shows the activation of the back wall (8 * 8 mm) with the site of pacing being indicated. The upper panel gives isochrones of the left ventricular wall under control conditions (0.5 ms isochrones), the lower panel after application of palmitoleic acid (10 μM) (1,25 ms isochrones). Note the change in the length to width ratio of the elliptic isochrones. The stimulation point is indicated. The horizontal bar represents 5 mm. The macroscopic fibre orientation in this area is indicated by the arrow.
Experiments on papillary muscles
In rabbit papillary muscles application of palmitoleic acid led to a significant prolongation of the stimulus-response interval starting at a concentration of 0.2 μM. However, the effect was saturated at 2 μM. The conduction velocity was concentration-dependently decreased by the drug. In parallel to this action, the maximum upstroke velocity was slightly (n.s.) enhanced by palmitoleic acid application. There was no influence of the drug on the other parameters of the transmembrane action potential, such as resting membrane potential, overshoot potential and action potential duration (Table 2; original registrations are given in Figure 5).
Table 2.
Effect of cumulative concentrations of palmitoleic acid on transmembrane action potentials recorded from rabbit papillary muscles
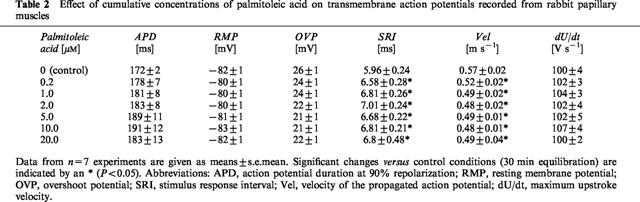
Figure 5.
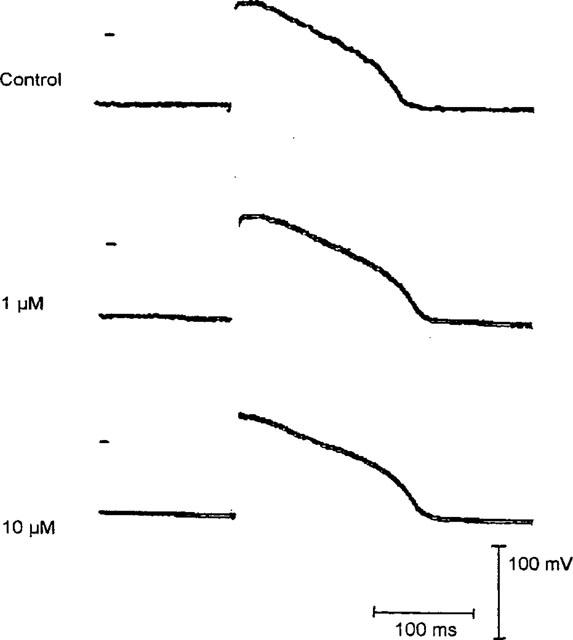
Influence of palmitoleic acid on the shape of rabbit papillary muscle action potentials. The original registrations are recorded under control conditions and after application of 1 and 10 μM palmitoleic acid at a stimulation rate of 1 Hz. The resting membrane potential in these recordings was −81 mV. The bars indicate 100 mV and 100 ms. The zero voltage point is indicated for each trace. Further details are given in the Methods sections.
Experiments on isolated cardiomyocytes
We obtained normally shaped action potentials from the cardiomyocytes with a duration of 350±12 ms (measured at 90% repolarization). After application of palmitoleic acid action potential duration remained unaltered: 345±15 ms at 1 μM palmitoleic acid, 348±10 ms at 10 μM palmitoleic acid, and 352±11 ms at 20 μM palmitoleic acid. Resting membrane potential remained stable at −78±2 mV. An example of an original experiment showing action potentials from a single cardiomyocyte with and without palmitoleic acid is given in Figure 6.
Figure 6.
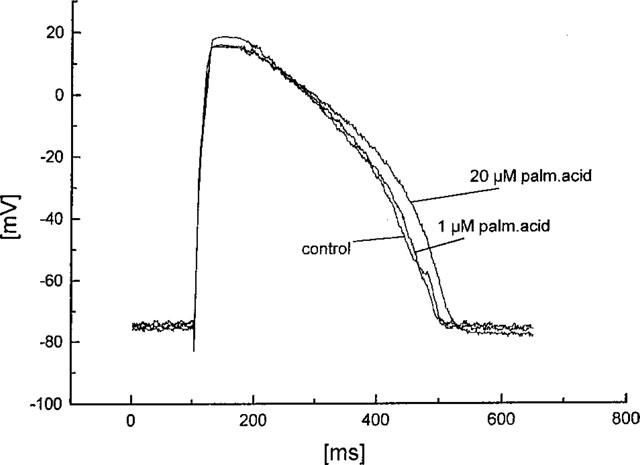
Influence of palmitoleic acid on the shape of action potentials of isolated cardiomyocytes. The original registrations are recorded under control conditions and after application of 1 and 20 μM palmitoleic acid as described in the text.
