Abstract
This study was carried out to investigate novel cardioprotective effects of urea and the underlying mechanisms. The cardiac functions under oxidative stress were evaluated using Langendorff perfused isolated heart.
Isolated dogfish shark hearts tolerated the oxidative stress generated by electrolysis (10 mA, 1 min) of the perfusion solution (n=4), and also showed normal cardiac functions during post-ischaemia reperfusion (n=4). The high concentration of urea (350 mM) in the heart perfusate was indispensable for maintaining the normal cardiac functions of the shark heart.
Urea at 3–300 mM (n=4 for each group) protected the isolated rat heart against both electrolysis-induced heart damage and post-ischaemia reperfusion-induced cardiac injury.
A concentration-dependent scavenging effect of urea (3–300 mM, n=4 for each group) against electrolysis-induced reactive oxygen species was also demonstrated in vitro.
Urea derivatives as hydroxyurea, dimethylurea, and thiourea had antioxidant cardioprotective effect against the electrolysis-induced cardiac dysfunction of rat heart, but were not as effective as urea in suppressing the post-ischaemia reperfusion injury.
Our results suggest that urea and its derivatives are potential antioxidant cardioprotective agents against oxidative stress-induced myocardium damage including the post-ischaemia reperfusion-induced injury.
Keywords: Urea, ischaemia, reperfusion, heart, oxidative stress, arrhythmia
Introduction
Cardiac dysfunction during post-ischaemia reperfusion has been largely attributed to the detrimental effects of reactive oxygen species (ROS) (Simpson & Lucchesi, 1987; Manning, 1988). Among the possible mechanisms involved in the ROS generation, xanthine oxidase was considered to play a central role (McCord et al., 1985; Thompson-Gorman & Zweier, 1990). Inhibitors of this enzyme were used to prevent ROS-induced heart injury (Manning & Hearse, 1984; Akizuki et al., 1985), but side effects were observed due to either the inhibitors themselves or to the accumulated substrates of xanthine oxidase (Thompson-Gorman & Zweier, 1990). Antioxidants, such as mannitol and vitamin E (Bernier et al., 1986; Opie, 1989), and ROS scavengers, such as superoxide dismutase (SOD, EC 1.15.1.1) (Meerson et al., 1987; Woodward & Zakaria, 1995), have also been shown to reduce the severity of the post-ischaemia reperfusion injury. However, some properties of these agents such as cytotoxicity (Visweswaran et al., 1997), prooxidant activity (Edge & Truscott, 1997), or high molecular weight in the case of SOD, limited their application as cardioprotective agents. Recently, it was reported that copper proteins such as ceruloplasmin (EC 1.16.3.1) (Atanasiu et al., 1995; Mateescu et al., 1995) and serum amine oxidase (EC 1.16.3.6) (Mondovi et al., 1997) also exerted cardioprotective effects against post-ischaemia reperfusion-induced heart injury. But as proteins, some difficulties of their pharmacological formulation and administration may occur.
Urea is the most common nitrogen-containing end product of protein catabolism (Wright, 1995), mainly resulting from the detoxification of the ammonia derived from amino acids deamination (Meijer et al., 1990). Urea crosses cell membranes by two ways: specialized transporters or nonspecific pores (Marsh & Knepper, 1992). Urea synthesis is involved in the regulation of chronic acid-base disturbances (Atkinson, 1992). Whether the accumulated urea in the plasma in hyperuremia contributes to the uremic syndrome has been an issue of controversy (Giovannetti & Barsotti, 1975). Recently, it was found that urea stimulated gene transcription and expression (Cohen et al., 1996). While some urea derivatives, such as thiourea, dimethylurea and dimethylthiourea, were recognized as potent antioxidants, urea itself was conventionally considered as a metabolic waste or at best an inert substance used as negative control in some antioxidant studies (Halliwell, 1978). However, Lukash et al. (1980) reported that urea, inhibiting the oxidation of divalent iron, might have an antioxidant effect protecting brain and liver tissue homogenates from peroxidation of lipids. Its inhibitory effect on NADPH-dependent lipid peroxidation in rat heart tissue was also described (Shveta & Davydov, 1994).
Dogfish shark (Squalus acanthias) plasma contains a particularly high level of urea. This suggested that shark could probably be oxidative stress-resistant. In this study, the cardiac functions of the isolated dogfish shark heart under oxidative stress induced by both electrolysis and post-ischaemia reperfusion were investigated, ex vivo, and the results compared with those obtained from isolated rat hearts. The potencies of urea and several urea derivatives in protecting hearts from post-ischaemia reperfusion damage and in scavenging ROS were also evaluated. Furthermore, the direct ROS scavenging activity of urea was examined in vitro. To our knowledge, this is the first study revealing high antioxidant cardioprotective capacity of urea. This newly observed effect of urea might have broad applications towards many oxidative stress-related pathophysiological situations.
