Abstract
Neurotrophic factors have been used for the treatment of several neurodegenerative diseases. However, their use is limited by their inability to cross the blood-brain barrier, their short half life and their side effects. SR 57746A is a new orally active compound that exhibits in vivo and in vitro neurotrophic effects in several experimental models.
We show here that SR 57746A (1 μM) increases the phenotypic survival of embryonic purified mouse motoneurons in vitro to the same extent as brain-derived neurotrophic factor (100 ng ml−1), and increases the outgrowth and number of their neurites. It acts in a dose-dependent manner up to 1 μM which is the optimal concentration. Above this concentration, its neurotrophic effect decreases.
Genistein (10 μM), a protein tyrosine kinase inhibitor, also increases the phenotypic survival and differentiation of mouse motoneurons. It does not act in a synergistic or additive manner with SR 57746A. However, at concentrations equal or superior to 25 μM, it decreases the survival of motoneurons. This suggests that the neurotrophic effect of genistein is due to a favourable alteration of equilibrium between phosphorylated and dephosphorylated states of proteins involved in survival and differentiation of motoneurons.
Like genistein, SR 57746A should be used at a critical concentration (1 μM) to exert its optimal effects. Since SR 57746A does not act synergistically with genistein, it is likely that its mechanism of action involves a pathway similar to that affected by this tyrosine kinase inhibitor.
At the present time, SR 57746A is the only orally active compound and the only synthetic compound shown to be active on motoneurons in vitro. It should thus be considered as a good candidate for the treatment of motoneuron diseases.
Keywords: BDNF, genistein
Introduction
Neurotrophins (NT)–such as NGF (nerve growth factor), BDNF (brain-derived neurotrophic factor), NT-3 (neurotrophin 3), NT-4/5 (Korsching, 1993; Lindsay et al., 1994), and NT-6 (Götz et al., 1994)–constitute a family of functionally and structurally related factors which promote survival and differentiation of neurons from peripheral and central nervous tissues (Barde, 1989).
For instance, NGF prevents the developmental death of sensory and sympathetic peripheral neurons (Levi-Montalcini, 1987), BDNF prevents the injury-induced degeneration of adult motoneurons (Yan et al., 1994; Friedman et al., 1995), and NT-3 partially rescues neonatal spinal motoneurons from injury-induced degeneration (Yan et al., 1993). These observations and many others have prompted neurologists to start studies on the therapeutic effects of these factors in neurodegenerative disorders and nerve injury (Olson, 1994). It appeared rapidly that their use was limited by their short half-life, their inability to cross the blood-brain barrier, and their side effects.
To overcome these difficulties, synthetic, nonpeptide compounds have been developed and studied for their neurotrophic properties, their ability to reach the nervous system, and their toxicity. SR 57746A {1-[2-(naphth-2-yl) ethyl] -4- (3-trifluoromethylphenyl) -1,2,5,6-tetra-hydropyridine hydrochloride} is one of these molecules. It is able to cross the blood-brain barrier. It displays neurotrophic activity both in vitro and in vivo. In vitro, it potentiates the promoting effect of NGF on neurite outgrowth of PC12 cells (Fournier et al., 1992; Pradines et al., 1995) and attenuates the cytostatic-induced reduction of neurite outgrowth in cocultures of rat dorsal root ganglia and Schwann cells (Ruigt et al., 1996). In vivo, it prevents the decrease of choline acetyltransferase (ChAT) and acetylcholinesterase (AChE) activities in a model of neurodegenerative in rats (Fournier et al., 1993). Recently, it was shown in our laboratory that SR 57746A increased the mean survival time and the motor performances of pmn mice (progressive motor neuronopathy) (Duong et al., 1998), a strain which displays an inherited axonopathy–resembling Charcot Marie-Tooth type II disease (Kennel et al., 1996). The molecular mechanisms of action of SR 57746A remains poorly understood. However, Pradines et al. (1995) have reported that tyrosine kinase inhibitors could mimic the effects of this compound in PC12 cells. It is the reason why we compared the effects of SR 57746A and those of genistein (4′,5,7-trihydroxyisoflavone), a tyrosine kinase inhibitor, on survival and morphology of purified spinal motoneurons from healthy mouse embryo. Genistein is a member of a large family of molecules which inhibits the tyrosine kinase activity of epidermal growth factor (EGF) receptor, pp60src and pp110gag-fes (Akiyama et al., 1987). It has a weak inhibitory activity on serine/threonine kinases, cyclic AMP-dependent protein kinases and protein kinase C. Morever, several studies have shown that genistein stimulates the effects of glial cell line-derived neurotrophic factor (GDNF) on neurite outgrowth (Hiwasa et al., 1997) and acts synergistically with nerve growth factor on survival of chick embryonic sensory neurons (Bartlett, 1997).
We present here results showing that SR 57746A, genistein, and SR 57746A plus genistein increase phenotypic survival of mouse purified motoneurons and induce morphological changes possibly by acting on phosphorylation cascades.
Methods
Drugs
SR 57746A (Sanofi Recherche, Montpellier, France) (Mol wt 417.90) was dissolved in dimethyl sulphoxide (DMSO; Sigma, l'Isle d'Abeau, France), then diluted in motoneuron culture medium and added to the cells to give a final concentration from 0.25–5 μM. Human brain-derived neurotrophic factor (BDNF; Tebu, Le Perray-en-Yvelines, France) was used at final concentrations of 50 ng ml−1 or 100 ng ml−1. Genistein (Ref. G-6649, Sigma) was dissolved in DMSO and used at final concentrations ranging between 10 and 100 μM.
