Abstract
The endothelial modulation of the relaxant responses to the nitric oxide (NO) donor sodium nitroprusside (SNP) and the KATP channel opener levcromakalim (LEM) and the interactions between these agents were analysed in isolated rat aorta.
LEM-induced relaxation was unchanged by endothelium removal or by the presence of L-NAME (10−4 M) or ODQ (10−6 M). In contrast, in KCl- (25 mM), but not in noradrenaline- (NA, 10−6 M) contracted arteries, SNP-induced relaxation was augmented by endothelium removal but not by L-NAME, indomethacin, glibenclamide nor charybdotoxin plus apamin.
The isobolographic analysis of the interactions between exogenously activated KATP channels and cyclic GMP using mixtures of SNP and LEM revealed that there were no interactions between both drugs at the proportions at which both drugs were active. However, the points for the SNP : LEM mixtures in proportions 10 : 1 and 1 : 10,000 (i.e. at concentrations at which LEM and SNP were inactive, respectively) fell significantly above the line of additivity indicating that there were negative interactions between both drugs at these selected proportions (about 5- and 2 fold inhibition, respectively). The former interaction was sensitive to glibenclamide, whereas the latter was insensitive ODQ. The magnitude of the 10 : 1 SNP : LEM interaction was smaller in endothelium-intact arteries and was absent in arteries stimulated by NA.
In conclusion, the relaxations induced by LEM and SNP were additive. However, the presence of endothelium and low concentrations of LEM inhibited SNP-induced relaxation. Both inhibitory effects were not additive and were only observed in KCl- and not in NA-contracted aortae.
Keywords: Nitroprusside, levcromakalim, isobolographic analysis, rat aorta, endothelium
Introduction
The endothelium plays a major role in regulating vascular smooth muscle tone through the release of a variety of vasoactive factors. Among the endothelium derived-vasodilators, nitric oxide (NO), probably the primary mediator of endothelium-dependent relaxation in most blood vessels, induces its vasorelaxant effects by an activation of soluble guanylate cyclase and the subsequent rise of intracellular cyclic GMP levels (Moncada et al., 1991; Warner et al., 1994). In addition, endothelium-derived hyperpolarizing factor (EDHF) is also an important mediator of vasodilatation in various vascular beds which induces vascular smooth muscle hyperpolarization mainly by activation of K+ channels (Cohen & Vanhoutte, 1995; Edwards et al., 1998).
K+ channels also regulate the membrane potential and modulate vascular smooth muscle tone (Nelson & Quayle, 1995). ATP-sensitive potassium channels (KATP) are expressed in vascular smooth muscle and their activation by hypoxia, endogenous mediators or potassium channel openers such as levcromakalim (LEM) results in hyperpolarization and relaxation (Edwards & Weston, 1993).
Recently, the cross talk between endogenous NO or NO donors and EDHF or potassium channel activators has received a great deal of attention. Under some circumstances, removal of basal NO activity results in augmented potassium channel openers- or EDHF-induced vasodilatation, whereas activation of the cyclic GMP pathway with NO donors or cyclic GMP mimetics produces opposite effects (Randall & Griffith, 1993; McCulloch & Randall, 1996; Bauersachs et al., 1996; Deka et al., 1998; McCulloch, et al., 1997). Therefore, it has been suggested that EDHF may function as a back up system which is upregulated when NO synthesis is impaired (Kilpatrick & Cocks, 1994; Kemp et al., 1995). However, this interaction does not appear to be a general rule because other authors have reported that endothelial derived NO or NO donors do not affect the vasodilatation mediated by EDHF or potassium channel openers (Gardiner et al., 1991; Zygmunt et al., 1998; Pérez-Vizcaíno et al., 1998). Furthermore, endothelium removal and inhibition of NO synthesis has been reported to reduce the relaxant responses to potassium channel openers (Drieu La Rochelle et al., 1992; Feleder & Adler-Graschinsky, 1997). On the other hand, activation of KATP channels does not appear to modify NO-induced vasodilatation (White & Hilley, 1998b; Pérez-Vizcaíno et al., 1998).
In the present study, we have analysed the endothelial modulation of the relaxant responses to the NO donor sodium nitroprusside (SNP) and the KATP channel opener LEM in isolated rat aorta. The possible cross talk between both pathways at the level of smooth muscle was analysed in endothelium denuded arteries by an isobolographic analysis using a wide range of mixtures of both drugs.
Methods
Tissue preparation
Male Wistar rats (300–350 g), were killed by a blow on the head and then exsanguinated. The descending thoracic aorta was rapidly dissected and placed in Krebs solution of the following composition (mM): NaCl 118, KCl 5, NaHCO3 25, MgSO4 1.2, CaCl2 2, KH2PO4 1.2 and glucose 11 at pH 7.4. After excess of fat and connective tissue had been removed, the aorta was cut into rings (2–3 mm). In some arteries the endothelium was mechanically removed by gently rubbing the intimal surface of the rings with a metal rod. The rings were suspended horizontally by means of two parallel L-shaped stainless steel holders inserted into the lumen in 5 ml organ baths filled with Krebs and bubbled with a 95% O2–5% CO2 gas mixture and maintained at 37°C. One holder served as anchor and the other was attached to an isometric force-displacement transducer coupled to a signal amplifier (Model PRE 206-4, Cibertec, Madrid, Spain) and connected to a computer via an A/D interface. Contractile tension was recorded by a REGXPC computer program (Cibertec, Madrid, Spain) as previously described (Pérez-Vizcaíno et al., 1993; 1998). Each ring was stretched to a resting tension of 2 g and allowed to equilibrate for 60–90 min. During this period tissues were re-stretched and washed every 30 min with warm Krebs solution. The procedure of endothelium removal was tested by the lack of relaxant effects of acetylcholine (10−6 M) in rings pre-contracted with noradrenaline (10−6 M).