Methods
Materials
Urea, trimethylamine N-oxide (TMAO), N,N′-diethyl-p-phenylenediamine (DPD), and the other chemicals were from Sigma Chem. Co. (St-Louis, MO, U.S.A.). All chemicals were of reagent grade and used without further purification.
Evaluation of cardiac functions of isolated shark heart under the oxidative stress induced by electrolysis or post-ischaemia reperfusion
Dogfish sharks (2–4 kg, male or female) were gillnetted in Frenchman Bay (ME, U.S.A.) by the collector of the Mt. Desert Island Biological Laboratory and maintained, without feeding, in submerged wooden live cars until use (1–2 days). To evaluate cardiac functions, the sharks were pithed and heart removed, washed in iced elasmobranch physiological saline (EPS) and mounted on a modified Langendorff perfusion system. The heart of dogfish shark consists of a ventricle and an atrium. A pair of coronary arteries ramifies over the ventricle and sends small branches to the walls of the pericardial cavity. The isolated hearts were perfused at a constant pressure of 5 kPa (50 cmH2O) at 25°C with EPS (pH 7.4) containing (in mM): NaCl 270, KCl 4, TMAO 150, urea 350, MgCl2 3, HEPES 10, CaCl2 3, Na2SO4 0.5, KH2PO4 0.5 and heparin (50 units ml−1). The EPS was continuously gassed with a mixture of 95% O2 and 5% CO2. Ventricular developed pressure (VDP) was detected by a pressure sensor connected to a three-way valve, 10 cm above the aorta. Epicardial electrograms (EPI-ECG) were recorded with two electrodes positioned one on the aorta and the other on the apex of the heart. Data acquisition and analysis were accomplished using a Biopac system (Biopac System Inc., Golata, U.S.A.), including MP 100 WS acquisition units, TCI 100 amplifiers, an Acknowledge software (3.01) and universal modules (Macintosh system).
The exogenous generation of ROS was realized by placing two platinum wire electrodes in the inflow cannula: the anode at 12 cm and the cathode at 15 cm away from the entry port of the atrium (Jackson et al., 1986). A direct current (DC), generated under various current intensities (10–30 mA) and different duration (1–3 min) by a Grass stimulator (Quincy, U.S.A.), was applied. A bubble trap placed above aorta prevented bubbles from getting into the heart. Endogenous ROS were generated by the reperfusion of the regional ischaemia, induced by occluding one coronary artery by ligation as previously described (Atanasiu et al., 1995).
The experimental protocols were divided into two groups.
Electrolysis group
The total recording time was set for 35 min (Figure 1A). The first 15 min of period served as control. Electrolysis started at the 16th min and lasted for 1, 2 or 3 min, respectively, with 10 mA DC. Alternatively, the electrolysis duration was kept constant for 1 min with different intensity of DC (10, 20 and 30 mA). The EPI-ECG and VDP were continuously recorded during the experiment.
Figure 1.
The experimental protocol for the isolated shark heart perfusion under the oxidative stress induced by electrolysis (A) and post-ischaemia reperfusion (B). T, time in minutes.
Post-ischaemia reperfusion group
The total recording time was set for 40 min (Figure 1B). The first 15 min served as control and the partial ischaemia was induced at the 16th min by occlusion of one of the two coronary arteries, and maintained for 10 min. Subsequently, the shark heart was reperfused for 15 min. During the whole process, EPI-ECG and VDP were continuously recorded.
Evaluation of cardiac functions of isolated rat heart under the oxidative stress induced by electrolysis or post-ischaemia reperfusion
Male Wistar rats (250–275 g) were anaesthetized, i.p. with Somnitol (0.1 ml 100 g−1 body weight) and heparinized (500 IU intravenously). The hearts were excised, washed in cold (4°C) Krebs-Henseleit (KH) physiological buffer, mounted on a modified Langendorff organ perfusion system, and perfused at a constant pressure of 10 kPa (100 cmH2O) at 37°C with the modified KH solution (pH 7.4). The modified KH solution contained in mM: NaCl 118, KCl 4.8, CaCl2 1.8, MgSO4 0.86, KH2PO4 1.2, NaHCO3 2.54, glucose 11 and EDTA 0.027. The KH solution was first filtered through a 0.5 μm cellulose acetate membrane and then continuously gassed with a mixture of 95% O2 and 5% CO2. Left ventricular developed pressure (LVDP) was monitored using a saline filled latex balloon inserted into the left ventricle by way of the left atrium and connected to a pressure transducer. EPI-ECG was recorded with two electrodes positioned one on the aorta and the other on the apex of the heart. Perfused volumes were collected manually and CF measured.