Mouse motoneuron cultures
Motoneurons were prepared according to the method described by Martinou et al. (1992) for rat embryos, after being adapted to mouse embryos in our laboratory. Briefly, spinal cord of E13-E14 Swiss mouse embryos were dissected and incubated for 20 min at room temperature in 0.025% (weight [W]/volume [V]) isotonic trypsin solution (LPCR, Schiltigheim, France). They were then mechanically dissociated by several passages through the 21 gauge needle of a syringe. The resulting suspension was first enriched in motoneurons by centrifugation for 10 min at 120×g at room temperature on a layer of 3.5% solution of bovine serum albumin (BSA) in Ca2+-and Mg2+-free Hanks balanced salt solution (HBSS−). The supernatant was discarded, the pellet was resuspended in HBSS−, the suspension was layered over a cushion of Optiprep (d: 1.06 g ml−1; Nycomed Pharma AS, Oslo, Norway) and centrifuged for 25 min, at 400×g, at room temperature. The upper phase, containing the purified motoneurons, was collected, centrifuged for 10 min at 700×g at room temperature, then the pellet was resuspended in 3 ml of HBSS− and centrifuged again for 10 min, at 700×g, at room temperature. The cell pellet was finally suspended in a defined culture medium which consisted of a mixture (v v−1) of Dulbecco's modified basal medium of Eagle (DMEM) and Ham F12 (Gibco-BRL, Life Technologies, Cergy Pontoise, France) supplemented with 5 μg ml−1 insulin, 10 μg ml−1 human transferrin, 0.1 mM putrescein, 1 pM oestradiol, 20 nM progesterone, 300 μl of a solution of 175 μg ml−1 sodium selenite prepared in phosphate-buffered saline (PBS in mM): pH 7.4, glucose 33, glutamine 2, sodium bicarbonate 14 (all from Sigma), 25 μg ml−1 gentallin (Gibco-BRL). Cells were seeded in 24-well plates (Nalge Nunc, Polylabo, Illkirch, France), precoated for 3 h with a 0.3 mg ml−1 aqueous solution of poly-L-lysine (Sigma) and cultured at 37°C in a humidified air (95%)–CO2 (5%) atmosphere. SR 57746A, BDNF and/or genistein were added to the culture medium immediately after seeding. Whichever the treatments or experimental conditions used, all the wells contained DMSO at a final concentration of 0.02%. Approximately 60% of the medium was replaced every 2 days with fresh medium.
Motoneuron identification
Motoneurons express high levels of ChAT activity (Camu & Henderson, 1992) and this property allows it to distinguish them from other spinal cells which express much less of this enzyme. It is therefore possible to identify motoneurons by immunolabelling with an anti-ChAT antibody. For this purpose, cultures were fixed with a 4% (v v−1) solution of paraformaldehyde in PBS for 30 min, and then treated overnight with a goat anti-choline acetyltransferase antibody (AB144, Chemicon, Temecula, CA, U.S.A.). After being washed, cells were incubated for 1 h with a biotin-labelled rabbit anti-goat antiserum (Sigma) and then with streptavidin-peroxidase (Immunotech, Marseille, France). The purity of a culture was assessed by determining the percentage of immunolabelled cells using a phase contrast microscope (Nikon, Tokyo, Japan). Usually, more than 90% of cells were positive for ChAT.
Cell counting and neurite measurement
Motoneurons were counted 2 h after the culture and then every 2 days by examining under a phase contrast microscope five randomly selected microscopic fields per well. Results were expressed as percentage of phenotypic surviving motoneurons or as a ratio of percentages (percentage of surviving motoneurons in treated cultures/percentage of surviving motoneurons in control cultures). Each experimental condition was tested in duplicate and experiments were repeated at least twice.
Neurite number and length were determined after immunolabelling cells with an anti-β-tubulin antibody. Briefly, motoneurons were fixed with a mixture of methanol (95 V) and glacial acetic acid (5 V) for 10 min, washed with PBS, and treated for 30 min with PBS containing 0.1% triton and 3% BSA. They were then treated for 1 h with a mouse monoclonal anti-β-tubulin antibody (T-4026; Sigma) diluted in 0.5% BSA–0.1% Triton X-100–PBS to 1/1000 and finally with a Cy3-labelled goat anti-mouse serum (115-165-044, Interbiotech, Montluçon, France) diluted in 0.5% BSA–0.1% Triton X-100–PBS to 1/800. The preparation was observed under a fluorescence microscope. Neurite length was measured using an objective micrometer. Results were expressed as mean number and mean length of neurites per cell and per experimental condition±standard error of the mean (s.e.mean). For each condition, 100 motoneurons were observed and only the length of the longest neurite was measured.
Statistical analysis
Differences between treated and control cultures were studied as follows. For each time point, motoneuron survival was evaluated using the one group t-test. The number and length of neurites were compared using a one way ANOVA test, followed by Fisher test for multiple comparisons. All statistical analysis were performed using Statview Student software (Abacus Concepts Inc., Berkeley, CA, U.S.A.).
Results
Effects of DMSO and BDNF on phenotypic motoneuron survival
Under our experimental conditions, we observed that our culture contained more than 90% motoneurons (91.6±2.4%; n=2), and that DMSO did not modify their survival time (Figure 1).
Figure 1.
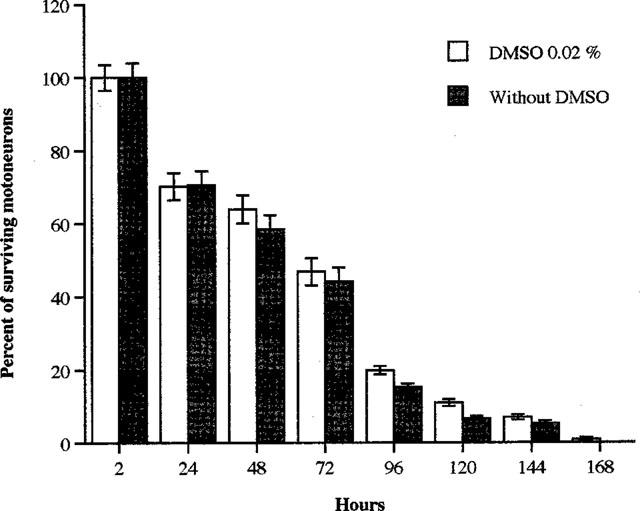
Dimethyl sulphoxide does not promote mouse purified motoneuron survival. DMSO (0.02%) was added to the culture immediately after seeding. Cell counting was performed at 2, 24, 48, 72, 96, 120, 144 and 168 h as described in Methods. Results are expressed as a percentage of surviving motoneurons±s.e.mean. No statistical differences were observed (Student's t-test).