Experimental procedures
After equilibration, aortic rings were contracted by 25 mM KCl or NA (10−6 M). When the contractile response reached a stable tension, some arteries were treated with glibenclamide (3×10−6 M), ODQ (10−6 M), L-NAME (10−4 M), indomethacin (10−6 M) or the combination of charybdotoxin (10−7 M) plus apamin (10−7 M) for 15 min. Thereafter, concentration-response curves to SNP (10−10–3×10−6 M), LEM (10−8–3×10−5 M) or mixtures of SNP : LEM were constructed by cumulative addition of the drugs. Parallel time-matched controls decayed less than 10% over the period of the study.
Drugs
The following drugs were used: sodium nitroprusside, glibenclamide, acetylcholine chloride, charybdotoxin, apamin, L-NAME, indomethacin and noradrenaline (Sigma Chemical, Alcobendas, Madrid, Spain), 1H-[1,2,4]oxadiazolo[4,3-a]quinoxalin-1-one (ODQ, Tocris Cookson, Bristol, U.K.) and levcromakalim (Smith Kline Beecham Pharmaceuticals, U.K.). All drugs were dissolved in distilled deionized water to prepare stock solutions (except glibenclamide and ODQ which were dissolved in DMSO), and further dilutions were made in Krebs solution. Ascorbic acid (10−4 M) was present in the stock solution of noradrenaline to prevent oxidation. The mixtures of SNP : LEM (mol : mol) were prepared by mixing the stock solutions of both drugs in the appropriate proportions.
Analysis of the results
Results are expressed as means±s.e.mean of measurements in n arteries. Individual cumulative concentration-response curves were fitted to the following logistic equation:
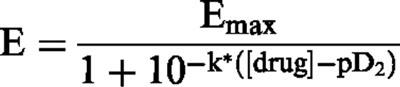 |
where E is the relaxant effect of the drug, Emax is the maximal relaxant effect (an index of the efficacy of the vasodilator) expressed as a percentage of the initial contractile response, k is a factor which represents the slope of the curve, and pD2 is the drug concentration exhibiting 50% of the Emax expressed as negative log molar (an index of the potency of the vasodilator). The calculated pD2 values of drug mixtures are expressed considering either the concentration of LEM or of SNP. These pD2 values reflect the estimated concentration (as negative log molar) of drug present in the mixture when a relaxation of 50% of the Emax is achieved and are not an indication of the potency of the component. Statistically significant differences were calculated by an ANOVA analysis followed by a Newman Keuls test. P<0.05 was considered statistically significant. The interaction between SNP and LEM was evaluated by an isobolographic analysis using mixtures of both drugs (Gessner, 1995). Statistically significant deviations from the line of additivity in the isobologram were analysed as described by Tallarida et al. (1989). Since the slopes of the curves to SNP and LEM (k values from the logistic equation) were statistically different, the analysis for curves with statistically different slopes was used (Tallarida et al., 1989).
Results
Influence of endothelium, basal NO and cyclic GMP on KATP channel mediated relaxation
KCl (25 mM) induced a contractile response in endothelium-intact and endothelium-denuded arteries of 1406±181 mg (n=10) and 1382±152 mg (n=8), respectively (P>0.05, intact vs denuded). LEM induced a concentration-dependent but endothelium-independent relaxation (Figure 1A, Table 1). In endothelium-intact arteries, inhibition of basal NO by addition of L-NAME (10−4 M) on the steady state contraction further increased tone by 19±6% (P<0.05, n=10) but had no effect on LEM-induced relaxation (Figure 1, Table 1). Furthermore, inhibition of basal cyclic GMP synthesis by ODQ (10−6 M), which per se had no effect on vascular tone in endothelium-denuded arteries, was also unable to modify the relaxant response to LEM (n=10).
Figure 1.
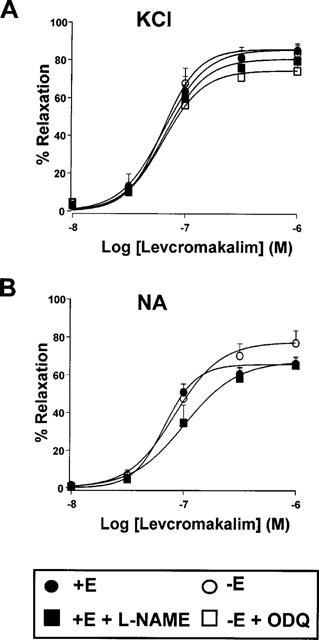
Relaxant effects of levcromakalim in endothelium intact (+E) or endothelium denuded (−E) aortic rings pre-contracted by 25 mM KCl (A) or 10−6 M NA (B) in the absence or the presence of L-NAME (10−4 M) or ODQ (10−6 M). Each symbol represents the mean±s.e.mean of 6–10 experiments.
Table 1.
