Abstract
In this study we report a new assay of heterodimeric γ-amino-butanoic acid subtype B (GABAB) receptors where either GABABR1a or GABABR1b are co-expressed with GABABR2 and the chimeric G-protein Gαq-z5 in tsA cells. In this manner we obtained a robust response to GABAB agonists measured as increase in phosphoinositide hydrolysis.
We used this assay to characterize a number of commonly used GABAB receptor ligands. Both splice variants displayed the same rank order of agonist potency; 3-aminopropyl(methyl)phosphinic acid (SKF-97541)>GABA>(R)-4-amino-3-(4-chlorophenyl)butanoic acid ((R)-baclofen)>(RS)-4-amino-3-(5-chloro-2-thienyl)butanoic acid (BCTG)>3-aminopropylphosphonic acid (3-APPA) and furthermore, the absolute agonist potency values were very close to each other.
3-APPA was a partial agonist displaying maximal responses of 41 and 61% compared to GABA at GABABR1a and GABABR1b, respectively. The antagonist (RS)-3-amino-2-(4-chlorophenyl)-2-hydroxypropylsulphonic acid (2-OH-saclofen) displayed KB values of 15 and 7.8 μM at GABABR1a and GABABR1b, respectively.
The rank order of agonist potency as well as the absolute ligand potencies correspond very well with those previously reported in different tissues, and this study thus provides a functional assay of cloned GABAB receptors which should be a valuable tool for further characterization of GABAB ligands. Finally, we can conclude that the functional pharmacological profiles of the two GABABR1 splice variants are very similar.
Keywords: GABAB receptors, GABABR1a, GABABR1b, GABABR2, heterodimeric receptors, SKF-97541, (R)-baclofen, (RS)-4-amino-3-(5-chloro-2-thienyl)butanoic acid, 3-APPA, 2-OH-saclofen
Introduction
γ-Aminobutanoic acid (GABA) is the major inhibitory amino acid in the central and peripheral nervous system. The physiological effects of GABA are mediated by ionotropic GABAA receptors and metabotropic GABAB receptors, the latter being coupled to the Gαi class of G-proteins (Morishita et al., 1990). The GABAB receptor was first defined by its selective activation by the GABA analogue (R)-4-amino-3-(4-chlorophenyl)butanoic acid ((R)-baclofen) (Bowery et al., 1980; Hill & Bowery, 1981) and more recently a number of selective and very potent antagonists have been developed (Bittiger et al., 1993). Using one of these radiolabelled antagonists, two different splice variants of the GABAB receptor (named GABABR1a and GABABR1b, respectively) were isolated by expression cloning (Kaupmann et al., 1997) which led several groups to identify a second subunit (Jones et al., 1998; White et al., 1998; Kaupmann et al., 1998a; Kuner et al., 1999; Ng et al., 1999a; Martin et al., 1999). Interestingly, although some of these groups have reported functional responses from cells transfected with either GABABR1 or GABABR2 alone (Kaupmann et al., 1997; 1998b; Kuner et al., 1999; Martin et al., 1999), several lines of evidence have shown that the two receptor subunits dimerize in order to form fully functional receptors with the expected pharmacological responses by agonists (Jones et al., 1998; White et al., 1998; Kaupmann et al., 1998a; Kuner et al., 1999; Ng et al., 1999b; Möhler & Fritschy, 1999). However, although transient expression functional assays are now available, the assays reported so far have either been limited by a low throughput or relatively weak responses. Thus, to this point only a couple of ligands have been characterized in functional assays based on heterodimeric cloned GABAB receptors compared to a much larger number of compounds characterized in binding experiments (Kaupmann et al., 1997; 1998a; Jones et al., 1998; White et al., 1998; Kuner et al., 1999; Ng et al., 1999b). In order to overcome these limitations on functional assays by designing a ‘more sensitive and much-needed' functional assay system (Bettler et al., 1998), we used a strategy of co-transfection of the receptors with the chimeric G-protein named Gαq-z5 in which the five C-terminal aminoacids of Gαq have been replaced by the C-terminus of Gαz (a member of the Gαi subgroup). In this manner, receptors coupled to the Gαi class of G-proteins have previously been shown to mediate robust stimulative responses with the expected pharmacological profiles on e.g., monoamine receptors (Conklin et al., 1993; Bräuner-Osborne & Brann, 1996) and metabotropic glutamate receptors (Gomeza et al., 1996) and as shown in this paper a similar robust signal (generation of inositol phosphates) can be obtained from heterodimeric GABAB receptors when these are co-transfected with Gαq-z5.