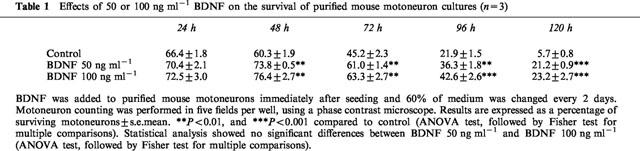
BDNF, a reference neurotrophic substance, used at 50 and 100 ng ml−1 significantly increased the percentage of phenotypic surviving motoneurons, as compared with control cultures. At all time points studied, this percentage was slightly, but not significantly, higher in cultures treated with 100 ng ml−1 BDNF than with 50 ng ml−1 (Table 1).
Table 1.
Effects of 50 or 100 ng ml−1 BDNF on the survival of purified mouse motoneuron cultures (n=3)
Effects of SR 57746A on phenotypic motoneuron survival
Given at a concentration range of 0.25 μM–1 μM, SR 57746A increased the percentage of surviving motoneurons in a dose-dependant manner (Figure 2). At 5 μM, this percentage decreased but remained significantly higher than in control cultures.
Figure 2.
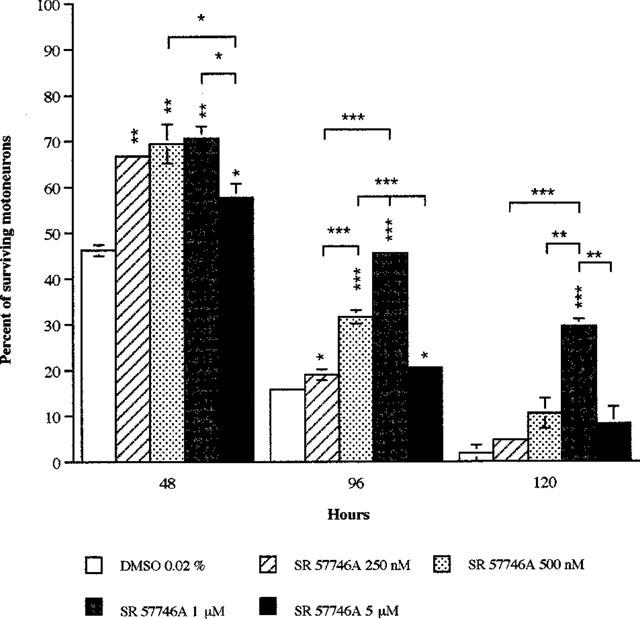
Dose-dependant effects of SR 57746A on survival of purified mouse motoneurons. Purified mouse motoneurons were incubated at 37°C with a range of SR 57746A concentrations from 0.25–5 μM immediately after seeding. For control cultures, DMSO (0.02%) was added to the medium instead of the drug. Motoneuron counting was performed at 48, 96 and 120 h as described in Methods. Results are expressed as a mean percentage of surviving motoneurons±s.e.mean for two independent experiments. *P<0.05, **P<0.01, ***P<0.001 (ANOVA test, followed by Fisher test for multiple comparisons).
When treated with 1 μM SR 57746A, motoneurons responded as well as when treated with 100 ng ml−1 BDNF (Figure 3). After 48 and 120 h of treatment both agents significantly increased (P<0.05; treated cultures versus control) the percentage of surviving cells. After 96 h, the only significant difference (P<0.05) was observed with BDNF, although SR 57746A also increased, non significantly, this percentage. Six days after seeding, the percentage of phenotypic surviving motoneurons in SR 57746A-treated cultures was approximately 20% whereas no surviving motoneurons could be detected in control cultures.
Figure 3.
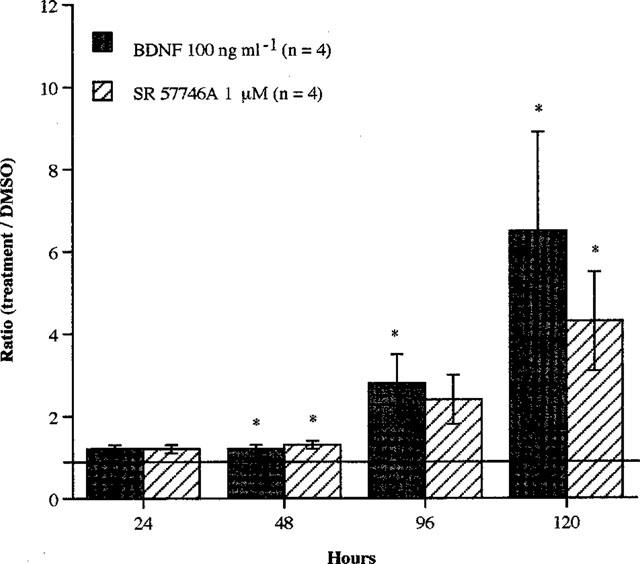
Effects of BDNF or SR 57746A on survival of purified mouse motoneurons. Purified mouse motoneurons were incubated at 37°C with BDNF (100 ng ml−1) or SR 57746A (1 μM) immediately after seeding. For control cells, DMSO (0.02%) was added to the culture medium instead of the drugs. Motoneuron counting was performed at 24, 48, 96 and 120 h as described in Methods. Data shown are the ratio (percentage of surviving motoneurons in treated cultures compared to control cultures) ±s.e.mean for four independent experiments, each conducted with duplicate determinations. *P<0.05 compared to control (ANOVA one way test, followed by Fisher test for multiple comparisons).
Combining SR 57746A (1 μM) and BDNF (100 ng ml−1) did not increase the survival of motoneurons as compared with that of motoneurons treated either with SR 57746A or BDNF alone. These two agents acted neither in additive nor synergistic manner (data not shown).