Parameters (pD2 and Emax) of the concentration-response curves to levcromakalim in endothelium intact (+E) and denuded (−E) isolated rat aorta contracted by KCl and NA
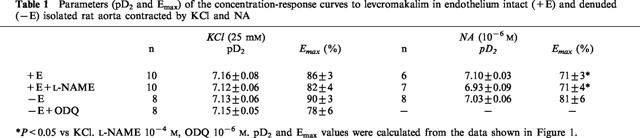
NA (10−6 M) induced a contractile response in endothelium-intact and endothelium-denuded arteries of 1633±145 mg (n=6) and 1728±204 mg (n=8), respectively (P>0.05, intact vs denuded). Addition of L-NAME to endothelium intact arteries increased tone by 5±2% (n=7). Under these conditions, LEM induced a concentration-dependent relaxation which was similar in intact and denuded arteries and unaffected by L-NAME (Figure 1B). No differences were found between the pD2 values of LEM for NA- and KCl-induced contractions but LEM showed a significantly greater Emax on KCl-induced contractions (Table 1).
Influence of endothelium, basal activity of KATP and KCa channels on SNP-mediated relaxation
SNP induced a concentration-dependent relaxation in rings contracted with 25 mM KCl which was enhanced after endothelium removal (Figure 2A, Table 2). In endothelium-intact arteries, addition of L-NAME (10−4 M) on the steady state contraction further increased tone by 14±3% (P<0.05, n=10), whereas addition of glibenclamide (3×10−6 M), indomethacin (10−6 M) or the combination of charybdotoxin (10−7 M) plus apamin (10−7 M) had no effect on vascular tone. However, none of these drugs affected SNP-induced relaxation (Figure 2A, Table 2).
Figure 2.
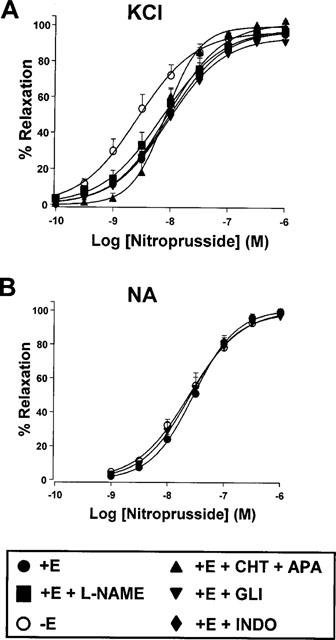
Relaxant effects of sodium nitroprusside in endothelium intact (+E) or endothelium denuded (−E) aortic rings pre-contracted by 25 mM KCl (A) or 10−6 M NA (B) in the absence or the presence of L-NAME, glibenclamide (GLI, 3×10−6 M), indomethacin (INDO, 10−6 M) or the combination of charybdotoxin (10−7 M) plus apamin (10−7 M) (CHT+APA). Each symbol represents the mean±s.e.mean of 5–19 experiments.
Table 2.
Parameters (pD2 and Emax) of the concentration-response curves to nitroprusside in endothelium intact (+E) and denuded (−E) isolated rat aorta contracted by KCl and NA
In NA-contracted arteries, SNP showed a significantly greater pD2 value (but similar Emax) than in KCl-induced contractions. In contrast to the results obtained in KCl-contracted arteries, in NA-contracted arteries SNP-induced vasodilatation was unaffected by endothelium removal. Addition of glibenclamide was also without effect on SNP-induced relaxations (Figure 2B, Table 2).
Interactions between SNP and LEM
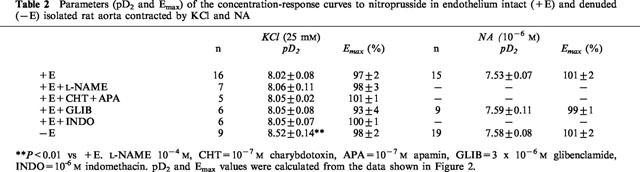
In endothelium denuded arteries stimulated by 25 mM KCl, SNP : LEM mixtures (100 : 1, 10 : 1, 1 : 1, 1 : 10, 1 : 30, 1 : 100, 1 : 1000, 1 : 10,000, 1 : 100,000) induced monophasic relaxant effects which could be fitted to a logistic equation as if they were single drugs (Figure 3A, Table 3). To further analyse the interactions between both drugs an isobolographic analysis (Figure 3B) was carried out from the curves shown in Figure 3A at the level of 50% relaxation. The dotted line in Figure 3B represents the line of additivity, so that deviations above and below this line mean negative and positive interactions, respectively. A novel logarithmic representation of the isoboles shown in the inset of Figure 3B allows a better visualization of the results. It can be observed that most of the points of the SNP : LEM mixtures were not significantly different from the line of additivity indicating that there were no interactions between the two drugs. However, the points for the 10 : 1 and 1 : 10,000 SNP : LEM mixtures fell significantly above the line of additivity indicating that there were negative interactions between both drugs at these selected proportions. Similar results were obtained when the analysis was performed at the level of 30 or 70% of relaxation except that the negative interaction of the 1 : 10,000 mixture did not reach statistical significance at the level of 70% relaxation (not shown).
Figure 3.