As pointed out previously, two different splice variants of GABABR1 have been identified differing in the distal amino terminal part of the amino-terminal of the receptor. The two splice variants show distinct expression patterns and different developmental regulation, indicating that they could have different physiological functions (Kaupmann et al., 1998b; Malitschek et al., 1998). Since it has recently been shown that GABA binds to the amino terminal domain (ATD) of the receptor (Galvez et al., 1999), it is tempting to speculate that the pharmacology of the splice variants could differ. So far, binding studies of the GABABR1 splice variants expressed alone (Kaupmann et al., 1997; 1998b) or in the presence of GABABR2 (Kaupmann et al., 1998a) have not revealed any such difference in binding affinity between the two splice variants. However, this does not rule out that the splice variants could differ in their pharmacological profiles in functional assays. Thus, in order to investigate this possibility we have also compared the pharmacology of a number of commonly used GABAB ligands (Figure 1) on the two splice variants of GABABR1 co-expressed with GABABR2 and Gαq-z5.
Figure 1.

Structure of the GABAB ligands used in this study.
Methods
Cell culture and second messenger assay
tsA cells (a transformed HEK 293 cell line (Chahine et al., 1994)) were maintained at 37°C in a humidified 5% CO2 incubator in Dulbecco's modified Eagle medium (DMEM) supplemented with penicillin (100 U ml−1), streptomycin (100 mg ml−1) and 10% foetal calf serum. Based on previously published protocols we decided to transfect the cells with a 5 fold excess of GABABR2 as compared to the GABABR1 splice variants (White et al., 1998; Kaupmann et al., 1998a). Two million cells were split into a 15 cm tissue culture plate and transfected with 1.4 μg GABABR1a-pcDNA3.1 or GABABR1b-pcDNA3.1, 7 μg GABABR2-pcDNA3.1 and 1.4 μg Gαq-z5-pcDNA the following day using SuperFect as a DNA carrier according to the protocol by the manufacturer (Qiagen, Hilden, Germany). In experiments where cells were only transfected with a subset of the plasmids, total DNA was maintained at the same level with empty pcDNA3 vector. The day after transfection, cells were split into two poly-D-lysine coated 24-well tissue culture plates in inositol-free growth medium containing 1 μCi ml−1 myo-[2-3H]inositol (Amersham, Buckinghamshire, U.K.). Sixteen to twenty hours later, cells were washed with phosphate buffered saline solution (PBS) and incubated for 20 min in PBS containing 0.9 mM CaCl2 and 1.05 mM MgCl2 (PBS++). Cells were then incubated for another 20 min in PBS++ containing 10 mM LiCl (PBS++-LiCl). Finally, after the cells had been incubated in PBS++-LiCl for 40 min with the indicated amounts of agonists, reactions were terminated by ice-cold 10 mM formic acid and total inositol phosphate (IP) generation was determined by ion-exchange chromatography as described previously (Nanevics et al., 1996). In the case of antagonist assays, cells were incubated in PBS++ for 20 min then in PBS++-LiCl containing antagonist for 20 min and finally in PBS++-LiCl containing both antagonist and 5 μM GABA for 40 min.
