Abstract
The blood glucose-lowering efficacy of rosiglitazone (RSG) and the mechanisms of associated weight gain were determined in dietary obese rats (DIOs). DIO and chow-fed rats received RSG 0.3–30 mg kg−1 daily for 21 days.
In DIOs, plasma glucose and insulin concentrations were reduced by RSG at dosages of 3 and 10 mg kg−1, respectively. Homeostasis model assessment (HOMA) indicated the threshold for a reduction of insulin resistance was 1 mg kg−1. Neither glucose nor insulin levels were affected by treatment in chow-fed rats.
RSG 0.3 mg kg−1 lowered free fatty acids (FFAs) in DIOs, whereas for plasma triglycerides (TGs), the threshold was 3 mg kg−1. By contrast, the threshold for reducing packed red cell volume (PCV) and increasing cardiac mass was 10 mg kg−1. Thus, the therapeutic index for RSG in DIOs was >3 and ⩽10.
Energy intake and weight gain increased in treated DIOs (by 20% and 50 g, at 30 mg kg−1) and chow-fed rats (by 25% and 35 g, at 30 mg kg−1). In DIOs, these increases coincided with falls in plasma leptin (40% lower at 30 mg kg−1) and insulin (43% lower at 30 mg kg−1). By contrast, in chow-fed rats, weight gain and hyperphagia occurred without changes in either leptin or insulin. However, reductions in FFAs below 0.4–0.3 mM were associated with hyperphagia and weight gain in DIO and chow-fed rats.
We conclude that increased energy intake and body weight did not attenuate the improved metabolism evoked by RSG in DIO rats, and that insulin action was enhanced at a dose >3 fold below the threshold for causing haemodilution and cardiac hypertrophy in DIO rats.
Keywords: Rosiglitazone, dietary obesity, insulin resistance, leptin
Introduction
The thiazolidinedione (TZD) chemical class, whose members include troglitazone, rosiglitazone (RSG) and pioglitazone, represent a promising new therapeutic approach to the treatment of type 2 diabetes mellitus (Henry, 1997; Kaneko, 1997; Saltiel & Horikoshi et al., 1995; Saltiel & Olefsky 1996). The primary molecular target for TZDs is the nuclear transcription factor, peroxisome proliferator activated receptor gamma (PPARγ) (Adams et al., 1997; Berger et al., 1996; Lambe & Tugwood, 1996; Lehmann et al., 1995; Shao et al., 1998; Willson et al., 1996; Young et al., 1998), activation of which results in increased gene transcription and the de novo synthesis of proteins involved in glucose and lipid metabolism (Gimble et al., 1996; Ibrahimi et al., 1994; Lefebvre et al., 1997; Martin et al., 1997; Paulik & Lenhard, 1997; Pearson et al., 1996; Tai et al., 1996). In isolated cells and tissues of animal models and clinical studies, the relative potency at PPARγ is correlated with potency in reducing insulin resistance and improving glycaemic control (Adams et al., 1997; Berger et al., 1996; Henry, 1997; Kaneko, 1997; Kawamori et al., 1997a,1997b; Patel et al., 1997; 1998; Wasada et al., 1996; Willson et al., 1996; Young et al., 1998).
Whilst the effects of TZDs on PPARγ in adipose tissue and the subsequent improvement in insulin sensitivity is established, it is somewhat less clear what long-term side effect liability is to be expected with these agents in man. At one extreme, hepatic toxicity is an acknowledged and potentially lethal rare complication of troglitazone therapy in humans (Shibuya et al., 1998; Watkins & Whitcomb, 1998), although at present there is no evidence that this is a class effect of TZDs.
By contrast, haemodilution appears as a common side-effect of TZDs in animal studies (Cantello et al., 1994) and clinical usage (Patel et al., 1998; Rezulin Package Insert, 1999). A potential outcome of prolonged haemodilution, if caused by significant plasma volume expansion, is cardiac hypertrophy, and this is cited as a side-effect of TZDs in animals (e.g., Ghazzi et al., 1997; Rezulin Package Insert, 1999) without explanation of the mechanism. Nonetheless, cardiac hypertrophy has not been observed in patients taking troglitazone, even after 96 weeks of follow-up (Driscoll et al., 1997; Ghazzi et al., 1997), suggesting that the degree of haemodilution being observed in man is devoid of pathological importance.
Weight gain is a further side-effect of troglitazone treatment in man (Rezulin Package Insert, 1999) and has also been demonstrated for several TZDs in animal studies (Arakawa et al., 1998; de Souza et al., 1995; Hirshman et al., 1995; Ikeda et al., 1990; Inoue et al., 1995; Yoshioka et al., 1993; Zhang et al., 1996). The mechanism(s) of weight gain may have several putative components; e.g., reduced urinary glucose excretion, expansion of plasma volume, antilipolytic (Oakes et al., 1994; 1997; Souza et al., 1998) and adipogenic actions (Gimble et al., 1996; Ibrahimi et al., 1994; Lefebvre et al., 1997; Martin et al., 1997; Paulik et al., 1997; Pearson et al., 1996; Tai et al., 1996) and changes to energy intake (Shimizu et al., 1998; Wang et al., 1997) or expenditure (Oakes et al., 1997). However, appropriate animal studies on TZDs, to define the contribution of each of these components, have not been published.
Even accepting that haemodilution and some weight gain may thus be accompanying side-effects of TZD usage in man, it is by no means clear at this point whether individual drugs differ in their liability for these effects at an appropriately antihyperglycaemic dosage. Whilst such information may ultimately become available from long-term clinical trials, it is of considerable interest to study the dose-effect relationship for the disparate actions of TZDs (e.g., insulin action-enhancing, haemodilution, cardiac hypertrophy and weight gain) in a single, appropriate, animal model.
Since the majority of human obesity is characterized by increased thermogenesis and is associated with a Western lifestyle, including ready availability of highly palatable food, we chose the dietary obese (DIO) rat as a relevant animal model. The choice of a non-diabetic model was based on the premise that in the diabetic state, a difference in glycemic control between placebo and drug-treated animals constitutes a confounding factor for food intake and plasma volume regulation. The DIO rat model is characterized by hyperphagia, increased thermogenesis, hyperleptinaemia and mild insulin resistance (McCormack et al., 1989; Widdowson et al., 1997; Wilding et al., 1992). RSG, the most potent of the TZDs in late stage clinical development, was selected for the present study.