Effects of genistein on phenotypic motoneuron survival
SR 57746A could exert its action through activation of tyrosine kinases. To explore this hypothesis, we studied the effect of genistein, a tyrosine kinase inhibitor, on phenotypic motoneuron survival. Table 2 shows that at concentrations of 25 μM and more, genistein reduced the percentage of surviving motoneurons in a dose-dependent manner. By contrast, at 10 μM, genistein significantly increased this percentage 48, 72 and 96 h after the beginning of the treatment.
Table 2.
Effects of a protein tyrosine kinase inhibitor, genistein on the survival of purified mouse motoneuron cultures

Comparison of SR 57746A and genistein on phenotypic motoneuron survival
Figure 4 shows that SR 57746A (1 μM) significantly increased the percentage of surviving cells, by the same order of magnitude as genistein (10 μM) (48 h: P<0.01 [genistein versus control]; P<0.05 [SR 57746A versus control]; 120 h: P<0.05 [genistein and SR 57746A versus control]). When used in combination with SR 57746A (1 μM), genistein (10 μM) was not more active than when used alone or than SR 57746A alone. Both neuroprotective agents did not act in additive or synergistic manner.
Figure 4.
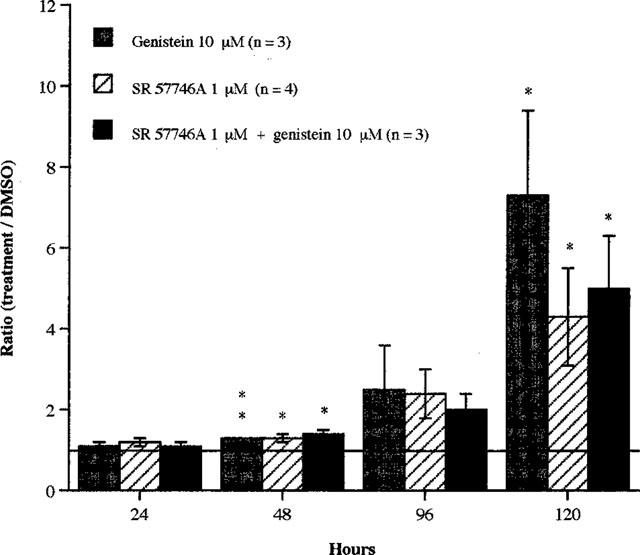
Effects of SR 57746A and/or genistein on survival of purified mouse motoneurons. Purified mouse motoneurons were incubated at 37°C with SR 57746A (1 μM) and/or genistein (10 μM) immediately after seeding. For control cells, DMSO (0.02%) was added to the culture medium instead of the drugs. Motoneuron counting was performed at 24, 48, 96 and 120 h as described in Methods. Data shown are the ratio (percentage of surviving motoneurons in treated cultures compared to control cultures) ±s.e.mean for three or more independent experiments, each conducted with duplicate determinations. *P<0.05, **P<0.01 compared with control (ANOVA one way test, followed by Fisher test for multiple comparisons).
Effect of BDNF, SR 57746A and genistein on neuritogenesis
Figure 5 shows the appearance of control motoneurons or motoneurons treated for 2, 4 or 5 days with SR 57746A (1 μM) or BDNF (100 ng ml−1). In all the culture conditions, 48 h after being seeded, the motoneurons emitted short neurites. At that time, there was no clear-cut difference between control and treated cells. By contrast, after 96 h of culture, neurites of control motoneurons disappeared whereas in SR 57746A-(1 μM), BDNF (100 ng ml−1) or SR 57746A plus BDNF-treated cultures, neurites continued to grow and remained intact up to 120 h after the beginning of the treatment.
Figure 5.
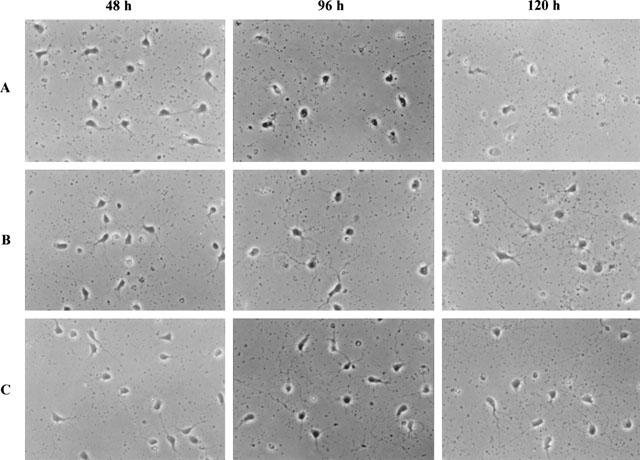
Morphology of motoneurons after 48, 96 and 120 h treatment with BDNF or SR 57746A. 0.02% DMSO (A), 100 ng ml−1 BDNF (B) or 1 μM SR 57746A (C) was added to the culture immediately after seeding. Observations were made by using a phase contrast microscope at 48, 96 and 120 h. Original magnification, 400×.
When motoneurons were treated for 24 h with BDNF (100 ng ml−1) their neurites were significantly more numerous (P<0.001) and longer (P<0.05) than those of untreated motoneurons (Figure 6A,B). Treating motoneurons with either SR 57746A (1 μM), genistein (10 μM) or both (respective concentrations as above), resulted in a striking increase in neurite length (P<0.001; treated versus control cells). No significant differences were noticed between SR 57746A (1 μM) and genistein (10 μM), or SR 57746A and SR 57746A plus genistein-treated motoneurons. Moreover, SR 57746A, and genistein combined to SR57746A, did not exert such effects.
Figure 6.