Materials
GABA and (R)-baclofen were obtained from Sigma (St. Louis, MO, U.S.A.). 3-Aminopropyl(methyl)phosphinic acid (SKF-97541), (RS) -4- amino-3 -(5- chloro-2 - thienyl)- butanoic acid (BCTG), 3-aminopropylphosphonic acid (3-APPA) and (RS) - 3 - amino - 2 - (4 - chlorophenyl)-2-hydroxypropylsulphonic acid (2-OH-saclofen) were obtained from Tocris Cookson (Bristol, U.K). Culture media, serum and antibiotics for cell culture were obtained from Life Technologies (Paisley, U.K.). The rat GABAB receptor plasmids and the Gαq-z5 construct were generous gifts from Dr Janet Clark (National Institute of Health, Bethesda, MD, U.S.A.) and Dr Bruce Conklin (University of California, San Francisco, CA, U.S.A.), respectively. The pcDNA3 vector was obtained from Stratagene (La Jolla, CA, U.S.A.). The tsA cells were a generous gift from Dr Penelope S.V. Jones (University of California, San Diego, CA, U.S.A.).
Data analysis
All experiments were performed in triplicate and the results are given as mean±s.e.mean of 3–5 experiments. Antagonist potencies were calculated from inhibition curves using the ‘functional equivalent' of the Cheng-Prusoff equation KB=IC50/(1+([A]/EC50)) (Craig, 1993), where [A] is the fixed agonist concentration.
Results
Initially, we tested whether it was necessary to express GABABR1, GABABR2 and Gαq-z5 simultaneously in order to stimulate IP formation. As expected, cells transfected with GABABR1a and GABABR2 were unable to stimulate IP formation when exposed to the agonist (R)-baclofen (Figure 2). In contrary, cells further co-transfected with Gαq-z5 gave a robust response to the agonist as a 4–6 fold increase in IP formation. As seen in Figure 2, the monomeric GABABR1a or GABABR2 co-expressed with Gαq-z5 were also unable to respond to the agonist. Thus, in conclusion GABABR1, GABABR2 and Gαq-z5 all needed to be expressed in order to obtain a functional IP response. Cells transfected with Gαq-z5 alone did also not show any increase in IP formation to (R)-baclofen or any of the other ligands used in this study (data not shown).
Figure 2.
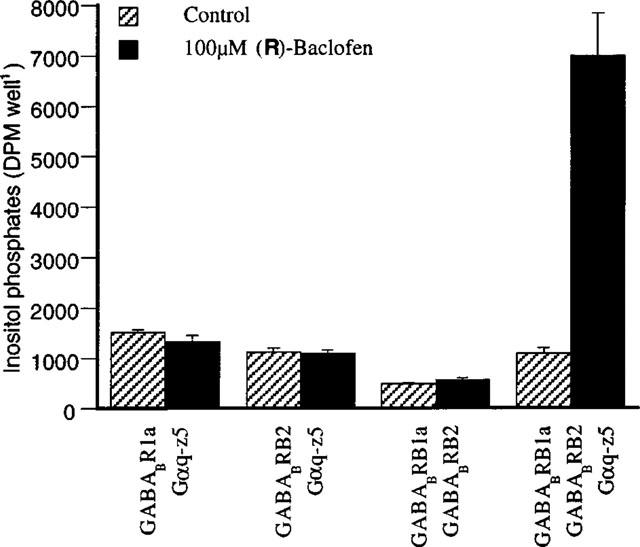
Generation of inositol phosphates by the GABAB agonist (R)-baclofen in tsA cells transfected with varying subsets of the GABAB receptor subunits and the chimeric G-protein Gαq-z5. After a 20 min preincubation in phosphate-buffered saline containing 0.9 mM CaCl2 and 1.05 mM MgCl2 (PBS++) cells were incubated for further 20 min in the same buffer containing 10 mM LiCl (PBS++-LiCl). Finally, after the cells had been incubated in PBS++-LiCl for 40 min in the absence or presence of 100 μM (R)-baclofen, reactions were terminated by ice-cold 10 mM formic acid and total inositol phosphate generation was determined by ion-exchange chromatography. Data are the means±s.d.mean of representative experiments performed in triplicate.