Discussion
As a main outcome of this study it became obvious that palmitoleic acid dose-dependently altered the epicardial activation pattern, increased the dispersion of the epicardial activation recovery intervals and prolonged the atrioventricular conduction time. Regarding the EC50 values and the maximum effect these parameters were the most sensitive parameters for the action of palmitoleic acid, whereas the breakthroughpoint similarity and the mean epicardial ARI itself remained unaffected.
A first important issue to discuss is the specificity of palmitoleic acid. It is known from previous investigations that palmitoleic acid dose-dependently uncouples gap junctional channels and the substance often is used for that purpose (Burt et al., 1991; Rohr et al., 1997). From the study of Burt and coworkers (1991), an EC50 value for gap junctional uncoupling in pairs of neonatal rat cardiomyocytes in the order of 5 μM is evident. This is in good accordance with the experiments reported in this study; we found a prolongation of the stimulus-response interval in rabbit papillary muscles with a similar EC50. As could be expected the maximum upstroke velocity slightly increased in parallel indicating a reduced current loss to the neighbouring cells. If the cellular coupling is low, i.e. if the intercellular resistance is high, the electrical load of a cell will be low and Vmax will increase and, oppositely, if cellular coupling is high, the electrical load will be high and, thus, Vmax will decline (Spach et al., 1992). Other parameters, such as resting membrane potential, overshoot potential, amplitude and action potential duration were not affected by palmitoleic acid. Thus, in our experiments there was no indication of an influence of palmitoleic acid on transmembrane action potential except uncoupling in the investgated concentration range. It may be noted that with increasing concentration of palmitoleic acid, the standard error or mean of the action potential duration is also slightly, although not significantly, increasing (see Table 2), which might reflect the increase in dispersion observed in the more compex whole heart preparation. However, in papillary muscle the action potential configuration may be affected by electronic interactions because of the multicellular character of this preparation. Thus, we carried out additional experiments in isolated epicardial ventricular myocytes. These single cell experiments confirmed the finding, that palmitoleic acid did not affect action potential duration in the concentration range used in our study.
In the whole heart preparation the duration of the QRS complex was prolonged, as was the total activation time. This indicates a slowing of ventricular conduction. If so, one could expect the peak-to-peak amplitude of the ventricular activation signal to be decreased since according to Spach & Dolber (1986) this parameter reflects the velocity of the activation wave beneath the electrode. The present study indeed showed dose-dependent decrease of the peak-to-peak amplitude. Regarding the anisotropy, we showed in accordance with the results of Brugada and colleagues (1991) that transversal conduction was more affected than longitudinal conduction. It might be speculated that the smaller number of gap junctions at the side-to-side border as compared to the intercalated disks might form the basis for a higher sensitivity of transverse propagation to uncoupling.
Comparing the action of palmitoleic acid on the ventricular conduction as quantitatively measured by its action on the parameters TAT, QRS and PTP, with the effect on atrioventricular conduction time PQ, both total activation time TAT and atrioventricular conduction time were prolonged about 2 fold by application of the highest concentration with similar EC50 values. Interestingly, supraventricular activation of the heart did not fail. The basis of this finding remains uncertain presently, but it might be related to the different isoforms of connexins expressed in the ventricles (mainly Cx43) and the AV-node and AV-bundle (mainly Cx40 (van Kempen et al., 1995); some authors reported low expression of Cx43 also). However, in the AV-nodal region there is only scarce expression of connexins with the gap junctions in end-to-end and side-to-side position (Oesthoek et al., 1993). Thus, one could imagine that conduction in this region is less anisotropic and may be less sensitive to gap junction blockade. If in theory the current source is high enough, propagation can be successfull although resistivity is rather high between the cells. This has been experimentally shown by Rohr and colleagues (1997): if these authors uncoupled a preparation of a strand and a sheet of neonatal cardiomyocytes with palmitoleic acid they found successful propagation from the strand to the sheet. In contrast, if this preparation was well coupled propagation failed due to the lowered current source. Since in our experiments the current source was not reduced, as it would be if sodium channels or calcium channels (in the AV-node) would have been blocked, propagation can be successful in spite of gap junctional uncoupling as shown by Rohr et al. (1997).