To generate ROS by electrolysis, a constant 10 mA DC was applied for 1 min. A bubble trap (placed above aorta) prevented bubbles from getting into the heart. The duration of each experiment was 35 min (Figure 2A) which began when the heart-beat was stabilized (after completion of balloon insertion and electrodes connection). The whole paradigm was divided into three periods: Heart was first perfused with normal KH solution for 10 min (period I) and then with KH solution containing urea (0, 3, 30 and 300 mM) for 10 min (period II) before the start of electrolysis. The beginning of electrolysis (10 mA DC, lasted for 1 min) represented the start of period III (15 min). During this period, the heart was continuously perfused with the same urea-containing KH solution as in period II. The LVDP, CF and HR were continuously monitored during the whole experiment.
Figure 2.
The experimental protocol for the isolated rat heart perfusion under the oxidative stress induced by electrolysis (A) and post-ischaemia reperfusion (B). T, time in minutes.
Regional ischaemia was induced by occluding the left anterior descending coronary artery by ligation as described above. The recording protocol of each experiment was set for 45 min (Figure 2B) and divided into four periods. The heart was first stabilized for 10 min with KH buffer perfusion as control (period I), then for another 10 min (period II) with KH buffer containing urea (0, 30, 150 and 300 mM). Regional ischaemia lasted for 10 min (period III) and was followed by 15 min of reperfusion (period IV) by re-opening the closed artery. The cardiodynamic parameters were continuously monitored over the whole paradigm. A typical ischaemia was marked by 40–50% reduction of LVDP and of CF accompanied by substantial elevation of left ventricular end diastolic pressure in period III, compared with pre-ischaemia values (period II). The cardioprotective effect of urea was evaluated by its ability to reduce the incidence of irreversible ventricular fibrillations (IVF) and to increase the accumulated duration of normal sinus rhythm at reperfusion during the quantification time. Arrhythmia was defined and analysed according to the Lambeth conventions (Walker et al., 1988) over the first 5 min of reperfusion. Two parameters were used to quantify the arrhythmia: (a) incidence of total ventricular fibrillations which were classified into reversible (lasting for less than 2 min) and irreversible (persisting for more than 2 min) expressed in percentage of hearts presenting such fibrillations; and (b) the total accumulated time of normal sinus rhythm during the first 5 min of reperfusion.
Antioxidant effect of urea in vitro against electrolysis-induced ROS
ROS were generated by electrolyzing (10 mA, 1 min) the modified KH solution. The electrolysis cell contained 3 ml of modified KH buffer in the presence of different concentrations of urea (0, 3, 30 and 300 mM). The electrolysis-induced ROS were determined indirectly by measuring colorimetrically (Anonymous, 1985; Dumoulin et al., 1996) their ability to induce oxidation of N,N′-diethyl-p-phenylenediamine (DPD). Practically, a volume of 0.2 ml of the electrolyzed solution was added to 0.8 ml DPD reagent (25 mg ml−1 in KH solution). The oxidant species react rapidly with DPD generating an oxidation product with a specific absorbency at 515 nm. The colorimetric reaction was calibrated with a potassium permanganate (KMnO4) solution. The oxidative capacity of 1 μM of free chlorine solution corresponds to that of 0.4 μM KMnO4 solution. The antioxidant capacity of urea was expressed as percentage of ROS scavenged. For instance, an antioxidant capacity of 100% represents the capacity of an antioxidant to scavenge the total amount of ROS generated.
Statistical analysis
Statistical significance of differences between values of LVDP, HR and CF under different treatments was evaluated with Student's t-test (P<0.05) and the differences between the values of VF and IVF for control and treated groups were evaluated with Fisher's exact test (P<0.05). All results were expressed as mean±s.e.mean. Rat hearts were rejected if (i) LVDP<70 mmHg; or (ii) HR<230 beats min−1; or (iii) during the period of ischaemia, the decrease of LVDP and flow rate was less than 40%. For the shark hearts, the rejection criteria were: (i) VDP<15 mmHg; or (ii) HR<35 beats min−1.