We then went on to test a series of commonly used GABAB receptor ligands (shown in Figure 1) in greater detail on tsA cells transiently transfected with either GABABR1a, GABABR2 and Gαq-z5 or GABABR1b, GABABR2 and Gαq-z5. As can be seen on the examples of concentration-response curves shown in Figure 3 and the derived pEC50 and pKB values shown in Table 1, the pharmacological profiles of the two GABABR1 splice variants were almost identical. They both showed a similar rank order of agonist potency; SKF-97541>GABA>(R)-baclofen>BCTG>3-APPA. Interestingly, whereas SKF-97541, (R)-baclofen and BCTG were full agonists, 3-APPA was a partial agonist producing responses of 41 and 61% of a full GABA response on the GABABR1a and GABABR1b splice variants, respectively (Figure 3 and Table 1).
Figure 3.

Concentration-response curves of some GABAB receptor agonists at tsA cells transfected with GABABR1a, GABABR2 and Gαq-z5 (a) or GABABR1b, GABABR2 and Gαq-z5 (b). Cell treatment and measurement of total inositol phosphates generated was performed as described in Figure 2.
Table 1.
Agonist and antagonist potencies on heterodimeric GABABR1 / GABABR2 receptors in functional assay

Discussion
The first goal of these studies was to develop a functional assay of cloned GABAB receptors which could circumvent some of the limitations (e.g. low throughput and low signal) of previously reported assays (Kaupmann et al., 1997; 1998a; Jones et al., 1998; White et al., 1998; Kuner et al., 1999; Ng et al., 1999b; Martin et al., 1999) by using a strategy of co-transfection with the chimeric G-protein Gαq-z5. We and others have previously used this strategy to obtain robust stimulative responses from receptors coupled to the Gαi class of G-proteins on monoamine receptors (Conklin et al., 1993; Bräuner-Osborne & Brann, 1996) and metabotropic glutamate receptors (Gomeza et al., 1996), and in agreement with these results we obtained 4–6 fold increases in IP generation by agonist treatment of cells transfected with both subunits of the GABAB receptor and the chimeric G-protein (Figures 2 and 3). In agreement with several groups, we were only able to detect agonist responses in cells transfected with both subunits of the GABAB receptor (Jones et al., 1998; White et al., 1998; Kaupmann et al., 1998a; Ng et al., 1999b) whereas no response could be detected in cells transfected with either GABABR1/Gαq-z5 or GABABR2/Gαq-z5. Some groups have reported that weak functional responses can be obtained from cells transfected with either GABABR1 or GABABR2 alone (Kaupmann et al., 1997; 1998b; Martin et al., 1999; Kuner et al., 1999), but most results point to the fact that both GABAB receptor subunits have to be co-expressed in order to obtain the expected agonist potency and effector coupling (Jones et al., 1998; White et al., 1998; Kaupmann et al., 1998a; Kuner et al., 1999; Ng et al., 1999b; Möhler & Fritschy, 1999). Our results are in agreement with these observations as correct coupling to Gαq-z5 only occur when both GABAB subunits are present.