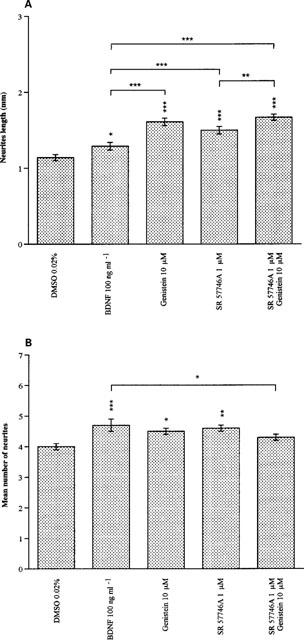
Effects of BDNF, SR 57746A and genistein on motoneuron morphological changes after 24 h culture. Purified mouse motoneurons were incubated with BDNF (100 ng ml−1), genistein (10 μM), SR 57746A (1 μM) or genistein (10 μM) combined with SR 57746A (1 μM) immediately after seeding. For control cells, DMSO (0.02%) was added to the culture medium instead of the drugs. Cells were immunolabelled with an antiboby anti β-tubulin 24 h after treatment. (A) Neurite length was measured by using a micrometer coupled with a fluorescent microscope. For each culture condition, 100 motoneurons were observed and the longest neurite of each was measured. Results are expressed as the mean length (mm) ±s.e.mean. (B) Neurites were counted using a fluorescent microscope. Data shown are the mean number of neurites ±s.e.mean. *P<0.05, **P<0.01 and ***P<0.001 (ANOVA test, followed by Fisher test for multiple comparisons).
Figure 7 shows control and treated motoneurons, immunolabelled for β-tubulin. It confirms that SR 57746A and genistein promoted neuritogenesis.
Figure 7.
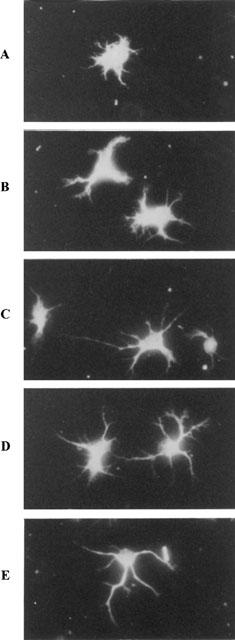
Morphology of motoneurons after 24 h treatment with BDNF, genistein, SR 57746A or SR 57746A plus genistein. Purified mouse motoneurons were incubated with 0.02% DMSO (A), 100 ng ml−1 BDNF (B), 10 μM genistein (C), 1 μM SR 57746A (D) or 10 μM genistein combined with 1 μM SR 57746A (E) immediately after seeding. Cells were immunolabelled with an anti β-tubulin antibody 24 h after treatment. Photographs were made using a fluorescence microscope. Original magnification, 400×.
Therefore beside their effects on phenotypic motoneuron survival, SR 57746A and genistein are also able to stimulate neurite outgrowth and elongation as reflected by increase in neurite number and length respectively.
Discussion
The present results first show that it is possible to prepare enriched mouse motoneuron suspensions containing more than 90% of ChAT positive cells, by starting from E13-E14 embryos–instead of E15 embryos as recommended by Schnaar & Schaffner (1981)–and using a method simpler than that described by Ang et al. (1993) who used metrizamide gradients. Under our experimental conditions, mouse motoneurons could survive in special culture medium often up to 144 h, with 50% of them usually surviving 48–72 h after seeding.
We also show that, as expected, BDNF increased the percentage of phenotypic surviving motoneurons. This observation confirms results obtained in vivo and in vitro by several authors. For instance, it has been shown, in vivo, that BDNF prevents the natural death of chick motoneurons (Oppenheim et al., 1993), injury-induced loss of cholinergic neurons (Tuszynski et al., 1996) and motoneuron degeneration occurring after sciatic nerve section (Li et al., 1994). In vitro, BDNF was shown to increase the survival of rat embryonic motoneurons in culture (Hughes et al., 1993). BDNF can be considered as a reference neurotrophic factor for motoneurons to whom all putative neurotrophic agents should be compared, and this is the reason why BDNF was included in the present study.
SR 57746A was shown here to promote phenotypic survival and neuritogenesis of purified mouse motoneurons, in a dose dependant manner between 0.25 and 1 μM. At 1 μM, it was as active as 100 ng ml−1 BDNF but was unable to act in an additive or synergistic manner with this factor. This suggests that both agents could partly act through a common, parallel or branched pathway. Finally, genistein, a potent inhibitor of tyrosine kinases, used at a concentration of 10 μM, was as active as SR 57746A (1 μM) or BDNF (100 ng ml−1) but did not act in an additive or synergistic way with the former agent. Here again, this suggests that genistein and SR 57746A could partly act through the same pathway. Above 10 μM, genistein was detrimental for mouse motoneurons. Thus, as for SR 57746A, genistein should be used at an optimal concentration.
BDNF, the effect of which is partly mimicked by SR 57746A and genistein, binds to tropomyosin receptor kinase B (trkB), a receptor possessing a cytoplasmic tyrosine kinase domain. When recognized by its ligand, trkB undergoes autophosphorylation which in turn triggers phosphorylation of numerous intracellular proteins (Iwasaki et al., 1997; Maher, 1988). SR 57746A could act like BDNF either by binding to trkB–which seems very unlikely and is not supported by experimental facts–or by inducing biosynthesis of endogenous BDNF. Recently, indeed, Labie et al. (1998) reported that SR 57746A induced a specific increase in BDNF mRNA levels in rat hippocampal astrocytes, 6–9 h after addition of the compound to the culture. We can practically exclude this possibility in the present study, since motoneurons do not produce BDNF and our preparations contained more than 90% of these cells. The most likely targets of SR 57746A may be intracellular kinases and phosphatases. Pradines et al. (1995) indeed have shown that incubation of protein tyrosine kinases inhibitors such as genistein (200 μM) and lavendustin (10 μM) is unable to inhibit the response of PC12 cells to SR 57746A or NGF, whereas sodium orthovanadate (250 μM) but not okadaic acid (400 nM), inhibitors of protein tyrosine phosphatases, were able to do so. They concluded that one or more endogenously activated protein phosphatases were involved in the early stages of signal transduction in SR 57746A- and NGF-induced PC12 functional responses. Moreover, Bartlett (1997) has reported that genistein (2.5–10 μM) synergized with NGF on sensory neuron survival, and stimulated GDNF-induced neurite formation.