Methods
Animals and treatment
Thirty-six male Wistar rats (220 g; Charles River U.K. Ltd., Margate, U.K.) were fed a highly palatable diet for 8 weeks to induce mild obesity, and 36 age-matched controls were fed rodent pelleted chow, comprising 60% of energy as carbohydrate, 30% as protein and 10% as fat (CRM (P), Special Diet Services, Essex, U.K.). The palatable diet consisted of 33% chow, 33% condensed milk (Nestlé U.K. Ltd., York, U.K.) and 7% sucrose (Tate & Lyle, London, U.K.) by weight, with the remainder being added water. This provided 65% of energy as carbohydrate, 19% as protein and 16% as fat. This diet is designed to promote weight gain through hyperphagia, without employing major changes in macronutrient composition, compared with normal rat chow. We have previously found this to be a reliable method of inducing weight gain and insulin resistance (Widdowson et al., 1997; Wilding et al., 1992). Rats were allowed free access to water throughout the study and were maintained on a 12 : 12 h light:dark phase schedule.
At the end of the 8-week period, when the palatable diet-treated rats had developed significant obesity, vehicle (1% carboxymethyl cellulose at 1 ml kg−1 body weight; Sigma, Poole, Dorset, U.K.) or RSG ((+/−)-5-([4-[2-methyl-2-(pyridylamino)ethoxy] phenyl]methyl) 2,4-thiazolidinedione; SmithKline Beecham Pharmaceuticals) at five doses (0.3, 1, 3, 10 or 30 mg kg−1) was given by gavage daily for 21 days to six rats per dose group, for both DIO animals and chow-fed rats. Food intake and body weight were measured daily throughout the treatment period. All procedures were performed in strict compliance with U.K. Home Office regulations governing animal experimentation.
Metabolic data and tissue mass
At the end of the study, and after a 4-h fast, rats were killed by CO2 inhalation. Blood was collected by cardiac puncture and, in the case of all DIO rats and of chow-fed rats treated with vehicle, the packed red cell volume (PCV) was measured. Also, hearts were excised, cut open, blotted and weighed in these animals. For all rats, plasma was separated by centrifugation and stored at −40°C until assayed. Plasma glucose concentration was determined using a glucose oxidase method, and FFA and TG concentrations were measured using commercial diagnostic kits (Boehringer Mannheim, Milton Keynes, Bucks and Sigma Diagnostics, Poole, Dorset, U.K.). Insulin and leptin concentrations were measured by radioimmunoassay (RIA) kits (Pharmacia/Upjohn Diagnostics, Lewes, Sussex and Linco Research, Biogenesis, Poole, Dorset, U.K., respectively). Body fat content was estimated by dissection and weighing of epididymal and perirenal fat pads and expressed as a percentage of total body weight. The latter was considered a useful measure of the fat-to-lean ratio, as the animals in the different groups were of different weights.
Homeostasis Model Assessment (HOMA)
This model, which incorporates measures of both fasting plasma concentrations of glucose and insulin, was used to calculate an index of insulin resistance as insulin (μU ml−1)×glucose (mM)/22.5 (Matthews et al., 1985).
Statistical analyses
Data are presented as mean±s.e.mean. The DIO and chow-fed vehicle controls were compared using 2-sample t-tests for all parameters. The RSG dose-response relationship was studied separately for the DIO and chow-fed rats. Dose-response curves were constructed for each of the parameters, and the significance of dose determined by one-way ANOVA. Between-dose comparisons for all parameters, comparing each dose to vehicle control, were made by post-hoc Bonferroni modified t-tests. Correlation coefficients were calculated for the relationships between plasma leptin, FFA and insulin concentrations and body weight gain and calorie intake during the dosing period. All statistical analysis was carried out using Arcus Pro-stat (Version 3.23; Iain Buchan, Liverpool, U.K.). Results were considered statistically significant at the P<0.05 level.
Results
Dietary obesity
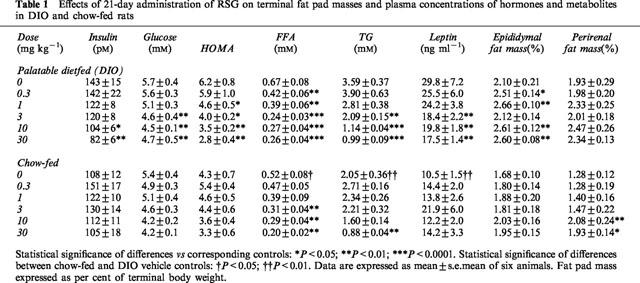
At baseline, the groups destined for chow feeding or to be given the palatable diet were of similar weight (227±2 vs 226±2 g, respectively). After being fed the palatable diet for 8 weeks prior to drug treatment, rats developed mild obesity, becoming 4% heavier than chow-fed rats (540±6 vs 520±6 g; P<0.05). At termination, DIO vehicle control rats were 9% heavier (596±9 g vs 547±10 g; P<0.005) and had greater absolute perirenal and epididymal fat masses than chow-fed vehicle controls (perirenal: 11.5±1.8 vs 7.0±0.7 g; epididymal: 12.6±1.4 vs 9.2±0.6 g; both P<0.05). The mean value of insulin resistance, as shown by an increase in HOMA, in DIO vehicle control rats was 45% higher than that of chow-fed vehicle controls, but this was not statistically significant (P=0.1; Figure 1a). Changes in lipid metabolism were also apparent in the DIO vehicle controls with 29% higher plasma FFA (P<0.05), 75% higher TG and nearly 3 fold higher plasma leptin levels than chow-fed vehicle controls (both P<0.01; Table 1).
Figure 1.