Another interesting aspect in this context is the role of ICa.L if gap junctions are uncoupled. Shaw & Rudy (1997) showed in a computer simulation that decreased gap junction coupling can change the dynamics of membrane ionic currents, so that under decreased coupling ICa.L becomes an important component of microscopic propagation and contributes to successfull conduction. If in neonatal cells grown as a strand contacted to a large zone conduction from a small strand to a large area failed and block occurred, enhancement of ICa.L with Bay k 8644 re-established the successfull conduction (Rohr & Kucera, 1995; Rohr et al., 1997). Thus, under the condition of gap junction coupling might critically depend on ICa.L and even very small changes in this current might become relevant, for example in tissues with slowed conduction such as the atrioventricular node. It remains to be elucidated in future investigations whether these effects may contribute to the changes seen in this study.
Regarding the alteration of ventricular activation patterns, one can imagine that this probably is the consequence of the different alteration of transverse and longitudinal conduction. A differential effect on these conduction velocities causes an alteration of the isochrone pattern as can be seen from Figure 4, and this means an alteration of the activation pattern as quantitatively assessed by the vectorfield similarity. Interestingly, we found the same EC50 for the reduction in PTP, i.e. a conduction velocity dependent parameter, and in vectorfield similarity.
The parameter most distinctly affected by palmitoleic acid was the dispersion of ventricular activation recovery intervals (about 6 fold increase). It is known from freshly isolated cells, that the intrinsic action potential duration of cardiomyocytes exhibits some variability. If two adjacent cells in the tissue develop action potentials of different duration thus repolarizing at different timepoints, the consequence is a potential gradient between both cells, which normally is compensated for by a current flowing from one cell to the other via the intercellular gap junction channels (Lesh et al., 1989). Thus, the gap junctional coupling helps to minimize inhomogeneities in the action potential duration, and, vice-versa, uncoupling enhances or unmasks these differences and results in an increase in dispersion. The finding of an enhanced dispersion during uncoupling is in good accordance with the predictions from computer simulation studies (Lesh et al., 1989; Müller & Dhein, 1993). However, besides epicardial dispersion a transmural dispersion may also occur. Intrinsic transmural dispersion or heterogeneity of action potential duration as described by Litovsky & Antzelevitch (1988) is considered to be caused by differences in the expression of repolarizing currents such as IKr, IKs (Antzelevitch et al., 1996; Sicouri et al., 1997) or by differences in It.o. (Litovsky & Antzelevitch, 1988; Fedida & Giles, 1991). This transmural dispersion may be enhanced if gap junction coupling is reduced as was suggested by a computer simulation study of a transmural strand by Visnawathan et al. (1999). Lowering gap junction coupling between the simulated cells (which exhibited different density of IKs or of IKr) maximized transmural action potential duration heterogeneity. This may play a role in our study as well. Unfortunately, this type of dispersion could not be determined in our study for technical reasons.
What is the meaning of these findings for arrhythmogenesis? Enhanced dispersion of action potential duration has been considered a risk factor for arrhythmia since the differential timepoints of repolarization result in a close vicinity of still activated cells and repolarized cells which can be activated again thereby making the heart more prone to reentrant arrhythmia (Kuo et al., 1983). This arrhythmogenic effect is enhanced by slowing of conduction velocity (Pogwizd & Corr, 1987, 1990; Janse & Wit, 1989), which also is seen as a prerequisite of reentry. The arrhythmogenic action is reflected by the marked alteration of the activation patterns with a reduction of VEC to 10%, the threshold for arrhythmogenesis by hypokalemia or digitalis-glycosides as determined in previous studies (Dhein et al., 1990). Thus, one might ask, why didn't the hearts in this study develop arrhythmia? First of all, the rabbit heart is rhythmically rather stable. Secondly, gap junction uncoupling in this study occurred as a global effect. According to many investigators a regional slowing and regionally enhanced dispersion will cause an arrhythmogenic focus, which exhibits altered electrophysiological behaviour than its surrounding, but can activate the electrophysiologically normal adjacent tissue so that the activation wave of the focus can be transferred to the whole heart. In addition, the pattern of breakthroughpoints was not altered by application of palmitoleic acid, so that the fixpoints of activation remained stable. This may be due to the fact, that these points are anatomically defined (Arisi et al., 1983). These observations suggest that pharmacological uncoupling in this study occurred mainly in the ventricular free wall and that this is, if it is a global effect and the breakthroughpoints of epicardial activation are not altered, not (or less) arrhythmogenic. Thus, the induction of conduction arrhythmia in the ventricular myocardium in this model probably requires alteration of the ventricular specific conduction system, too. Since the free area between the unchanged breakthroughpoints is small in a rabbit heart, it could be speculated, that there might be a minimum length required, in which a certain degree of conduction slowing has to be present, to induce arrhythmia in the ventricle under these conditions.