In order to determine whether the GABAB receptors were able to activate Gαq-z5 in the same manner as the endogenous G-proteins, we tested a number of commonly used GABAB ligands in greater detail. Furthermore, we also wanted to test whether the two GABABR1 splice variants differed in their functional pharmacological profile. First of all we found that both splice variants displayed very similar pharmacology when co-expressed with GABABR2 and Gαq-z5. This is in agreement with binding studies on cells expressing either GABABR1a or GABABR1b alone or in the presence of GABABR2 which were also unable to detect any pharmacological difference between the two splice variants (Kaupmann et al., 1997; 1998a,1998b; Malitschek et al., 1998). Thus, the function of the splice variation in the distal part of the ATD is still unknown. As pointed out recently, it is also possible that the splice variants interact with different membrane anchoring proteins causing distinct localization at the subcellular level (Möhler & Fritschy, 1999), but this remains to be proven by a different set of experiments than those presented in this study. The rank order of agonist potency was thus similar on both splice-variants; SKF-97541>GABA>(R)-baclofen>BCTG >3-APPA. Although, no previous study has looked at all these agonists simultaneously in functional experiments it can still be concluded that the rank order is in agreement with studies using tissue pharmacology or receptor binding. Thus, SKF-97541 has been reported to be more potent than baclofen (Knight & Bowery, 1996; Seabrook et al., 1990), GABA has been reported to be more potent than baclofen (Berthelot et al., 1991) which is more potent than BCTG (Berthelot et al., 1991; Lacey et al., 1993; Ong et al., 1997), and 3-APPA has been shown to be less potent than baclofen (Luzzi et al., 1986; Kerr et al., 1987). The actual pEC50 values of our study are also in agreement with those reported in tissues previously. Furthermore, 3-APPA has been shown to be a partial agonist in guinea-pig ileum where it both displayed agonist and antagonist actions (Luzzi et al., 1986; Kerr et al., 1987), which is in agreement with our findings of 3-APPA being a partial agonist. It should be noted that 3-APPA is a full agonist in the cat spinal cord (Kerr et al., 1987). We have previously shown that the intrinsic activity of partial agonists is highly dependent on the expression levels of receptors and G-proteins (Bräuner-Osborne et al., 1996) and this could explain why 3-APPA is a partial and full agonist in the ileum and spinal cord, respectively. We also tested the classical GABAB competitive antagonist 2-OH-saclofen. As shown in Table 1, this compound could also not discriminate between the GABABR1a (pKB=4.9) and GABABR1b (pKB=5.1) splice variants. However, the potency of 2-OH-saclofen found in our assay is very similar to those found in tissue preparations such as the guinea-pig ileum (pA2=5.0) (Kerr et al., 1988).
In conclusion, we have reported a new method for functional testing of cloned heterodimeric GABAB receptors expressed in mammalian cells. The results using the method are in very nice agreement with previously published results from tissue preparations and since the assay has a fairly high throughput and a robust signal, the assay seems to be superior to previously reported functional assays. We can also conclude that at least in our functional assay, we were unable to detect any significant difference between the two GABABR1 splice variants. This is in agreement with previous reports using binding assays (Kaupmann et al., 1997; 1998a,1998b; Malitschek et al., 1998) and the physiological significance of the splice variation in the amino-terminal thus remains a puzzle which warrants further experiments to solve.
Acknowledgments
We would like to thank Drs Janet Clark, Bruce Conklin and Penelope S.V. Jones for their kind gifts of plasmids and cell lines and Ms Heidi Petersen for technical assistance. This work was supported by grants from the H. Lundbeck A/S, The Lundbeck foundation and the NeuroScience PharmaBiotec Research Centre.