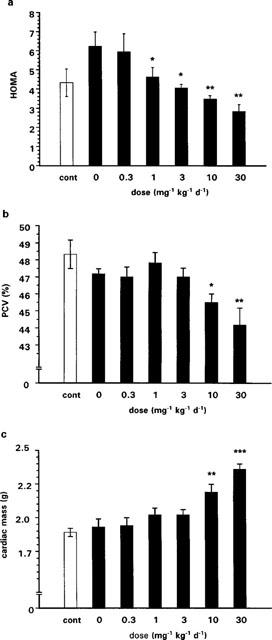
Final HOMA values (a), PCV (b) and cardiac mass (c) in chow-fed, vehicle-treated controls (cont) and DIO rats treated with RSG at various doses for 3 weeks (n=6/group/dose). There were no significant differences between untreated chow-fed and DIO rats. ANOVA followed by Bonferroni modified t-tests in the DIO group showed that insulin sensitivity (a) improved at a threshold dose of 1 mg kg−1, whereas significant haemodynamic side-effects (b,c) were present only at 10 mg kg−1 and above (*P<0.05; **P<0.01; ***P<0.0001). Data are expressed as mean±s.e.mean.
Table 1.
Effects of 21-day administration of RSG on terminal fat pad masses and plasma concentrations of hormones and metabolites in DIO and chow-fed rats
Effects of RSG on metabolic parameters
In DIO rats, the concentration of plasma glucose was significantly reduced at 3 mg kg−1 of RSG (by 21%; P<0.01), whereas the plasma insulin concentration was lowered at 10 mg kg−1 (by 27%; P<0.05; Table 1). Neither plasma glucose nor insulin levels were affected by RSG treatment in chow-fed rats (P>0.05; Table 1). The HOMA model showed that RSG induced a dose-related improvement in insulin sensitivity in DIO rats with a threshold of 1 mg kg−1 (P<0.05; Table 1 and Figure 1a), but drug treatment did not significantly alter insulin sensitivity in chow-fed rats (P>0.05; Table 1). Fasting plasma FFA levels were significantly reduced by drug treatment in DIO rats, although at a lower dose (0.3 mg kg−1; 37% fall; P<0.01) than that required for improving insulin sensitivity. Plasma TG levels were also reduced by RSG in DIO rats, decreasing by 42% at a threshold dose of 3 mg kg−1 (P<0.01). Both plasma FFA (by 69%) and TG (by 57%) levels were also reduced by RSG in chow-fed rats, but at 10 fold higher threshold doses than those required in DIO rats; i.e., 3 and 30 mg kg−1, respectively (both P<0.01; Table 1).
Haemodynamic factors
Effects of RSG on PCV and cardiac mass in DIO rats were only apparent at doses of 10 or 30 mg kg−1. At the threshold dose, there was a 3.5% fall in PCV (P<0.05; Figure 1b) and a 13% increase in cardiac mass (P<0.001; Figure 1c). Thus, the therapeutic index for RSG in DIO rats (the ratio of the threshold dose for causing haemodilution/cardiac hypertrophy to that for improving insulin sensitivity) was >3 and ⩽10 (also compare panels b and c with panel a in Figure 1).
Food intake and body weight
RSG increased food intake dose-dependently in DIO rats (P<0.05). In chow-fed rats, however, this effect was only seen at doses ⩾3 mg kg−1 (P<0.01; Table 2). RSG also augmented weight gain in DIO rats (P<0.0001; Table 2), an effect which coincided with a 20–24% increase in gonadal fat mass (P<0.05; Table 1). Chow-fed rats did not significantly gain body weight at doses of RSG below 3 mg kg−1, and their gonadal fat pad mass did not increase at any dose. However, the mass of the perirenal depot did increase significantly at 10 and 30 mg kg−1 (P<0.01; Table 1). Plasma FFAs were significantly negatively correlated with weight gain during the 3 week dosing period in both DIO (R2=0.89; P<0.005) and chow-fed rats (R2=0.87; P<0.005; Figure 2a). Similarly, plasma FFAs were also significantly negatively correlated with energy intake in these groups (DIO rats: R2=0.76; P<0.02; chow-fed: R2=0.89; P<0.005). By contrast, whilst plasma insulin concentrations were significantly negatively correlated with both weight gain (R2=0.71; P<0.05; Figure 2b) and energy intake (R2=0.72; P<0.02) in DIO rats, no such correlations were seen in chow-fed rats (P=0.43 for weight gain; Figure 2b; P=0 for energy intake).
Table 2.
Effects of 21-day administration of RSG on cumulative energy intake and body weight gain in DIO and chow-fed rats
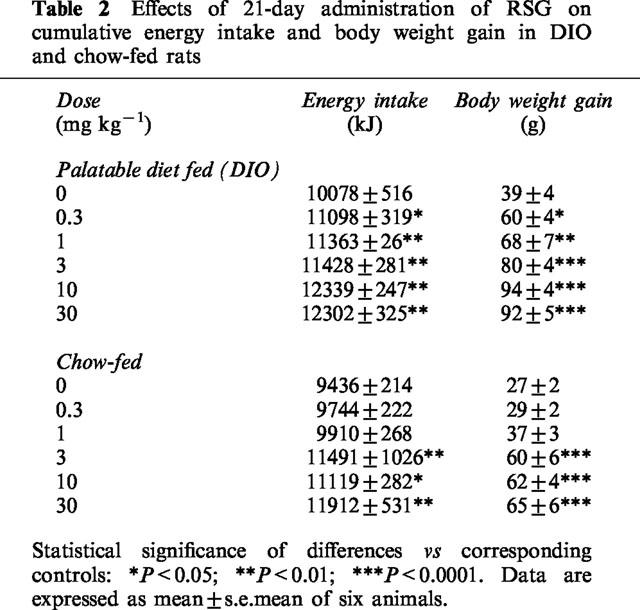
Figure 2.
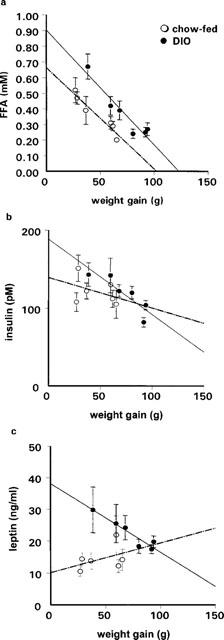
Relationship between final plasma concentrations of FFA (a), insulin (b), leptin (c) and weight gain in chow-fed and DIO rats after 3 weeks of treatment with RSG at various doses (0–30 mg kg−1). There was a significant relationship between FFA levels and body weight gain in both groups (chow: R2=0.87; P<0.005; DIO: R2=0.89; P<0.005). Insulin levels were negatively correlated with weight gain in DIO rats (R2=0.71; P<0.05), but not in chow-fed rats (R2=0.1; P=0.43). Leptin levels were negatively correlated with body weight gain (R2=0.9; P<0.005) in DIO rats, but not in the chow-fed group (R2=0.17, P=0.4).