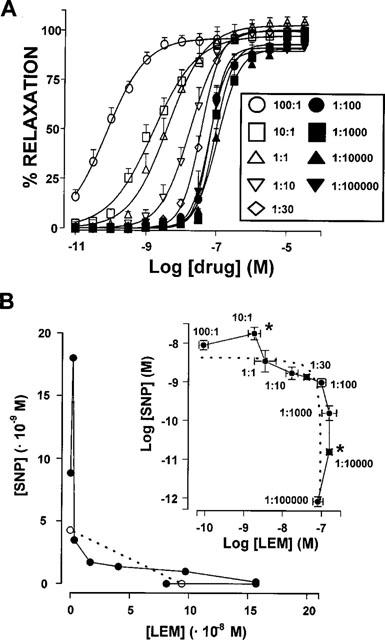
Interactions between SNP and LEM. (A) Relaxant effects of SNP : LEM mixtures in endothelium denuded aortic rings pre-contracted by 25 mM KCl. Drug concentration in the abscissa represents the concentration of LEM present in the mixture. Each symbol represents the mean±s.e.mean of 5–15 experiments. (B) Isobologram for the interaction between SNP and LEM in causing relaxation in endothelium-denuded aortic rings pre-contracted by 25 mM KCl. Each solid circle represents the estimated concentration of drug present in the mixture when a 50% relaxation is achieved, calculated from the data in (A). The concentrations of pure SNP and pure LEM inducing 50% relaxation are shown in open circles. The inset shows a novel logarithmic representation of the same results. The s.e.means, statistical significance and correspondence of symbols with drug mixtures are only shown in the inset for clarity. The dotted line in both the main panel and the inset represents the line of additivity, deviations above and below the line mean negative and positive interactions, respectively. Results are expressed as means±s.e.means. *Significantly different (P<0.05) from the line of additivity.
Table 3.
Parameters (pD2 and Emax) of the concentration-response curves to SNP:LEM mixtures in endothelium-denuded isolated rat aorta contracted by 25 mM KCl (from Figure 3).
Negative modulation of SNP-induced relaxation by KATP channels (10 : 1 SNP : LEM mixture)
In order to analyse the involvement of KATP channels in the deviation from the line of additivity for the 10 : 1 mixture, another series of experiments were performed with SNP and the 10 : 1 mixture in the presence and absence of 3×10−6 M glibenclamide in rings contracted by KCl or NA. In Figure 4, the drug concentration in the abscissa for the 10 : 1 mixture represents the concentration of SNP present in the mixture. In endothelium-denuded arteries contracted by 25 mM KCl, the curve for the 10 : 1 mixture was significantly shifted rightwards by about 6 fold (pD2 expressed as SNP=7.56±0.19, n=5) as compared to SNP (pD2=8.34±0.16, n=6, P<0.05), but this effect was blunted by glibenclamide (pD2 expressed as SNP=8.33±0.08, n=6), (Figure 4A). In endothelium intact arteries (Figure 4B), the curve to the 10 : 1 mixture was also shifted rightwards (pD2 expressed as SNP=7.66±0.15, n=6) as compared to SNP (pD2=8.02±0.07, n=10, P<0.05). However, this shift, which was also blunted by glibenclamide (pD2 expressed as SNP=8.09±0.09, n=8), was smaller than in endothelium denuded arteries (only about 2 fold). In contrast, when the arteries were contracted by NA (10−6 M) similar relaxant responses were obtained for SNP and the 10 : 1 mixture either in the presence or in the absence of glibenclamide (Figure 4C).
Figure 4.
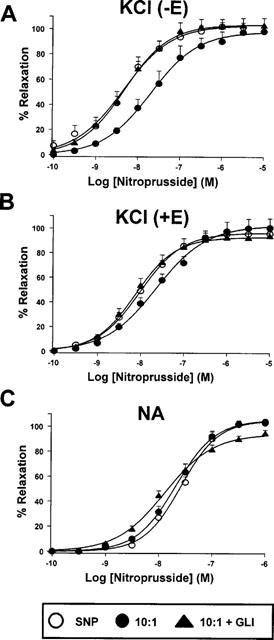
Relaxant effects of SNP and the 10 : 1 SNP : LEM mixture in endothelium intact (+E) and denuded (−E) aortic rings pre-contracted by 25 mM KCl (A and B) or 10−6 M NA (C) in the presence or absence of glibenclamide (3×10−6 M, GLI) or ODQ (10−6 M). In the case of the 10 : 1 mixture, the drug concentration in the abscissa represents the concentration of SNP present in the mixture. Each symbol represents the mean±s.e.mean of 6–10 experiments.
To further confirm the interaction observed with the 10 : 1 SNP : LEM mixture we also performed experiments where the effect of the sequential addition of single concentrations of the two drugs at the 10 : 1 proportion instead of concentration-response curves with the premixed drugs. In endothelium denuded arteries, SNP (3×10−9 M) relaxed the contractions induced by 25 mM KCl-induced contractions by 50±7% (n=9). In parallel experiments, pretreatment with LEM (3×10−10 M) for 10 min after KCl-induced contractions reached steady-state had no effect on vascular tone but inhibited SNP-induced relaxation (25±3%, n=11, P<0.01) and this inhibitory effect was prevented by 10−7 M glibenclamide added 10 min before LEM (40±7%, n=9, P<0.05 vs LEM alone).
Negative modulation of LEM-induced relaxation by SNP (1 : 10,000 SNP : LEM mixture)
The negative interaction observed for the 1 : 10,000 SNP : LEM mixture was very weak (about 2 fold shift) and was accompanied by a significant reduction in the slope of the curve (P<0.05). Furthermore, as shown in Figure 5, the relaxant effect of this mixture was not modified by pretreatment with ODQ (10−6 M, n=6). Moreover, this interaction was not observed when using single concentrations of the drugs. LEM (10−7 M) induced a relaxation of 51±9% (n=9) in aortae contracted by 25 mM KCl. Pretreatment with SNP (10−11 M) had no effect on tone and did not modify the relaxant effect of LEM (60±10%, n=9, P>0.05 vs LEM alone).
Figure 5.