Results
Isolated rat and shark hearts were found to have very different reactions to oxidative stress. ROS generated by electrolysis (10 mA, 1 min) induced dysfunction of the isolated rat heart: LVDP, HR and CF, measured 15 min after electrolysis, fell to 17–28% of the control values before electrolysis (Table 1, Figures 3 and 4). However, no significant dysfunction of isolated shark hearts was found under the same or even more intensive conditions of perfusate electrolyte (30 mA, 1 min) (Table 1). Exposure of isolated hearts to post-ischaemia reperfusion caused an incidence of 100% of ventricular fibrillation (VF) and 83% IVF in rat hearts while no VF was recorded for shark hearts under the same conditions. During the first 5 min of reperfusion, the time of sinus rhythm was 0.4±0.2 min (8±0.6% of the total time) for rat heart and 4.9±0.8 min (98±6% of the total time) for shark heart (Table 1).
Table 1.
Reactions of the isolated rat and shark hearts to the oxidative stress induced by electrolysis and post-ischaemia reperfusion
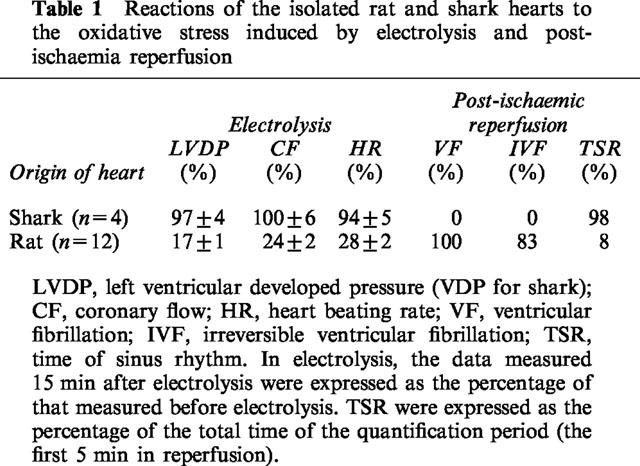
Figure 3.
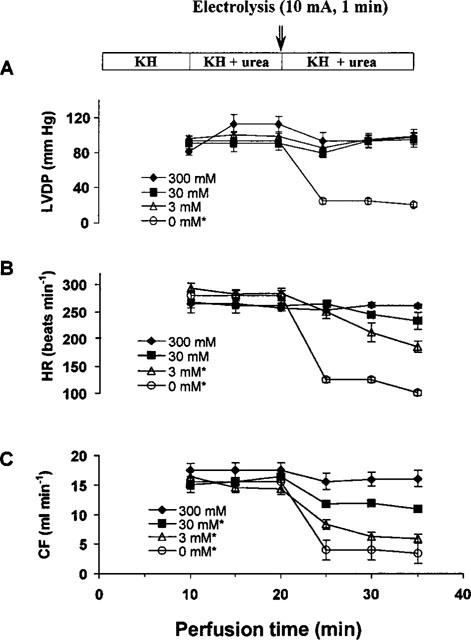
The concentration- and time-dependent effects of urea on the isolated rat heart. *Indicates that the data measured 15 min after electrolysis (T35) were significantly different from that measured before electrolysis (T20) (P<0.01). The concentrations (mM) shown in inserts are those of urea used in the experiments.
Figure 4.
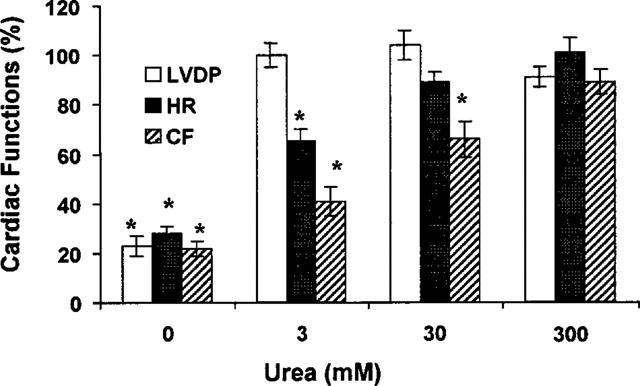
Relative change in cardiac functions after electrolysis damage. Left ventricular developed pressure (LVDP), heart beating rate (HR) and coronary flow (CF) of the isolated rat heart recorded 15 min after the electrolysis (10 mA, 1 min) were compared with the control values measured in the same heart before electrolysis (100%). n=4 for each group, *P<0.01 vs control.
To verify if the oxidative stress resistant properties of isolated shark hearts were related to the presence of urea or TMAO in EPS, the heart function was re-evaluated after urea or TMAO in EPS was equimolarly replaced by an inert compound, sucrose. The heart was first perfused with normal EPS (350 mM urea) for 10 min and then with the urea- or TMAO-replaced EPS for 10 min followed by 1 min of electrolysis (10 mA) and 9 min continuous perfusion.