Leptin
Plasma leptin concentrations (Table 1) were correlated overall with body weight across all DIO and chow-fed groups (R2=0.46; P<0.01). In DIO vehicle control rats, the plasma leptin concentration was 65% higher than in chow-fed vehicle controls (P<0.01). There was also a pattern of decreasing plasma leptin concentration with increasing dose of RSG in DIO rats (P<0.01), which was not seen in chow-fed rats (P>0.05; Table 1). Indeed, there was a significant negative correlation between plasma leptin levels and body weight gain (R2=0.9; P<0.005; Figure 2c) or energy intake (R2=0.73; P<0.02) during the 3 weeks of RSG administration in the DIO group, but not in the chow-fed group (for body weight: P=0.4; Figure 2c; for energy intake: P=0.3).
Discussion
These results show that RSG effectively improves insulin sensitivity and lipid abnormalities in DIO rats, but this effect is not apparent in chow-fed rats. In essence, our data in DIO rats support the hypothesis that the insulin-sensitizing effect of RSG is closely linked to the reduced availability of fatty acids as a fuel for skeletal muscle (Oakes et al., 1994, 1997) via the Randle cycle (Randle et al., 1963). Thus, in the present study in DIO rats, plasma FFAs fell at a dose of RSG (0.3 mg kg−1) slightly lower than that required to detect improved insulin sensitivity (1 mg kg−1). Coincidentally, gonadal fat mass increased significantly at the lower dose, suggesting either enhanced fatty acid clearance and deposition as triglyceride in adipose tissue and/or reflecting the antilipolytic action of the drug (Oakes et al., 1994; 1997; Souza et al., 1998).
Additionally, the observation that the threshold dose for lowering plasma TGs in DIO rats was 3 mg kg−1 (i.e., 10 fold higher than the lowest dose used, where FFAs were already reduced), suggests that changes in the plasma FFA level represent the more important trigger for improving insulin action. This discordance between effects of RSG on plasma FFAs and TGs may reflect a complex series of events. Reduced lipolysis will decrease the supply of FFAs and should lower TGs, but this may be offset by increased food consumption and the generation of additional circulating TG-rich lipoproteins. Furthermore, TZDs may directly affect TG clearance from the circulation, although there is some controversy about this effect. In one ex vivo study (Lefebvre et al., 1997), epididymal fat lipoprotein lipase (LPL) mRNA and activity were increased by RSG, but in a study using rat adipocytes in culture, both troglitazone and RSG inhibited LPL activity (Ranganathan & Kern, 1998).
Importantly, the insulin action-enhancing effect of RSG was observed at a dose between 3 and 10 fold lower than that required to cause haemodilution and cardiac hypertrophy. Haemodilution with TZDs is also commonly encountered in man (Patel et al., 1998; Rezulin Package Insert, 1999), though its mechanism has not been defined in the literature. Troglitazone does not influence red blood cell mass, as estimated using 51Cr labelling of erythrocytes (Young et al., 1997). This suggests that plasma volume expansion is the key component of haemodilution. A known property of TZDs, independent of their effect on PPARγ, is their ability to inhibit vascular smooth muscle cell Ca2+ channels and to reduce vascular tone (Buchanan et al., 1995; Fujiwara et al., 1995; Nakamura et al., 1998; Song et al., 1997; Verma et al., 1998; Walker et al., 1998; Walsh et al., 1996; Zhang et al., 1994). By extrapolation, it is possible that haemodilution in both rat and man, and cardiac hypertrophy in rat, though not seemingly in man (Driscoll et al., 1997; Ghazzi et al., 1997), can be traced back, at least partly, to this inhibitory effect on vascular smooth muscle cell Ca2+ ion flux. The ingredients of a potentially relevant haemodynamic action, including reduced diastolic blood pressure and peripheral vascular resistance, and increased cardiac index and stroke volume index, have been reported during the clinical use of troglitazone (Ghazzi et al., 1997).
Whilst the present study suggests that RSG exhibits dose-differentiation for its effects on insulin action and haemodilution in the DIO rat, the situation is unresolved for troglitazone and pioglitazone. Troglitazone is a more potent calcium channel blocker than pioglitazone (Nakamura et al., 1998), and an earlier study indicated that troglitazone, but not RSG, relaxes human subcutaneous arterial resistance vessels, albeit apparently by a prostaglandin-related mechanism (Walker et al., 1998). Nonetheless, a recent report that RSG also causes haemodilution in man at antihyperglycemic doses (Patel et al., 1998), suggests that the situation may be more complex than the picture presented here for DIO rats. Clearly this is an issue which requires additional evaluation.
That TZDs cause hyperphagia in rats has been known for some time (De Vos et al., 1996; Wang et al., 1997; Zhang et al., 1996), but the finding that increased appetite may also occur in man with troglitazone is noteworthy (Shimizu et al., 1998). In the present study, food consumption in DIO rats was significantly increased even at the lowest dose of RSG tested and there was a highly significant negative correlation between plasma leptin concentration and both energy intake and weight gain in these animals. Like leptin, however, insulin itself is a satiety signal in rodents (Schwartz et al., 1992), suggesting that RSG-evoked falls in the plasma levels of both these hormones may have contributed to hyperphagia and weight gain. Indeed, as with leptin, the plasma insulin concentration in DIO rats correlated negatively with energy intake and weight gain. The decrement in plasma leptin caused by RSG may also have been at least partly due to the fall in plasma insulin, since insulin stimulates ob gene expression (Leroy et al., 1996) and leptin secretion from adipocytes (Hardie et al., 1996). Nonetheless, direct effects of TZDs on OB gene expression (Kallen & Lazar, 1996) and leptin secretion (Nolan et al., 1996) have also been documented. Other studies have shown that the effects of TZDs translate to a reduction in the plasma leptin concentration in type 2 diabetic animals (McCormack et al., 1989; Zhang et al., 1996) and man (Walsh et al., 1996). As for the hypothalamic signal transducing the hyperphagic signal, NPY is not a prime candidate, at least as has been demonstrated in Zucker fatty rats given a low, insulin-sensitizing dose of RSG (Wang et al., 1997).