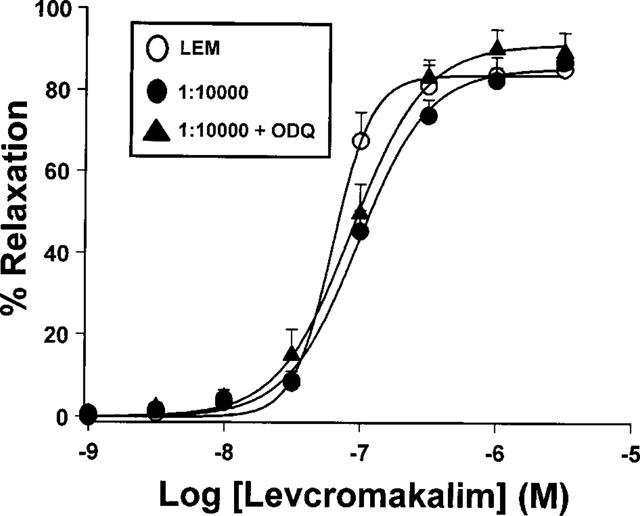
Relaxant effects of LEM and the 1 : 10,000 SNP : LEM mixture in endothelium denuded aortic rings pre-contracted by 25 mM KCl) in the presence or absence of ODQ (10−6 M). In the case of the 1 : 10,000 mixture, the drug concentration in the abscissa represents the concentration of LEM present in the mixture. Each symbol represents the mean±s.e.mean of 6–10 experiments.
Discussion
In the present study we have analysed the endothelial modulation of the relaxant responses to the NO donor sodium nitroprusside (SNP) and the KATP channel opener LEM in isolated rat aorta and the possible interactions between both pathways. LEM-induced relaxation in aortic rings contracted by either KCl or NA was not influenced by endothelium removal or by inhibition of basal NO or cyclic GMP synthesis because it persisted in rings pretreated with L-NAME and ODQ, respectively. In contrast, in KCl- but not in NA-contracted arteries, SNP-induced relaxation was augmented by endothelium removal, whereas the inhibition of KATP channels by glibenclamide or the inhibition of KCa channels by the combination of charybdotoxin plus apamin were without effect. The interactions between exogenously activated KATP channels and SNP were analysed using mixtures of SNP : LEM and revealed that the vasodilator effects of both drugs were additive when added at proportions at which both drugs were active. However, in KCl-contracted arteries very small concentrations of LEM (in the range of high pM to low nM), which per se have no effect on arterial tone, negatively modulate the response to SNP. Conversely, a very small concentration of SNP (in the pM range) inhibited LEM-induced relaxation. The former interaction was sensitive to glibenclamide, whereas the later was insensitive to ODQ.
In the rat aorta, EDHF appears to play a minor role in endothelium-dependent relaxation (Chen et al., 1988; Shimokawa et al., 1996). However, hyperpolarization induced by potassium channel openers is very effective in inducing vasodilatation in this tissue (Pérez-Vizcaíno et al., 1993). The role of the endothelium in modulating the relaxant effects of potassium channel openers in vascular smooth muscle is controversial because enhanced, unchanged or reduced relaxant responses have been reported after endothelium removal (White & Hilley, 1998a; Cavero et al., 1989; Drieu La Rochelle et al., 1992; Feleder & Adler-Graschinsky, 1997). The picture is further complicated since, in a given vessel under the same experimental conditions, endothelium removal increased and decreased the relaxant responses to the potassium channel openers, LEM and pinacidil, respectively (Deka et al., 1998). The enhanced relaxant effects after endothelium removal have been attributed to a negative modulatory effect of basally released NO from the endothelium (McCulloch & Randall, 1996), whereas the reduced relaxant effects might be explained by a hyperpolarizing effect of potassium channel openers in endothelial cells leading to increased endothelial intracellular Ca2+ concentration, activation of endothelial NO synthase and release of NO (Feleder & Adler-Graschinsky, 1997). In rat aortic endothelium-intact rings, L-NAME induced a further contractile response (5–19% over the previous value) indicating that NO is being released from the endothelium and that it negatively modulates the contractile response to NA and KCl. However, endothelium removal and inhibition of NO or cyclic GMP synthesis had no effect on LEM-induced relaxation indicating that endothelial derived NO does not appear to modulate this vasodilator response.
In the rat aorta, SNP-induced relaxation is strongly inhibited by the soluble guanylate cyclase inhibitor ODQ, indicating that this effect is due to an increase in cyclic GMP (Cogolludo et al., 1999). Endothelial denudation results in either unchanged or increased relaxant responses to NO donors (White & Hilley, 1998a; Furchgott et al., 1981; Rubanyi & Vanhoutte, 1985; Shirasaki & Su, 1985). In the present study, endothelium removal enhanced the relaxant response to SNP in aortae contracted by 25 mM KCl suggesting that the endothelium releases a factor which inhibits the effects of SNP. However, this endothelial inhibitory factor was not NO, cyclooxygenase (COX) derived metabolites or other factors activating KATP or KCa channels because L-NAME, indomethacin, the KATP channel inhibitor glibenclamide or the combination of the KCa channel blockers charybdotoxin (blocker of the high and intermediate conductance KCa channels, BKCa and IKCa, respectively) plus apamin (blocker of the small conductance KCa channel, SKCa) were without effect on the relaxant effect of SNP in endothelium-intact arteries. Accordingly, other NO synthesis inhibitors did not modify SNP-induced relaxation (Sakuma et al., 1988; Wang et al., 1995). In contrast to the results obtained in KCl-contracted arteries, in rings stimulated by NA, SNP-induced relaxation was unaffected by endothelium removal suggesting that KCl-induced depolarization of endothelial or smooth muscle cells might be a prerequisite.