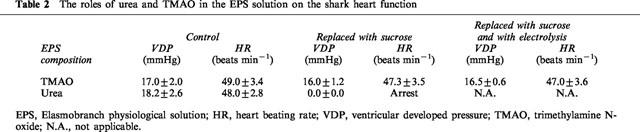
Table 2 shows that TMAO could be replaced by sucrose without changing significantly the shark heart function (n=4, P>0.05). The resistance of shark heart to the oxidative stress induced by electrolysis was also not changed in the absence of TMAO (n=4, P>0.05). On the other hand, the replacement of urea resulted in a gradual decrease of heart beating rate and eventually arrested the heart (n=4). Therefore, it was not feasible to further evaluate if the shark heart perfused with the urea-free EPS was still resistant to oxidative stress. It seemed that urea was indispensable for the normal shark cardiac functions. These results also indicated that the protection offered by urea in ESP was not due to an osmolality factor. The electrolysis of the modified EPS, in which urea was equimolarly replaced by sucrose, generated in vitro the same amount of ROS as the electrolysis of KH solution, while the normal urea-containing EPS only generated 6±0.4% of that of KH solution.
Table 2.
The roles of urea and TMAO in the EPS solution on the shark heart function
The electrolysis-induced cardiac dysfunction of isolated rat heart was decreased by the addition of urea to KH buffer. The concentration-dependent and time-dependent effects of urea on isolated rat hearts are shown in Figure 3. The relative potencies of urea at different concentrations in protecting rat heart functions from electrolysis damage are shown in Figure 4. When the electrolyzed urea-free KH solution was used, LVDP dropped to 17±1% of the control value obtained before electrolysis. With 3 mM of urea included in the electrolyzed KH solution, LVDP was totally recovered, and HR was increased from 28±2 to 65±5% and CF from 23±2 to 41±3%. When 300 mM of urea was included in the electrolyzed KH solution, the recoveries of LVDP, HR and CF were 87–97% and not statistically different from the values before electrolysis (n=4, P>0.05).
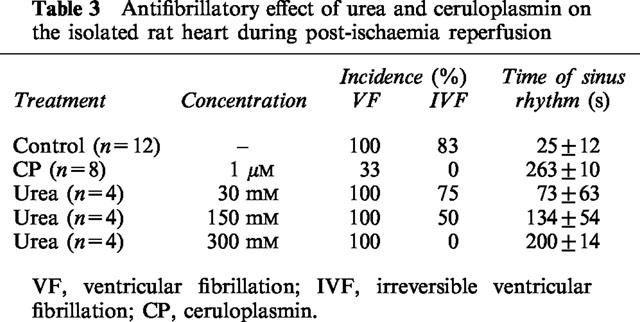
In isolated rat hearts, urea had a direct cardiac effect. At a high concentration of 300 mM, urea induced an initial 59±6% LVDP depression (lasted 30 s) followed by a rebounded increase of LVDP to a stable level of 35±4% (n=4) higher than the control. Meanwhile, HR and CF remained unchanged. At concentrations equal to or lower than 30 mM, urea only induced a transient decrease (less than 1 min), but not the rebounded increase, in LVDP. Urea also protected the isolated rat heart against the damage of post-ischaemia reperfusion (Table 3). At 300 mM, urea fully protected the heart from irreversible fibrillation (IVF 0%). The cardiac protective effect of ceruloplasmin (Atanasiu et al., 1995), a ROS scavenger, was also shown in Table 3 for comparison.
Table 3.
Antifibrillatory effect of urea and ceruloplasmin on the isolated rat heart during post-ischaemia reperfusion
Our in vitro study showed that urea possessed an antioxidant effect against electrolysis-generated ROS in a concentration dependent mode (Figure 5). The concentrations tested were in the range from the physiological concentration of urea in human plasma (3 mM) (Guyton, 1976) to that of shark (300 mM) (Smith, 1936). The antioxidant capacities of 3 and 30 mM of urea were 56±4 and 94±6%, respectively.
Figure 5.
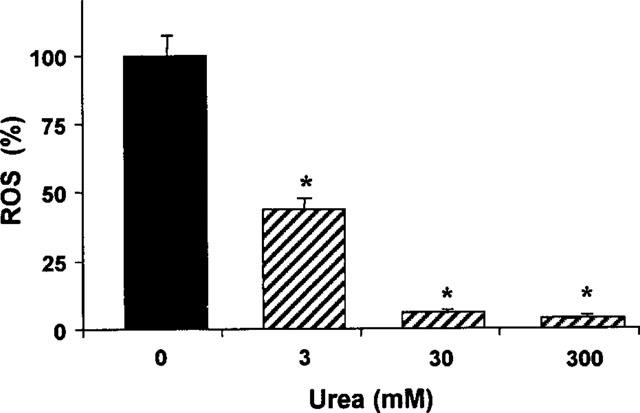
The relative level of ROS induced by electrolysis (10 mA, 1 min) of KH solution in the absence and the presence of different concentrations of urea (n=4 for each concentration, *P<0.01 vs control).