In summary, the present study shows that global uncoupling leads to (1) delayed atrioventricular conduction, (2) increase in dispersion, (3) reduction in conduction velocity, and (4) alteration of activation pattern. With regard to the maximum effect dispersion seems to be a sensitive indicator of uncoupling. In addition, palmitoleic acid might be an interesting substance for investigating the effects of gap junction uncoupling in isolated organ systems.
Limitations of this study
The papillary muscle is known to consist of fibres mainly parallel to each other, thus being activated mainly by longitudinal conduction (Dhein et al., 1997). Consequently, the sensitivity of the papillary muscle to gap junctional uncoupling may be limited since in the heart transversal conduction is more affected by the drug than longitudinal as was shown by Delmar et al. (1987) and in this study.
The use of a mapping system with distinct electrodes has the disadvantage of spatially discrete measurements, which could be overcome with the use of potential-sensitive dyes. However, since these dyes can exert toxic effects and since it is difficult to exclude that the dye may chemically interact with the drug or substance under investigation, we decided to use a multielectrode system for this pharmacological approach.
In addition, the use of palmitoleic acid and its specificity may be a matter of debate. From the papillary muscle experiments a possible effect on epicardial cells cannot be totally ruled out, since it was shown that there is a marked difference in the expression of It.o. between endocardial and epicardial cells (Litovsky & Antzelevitch, 1988; Fedida & Giles, 1991) as well as differences in other potassium currents (Antzelevitch et al., 1996; Sicouri et al., 1997). In addition, an electrotonic interaction masking effects of palmitoleic acid on the intrinsic action potential duration can not be completely excluded by the papillary muscle experiments. However, in the single cell experiments there was no effect of palmitoleic acid on action potential duration. We also did not see a significant effect on action potential amplitude. A small reduction in action potential amplitude might be indicative of a reduction in slow inward current, which could be critical for propagation under decreased gap junction coupling especially in the atrioventricular node. At present we can not fully exclude such a small effect on ICa.L.
Palmitoleic acid is supposed to block gap junction channels by incorporation into the plasma membrane thus disturbing the correct conformation of the channels and probably disturbing the docking as well. Thus, it can be considered a physical uncoupling agent and does not regulatively uncouple (like phorbol esters or others). However, although its mechanism of action is not specific for gap junctions, it seems to be rather specific in the concentration range mainly considered here (0.2–10 μM), since in these concentrations an effect on other parameters of the action potential was not seen.
Since because of technical reasons the intracellular action potentials could not be recorded at the physiological beating rate of a rabbit heart (3–3.5 Hz), the results from the papillary muscle experiments cannot be uncritically transferred to the findings in the whole heart (supported by the Deutsche Forschungsgemeinschaft, Grant Dh 3/6-1 to S.D.).