Our data in chow-fed rats, however, suggest that changes in plasma leptin and insulin do not entirely explain the RSG-induced hyperphagia and weight gain. Whilst RSG did not significantly influence food intake in chow-fed rats until the dose was raised to 3 mg kg−1, neither plasma insulin nor leptin were reduced in these rats even at 30 mg kg−1. Moreover, unlike DIO rats, there was no negative correlation between plasma leptin or insulin and calorie intake or weight gain in chow-fed rats. The difference in dose level for causing hyperphagia in DIO and chow-fed rats thus constitutes a conundrum and may suggest that, at least at higher dosages of RSG, an additional mechanism may be operating. Whether this mechanism is of peripheral or central identity is not certain from present data, but it is of interest that the lowest doses that resulted in weight gain in both chow-fed and DIO animals were identical to those that reduced FFA concentrations. Furthermore, there was a highly significant negative correlation between terminal plasma FFA and energy intake or weight gain in both DIO and chow-fed rats.
In summary, we have shown that RSG improves the metabolic profile and insulin resistance in the DIO rat model, and we have also demonstrated clear dose separation of these beneficial effects from the adverse effects of haemodilution and cardiac hypertrophy. Weight gain, whilst seemingly closely related to the FFA lowering and insulin-sensitizing effects of RSG in DIO rats, did not conspicuously attenuate the marked improvement in metabolic status which was observed, but we do not yet know if these findings apply to man.
Acknowledgments
This work was supported financially by a grant from SmithKline Beecham Pharmaceuticals. We would like to thank Dr Tom Leonard for his helpful comments on the manuscript. We also thank Miss Karen Owens and Mrs Pamela Vincent for their technical assistance and the staff of the Biomedical Services Unit at the University of Liverpool for their consciencious care of the animals.
Abbreviations
- DIO
dietary obese
- FFA
free fatty acid
- HOMA
homeostasis model assessment
- PCV
packed cell volume
- PPARγ
peroxisome proliferator activated receptor gamma
- RSG
rosiglitazone
- TZD
thiazolidinedione
- TG
triglyceride
References
- ADAMS M., MONTAGUE C.T., PRINS J.B., HOLDER J.C., SMITH S.A., SANDERS L., DIGBY J.E., SEWTER C.P., LAZAR M.A., CHATTERJEE K.K., O'RAHILLY S. Activators of peroxisome proliferator-activated receptor γ have depot-specific effects on human preadipocyte differentiation. J. Clin. Invest. 1997;100:3149–3153. doi: 10.1172/JCI119870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ARAKAWA K., ISHIHARA T., AOTO M., INAMASU M., SAITO A., IKEZAWA K. Actions of novel antidiabetic thiazolidinedione, T-174, in animal models of non-insulin-dependent diabetes mellitus (NIDDM) and in cultured muscle cells. Br. J. Pharmacol. 1998;125:429–436. doi: 10.1038/sj.bjp.0702066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BERGER J., BAILEY P., BISWAS C., CULLINAN C.A., DOEBBER T.W., HAYES N.S., SAPERSTEIN R., SMITH R.G., LEIBOWITZ M.D. Thiazolidinediones produce a conformational change in peroxisomal proliferator-activated receptor-γ: binding and activation correlate with antidiabetic actions in db/db mice. Endocrinology. 1996;137:4189–4195. doi: 10.1210/endo.137.10.8828476. [DOI] [PubMed] [Google Scholar]
- BUCHANAN T.A., MEEHAN W.P., JENG Y.Y., YANG D., CHAN T.M., NADLER J.L., SCOTT S., RUDE R.K., HSUEH W.A. Blood pressure lowering by pioglitazone. Evidence for a direct vascular effect. J. Clin. Invest. 1995;96:354–360. doi: 10.1172/JCI118041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CANTELLO B.C.C., CAWTHORNE M.A., COTTAM G.P., DUFF P.T., HAIGH D., HINDLEY R.M., LISTER C.A., SMITH S.A., THURLBY P.L. [[ω-(heterocyclylamino)alkoxy]benzyl]-2,4-thiazolidinediones as potent antihyperglycemic agents. J. Med. Chem. 1994;37:3977–3985. doi: 10.1021/jm00049a017. [DOI] [PubMed] [Google Scholar]
- DE SOUZA C.J., YU J.H., ROBINSON D.D., ULRICH R.G., MEGLASSON M.D. Insulin secretory defect in Zucker fa/fa rats is improved by ameliorating insulin resistance. Diabetes. 1995;44:984–991. doi: 10.2337/diab.44.8.984. [DOI] [PubMed] [Google Scholar]
- DE VOS P., LEFEBVRE A.-M., MILLER S.G., GUERRE-MILLO M., WONG K., SALADIN R., HAMANN L.G., STAELS B., BRIGGS M.R., AUWERX J. Thiazolidinediones repress ob gene expression in rodents via activation of peroxisome proliferator-activated receptor γ. J. Clin. Invest. 1996;98:1004–1009. doi: 10.1172/JCI118860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DRISCOLL J., GHAZZI M., PEREZ J., HUANG S., WHITCOMB R. A 96-week follow-up on cardiac safety in patients with Type II diabetes treated with troglitazone. Diabetes. 1997;46 Suppl. 1:149A. [Google Scholar]