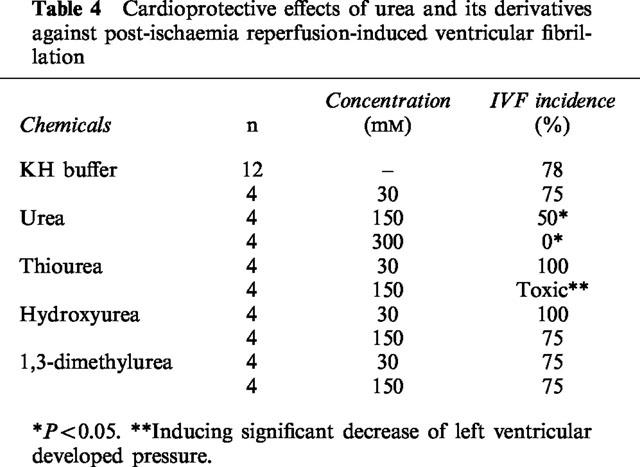
Hydroxyurea, dimethylurea, thiourea, 1,1-dimethylurea, and 1,3-dimethylurea (N,N′-dimethylurea) at a concentration of 3 mM also had cardioprotective effects on the isolated rat hearts against the damage induced by electrolysis (Figure 6). It appears that urea derivatives were more effective than urea in protecting HR and CF. However, these derivatives failed to protect the rat heart from IVF even at 150 mM (Table 4).
Figure 6.
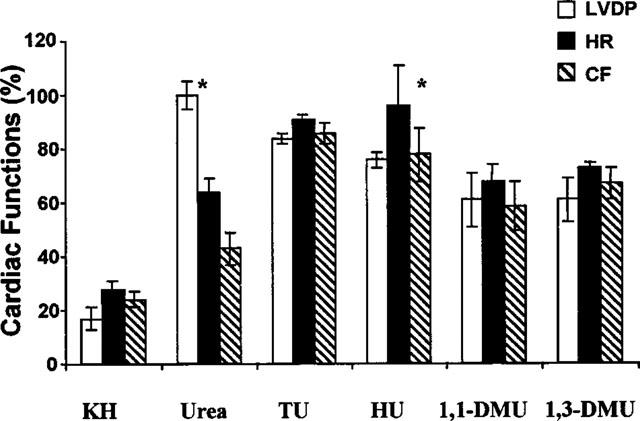
Cardioprotective effects of urea and urea-derivatives (3 mM) against the electrolysis (10 mA, 1 min) induced oxidative stress on isolated rat heart. TU, thiourea; HU, Hydroxyurea; 1,1-DMU, 1,1-dimethylurea; 1,3-DMU, 1.3-dimethylurea; KH, Krebs-Henseleit buffer (n=4 for each group. *P>0.05 vs control).
Table 4.
Cardioprotective effects of urea and its derivatives against post-ischaemia reperfusion-induced ventricular fibrillation
Discussion
It was reported that urea could protect brain and liver tissue homogenates from lipid peroxidation (Lukash et al., 1980). In this context, several interesting questions need to be answered. First, does urea protect intact isolated organs such as heart from ROS injury? Second, does the isolated heart from species with high plasma urea content, such as dogfish shark, tolerate the impact of ROS? Finally, does urea possess ROS scavenging properties? This study tentatively answered these questions.