Abbreviations
- 2-OH-saclofen
(RS)-3-amino-2-(4-chlorophenyl)-2-hydroxypropylsulphonic acid
- 3-APPA
3-aminopropylphosphonic acid
- ATD
amino terminal domain
- (R)-baclofen
(R)-4-amino-3-(4-chlorophenyl)butanoic acid
- BCTG
(RS)-4-amino-3-(5-chloro-2-thienyl)butanoic acid
- DMEM
Dulbecco's modified Eagle medium
- IP
inositol phosphate
- GABA
γ-amino-butanoic acid
- PBS
phosphate buffered saline solution
- SKF-97541
3-aminopropyl(methyl)phosphinic acid
References
- BERTHELOT P., VACCHER C., FLOUQUET N., DEBAERT M., LUYCKX M., BRUNET C. 3-Thienyl- and 3-furylaminobutyric acids. Synthesis and binding GABAB receptor studies. J. Med. Chem. 1991;34:2557–2560. doi: 10.1021/jm00112a033. [DOI] [PubMed] [Google Scholar]
- BETTLER B., KAUPMANN K., BOWERY N. GABAB receptors: drugs meet clones. Curr. Opin. Neurobiol. 1998;8:345–350. doi: 10.1016/s0959-4388(98)80059-7. [DOI] [PubMed] [Google Scholar]
- BITTIGER H., FROESTL W., MICKEL S.J., OLPE H.R. GABAB receptor antagonists: from synthesis to therapeutic applications. Trends Pharmacol. Sci. 1993;14:391–394. doi: 10.1016/0165-6147(93)90056-p. [DOI] [PubMed] [Google Scholar]
- BOWERY N.G., HILL D.R., HUDSON A.L., DOBLE A., MIDDLEMISS D.N., SHAW J., TURNBULL M. (−)Baclofen decreases neurotransmitter release in the mammalian CNS by an action at a novel GABA receptor. Nature. 1980;283:92–94. doi: 10.1038/283092a0. [DOI] [PubMed] [Google Scholar]
- BRÄUNER-OSBORNE H., BRANN M.R. Pharmacology of muscarinic acetylcholine receptor subtypes (m1-m5): High throughput assays in mammalian cells. Eur. J. Pharmacol. 1996;295:93–102. doi: 10.1016/0014-2999(95)00639-7. [DOI] [PubMed] [Google Scholar]
- BRÄUNER-OSBORNE H., EBERT B., BRANN M.R., FALCH E., KROGSGAARD-LARSEN P. Functional partial agonism at cloned muscarinic acetylcholine receptors. Eur. J. Pharmacol. 1996;313:145–150. doi: 10.1016/0014-2999(96)00501-8. [DOI] [PubMed] [Google Scholar]
- CHAHINE M., BENNETT P.B., GEORGE A.L., JR, HORN R. Functional expression and properties of the human skeletal muscle sodium channel. Pflügers. Arch. 1994;427:136–142. doi: 10.1007/BF00585952. [DOI] [PubMed] [Google Scholar]
- CONKLIN B.R., FARFEL Z., LUSTIG K.D., JULIUS D., BOURNE H.R. Substitution of three amino acids switches receptor specificity of Gq alpha to that of Gi alpha. Nature. 1993;363:274–276. doi: 10.1038/363274a0. [DOI] [PubMed] [Google Scholar]
- CRAIG D.A. The Cheng-Prusoff relationship: something lost in the translation. Trends Pharmacol. Sci. 1993;14:89–91. doi: 10.1016/0165-6147(93)90070-z. [DOI] [PubMed] [Google Scholar]
- GALVEZ T., PARMENTIER M.L., JOLY C., MALITSCHEK B., KAUPMANN K., KUHN R., BITTIGER H., FROESTL W., BETTLER B., PIN J.P. Mutagenesis and modeling of the GABAB receptor extracellular domain support a Venus flytrap mechanism for ligand binding. J. Biol. Chem. 1999;274:13362–13369. doi: 10.1074/jbc.274.19.13362. [DOI] [PubMed] [Google Scholar]