Interactions between NO donors and potassium channel openers have been analysed by several authors with conflicting results (McCulloch & Randall, 1996; Deka et al., 1998; White & Hilley, 1998a,1998b; Pérez-Vizcaíno et al., 1998). The differences in the results may be explained, not only by the different vessels studied, but also by the protocols employed. The interactions have been analysed by comparing the effects of a drug (normally a concentration-response curve) in the absence or presence of a single concentration of the second drug. This approach has the disadvantage that the tone at which the concentration-response curve is performed is lower in the presence of the second drug. The problem can be solved by increasing the concentration of vasoconstrictor to induce a similar tone in both cases, but again the different concentrations of contracting agonist may influence the results. Likewise, vasoconstrictors (including α1-adrenergic receptor agonists) inhibit KATP channel activity through the activation of protein kinase C (Bonev & Nelson, 1996). Furthermore, the interactions may depend on the concentration of the second drug; e.g. 100 nM but not 30 nM SNAP (which induced about 50 and 30% relaxation, respectively) shifted rightwards the concentration-response curve to LEM (White & Hilley, 1998a). To avoid these problems, in the present study we have analysed the interactions between SNP and LEM using mixtures of a wide range of proportions of both drugs. Using an isobolographic analysis, we found that most of the points of the SNP : LEM mixtures were not significantly different from the line of additivity when the analysis was performed at the level of 30, 50 and 70% relaxation which indicated that there were no interactions between the two drugs, i.e. their effects were additive. Particularly, there were no interactions at the proportions 1 : 1, 1 : 10, 1 : 30, 1 : 100 and 1 : 1000 at which both components were expected to be active (the relative expected potency of the two components at these proportions, i.e. the ratio of the EC50 values of the single drugs corrected by the proportion factor, were 24.5, 2.45, 0.82, 0.245 and 0.024, respectively). However, the points for the 10 : 1 and 1 : 10,000 SNP : LEM mixtures fell significantly above the line of additivity indicating that there were negative interactions between both drugs at these selected proportions. Thus, as reported by White & Hilley (1998a), interactions between KATP channels and NO donors depend on the degree of activation of each pathway.
The negative interaction at the 1 : 10,000 mixture indicates that a very small concentration of SNP (in the pM range) inhibited LEM-induced relaxation. However, several facts raise some doubts about the significance of this interaction: (a) its magnitude was small (only 2 fold shift), (b) it did not appear when using single concentrations of the drugs, and (c) it was not reverted by the guanylate cyclase inhibitor ODQ. Thus, it might reflect a direct effect of SNP or NO on the KATP channels.
The negative interaction at the 10 : 1 mixture indicates that very small concentrations of LEM (in the range of high pM to low nM), which per se have no effect on arterial tone, negatively modulate the response to SNP. The restoration of the expected relaxant response of the 10 : 1 mixture by glibenclamide indicates that the inhibitory action of LEM on SNP-induced relaxation is mediated by an activation of KATP channels. We speculate that the interaction may involve effects in spatial microdomains, probably within the membrane or the sarcoplasmic reticulum. LEM can activate both sarcolemmal and sarcoplasmic reticulum KATP channels. Thus, it might be that the activation of these KATP channels to an extent insufficient to modify whole cell membrane potential and tone may alter the activity of a nearby protein involved in the signalling pathway of SNP (e.g. membrane Na+/K+ ATPase or the sarcoplasmic reticulum Ca2+ATPase). This negative interaction was also observed in endothelium-intact arteries, although the degree of the rightward shift of the curves was much smaller than in endothelium-denuded arteries.
Our results indicate there is not an overall interaction between these two important pathways for both physiological and pharmacological vascular smooth muscle relaxation. The significance of the negative interactions observed at some selected concentrations is not so clear. One could speculate that both NO and KATP channel activation exert a mutual inhibition so that a weak activation of one of them negatively controls the other and vice versa, whereas the vasodilatory system is fully working when both are activated. However, the finding that this interaction occurs only in arteries constricted with 25 mM KCl indicates that this is not a general mechanism. Nevertheless, there were some similarities between the inhibitory action of endothelium and low concentrations of LEM: (a) they only occur in KCl- but not in NA-contracted arteries and (b) their inhibitory effects were not additive, i.e. in the presence of LEM, the endothelium is unable to further inhibit the effects of SNP, probably because a common inhibitory mechanism is already activated. Therefore, there might be a common link between both effects.
In conclusion, in the rat aorta, LEM-induced relaxation was not influenced by endothelium removal or by inhibition of basal NO or cyclic GMP synthesis. In contrast, SNP-induced relaxation was inhibited by the endothelium through a mechanism independent of NO and KATP or KCa channels. The relaxations induced by LEM and SNP were additive. However, low concentrations of LEM inhibited SNP-induced relaxation through a KATP channel-dependent mechanism. Endothelium- and LEM-induced inhibition were not additive and were only observed in KCl- but not in NA-contracted aortae.
Acknowledgments
The authors are grateful to C. Rivas for excellent technical assistance. Supported by SAF 99/0069 and CAM 08.4/0019/1998 Grants. F. Pérez-Vizcaíno and A.L. Cogolludo are supported by grants from the Comunidad Autónoma de Madrid, F Zaragozá-Arnáez by a grant from the Consejo Superior de Investigaciones Científicas and M. Ibarra by the Junta de Castilla La Mancha.