Abbreviations
- APD
action potential duration
- ARI
activation-recovery interval
- BCL
basic cycle length
- BTP
breakthroughpoint similarity
- CF
coronary flow
- Cx
connexin
- LVP
left ventricular pressure
- OVP
overshoot potential
- PA
palmitoleic acid
- PTP
peak-to-peak voltage
- RMP
resting membrane potential
- SRI
stimulus response interval
- TAT
total activation time
- VEC
similarity of vector fields
- Vel
conduction velocity
References
- ANTZELEVITCH C., SUN Z.Q., ZHANG Z.Q., YAN G.X. Cellular and ionic mechanisms underlying erythromycin-induced long QT intervals and torsade de pointes. J. Am. Coll. Cardiol. 1996;28:1836–1848. doi: 10.1016/S0735-1097(96)00377-4. [DOI] [PubMed] [Google Scholar]
- ARISI G., MACCHI E., BARUFFI S., SPAGGIARI S., TACCARDI B. Potential field on the ventricular surface of the exposed dog heart during normal excitation. Circ. Res. 1983;52:706–715. doi: 10.1161/01.res.52.6.706. [DOI] [PubMed] [Google Scholar]
- BASTIDE B., NEYSES L., GANTEN D., PAUL M., WILLECKE K., TRAUB O. Gap junction protein connexin40 is preferentially expressed in vascular endothelium and conductive bundles of rat myocardium and is increased under hypertensive conditions. Circ. Res. 1993;73:1138–1149. doi: 10.1161/01.res.73.6.1138. [DOI] [PubMed] [Google Scholar]
- BEYER E.C. Molecular cloning and developmental expression of two chick embryo gap-junction proteins. J. Biol. Chem. 1990;265:14439–14443. [PubMed] [Google Scholar]
- BEYER E.C., PAUL D.L., GOODENOUGH D.A. Connexin43: a protein from rat heart homologous to a gap junctional protein from liver. J. Cell. Biol. 1987;105:2621–2629. doi: 10.1083/jcb.105.6.2621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BOERSMA L., BRUGADA J., ABDOLLAH H., KIRCHHOF C., ALLESSIE M. Effects of heptanol, class Ic, and class III drugs on reentrant ventricular tachycardia. Importance of the excitable gap fro the inducibility of double-wave reentry. Circulation. 1994;90:1012–1022. doi: 10.1161/01.cir.90.2.1012. [DOI] [PubMed] [Google Scholar]
- BRUGADA J., MONT L., BOERSMA L., KIRCHHOF C., ALLESSIE M.A. Differential effects of heptanol, potassium, and tetrodotoxin on reentrant ventricular tachycardia around a fixed obstacle in anisotropic myocardium. Circulation. 1991;84:1307–1318. doi: 10.1161/01.cir.84.3.1307. [DOI] [PubMed] [Google Scholar]
- BURT J.M., MASSEY K.D., MINNICH B.N. Uncoupling of cardiac cells by fatty acids: structure-activity relationships. Am. J. Physiol. 1991;260:C439–C448. doi: 10.1152/ajpcell.1991.260.3.C439. [DOI] [PubMed] [Google Scholar]
- DEKKER L.R.C., FIOLET J.W.T., VANBAVEL E., CORONEL R., OPTHOF T., SPAAN J.A.E., JANSE M.J. Intracellular Ca2+, intercellular electrical coupling and mechanical activity in ischemic rabbit papillary muscle. Effects of preconditioning and metabolic blockade. Circ. Res. 1996;79:237–246. doi: 10.1161/01.res.79.2.237. [DOI] [PubMed] [Google Scholar]
- DELMAR M., MICHAELS D.C., JOHNSON T., JALIFE J. Effects of increasing intercellular resistance on transverse and longitudinal propagation in sheep epicardial muscle. Circ. Res. 1987;60:780–785. doi: 10.1161/01.res.60.5.780. [DOI] [PubMed] [Google Scholar]
- DHEIN S. Gap Junction channels in the cardiovascular system: pharmacological and physiological modulation. Trends in Pharmacological Sciences. 1998;19:229–241. doi: 10.1016/s0165-6147(98)01192-4. [DOI] [PubMed] [Google Scholar]
- DHEIN S., HARTBAUER M., MÜLLER W., WINDISCH H., SALAMEH A., TRITTHART H.A. Flecainide alters the cardiac microscopic activation pattern. An in-vitro study using voltage sensitive dyes. Pharmacol. Res. 1997;34:125–130. doi: 10.1006/phrs.1996.0076. [DOI] [PubMed] [Google Scholar]