- FUJIWARA T., OHSAWA T., MIYAMOTO M., USHIYAMA S., MATSUDA K., HORIKOSHI H. Troglitazone (CS-045) acutely increases skin blood flow in dexamethasone-induced diabetic obese Zucker rats and normal rats. Diabetes. 1995;44 Suppl. 1:72A. [Google Scholar]
- GHAZZI M.N., PEREZ J.E., ANTONUCCI T.K., DRISCOLL J.H., HUANG S.M., FAJA B.W., WHITCOMB R.W. The Troglitazone Study Group: Cardiac and glycemic benefits of troglitazone treatment in NIDDM. Diabetes. 1997;46:433–439. doi: 10.2337/diab.46.3.433. [DOI] [PubMed] [Google Scholar]
- GIMBLE J.M., ROBINSON C.E., WU X., KELLY K.A., RODRIGUEZ B.R., KLIEWER S.A., LEHMANN J.M., MORRIS D.C. Peroxisome proliferator-activated receptor-γ activation by thiazolidinediones induces adipogenesis in bone marrow stromal cells. Mol. Pharmacol. 1996;50:1087–1094. [PubMed] [Google Scholar]
- HARDIE L.J., GUILHOT N., TRAYHURN P. Regulation of leptin production in cultured mature white adipocytes. Horm. Metab. Res. 1996;28:685–689. doi: 10.1055/s-2007-979878. [DOI] [PubMed] [Google Scholar]
- HENRY R.R. Thiazolidinediones. Endocrinol. Metab. Clin. North. Am. 1997;26:553–573. doi: 10.1016/s0889-8529(05)70267-x. [DOI] [PubMed] [Google Scholar]
- HIRSHMAN M.F., FAGNANT P.M., HORTON E.D., KING P.A., HORTON E.S. Pioglitazone treatment for 7 days failed to correct the defect in glucose transport and glucose transporter translocation in obese Zucker rat (fa/fa) skeletal muscle plasma membranes. Biochem. Biophys. Res. Commun. 1995;208:835–845. doi: 10.1006/bbrc.1995.1412. [DOI] [PubMed] [Google Scholar]
- IBRAHIMI A., TEBOUL L., GAILLARD D., AMRI E.-Z., AILHAUD G., YOUNG P., CAWTHORNE M.A., GRIMALDI P.A. Evidence for a common mechanism of action for fatty acids and thiazolidinedione antidiabetic agents on gene expression in preadipose cells. Mole. Pharmacol. 1994;46:1070–1076. [PubMed] [Google Scholar]
- IKEDA H., TAKETOMI S., SUGIYAMA Y., SHIMURA Y., SOHDA T., MEGURO K., FUJITA T. Effects of pioglitazone on glucose and lipid metabolism in normal and insulin resistant animals. Drug Res. 1990;40:156–162. [PubMed] [Google Scholar]
- INOUE I., TAKAHASHI K., KATAYAMA S., HARADA Y., NEGISHI K., ITABASHI A., ISHII J. Effect of troglitazone (CS-045) and benzofibrate on glucose tolerance, liver glycogen synthase activity, and β-oxidation in fructose-fed rats. Metabolism. 1995;44:1626–1630. doi: 10.1016/0026-0495(95)90085-3. [DOI] [PubMed] [Google Scholar]
- KALLEN C.B., LAZAR M.A. Antidiabetic thiazolidinediones inhibit leptin (ob) gene expression in 3T3-L1 adipocytes. Proc. Natl. Acad. Sci. U.S.A. 1996;93:5793–5796. doi: 10.1073/pnas.93.12.5793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KANEKO T. Troglitazone (CS-045): a new antidiabetic agent. Horm. Metab. Res. 1997;29:203–213. doi: 10.1055/s-2007-979023. [DOI] [PubMed] [Google Scholar]
- KAWAMORI R., KINOSHITA J., IKEDA M., KUBOTA M., WADA M., KANDA T., IKEBUCHI M., TODO R., YAMASAKI Y. Pioglitazone (AD-4833) enhances hepatic glucose uptake in NIDDM, an assessment by the euglycemic hyperinsulinemic clamp combined with oral glucose load. Diabetes. 1997a;46 Suppl. 1:242A. [Google Scholar]
- KAWAMORI R., KINOSHITA J., IKEDA M., KUBOTA M., WADA M., KANDA T., IKEBUCHI M., TODO R., YAMASAKI Y. Pioglitazone ameliorates insulin resistance in NIDDM. Diabetologia. 1997b;40 Suppl. 1:A306. [Google Scholar]
- LAMBE K.G., TUGWOOD J.D. A human peroxisome-proliferator-activated receptor-γ is activated by inducers of adipogenesis, including thiazolidinedione drugs. Eur. J. Biochem. 1996;239:1–7. doi: 10.1111/j.1432-1033.1996.0001u.x. [DOI] [PubMed] [Google Scholar]
- LEFEBVRE A.-M., PEINADO-ONSURBE J., LEITERSDORF I., BRIGGS M.R., PATERNITI J.R., FRUCHART J.-C., FIEVET C., AUWERX J., STAELS B. Regulation of lipoprotein metabolism by thiazolidinediones occurs through a distinct but complementary mechanism relative to fibrates. Arterioscler. Thromb. Vasc. Biol. 1997;17:1756–1764. doi: 10.1161/01.atv.17.9.1756. [DOI] [PubMed] [Google Scholar]
- LEHMANN J.M., MOORE L.B., SMITH-OLIVER T.A., WILKISON W.O., WILLSON T.M., KLIEWER S.A. An antidiabetic thiazolidinedione is a high affinity ligand for peroxisome pro-liferator-activated receptor γ. J. Biol. Chem. 1995;270:12953–12956. doi: 10.1074/jbc.270.22.12953. [DOI] [PubMed] [Google Scholar]
- LEROY P., DESSOLIN S., VILLAGEOIS P., CHUN MOON B., FRIEDMAN J.M., AILHAUD G., DANI C. Expression of ob gene in adipose cells. J. Biol. Chem. 1996;271:2365–2368. doi: 10.1074/jbc.271.5.2365. [DOI] [PubMed] [Google Scholar]
- MARTIN G., SCHOONJANS K., LEFEBVRE A.-M., STAELS B., AUWERX J. Co-ordinate regulation of the expression of the fatty acid transport protein and acyl-CoA synthetase genes by PPARα and PPARγ activators. J. Biol. Chem. 1997;272:28210–28217. doi: 10.1074/jbc.272.45.28210. [DOI] [PubMed] [Google Scholar]