Abbreviations
- COX
cyclooxygenase
- DMSO
dimethylsulphoxide
- EDHF
Endothelial derived hyperpolarizing factor
- Emax
maximal relaxant effect
- k
factor which represents the slope of the concentration-response curve
- KATP
ATP-dependent K+ channels
- KCa
Ca2+-dependent K+ channels
- LEM
levcromakalim
- NA
noradrenaline
- NO
nitric oxide
- ODQ
1H-[1,2,4]oxadiazolo[4,3-a]quinoxalin-1-one
- pD2
drug concentration exhibiting 50% of the Emax expressed as negative log molar
- SNP
sodium nitroprusside
References
- BAUERSACHS J., POPP R., HECKER M., SAUER E., FLEMMING I., BUSSE R. Nitric oxide attenuates the release of endothelium-derived hyperpolarizing factor. Circulation. 1996;94:3341–3347. doi: 10.1161/01.cir.94.12.3341. [DOI] [PubMed] [Google Scholar]
- BONEV A.D., NELSON M.T. Vasoconstrictors inhibit ATP-sensitive K+ channels in arterial smooth muscle through protein kinase C. J. Gen. Physiol. 1996;108:315–323. doi: 10.1085/jgp.108.4.315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CAVERO I., MONDOT S., MESTRE M. Vasorelaxant effects of cromakalim in rats are mediated by glibenclamide-sensitive potassium channels. J. Pharmacol. Exp. Ther. 1989;248:1261–1268. [PubMed] [Google Scholar]
- CHEN G., SUZUKI H., WESTON A.H. Acetylcholine releases endotheliumderived hyperpolarizing factor and EDRF from rat blood vessels. Br. J. Pharmacol. 1988;95:1165–1174. doi: 10.1111/j.1476-5381.1988.tb11752.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- COGOLLUDO A.L., PEREZ-VIZCAINO F., FAJARDO S., IBARRA M., TAMARGO J. Effects of nicorandil as compared to mixtures of sodium nitroprusside and levcromakalim in isolated rat aorta. Br. J. Pharmacol. 1999;126:1025–1033. doi: 10.1038/sj.bjp.0702375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- COHEN R.A., VANHOUTTE P.M. Endothelium-dependent hyperpolarization. Beyond nitric oxide and cyclic GMP. Circulation. 1995;87 suppl. V:V18–V25. doi: 10.1161/01.cir.92.11.3337. [DOI] [PubMed] [Google Scholar]
- DEKA D.K., RAVIPRAKASH V., MISHRA S.K. Basal nitric oxide release differentially modulates vasodilations by pinacidil and levcromakalim in goat coronary artery. Eur. J. Pharmacol. 1998;348:11–23. doi: 10.1016/s0014-2999(98)00066-1. [DOI] [PubMed] [Google Scholar]
- DRIEU LA ROCHELLE C., RICHARD V., DUBOIS-RANDE J.L., ROUPIE E., GUIDICELLI J.F., HITTINGER L., BERDEAUX A. Potassium channel openers dilate large epicardial coronary arteries in conscious dogs by an indirect, endothelium-dependent mechanism. J. Pharmacol. Exp. Ther. 1992;263:1091–1096. [PubMed] [Google Scholar]
- EDWARDS G., DORA K.A, , GARDENER M.J., GARLAND C.J., WESTONA H. K+ is an endothelium-derived hyperpolarizing factor in rat arteries. Nature. 1998;396:269–271. doi: 10.1038/24388. [DOI] [PubMed] [Google Scholar]
- EDWARDS G., WESTON A.H. The pharmacology of ATP-sensitive potassium channels. Annu. Rev. Pharmacol. Toxicol. 1993;33:597–637. doi: 10.1146/annurev.pa.33.040193.003121. [DOI] [PubMed] [Google Scholar]
- FELEDER E.C., ADLER-GRASCHINSKY E. Endothelium-mediated and Nω-nitro-arginine methyl ester-sensitive responses to cromakalim and diazoxide in the rat mesenteric bed. Eur. J. Pharmacol. 1997;319:229–238. doi: 10.1016/s0014-2999(96)00843-6. [DOI] [PubMed] [Google Scholar]
- FURCHGOTT R.F., ZAWADKI J.W., CHERRY P.D.Role of endothelium in the vasodilator response to acetylcholine Vasodilation 1981New York: Raven Press; 49–57.ed. Vanhoutte, P.M. & Leusen, I [Google Scholar]
- GARDINER S.M., KEMP P.A., BENNETT T. Effects of NG-nitro-L-arginine methyl ester on vasodilator responses to adrenaline or BRL 38227 in conscious rats. Br. J. Pharmacol. 1991;104:731–737. doi: 10.1111/j.1476-5381.1991.tb12496.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GESSNER P.K. Isobolographic analysis of interactions: an update on applications and utility. Toxicology. 1995;105:161–179. doi: 10.1016/0300-483x(95)03210-7. [DOI] [PubMed] [Google Scholar]