- GOMEZA J., MARY S., BRABET I., PARMENTIER M.L., RESTITUITO S., BOCKAERT J., PIN J.-P. Coupling of metabotropic glutamate receptors 2 and 4 to Gα15, Gα16, and chimeric Gαq/i proteins: characterization of new antagonists. Mol. Pharmacol. 1996;50:923–930. [PubMed] [Google Scholar]
- HILL D.R., BOWERY N.G. 3H-baclofen and 3H-GABA bind to bicuculline-insensitive GABAB sites in rat brain. Nature. 1981;290:149–152. doi: 10.1038/290149a0. [DOI] [PubMed] [Google Scholar]
- JONES K.A., BOROWSKY B., TAMM J.A., CRAIG D.A., DURKIN M.M., DAI M., YAO W.J., JOHNSON M., GUNWALDSEN C., HUANG L.Y., TANG C., SHEN Q., SALON J.A., MORSE K., LAZ T., SMITH K.E., NAGARATHNAM D., NOBLE S.A., BRANCHEK T.A., GERALD C. GABAB receptors function as a heteromeric assembly of the subunits GABABR1 and GABABR2. Nature. 1998;396:674–679. doi: 10.1038/25348. [DOI] [PubMed] [Google Scholar]
- KAUPMANN K., HUGGEL K., HEID J., FLOR P.J., BISCHOFF S., MICKEL S.J., McMASTER G., ANGST C., BITTIGER H., FROESTL W., BETTLER B. Expression cloning of GABAB receptors uncovers similarity to metabotropic glutamate receptors. Nature. 1997;386:239–246. doi: 10.1038/386239a0. [DOI] [PubMed] [Google Scholar]
- KAUPMANN K., MALITSCHEK B., SCHULER V., HEID J., FRöSTL W., BECK P., MOSBACHER J., BISCHOFF S., KULIK A., SHIGEMOTO R., KARSCHIN A., BETTLER B. GABAB-receptor subtypes assemble into functional heteromeric complexes. Nature. 1998a;396:683–687. doi: 10.1038/25360. [DOI] [PubMed] [Google Scholar]
- KAUPMANN K., SCHULER V., MOSBACHER J., BISCHOFF S., BITTIGER H., HEID J., FROESTL W., LEONHARD S., PFAFF T., KARSCHIN A., BETTLER B. Human gamma-aminobutyric acid type B receptors are differentially expressed and regulate inwardly rectifying K+ channels. Proc. Natl. Acad. Sci. U.S.A. 1998b;95:14991–14996. doi: 10.1073/pnas.95.25.14991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KERR D.I., ONG J., JOHNSTON G.A., ABBENANTE J., PRAGER R.H. 2-Hydroxy-saclofen: an improved antagonist at central and peripheral GABAB receptors. Neurosci. Lett. 1988;92:92–96. doi: 10.1016/0304-3940(88)90748-3. [DOI] [PubMed] [Google Scholar]
- KERR D.I.B., ONG J., PRAGER R.H., GYNTHER B.D., CURTIS D.R. Phaclofen: a peripheral and central baclofen antagonist. Brain Res. 1987;405:150–154. doi: 10.1016/0006-8993(87)90999-1. [DOI] [PubMed] [Google Scholar]
- KNIGHT A.R., BOWERY N.G. The pharmacology of adenylyl cyclase modulation by GABAB receptors in rat brain slices. Neuropharmacology. 1996;35:703–712. doi: 10.1016/0028-3908(96)84642-9. [DOI] [PubMed] [Google Scholar]
- KUNER R., KÖHR G., GRÜNEWALD S., EISENHARDT G., BACH A., KORNAU H.-C. Role of heteromer formation in GABAB receptor function. Science. 1999;283:74–77. doi: 10.1126/science.283.5398.74. [DOI] [PubMed] [Google Scholar]
- LACEY G., BERTHELOT P., VACCHER C., FLOUQUET N., VACCHER M.P., DEBAERT M., CURTIS D.R. Thienyl-GABA derivatives as specific baclofen agonists in the rat and cat spinal cord in vivo. Neurosci. Lett. 1993;159:64–66. doi: 10.1016/0304-3940(93)90799-q. [DOI] [PubMed] [Google Scholar]