- DHEIN S., MÜLLER A., GERWIN R., KLAUS W. Comparative study on the proarrhythmic effects of some antiarrhythmic agents. Circulation. 1993;87:617–630. doi: 10.1161/01.cir.87.2.617. [DOI] [PubMed] [Google Scholar]
- DHEIN S., MÜLLER A., KLAUS W. The potential of epicardial activation mapping for the assessment of arrhythmogenic and antiarrhythmic drug activity. J. Pharmacol. Meth. 1989;22:197–206. doi: 10.1016/0160-5402(89)90014-4. [DOI] [PubMed] [Google Scholar]
- DHEIN S., MÜLLER A., KLAUS W. Prearrhythmia: changes preceeding arrhythmia, new aspects by epicardial mapping. Basic Res. Cardiol. 1990;85:285–296. doi: 10.1007/BF01907117. [DOI] [PubMed] [Google Scholar]
- DHEIN S., RUTTEN P., KLAUS W. A new method for analysing the geometry and timecourse of epicardial potential spreading. Int. J. Biomed. Computing. 1988;23:201–207. doi: 10.1016/0020-7101(88)90014-1. [DOI] [PubMed] [Google Scholar]
- DURRER D., VAN DER TWEEL L.H. Spread of activation in the left ventricular wall of the dog. Activation conditions at the epicardial surface. Am. Heart. J. 1954;47:192–203. doi: 10.1016/0002-8703(54)90249-5. [DOI] [PubMed] [Google Scholar]
- FEDIDA D., GILES W.R. Regional variations in action potentials and transient outward current in myocytes isolated from rabbit left ventricle. J. Physiol. (Lond.) 1991;442:191–209. doi: 10.1113/jphysiol.1991.sp018789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GUERRERO P.A., SCHUESSLER R.B., DAVIS L.M., BEYER E.C., JOHNSON C.M., YAMADA K.A., SAFFITZ J.E. Slow ventricular conduction in mice heterozygous for a connexin43 null mutation. J. Clin. Invest. 1997;99:1991–1998. doi: 10.1172/JCI119367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HAVERKAMP W., VOGT B., HINDRICKS G., KOTTKAMP H., SHENASA M., BORGGREFE M., BREITHARDT G.Effects of flecainide, sotalol and other pharmacological interventions on anisotropic conduction Cardiac Mapping 1993Futura: Mount Kisco NY; 237–249.In: Shenasa, M., Borggrefe, M., Breithardt, G. (eds) [Google Scholar]
- HOYT R.H., COHEN M.L., SAFFITZ J.E. Distribution and three-dimensional structure of intercellular junctions in canine myocardium. Circ. Res. 1989;64:563–574. doi: 10.1161/01.res.64.3.563. [DOI] [PubMed] [Google Scholar]
- JANSE M.J., WIT A.L. Electrophysiological mechanisms of ventricular arrhythmias resulting from myocardial ischemia and infarction. Physiol. Rev. 1989;69:1049–1169. doi: 10.1152/physrev.1989.69.4.1049. [DOI] [PubMed] [Google Scholar]
- JOYNER R.W. Effects of the discrete pattern of electrical coupling on propagation through an electrical syncytium. Circ. Res. 1982;50:192–200. doi: 10.1161/01.res.50.2.192. [DOI] [PubMed] [Google Scholar]
- KANTER H.L., SAFFITZ J.E., BEYER E.C. Cardiac myocytes express multiple gap junction proteins. Circ. Res. 1992;70:438–444. doi: 10.1161/01.res.70.2.438. [DOI] [PubMed] [Google Scholar]
- KANTER H.L., SAFFITZ J.E., BEYER E.C. Molecular cloning of two human cardiac gap junction proteins, connexin 40 and connexin 45. J. Mol. Cell. Cardiol. 1994;26:861–868. doi: 10.1006/jmcc.1994.1103. [DOI] [PubMed] [Google Scholar]
- KUO C.S., MUNAKATA K., REDDY C.P., SURAWICZ B. Characteristics and possible mechanism of ventricular arrhythmia dependent on the dispersion of action potential durations. Circulation. 1983;67:1356–1367. doi: 10.1161/01.cir.67.6.1356. [DOI] [PubMed] [Google Scholar]
- LESH M.D., PRING M., SPEAR J.F. Cellular uncoupling can unmask dispersion of action potential duration in ventrcular myocardium. Circ. Res. 1989;65:1426–1440. doi: 10.1161/01.res.65.5.1426. [DOI] [PubMed] [Google Scholar]
- LITOVSKY S.H., ANTZELEVITCH C. Transient outward current prominent in canine ventricular epicardium but not endocardium. Circ. Res. 1988;62:116–126. doi: 10.1161/01.res.62.1.116. [DOI] [PubMed] [Google Scholar]