It is rather surprising that tyrosine kinase inhibitors exert on phenotypic motoneuron survival effects analogous to those of neurotrophic factors which are known to activate tyrosine kinases. However, in our experiments, genistein was used at a concentration which could provide the intracellular medium with a favourable balance between phosphorylated and dephosphorylated proteins and enzymes involved in motoneuron survival. This interpretation could explain why the phenotypic survival dose-response curve for SR57746A was bell-shaped (an analogous although less pronounced dose-response curve was observed by Fournier et al., 1997 with septal neurons from rat embryo in primary culture upon exposure to SR 57746A at doses ranging between 100 and 1000 nM; the decreased response occurring beyond 500 nM). By modifying the optimal equilibrium between phosphorylated and dephosphorylated survival factors, SR 57746A used at supraoptimal concentrations can decrease the survival response, before appearance of any sign of cytotoxicity. As noted by Charbonneau & Tonks (1992), indeed, tyrosine phosphorylation of certain proteins in response to mitogens or growth factors can trigger the translocation of protein tyrosine phosphatases containing specific domains (such as SH2) to specific intracellular sites and thereby restrict the substrates that they can dephosphorylate. Alternatively, if the activity of phosphatases is modulated by the binding of phosphotyrosyl residues to their SH2 domains, another level of control may exist which could be involved, for example by an auto-inhibition mechanism if the protein tyrosine phosphatase is itself phosphorylated on tyrosyl residues. By playing on phosphorylation-dephosphorylation cascades, it is therefore possible to mimic or to modulate effects of mitogens or growth factors.
Genistein (10 μM) also inhibits the expression of many proto-oncogenes such as c-src, c-yes, c-fps, c-ros, c-abl and c-erbB which all code for tyrosine kinases normally expressed in developing and regenerating neurons (Ignelzi et al., 1992). If motoneuron death results from inappropriate and unsuccessful attempts to re-enter the cell cycle (Ferrari & Greene, 1994; Park et al., 1996), then the inhibition of expression of proteins involved in cell division and proliferation might be able to rescue post-mitotic cells from death.
Beside its effects on phenotypic survival, SR 57746A promotes neuritogenesis. Here again, it is possible that this effect be mediated by an alteration of phosphorylation-dephosphorylation cascade. Miller et al. (1993), indeed, have observed that inhibition of tyrosine kinase activity in PC12 cells increased neurite outgrowth. They suggested that tyrosine kinase inhibition could enhance post-mitotic cell survival by maintaining their neurites, which otherwise would die in the absence of neurotrophic factors. They also pointed out that increase in the outgrowth rate of neurites results in decrease in adhesion of growth cone to substrate.
The nerve growth cone is a mobile structure containing large amounts of cytoskeletal proteins; in vivo, it guides developing neurites through the tissues up to their targets; in vitro, it guides neurites onto the culture substrate. The plasma membrane of the growth cone is very rich in pp60c-src, a protein kinase encoded by c-src gene. This tyrosine kinase plays an important role in growth cone-mediated axon elongation, by modifying its adherence on extracellular matrix or culture substrate and modulating the polymerization of cytoskeletal components (Maness et al., 1988). Miller et al. (1993) have shown that tyrosine kinase activity negatively regulates neurite elongation by affecting directly or indirectly surface dynamics at the growth cone. Pradines et al. (1995) have observed that SR 57746A induced a rapid redistribution of F-actin and enhanced the level of α-actinin in PC12 cells. Taken together with our results, these observations suggest that SR 57746A probably modulates motoneuron morphology by influencing pp60c-src activity, as does genistein, and induces changes in cytoskeletal protein content.
Since SR 57746A was added to culture immediately after plating, the effects observed on cells survival may be due to an increase initial adherence. Several reports (Heuertz et al., 1997; Detmers et al., 1998; Widmann et al., 1999) have shown that mitogen-activated protein kinases (MAPK) are implicated in cell adhesion. We have observed (unpublished results) that SR 57746A induces a rapid activation of MAPK in PC12 cells. Therefore, the main effect of this compound reported in this paper could be related to an increase adherence of cells. However, the fact that the increase of MAPK activation by SR 57746A is transient, and that the percentage of phenotypic survival of SR 57746A treated-motoneurons for 24 h is identical with those untreated, we can exclude the possibility that the entire observed effects are due to an increase initial adherence.
In summary, SR 57746A could increase phenotypic motoneuron survival by preventing them to inadequately re-enter the cell cycle and maintaining neurite integrity. Due to its neurotrophic properties, SR 57746A should be considered as a serious candidate for the treatment of motoneuron diseases.
Acknowledgments
The authors are very grateful to Olivier Dorchies and Nathalie Leclerc for their help with immunocytochemistry and cell culture. We wish to thank Dr Peter E. Keane, Dr Jean-Pierre Gies and Dr Kenneth Takeda for critical reading of our manuscript. This work was supported by the Association Française contre les Myopathies (AFM) and Sanofi Recherche.