- MATTHEWS D.R., HOSKER J.P., RUDENSKI A.S., NAYLOR B.A., TREACHER D.F., TURNER R.C. Homeostasis model assessment: insulin resistance and β-cell function from fasting plasma glucose and insulin concentration in man. Diabetologia. 1985;28:412–419. doi: 10.1007/BF00280883. [DOI] [PubMed] [Google Scholar]
- MCCORMACK J.G., DEAN H.G., JENNINGS G.J., BLUNDELL J.E. Effects of chronic low doses of d-Fenfluramine on weight gain and calorie intake, brown adipose tissue thermogenic parameters and brain neurotransmitter content in rats fed chow or palatable diets. Int. J. Obes. 1989;13:635–633. [PubMed] [Google Scholar]
- NAKAMURA Y., OHYA Y., ONAKA U., FUJII K., ABE I., FUJISHIMA M. Inhibitory action of insulin-sensitizing agents on calcium channels in smooth muscle cells from resistance arteries of guinea-pig. Br. J. Pharmacol. 1998;123:675–682. doi: 10.1038/sj.bjp.0701669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NOLAN J.J., OLEFSKY J.M., NYCE M.R., CONSIDINE R.V., CARO J.F. Effect of troglitazone on leptin production. Studies in vitro and in human subjects. Diabetes. 1996;45:1276–1278. doi: 10.2337/diab.45.9.1276. [DOI] [PubMed] [Google Scholar]
- OAKES N.D., CAMILLERI S., FURLER S.M., CHISHOLM D.J., KRAEGEN E.W. The insulin sensitizer, BRL 49653, reduces systemic fatty acid supply and utilization and tissue lipid availability in the rat. Metabolism. 1997;46:935–942. doi: 10.1016/s0026-0495(97)90083-4. [DOI] [PubMed] [Google Scholar]
- OAKES N.D., KENNEDY C.J., JENKINS A.B., LAYBUTT D.R., CHISHOLM D.J., KRAEGEN E.W. A new antidiabetic agent, BRL 49653, reduces lipid availability and improves insulin action and glucoregulation in the rat. Diabetes. 1994;43:1203–1210. doi: 10.2337/diab.43.10.1203. [DOI] [PubMed] [Google Scholar]
- PATEL J., MILLER E., HU J., GRANETT J. BRL 49653 (a thiazolidinedione) improves glycemic control in NIDDM patients. Diabetes. 1997;46 Suppl. 1:150A. [Google Scholar]
- PATEL J., MILLER E., PATWARDHAN R. The Rosiglitazone 011 Study Group: Rosiglitazone (BRL 49653) monotherapy has significant glucose lowering effect in type 2 diabetic patients. Diabetes. 1998;47 Suppl. 1:A17. [Google Scholar]
- PAULIK M.A., LENHARD J.M. Thiazolidinediones inhibit alkaline phosphatase activity while increasing expression of uncoupling protein, deiodinase, and increasing mitochondrial mass in C3H10T1/2 cells. Cell. Tissue Res. 1997;290:79–87. doi: 10.1007/s004410050910. [DOI] [PubMed] [Google Scholar]
- PEARSON S.L., CAWTHORNE M.A., CLAPHAM J.C., DUNMORE S.J., HOLMES S.D., MOORE G.B.T., SMITH S.A., TADAYYON M. The thiazolidinedione insulin sensitiser, BRL 49653, increases the expression of PPAR-γ and aP2 in adipose tissue of high-fat-fed rats. Biochem. Biophys. Res. Commun. 1996;229:752–757. doi: 10.1006/bbrc.1996.1876. [DOI] [PubMed] [Google Scholar]
- RANDLE P.J., HALES C.N., GARLAND P.B., NEWSHOLME E.A. The glucose fatty-acid cycle. Its role in insulin sensitivity and the metabolic disturbances of diabetes mellitus. Lancet. 1963;April 13:7285–7290. doi: 10.1016/s0140-6736(63)91500-9. [DOI] [PubMed] [Google Scholar]
- RANGANATHAN S., KERN P.A. Thiazolidinediones inhibit lipoprotein lipase activity in adipocytes. J. Biol. Chem. 1998;273:26117–26122. doi: 10.1074/jbc.273.40.26117. [DOI] [PubMed] [Google Scholar]
- REZULIN (TROGLITAZONE) PACKAGE INSERT . Warner-Lambert Company, Morris Plains, NJ; 1999. [Google Scholar]
- SALTIEL A.R., HORIKOSHI H. Thiazolidinediones are novel insulin-sensitizing agents. Curr. Opin. Endocrinol. Diabetes. 1995;2:341–347. [Google Scholar]
- SALTIEL A.R., OLEFSKY J.M. Thiazolidinediones in the treatment of insulin resistance and type II diabetes. Diabetes. 1996;45:1661–1669. doi: 10.2337/diab.45.12.1661. [DOI] [PubMed] [Google Scholar]
- SCHWARTZ M.W., FIGLEWICZ D.P., BASKIN D.G., WOODS S.C., PORTE D. Insulin in the brain: a hormonal regulator of energy balance. Endocrine Rev. 1992;13:387–413. doi: 10.1210/edrv-13-3-387. [DOI] [PubMed] [Google Scholar]
- SHAO D., RANGWALA S.M., BAILEY S.T., KRAKOW S.L., REGINATO M.J., LAZAR M.A. Interdomain communication regulating ligand binding by PPAR-γ. Nature. 1998;396:377–380. doi: 10.1038/24634. [DOI] [PubMed] [Google Scholar]
- SHIBUYA A., WATANABE M., FUJITA Y., SAIGENJI K., KUWAO S., TAKAHASHI H., TAKEUCHI H. An autopsy case of troglitazone-induced fulminant hepatitis. Diabetes Care. 1998;21:2140–2143. doi: 10.2337/diacare.21.12.2140. [DOI] [PubMed] [Google Scholar]
- SHIMIZU H., TSUCHIYA T., SATO N., SHIMOMURA Y., KOBAYASHI I., MORI M. Troglitazone reduces plasma leptin concentration but increases hunger in NIDDM patients. Diabetes Care. 1998;21:1470–1474. doi: 10.2337/diacare.21.9.1470. [DOI] [PubMed] [Google Scholar]
- SONG J., WALSH M.F., IGWE R., RAM J.L., BARAZI M., DOMINGUEZ L.J., SOWERS J.R. Troglitazone reduces contraction by inhibition of vascular smooth muscle cell Ca2+ currents and not endothelial nitric oxide production. Diabetes. 1997;46:659–664. doi: 10.2337/diab.46.4.659. [DOI] [PubMed] [Google Scholar]