- MAKOWSKY L., CASPAR D.L.D., PHILLIPS W.C., GOODENOUGH D.A. Gap junction structures. II. Analysis of the x-ray diffraction data. J. Cell. Biol. 1977;74:629–645. doi: 10.1083/jcb.74.2.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MILLAR C.K., KRALIOS F.A., LUX R.L. Correlation between refractory periods and activation recovery intervals from electrograms: effects of rate and adrenergic interventions. Circulation. 1985;72:1372–1379. doi: 10.1161/01.cir.72.6.1372. [DOI] [PubMed] [Google Scholar]
- MÜLLER A., DHEIN S. Sodium channel blockade enhances dispersion of the cardiac action potential duration. A computer simulation study. Basic Res. Cardiol. 1993;88:11–22. doi: 10.1007/BF00788526. [DOI] [PubMed] [Google Scholar]
- MÜLLER A., DHEIN S., KLAUS W. Hetergeneously distributed sensitivities to potassium as a cause of hypokalemic arrhythmias in isolated rabbit hearts. J. Cardiovasc. Electrophysiology. 1991;2:145–155. [Google Scholar]
- MÜLLER A., SCHAEFER T., GOTTWALD M., TUDYKA T., LINKE W., KLAUS W., DHEIN S. Effect of the antiarrhythmic peptide AAP10 on cellular coupling. Naunyn Schmiedeberg's Arch. Pharmacol. 1997;356:76–82. doi: 10.1007/pl00005031. [DOI] [PubMed] [Google Scholar]
- OOSTHOEK P., VIRAGH S., LAMERS W.H., MOORMAN A.F.M. Immunohistochemical delineation of the conduction system. I: The atrioventricular node and Purkinje fibers. Circ. Res. 1993;73:482–491. doi: 10.1161/01.res.73.3.482. [DOI] [PubMed] [Google Scholar]
- PERKINS G., GOODENOUGH D.A., SOSINSKY G. Three-dimensional structure of the gap junction connexon. Biophys. J. 1997;72:533–544. doi: 10.1016/s0006-3495(97)78693-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- POGWIZD S.M., CORR P.B. Reentrant and non-reentrant mechanisms contribute to arrhythmogenesis during early myocardial ischemia: results using three dimensional mapping. Circ. Res. 1987;61:352–371. doi: 10.1161/01.res.61.3.352. [DOI] [PubMed] [Google Scholar]
- POGWIZD S.M., CORR P.B. Mechanisms underlying the development of ventricular fibrillation during early myocardial ischemia. Circ. Res. 1990;66:672–695. doi: 10.1161/01.res.66.3.672. [DOI] [PubMed] [Google Scholar]
- QUAN W., RUDY Y. Unidirectional block and reentry of cardiac excitation: a model study. Circ. Res. 1990;66:367–382. doi: 10.1161/01.res.66.2.367. [DOI] [PubMed] [Google Scholar]
- REAUME A.G., DE SOUSA P.A., KULKARNI S., LANGILLE B.L., ZHU D., DAVIES T.C., JUNEJA S.C., KIDDER G.M., ROSSANT J. Cardiac malformation in neonatal mice lacking connexin43. Science. 1995;267:1831–1834. doi: 10.1126/science.7892609. [DOI] [PubMed] [Google Scholar]
- ROHR S., KUCERA J.P. The calcium inward current can play a critical role for the success of impulse propagation across abrupt expansions of cardiac tissue in the presence of the sodium inward current. Circulation. 1995;92 suppl. I:I–432. [Google Scholar]
- ROHR S., KUCERA J.P., FAST V.G., KLÉBER A.G. Paradoxical improvement of impulse conduction in cardiac tissue by partial cellular uncoupling. Science. 1997;275:841–844. doi: 10.1126/science.275.5301.841. [DOI] [PubMed] [Google Scholar]
- SAFFITZ J.E., DAVIS L.M., DARROW B.J., KANTER H.L., LAING J.G., BEYER E.C. The molecular basis of anisotropy: role of gap junctions. J. Cardiovasc. Electrophysiol. 1995;6:498–510. doi: 10.1111/j.1540-8167.1995.tb00423.x. [DOI] [PubMed] [Google Scholar]
- SAFFITZ J.E., KANTER H.L., GREEN K.G., TOLLEY T.K., BEYER E.C. Tissue specific determinants of anisotropic conduction velocity in canine atrial and ventricular myocardium. Circ. Res. 1994;74:1065–1070. doi: 10.1161/01.res.74.6.1065. [DOI] [PubMed] [Google Scholar]