- KEMP B.K., SMOLICH J.J, , RITCHIE B.C., COCKS T.M. Endothelium-dependent relaxations in sheep pulmonary arteries and veins: resistance to block by NG-nitro-L-arginine in pulmonary hypertension. Br. J. Pharmacol. 1995;116:2457–2467. doi: 10.1111/j.1476-5381.1995.tb15096.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KILPATRICK E.V., COCKS T.M. Evidence for differential roles of nitric oxide (NO) and hyperpolarization in endothelium-dependent relaxation of pig isolated coronary artery. Br. J. Pharmacol. 1994;112:557–565. doi: 10.1111/j.1476-5381.1994.tb13110.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MCCULLOCH A.I., BOTRILL F.E., RANDALL M.D., HILLEY R.C. Characterization and modulation of EDHF-mediated relaxations in the rat isolated superior mesenteric arterial bed. Br. J. Pharmacol. 1997;120:1431–1438. doi: 10.1038/sj.bjp.0701066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MCCULLOCH A.I., RANDALL M.D. Modulation of vasorelaxant responses to potassium channel openers by basal nitric oxide in the rat isolated superior mesenteric arterial bed. Br. J. Pharmacol. 1996;117:859–866. doi: 10.1111/j.1476-5381.1996.tb15272.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MONCADA S., PALMER R.M.J., HIGGS E.A. Nitric oxide: Physiology, pathophysiology, and pharmacology. Pharmacol. Rev. 1991;43:109–142. [PubMed] [Google Scholar]
- NELSON M.T., QUAYLE J.M. Physiological roles and properties of potassium channels in arterial smooth muscle. Am. J. Physiol. 1995;268:C799–C822. doi: 10.1152/ajpcell.1995.268.4.C799. [DOI] [PubMed] [Google Scholar]
- PEREZ-VIZCAINO F., CASIS O., RODRÍGUEZ R., GÓMEZ G.A., GARCÍA-RAFANELL J., TAMARGO J. Effects of the novel potassium channel opener UR-8225 in rat vascular smooth muscle. Br. J. Pharmacol. 1993;110:1165–1171. doi: 10.1111/j.1476-5381.1993.tb13936.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PEREZ-VIZCAINO F., COGOLLUDO A.L., VILLAMOR E., TAMARGO J. Role of K+ channel opening and stimulation of cGMP in the vasorelaxant effects of nicorandil in isolated piglet pulmonary and mesenteric arteries: Relative efficacy and interactions between both pathways. Br. J. Pharmacol. 1998;123:847–854. doi: 10.1038/sj.bjp.0701693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RANDALL M.D., GRIFFITH T.M. Modulation of vasodilatation to levcromakalim by hypoxia and EDRF in the rabbit isolated ear: a comparison with pinacidil, sodium nitroprusside and verapamil. Br. J. Pharmacol. 1993;109:386–393. doi: 10.1111/j.1476-5381.1993.tb13581.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RUBANYI G., VANHOUTTE P.M. Endothelium-removal decreases relaxations of canine coronary arteries caused by β-adrenergic agonists and adenosine. J. Cardiovasc. Pharmacol. 1985;7:139–145. doi: 10.1097/00005344-198501000-00023. [DOI] [PubMed] [Google Scholar]
- SAKUMA I., STUEHR D.J., GROSS S., NATHAN C., LEVI R. Identification of arginine as a precursor of endothelium-derived relaxing factor. Proc. Natl. Acad. Sci. U.S.A. 1988;85:8664–8667. doi: 10.1073/pnas.85.22.8664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SHIMOKAWA H., YASUTAKE H., FUJII K., OWADA M.K., NAKAIKE R., FUKUMOTO Y., TAKAYANAGI T., NAGAO T., EGASHIRA K., FUJISHIMA M., TAKESHITA A. The importance of the hyperpolarizing mechanism increases as the vessel size decreases in endotheliumdependent relaxations in rat mesenteric circulation. J. Cardiovasc. Pharmacol. 1996;28:703–711. doi: 10.1097/00005344-199611000-00014. [DOI] [PubMed] [Google Scholar]
- SHIRASAKI Y., SU C. Endothelium removal augments vasodilation by sodium nitroprusside and sodium nitrite. Eur. J. Pharmacol. 1985;114:93–96. doi: 10.1016/0014-2999(85)90527-8. [DOI] [PubMed] [Google Scholar]
- TALLARIDA R.J., PORRECA F., COWAN A. Statistical analysis of drug-drug and site-site interactions with isobolograms. Life Sci. 1989;45:947–961. doi: 10.1016/0024-3205(89)90148-3. [DOI] [PubMed] [Google Scholar]
- WANG Y-X., CHENG X., PANG C.C.Y. Vascular pharmacology of methylene blue in vitro and in vivo: a comparison with Nω-nitro-L-arginine and diphenyleneiodonium. Br. J. Pharmacol. 1995;114:194–202. doi: 10.1111/j.1476-5381.1995.tb14925.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WARNER T.D., MITCHELL J.A., SHENG H., MURAD F. Effects of cyclic GMP on smooth muscle relaxation. Adv. Pharmacol. 1994;26:171–194. doi: 10.1016/s1054-3589(08)60054-x. [DOI] [PubMed] [Google Scholar]
- WHITE R., HILLEY C.R. Modulation of relaxation to levcromakalim by S-nitroso-N-acetylpenicillamine (SNAP) and 8-bromo cyclic GMP in the rat isolated mesenteric artery. Br. J. Pharmacol. 1998a;124:1219–1226. doi: 10.1038/sj.bjp.0701973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WHITE R., HILLEY C.R. Effects of K+ channel openers on relaxations to nitric oxide and endotheliumderived hyperpolarizing factor in rat mesenteric artery. Eur. J. Pharmacol. 1998b;357:41–51. doi: 10.1016/s0014-2999(98)00538-x. [DOI] [PubMed] [Google Scholar]
- ZYGMUNT P.M., PLANE F., PAULSSON M., GARLAND C.J., HÖGESTÄTT E.D. Interactions between endothelium-derive relaxing factors in the rat hepatic artery: focus on regulation of EDHF. Br. J. Pharmacol. 1998;124:992–1000. doi: 10.1038/sj.bjp.0701893. [DOI] [PMC free article] [PubMed] [Google Scholar]