- SOUZA S.C., YAMAMOTO M.T., FRANCIOSA M.D., LIEN P., GREENBERG A.S. BRL 49653 blocks the lipolytic actions of tumor necrosis factor-α. A potential new insulin-sensitizing mechanism for thiazolidinediones. Diabetes. 1998;47:691–695. doi: 10.2337/diabetes.47.4.691. [DOI] [PubMed] [Google Scholar]
- TAI T.-A.C., JENNERMANN C., BROWN K.K., OLIVER B.B., MACGINNITIE M.A., WILKISON W.O., BROWN H.R., LEHMANN J.M., KLIEWER S.A., MORRIS D.C., GRAVES R.A. Activation of the nuclear receptor peroxisome proliferator-activated receptor γ promotes brown adipocyte differentiation. J. Biol. Chem. 1996;271:29909–29914. doi: 10.1074/jbc.271.47.29909. [DOI] [PubMed] [Google Scholar]
- VERMA S., BHANOT S., ARIKAWA E., YAO L., MCNEILL J.H. Direct vasodepressor effects of pioglitazone in spontaneously hypertensive rats. Pharmacology. 1998;56:7–16. doi: 10.1159/000028177. [DOI] [PubMed] [Google Scholar]
- WALKER A.B., NADERALI E.K., CHATTINGTON P.D., BUCKINGHAM R.E., WILLIAMS G. Differential vasoactive effects of the insulin sensitizers rosiglitazone (BRL 49653) and troglitazone on human small arteries in vitro. Diabetes. 1998;47:810–814. doi: 10.2337/diabetes.47.5.810. [DOI] [PubMed] [Google Scholar]
- WALSH M.F., BARAZI M., SYED A., IGWE R., ZHANG F., SOWERS J.R.Troglitazone induces both immediate and delayed relaxation in rat tail arteries Int. Congress of Endocrinology 1996P3–858.(San Francisco)
- WANG Q., DRYDEN S., FRANKISH H.M., BING C., PICKAVANCE L., HOPKINS D., BUCKINGHAM R., WILLIAMS G. Increased feeding in fatty Zucker rats by the thiazolidinedione BRL 49653 (rosiglitazone) and the possible involvement of leptin and hypothalamic neuropeptide Y. Br. J. Pharmacol. 1997;122:1405–1410. doi: 10.1038/sj.bjp.0701535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WASADA T., OMORI Y., SASAKI H., KAWAMORI R., YAMASAKI Y., BABA S., SHICHIRI M., KANEKO T. Effect of pioglitazone (AD-4833) on insulin-stimulated glucose disposal in NIDDM, an assessment by the euglycemic hyperinsulinemic clamp method. Diabetes. 1996;45 Suppl 2:73A. [Google Scholar]
- WATKINS P.B., WHITCOMB R.W. Hepatic dysfunction associated with troglitazone. New Engl. J. Med. 1998;338:916–917. doi: 10.1056/NEJM199803263381314. [DOI] [PubMed] [Google Scholar]
- WIDDOWSON P.S., UPTON R., BUCKINGHAM R., ARCH J., WILLIAMS G. Inhibition of food response to intracerebroventricular injection of leptin is attenuated in rats with diet-induced obesity. Diabetes. 1997;46:1782–1785. doi: 10.2337/diab.46.11.1782. [DOI] [PubMed] [Google Scholar]
- WILDING J.P.H., GILBEY S.G., MANNAN M., ASLAM N., GHATEI M.A., BLOOM S.R. Increased neuropeptide Y content in individual hypothalamic nuclei, but not neuropeptide Y mRNA, in diet-induced obesity in rats. J. Endocrinol. 1992;132:299–304. doi: 10.1677/joe.0.1320299. [DOI] [PubMed] [Google Scholar]
- WILLSON T.M., COBB J.E., COWAN D.J., WIETHE R.W., CORREA I.D., PRAKASH S.R., BECK K.D., MOORE L.B., KLIEWER S.A., LEHMANN J.M. Structure-activity relationship between peroxisome proliferator-activated receptor γ agonism and the antihyperglycemic activity of thiazolidinediones. J. Biol. Chem. 1996;39:665–668. doi: 10.1021/jm950395a. [DOI] [PubMed] [Google Scholar]
- YOSHIOKA S., NISHINO H., SHIRAKI T., IKEDA K., KOIKE H., OKUNO A., WADA M., FUJIWARA T., HORIKOSHI H. Antihypertensive effects of CS-045 treatment in obese Zucker rats. Metabolism. 1993;42:75–80. doi: 10.1016/0026-0495(93)90175-n. [DOI] [PubMed] [Google Scholar]
- YOUNG M.M.R., SQUASSANTE L., WERNER J. Troglitazone has no effect on the red cell mass or other erythropoietic parameters. Diabetologia. 1997;40 Suppl 1:A311. doi: 10.1007/s002280050602. [DOI] [PubMed] [Google Scholar]
- YOUNG P.W., BUCKLE D.R., CANTELLO B.C.C., CHAPMAN H., CLAPHAM J.C., COYLE P.J., HAIGH D., HINDLEY R.M., HOLDER J.C., KALLENDER H., LATTER A.J., LAWRIE K.W.M., MOSSAKOWSKA D., MURPHY G.J., ROXBEE-COX L., SMITH S.A. Identification of high-affinity binding sites for the insulin sensitizer rosiglitazone (BRL-49653) in rodent and human adipocytes using a radioiodinated ligand for peroxisomal proliferator-activated receptor γ. J. Pharmacol. Exp. Ther. 1998;284:751–759. [PubMed] [Google Scholar]
- ZHANG B., GRAZIANO M.P., DOEBBER T.W., LEIBOWITZ M.D., WHITE-CARRINGTON S., SZALKOWSKI D.M., HEY P.J., WU M., CULLINAN C.A., BAILEY P., LOLLMAN B., FREDERICH R., FLIER J.S., STRADER C.D., SMITH R.G. Down-regulation of the expression of the obese gene by an antidiabetic thiazolidinedione in Zucker Diabetic Fatty rats and db/db mice. J. Biol. Chem. 1996;271:9455–9459. doi: 10.1074/jbc.271.16.9455. [DOI] [PubMed] [Google Scholar]
- ZHANG F., SOWERS J.R., RAM J.L., STANDLEY P.R., PEULER J.D. Effects of pioglitazone on calcium channels in vascular smooth muscle. Hypertension. 1994;24:170–175. doi: 10.1161/01.hyp.24.2.170. [DOI] [PubMed] [Google Scholar]