Abbreviations
- AChE
acetylcholinesterase
- BDNF
brain derived neurotrophic factor
- BSA
bovine serum albumin
- ChAT
choline acetyltransferase
- DMEM
Dulbecco's modified basal medium of Eagle
- DMSO
dimethyl sulphoxide
- EGF
epidermal growth factor
- GDNF
glial cell line-derived neurotrophic factor
- HBSS−
Ca2+- and Mg2+-free Hanks balanced salt solution
- MAPK
mitogen-activated protein kinase
- NGF
nerve growth factor
- NT
neurotrophins
- NT-3
neurotrophin 3
- PBS
phosphate-buffered saline
- pmn
progressive motor neuronopathy
- trkB
tropomyosin receptor kinase B
References
- AKIYAMA T., ISHIDA J., NAKAWAGA S., OGAWARA H., WATANABE S-I., ITOHN, SHIBUYA M., FUKAMI Y. Genistein, a specific inhibitor of tyrosine-specific protein kinases. J. Biol. Chem. 1987;262:5592–5595. [PubMed] [Google Scholar]
- ANG L.C., BHAUMICK B., JUURLINK B.H. Neurite promoting activity of insulin-like growth factor I and nerve growth factor on spinal motoneurons is astrocyte dependent. Dev. Brain Res. 1993;74:83–88. doi: 10.1016/0165-3806(93)90086-p. [DOI] [PubMed] [Google Scholar]
- BARDE Y.A. Trophic factors and neuronal survival. Neuron. 1989;2:1525–1534. doi: 10.1016/0896-6273(89)90040-8. [DOI] [PubMed] [Google Scholar]
- BARTLETT S.E. Protein tyrosine kinase inhibitors synergize with nerve growth factor in embryonic chick sensory neuronal cell survival. Neurosci. Lett. 1997;227:87–90. doi: 10.1016/s0304-3940(97)00318-2. [DOI] [PubMed] [Google Scholar]
- CAMU W., HENDERSON C.E. Purification of embryonic rat motoneurons by panning on a monoclonal antibody to the low-affinity NGF receptor. J. Neurosci. Methods. 1992;44:59–70. doi: 10.1016/0165-0270(92)90114-s. [DOI] [PubMed] [Google Scholar]
- CHARBONNEAU H., TONKS N.K. 1002 protein phosphatases. Annu. Rev. Cell Biol. 1992;8:463–493. doi: 10.1146/annurev.cb.08.110192.002335. [DOI] [PubMed] [Google Scholar]
- DETMERS P.A., ZHOU D., POLIZZI E., THIERINGER R., HANLON W.A., VAIDYA S., BANSAL V. Role of stress-activated mitogen-activated protein kinase (p38) in beta 2-integrin-dependent neutrophil adhesion and the adhesion-dependent oxidative burst. J. Immunol. 1998;15:1921–1929. [PubMed] [Google Scholar]
- DUONG F., FOURNIER J., KEANE P.E., GUÉNET J. L., SOUBRIÉ P., WARTER J.M., BORG J., POINDRON P. The effect of the nonpeptide compound SR 57746A on the progression of the disease state of the pmn mouse. Br. J. Pharmacol. 1998;124:811–817. doi: 10.1038/sj.bjp.0701885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FERRARI G., GREENE L.A. Proliferative inhibition by dominant-negative Ras rescues naive and neuronally differentiated PC12 cells from apoptotic death. EMBO J. 1994;13:5922–5928. doi: 10.1002/j.1460-2075.1994.tb06937.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FOURNIER J., GAUTHIER T., KEANE P.E., COUDE F.X., GUZZI U., SOUBRIÉ P., LE FUR G. Neurotrophic effects of SR 57746A, a new non peptide compound: in vitro studies. Br. J. Pharmacol. 1992;106 Supp:120P. [Google Scholar]
- FOURNIER J., KEANE P.E., FERRARA P., SOUBRIE P. SR 57746A: an orally active non-peptide compound with neurotrophic and neuroprotective effects. CNS Drugs Reviews. 1997;3:148–167. [Google Scholar]
- FOURNIER J., STEINBERG R., GAUTHIER T., KEANE P.E., GUZZI U., COUDÉ F.X., BOUGAULT I., MAFFRAND J.P., SOUBRIÉ P., LE FUR G. Protective effects of SR 57746A in central and peripheral models of neurodegenerative disorders in rodents and primates. Neurosci. 1993;55:629–641. doi: 10.1016/0306-4522(93)90429-j. [DOI] [PubMed] [Google Scholar]
- FRIEDMAN B., KLEINFELD D., VERGE V.M.K., MOULTON R., BOLAND P., ZLOTCHENKO E., LINDSAY R.M., LIU L. BDNF and NT-4/5 exert neurotrophic influences on injured adult spinal motor neurons. J. Neurosci. 1995;15:1044–1056. doi: 10.1523/JNEUROSCI.15-02-01044.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GÖTZ R., KÖSTER R., WINKLER C., RAULF F., LOTTSPEICH F., SCHARTL M., THOENEN H. Neurotrophin-6 is a new member of the nerve growth factor family. Nature. 1994;372:266–269. doi: 10.1038/372266a0. [DOI] [PubMed] [Google Scholar]
- HEUERTZ R.M., HAMANN K.J., HERSHENSON M.B., LEFF A.R. Adhesion of bovine airway smooth muscle cells activates extracellular signal-regulated kinases. Am. J. Respir. Cell Mol. Biol. 1997;17:456–461. doi: 10.1165/ajrcmb.17.4.2702. [DOI] [PubMed] [Google Scholar]
- HIWASA T., KONDO K., HISHIKI T., KOSHIZAWA S., UMEZAWA K., NAKAGAWARA A. GDNF-induced neurite formation was stimulated by protein kinase inhibitors and suppressed by Ras inhibitors. Neurosci. Lett. 1997;238:115–118. doi: 10.1016/s0304-3940(97)00861-6. [DOI] [PubMed] [Google Scholar]
- HUGHES R.A., SENDTNER M., THOENEN H. Members of several gene families influence survival of rat motoneurons in vitro and in vivo. J. Neurosci. Res. 1993;36:663–671. doi: 10.1002/jnr.490360607. [DOI] [PubMed] [Google Scholar]
- IGNELZI M.A., PADILLA S.S., WARDER D.E., MANESS P.F. Altered expression of pp60c-src induced by peripheral nerve injury. J. Comp. Neurol. 1992;315:171–177. doi: 10.1002/cne.903150205. [DOI] [PubMed] [Google Scholar]
- IWASAKI Y., ISHIKAWA M., OKADA N., KOIZUMI S. Induction of a distinct morphology and signal transduction in TrkB/PC12 cells by nerve growth factor and brain-derived neurotrophic factor. J. Neurochem. 1997;68:927–934. doi: 10.1046/j.1471-4159.1997.68030927.x. [DOI] [PubMed] [Google Scholar]