Urea not only protects the tissue homogenates from oxidative damage, but also protects the whole organ, as shown in the present study, against oxidative stress. Cardioprotective effects of urea were tested in our study using a perfusate electrolysis model (Jackson et al., 1986). This system generates a variety of OFR and ROS, allowing the evaluation of cardioprotective properties of various antioxidants (Mateescu et al., 1995; Dumoulin et al., 1996). Under electrolysis-induced oxidative stress, LVDP remains essentially unchanged in the presence of 30 mM of urea (Figure 3). Without urea LVDP decreased to 17±1% of the control value before electrolysis. The other cardiodynamic variables, HR and CF, were also greatly protected from electrolysis damage in the presence of urea. The relative recovery of LVDP, HR and CF were not synchronic with the increase of urea concentration. For instance, 3 mM of urea fully recovered LVDP while 300 mM of urea was required for a full recovery of CF. These results are in agreement with data obtained with other antioxidants such as ceruloplasmin. In fact, it was observed that among the cardiodynamic variables, CF recovery was slower under antioxidant treatment in post-ischaemia reperfusion (Atanasiu et al., 1995; Xia et al., 1996; Mondovi et al., 1997). It is possible that the vascular tissues and cardiac tissues may react differently to ROS. The scavenging of ROS by urea or other antioxidants could suffice to recover the contractility of ventricular muscles but the constricted coronary arteries might remain at a partial constricted status due to the existence of a smooth muscle intrinsic ‘latch-bridge' contraction mechanism (Murphy, 1994). Moreover, urea can directly decrease the affinity of pyruvate kinase for pyruvate (Burg et al., 1996) which plays different roles in the energy metabolism of cardiac muscles and vascular smooth muscles. The full recovery of CF in the presence of 300 mM urea could also be explained by a direct vasodilating effect of urea at high concentrations (Vajragupta et al., 1996).
The cardioprotective effects of urea are probably not related to osmolality change of the perfusate. In fact, Abaurre et al. (1989) showed that the change of osmolality (by adding 17 mM urea) to the perfusate did not affect the mechanical and electrical properties of the isolated rat heart but decreased the systolic ventricular pressure. In our experiments, high concentration of urea only induced a transient change in LVDP, but not HR or CF, in isolated rat hearts. As urea is virtually free to permeate cell membranes, any osmolality difference between extracellular and intracellular solutions induced by adding urea to the extracellular solution would be eliminated within a short period of time. The notion that the cardioprotective effects of urea are not due to osmolality change is also supported by the data obtained with sucrose replacing urea in EPS (Table 2).
Urea appears to protect the isolated rat heart against post-ischaemia reperfusion injury. In the presence of 300 mM of urea, IVF were completely prevented. This effect is comparable to that observed with 1–2 μM ceruloplasmin as antiarrhythmic agent (Atanasiu et al., 1995) under similar conditions.
The isolated heart from dogfish shark, Squalus acanthias, was chosen as model in the present study because this fish has a high plasma urea concentration of approximately 350 mM (Smith, 1936). Our results show that the isolated shark heart could withstand up to 30 mA DC for 1 min or to 10 mA DC for 3 min electrolysis of the perfusate, as well as to damage induced by reperfusion after 10 min of regional ischaemia, without any significant change of the cardiodynamic variables (Table 1). In the case of rat heart, electrolysis under milder conditions (10 mA for 1 min) and post-ischaemia reperfusion resulted in a decline of the cardiodynamic variables to 17–28% of the normal values (Mateescu et al., 1997) and a 83% incidence of irreversible ventricular fibrillations (Atanasiu et al., 1995), respectively. Considering that arrhythmias of rat heart induced by electrolysis and by posti-schaemic reperfusion may be caused by ROS (Jackson et al., 1986; Simpson & Lucchesi, 1987; Manning, 1988), the high tolerance of the shark heart to the oxidative stress is likely to be ascribed to the protective antioxidant activities of urea (350 mM) in the EPS solution.
Finally, an important antioxidant effect of urea was demonstrated. It was observed that urea concentration-dependently scavenged the ROS induced by electrolysis of the KH solution with a scavenging capacity of 56±4% at 3 mM and 94±12% at 30 mM.
The importance of urea in maintaining the normal function of the shark heart, the cardiac protective effects of urea on the isolated rat heart, and the ROS scavenging by urea, support the hypothesis that urea plays a role in the antioxidant defence of the organisms. This hypothesis can explain, in part, the pathogenesis of the dialysis disequilibrium syndrome (DDS) characterized by neurologic deterioration and cerebral oedema after haemodialysis of patients with end stage renal failure (Silver et al., 1992). Complement-dependent granulocyte activation during haemodialysis causes the production of ROS (Himmelfarb & Hakim, 1997). The ROS are involved in the vascular injury with subsequent oedema and brain neuronal dysfunction (Demopoulos et al., 1980; Chan et al., 1982; Hammond et al., 1985). The effects of ROS generated by granulocyte activation and by other ROS producing systems may be limited and inhibited by high concentrations of urea. As a result of haemodialysis, antioxidant defence is rapidly decreased due to the clearance of plasma urea, resulting in the cerebral-oedema. In fact, the addition of urea into the haemodialysate to a level equal to the plasma urea level before dialysis was shown to prevent cerebral oedema during haemodialysis (Silver et al., 1992). Dysfunction of the isolated shark heart when perfused with urea free EPS may be explained in the same way. The removal of urea from EPS may result in a pro-oxidant–antioxidant unbalance allowing production and accumulation of ROS that cause heart dysfunction.