- SEVERS N.J. The cardiac gap junction and intercalated disk. Int. J. Cardiol. 1990;26:137–173. doi: 10.1016/0167-5273(90)90030-9. [DOI] [PubMed] [Google Scholar]
- SHAW R.M., RUDY Y. Ionic mechanisms of propagation in cardiac tissue. Roles of the sodium and L-type calcium currents during reduced excitability and decreased gap junction coupling. Circ. Res. 1997;81:727–734. doi: 10.1161/01.res.81.5.727. [DOI] [PubMed] [Google Scholar]
- SICOURI S., MORO S., LITOVSKY S., ELIZARI M.V., ANTZELEVITCH C. Chronic amiodarone reduces transmural dispersion of repolarization in the canine heart. J. Cardiovasc. Electrophysiol. 1997;8:1269–1279. doi: 10.1111/j.1540-8167.1997.tb01018.x. [DOI] [PubMed] [Google Scholar]
- SIMON A.M., GOODENOUGH D.A., PAUL D.L.Role of Cx40 in cardiac conduction Gap Junctions 1998IOS Press: Amsterdam; 299–303.In: Werner, R. (ed.) [Google Scholar]
- SPACH M.S., DOLBER P.C. Relating extracellular potentials and their derivatives to anisotropic propagation at a microscopic level in human cardiac muscle. Circ. Res. 1986;58:356–371. doi: 10.1161/01.res.58.3.356. [DOI] [PubMed] [Google Scholar]
- SPACH M.S., HEIDLAGE J.F. The stochastic nature of cardiac propagation at a microscopic level. Electrical description of myocardial architecture and its application to conduction. Circ. Res. 1995;76:366–380. doi: 10.1161/01.res.76.3.366. [DOI] [PubMed] [Google Scholar]
- SPACH M.S., HEIDLAGE J.F., DARKEN E.R., HOFER E., RAINES K.H., STARMER C.F. Cellular Vmax reflects both membrane properties and the load presented by adjoining cells. Am. J. Physiol. 1992;263:H1855–H1863. doi: 10.1152/ajpheart.1992.263.6.H1855. [DOI] [PubMed] [Google Scholar]
- THOMAS S.A., SCHUESSLER R.B., BERUL C.I., BEARDSLEE M.A., BEYER E.C., MENDELSOHN M.E., SAFFITZ J.E. Disparate effects of deficient expression of connexin43 on atrial and ventricular conduction: evidence for chamber-specific molecular determinats of conduction. Circulation. 1998;97:686–691. doi: 10.1161/01.cir.97.7.686. [DOI] [PubMed] [Google Scholar]
- VAN KEMPEN M.J.A., TEN VELDE I., WESSELS A., OOSTHOEK P.W., GROS D., JONGSMA H.J., MOORMAN A.F., LAMERS W.H. Differential connexin distribution accommodates cardiac function in different species. Microsc. Res. Tech. 1995;31:420–436. doi: 10.1002/jemt.1070310511. [DOI] [PubMed] [Google Scholar]
- VEENSTRA R.D., BERG K., WANG H.Z., WESTPHALE E.M., BEYER E.C.Molecular and biophysical properties of the connexins from developing chick heart Progress in Cell Research 19933Elsevier Science Publishers: Amsterdam, 1993; 89–95.In: Hall, J.E., Zampighi, G.A. & Davis, R.M. (eds) [Google Scholar]
- VERHEULE S., VAN KEMPEN M.J.A., TE WELSCHER P.H.J.A., KWAK B.R., JONGSMA H.J. Characterization of gap junction channels in adult rabbit atrial and ventricular myocardium. Circ. Res. 1997;80:673–681. doi: 10.1161/01.res.80.5.673. [DOI] [PubMed] [Google Scholar]
- VISWANATHAN P.C., SHAW R.M., RUDY Y. Effects of IKr and IKs heterogeneity on action potential duration and its rate dependence: a simulation study. Circulation. 1999;99:2466–2474. doi: 10.1161/01.cir.99.18.2466. [DOI] [PubMed] [Google Scholar]
- WILLECKE K., BUCHMANN A., BÜTZLER C., GABRIEL H.D., HAGENDORFF A., JUNG D., KIRCHHOFF S., KRÜGER O., NELLES E., SCHWARZ M., TEMME A., TRAUB O., WINTERHAGER E.Biological functions of gap junctions revealed by targeted inactivation of mouse connexin32, −26 and −40 genes Gap. Junctions 1998IOS Press: Amsterdam; 304–308.In: Werner, R. (ed.) [Google Scholar]
- WIT A.L., DILLON S.M.Anisotropic reentry Cardiac Mapping 1993Futura: Mount Kisco NY; 127–154.In: Shenasa, M., Borggrefe, M., Breithardt, G. (eds) [Google Scholar]