- KENNEL P.F., FONTENEAU P., MARTIN E., SCHMIDT J.M., AZZOUZ M., BORG J., GUENET J.L., SCHMALBRUCH H., WARTER J.M., POINDRON P. Electromyographical and motor performance studies in the pmn mouse model of neurodegenerative disease. Neurobiol. Dis. 1996;3:137–147. doi: 10.1006/nbdi.1996.0014. [DOI] [PubMed] [Google Scholar]
- KORSCHING S. The neurotrophic factor concept: a reexamination. J. Neurosci. 1993;13:2739–2748. doi: 10.1523/JNEUROSCI.13-07-02739.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LABIE C., LAFON C., FOURNIER J., KEANE P.E., LE FUR G., MAFFRAND J.P., SOUBRIÉ P. Effect of the non-peptide neuroprotective compound SR 57746A on NGF and BDNF synthesis in primary astrocytes in culture. Br. J. Pharmacol. 1998;123:80P. doi: 10.1038/sj.bjp.0702545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LEVI MONTALCINI R. The nerve growth factor: thirty-five years later. EMBO J. 1987;6:1145–1154. doi: 10.1002/j.1460-2075.1987.tb02347.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LI L., OPPENHEIM R.W., LEI M., HOUENOU L.J. Neurotrophic agents prevent motoneuron death following sciatic nerve section in the neonatal mouse. J. Neurobiol. 1994;25:759–766. doi: 10.1002/neu.480250702. [DOI] [PubMed] [Google Scholar]
- LINDSAY R.M., WIEGAND S.J., ALTAR C.A., DISTEFANO P.S. Neurotrophic factors: from molecule to man. Trends Neurosci. 1994;17:182–190. doi: 10.1016/0166-2236(94)90099-x. [DOI] [PubMed] [Google Scholar]
- MAHER P.A. Nerve growth factor induces protein-tyrosine phosphorylation. Proc. Natl. Acad. Sci. U.S.A. 1988;85:6788–6791. doi: 10.1073/pnas.85.18.6788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MANESS P.F., AUBRY M., SHORES C.G., FRAME L., PFENNINGER K.H. c-src gene product in developing rat brain is enriched in nerve growth cone membranes. Proc. Natl. Acad. Sci. USA. 1988;85:5001–5005. doi: 10.1073/pnas.85.14.5001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MARTINOU J.-C., MARTINOU I., KATO A.C. Cholinergic differentiation factor (CDF/LIF) promotes survival of isolated rat embryonic motoneurons in vitro. Neuron. 1992;8:737–744. doi: 10.1016/0896-6273(92)90094-t. [DOI] [PubMed] [Google Scholar]
- MILLER D.R., LEE G.M., MANESS P.F. Increased neurite outgrowth induced by inhibition of protein tyrosine kinase activity in PC12 pheochromocytoma cells. J. Neurochem. 1993;60:2134–2144. doi: 10.1111/j.1471-4159.1993.tb03498.x. [DOI] [PubMed] [Google Scholar]
- OLSON L. Neurotrophins in neurodegenerative disease: theoretical issues and clinical trials. Neurochem. Int. 1994;25:1–3. doi: 10.1016/0197-0186(94)90044-2. [DOI] [PubMed] [Google Scholar]
- OPPENHEIM R.W., PREVETTE D., HAVERKAMP L.J., HOUENOU L., YIN Q.W., MCMANAMAN J. Biological studies of a putative avian muscle-derived neurotrophic factor that prevents naturally occurring motoneuron death in vivo. J. Neurobiol. 1993;24:1065–1079. doi: 10.1002/neu.480240806. [DOI] [PubMed] [Google Scholar]
- PARK D.S., FARINELLI S.E., GREENE L.A. Inhibitors of cyclin-dependent kinases promote survival of post-mitotic neuronally differentiated PC12 cells and sympathetic neurons. J. Biol. Chem. 1996;271:8161–8169. doi: 10.1074/jbc.271.14.8161. [DOI] [PubMed] [Google Scholar]
- PRADINES A., MAGAZIN M., SCHILTZ P., LE FUR G., CAPUT D., FERRARA P. Evidence for nerve growth factor-potentiating activities of the nonpeptidic compound SR 57746A in PC12 cells. J. Neurochem. 1995;64:1954–1964. doi: 10.1046/j.1471-4159.1995.64051954.x. [DOI] [PubMed] [Google Scholar]
- RUIGT G.S.F., MAKKINK W.K., KONINGS P.N.M. SR 57746A attenuates cytostatic drug-induced reduction of neurite outgrowth in co-cultures of rat dorsal root ganglia and Schwann cells. Neurosci. Lett. 1996;203:9–12. doi: 10.1016/0304-3940(95)12251-6. [DOI] [PubMed] [Google Scholar]
- SCHNAAR R.L., SCHAFFNER A.E. Separation of cell types from embryonic chicken and rat spinal cord: characterization of motoneuron enriched fractions. J. Neurosci. 1981;1:204–217. doi: 10.1523/JNEUROSCI.01-02-00204.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TUSZYNSKI M.H., MAFONG E., MEYER S. Central infusions of brain-derived neurotrophic factor and neurotrophin-4/5, but not nerve growth factor and neurotrophin-3, prevent loss of the cholinergic phenotype in injured adult motor neurons. Neurosci. 1996;71:761–771. doi: 10.1016/0306-4522(95)00440-8. [DOI] [PubMed] [Google Scholar]
- WIDMANN C., GIBSON S., JARPE M.B., JOHNSON G.L. Mitogen-activated protein kinase: conservation of a three-kinase module from yeast to human. Physiol. Rev. 1999;79:143–180. doi: 10.1152/physrev.1999.79.1.143. [DOI] [PubMed] [Google Scholar]
- YAN Q., ELLIOTT J.L., MATHESON C., SUNJ, ZHANG L., MU X., REX K.L., SNIDERW D. Influences of neurotrophins on mammalian motoneurons in vivo. J. Neurobiol. 1993;24:1555–1577. doi: 10.1002/neu.480241202. [DOI] [PubMed] [Google Scholar]
- YAN Q., MATHESON C., LOPEZ O., MILLER J.A. The biological responses of axotomized motoneurons to brain-derived neurotrophic factor. J. Neurosci. 1994;14:5281–5291. doi: 10.1523/JNEUROSCI.14-09-05281.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]