- LUZZI S., FRANCHI-MICHELI S., CIUFFI M., PAJANI A., ZILLETTI L. GABA-related activities of amino phosphonic acids on guinea-pig ileum longitudinal muscle. J. Auton. Pharmacol. 1986;6:163–169. doi: 10.1111/j.1474-8673.1986.tb00641.x. [DOI] [PubMed] [Google Scholar]
- MALITSCHEK B., RÜEGG D., HEID J., KAUPMANN K., BITTIGER H., FRÖSTL W., BETTLER B., KUHN R. Developmental changes of agonist affinity at GABABR1 receptor variants in rat brain. Mol. Cell. Neurosci. 1998;12:56–64. doi: 10.1006/mcne.1998.0698. [DOI] [PubMed] [Google Scholar]
- MARTIN S.C., RUSSEK S.J., FARB D.H. Molecular identification of the human GABABR2: cell surface expression and coupling to adenylyl cyclase in the absence of GABABR1. Mol. Cell Neurosci. 1999;13:180–191. doi: 10.1006/mcne.1999.0741. [DOI] [PubMed] [Google Scholar]
- MORISHITA R., KATO K., ASANO T. GABAB receptors couple to G proteins Go, Go* and Gi1 but not to Gi2. FEBS Lett. 1990;271:231–235. doi: 10.1016/0014-5793(90)80413-d. [DOI] [PubMed] [Google Scholar]
- MÖHLER H., FRITSCHY J.-M. GABAB receptors make it to the top–as dimers. Trends Pharmacol. Sci. 1999;20:87–89. [Google Scholar]
- NANEVICS T., WANG L., CHEN M., ISHII M., COUGHLIN S.R. Thrombin receptor activating mutations. J. Biol. Chem. 1996;271:702–706. doi: 10.1074/jbc.271.2.702. [DOI] [PubMed] [Google Scholar]
- NG G.Y., MCDONALD T., BONNERT T., RIGBY M., HEAVENS R., WHITING P., CHATEAUNEUF A., COULOMBE N., KARGMAN S., CASKEY T., EVANS J., O'NEILL G.P., LIU Q. Cloning of a novel G-protein-coupled receptor GPR 51 resembling GABAB receptors expressed predominantly in nervous tissues and mapped proximal to the hereditary sensory neuropathy type 1 locus on chromosome 9. Genomics. 1999a;56:288–295. doi: 10.1006/geno.1998.5706. [DOI] [PubMed] [Google Scholar]
- NG G.Y.K., CLARK J., COULOMBE N., ETHIER N., HEBERT T.E., SULLIVAN R., KARGMAN S., CHATEAUNEUF A., TSUKAMOTO N., MCDONALD T., WHITING P., MEZEY E., JOHNSON M.P., LIU Q., KOLAKOWSKI L.F., JR, EVANS J.F., BONNER T.I., O'NEILL G.P. Identification of a GABAB receptor subunit, gb2, required for functional GABAB receptor activity. J. Biol. Chem. 1999b;274:7607–7610. doi: 10.1074/jbc.274.12.7607. [DOI] [PubMed] [Google Scholar]
- ONG J., KERR D.I.B., VACCHER C., BERTHELOT P. Actions of thienyl analogs of baclofen at GABAB receptors in rat neocortical slices. Eur. J. Pharmacol. 1997;329:133–136. doi: 10.1016/s0014-2999(97)00185-4. [DOI] [PubMed] [Google Scholar]
- SEABROOK G.R., HOWSON W., LACEY M.G. Electrophysiological characterization of potent agonists and antagonists at pre- and postsynaptic GABAB receptors on neurones in rat brain slices. Br. J. Pharmacol. 1990;101:949–957. doi: 10.1111/j.1476-5381.1990.tb14186.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WHITE J.H., WISE A., MAIN M.J., GREEN A., FRASER N.J., DISNEY G.H., BARNES A.A., EMSON P., FOORD S.M., MARSHALL F.H. Heterodimerization is required for the formation of a functional GABAB receptor. Nature. 1998;396:679–682. doi: 10.1038/25354. [DOI] [PubMed] [Google Scholar]