As an antioxidant, urea has several advantages over many other natural or synthetic antioxidants. Due to its low molecular weight and easy passing through cell membranes, urea appears to be more mobile compared to macromolecular antioxidants such as superoxide dismutase (SOD), catalase, and ceruloplasmin, whose total antioxidant capacity appears confined by their limited mobility and their compartmental distribution. Among the main natural antioxidants, the contribution of vitamin E to the total antioxidant activity of serum is very small (Stocks et al., 1974; Wayner et al., 1985). The low plasma concentration (34–111 μM) (Catherine, 1995) and the potency of vitamin C to act as an pro-oxidant (Chow, 1988) limit its contribution to the serum total antioxidant activity. Uric acid was suggested to have a biological role as an antioxidant contributing to the life span of primates with low urate oxidase activity (Seegmiller, 1979). However, serum concentrations of uric acid slightly higher than normal, due to its low solubility, are considered a risk factor of gout and heart disease (Reddy et al., 1982). Considering all the facts discussed above, urea can probably be regarded as an ideal plasma antioxidant. It is worth noting that, however, urea failed to show its antioxidant activity in some conditions, such as to prevent damage induced by OFR (mainly hydroxyl radicals) in xanthine–xanthine oxidase system (Halliwell, 1978). The underlying mechanism for the different effects of urea under different conditions should be further investigated, especially in terms of its scavenging capability.
In addition, the cardioprotective capacity of urea may also be due, in part, to its activity to inhibit the production of the gaseous free radical nitric oxide, NO. It was established that the amount of NO is significantly increased upon the reperfusion of the isolated ischaemic heart (Zweier et al., 1995; Wang & Zweier, 1996). There is strong evidence that NO is involved in the process of myocardial reoxygenation injury (Beckman et al., 1990; Matheis et al., 1992). Recently, it was found that urea was an inhibitor of nitric oxide synthase (Prabhakar et al., 1997). Therefore, the cardioprotective effect of urea can be reinforced by a decreased NO production from cardiac tissues during myocardial reperfusion. However, whether urea can inhibit the basal NO formation is still open for investigation. It is also worth noting that NO can be implicated both in myocardial reperfusion injury and in the protective ischaemic preconditioning (Takano et al., 1998). Nevertheless, our present study demonstrated the cardiac protecting effect of urea while it was present throughout the whole ischaemia-reperfusion process. If urea cannot be applied prior to ischaemia, a cardiac protective effect of urea applied prior to reperfusion would also be in force considering both the antioxidant effect of urea and the urea-induced inhibition of inducible NO synthase.
Acknowledgments
Xintao Wang was a recipient of a post-doctoral fellowship from Foundation of UQAM (Québec, Canada). Rui Wang is a McDonald scholar of Heart and Stroke Foundation of Canada. This study was supported by research grants from Natural Sciences and Engineering Research Council of Canada and from Heart and Stroke Foundation of Canada.
Abbreviations
- CF
coronary flow
- DPD
N,N′-diethyl-p-phenylenediamine
- ECG
electrocardiogram
- EPS
Elasmobranch physiological solution
- HR
heart beating rate
- IVF
irreversible ventricular fibrillation
- KH
Krebs-Henseleit
- LVDP
left ventricular developed pressure
- OFR
oxygen free radicals
- ROS
reactive oxygen species
- TMAO
trimethylamine N-oxide
- VDP
ventricular developed pressure
- VF
ventricular fibrillation
References
- ABAURRE P.F., STEFANON I., VASSALLO D.V., MILL J.G., LEITE C.M. Effect of urea on the mechanical and electrical activity of the perfused rat heart. Braz. J. Med. Biol. Res. 1989;22:1307–1310. [PubMed] [Google Scholar]
- AKIZUKI S., YOSHIDA S., CHAMBERS D.E., EDDY L.J., PARMLEY L.F., YELLON D.M., DOWNEY J.M. Infarct size limitation by the xanthine oxidase inhibitor, allopurinol, in closed-chest dogs with small infarcts. Cardiovasc. Res. 1985;19:686–692. doi: 10.1093/cvr/19.11.686. [DOI] [PubMed] [Google Scholar]
- ANONYMOUS DPD colorimetric method. Standard methods for the examination of water and wastewater. APHA, AWWA, WPCF 1985New York; 16th edn. p. 306 [Google Scholar]
- ATANASIU R., DUMOULIN M.J., CHAHINE R., MATEESCU M.A., NADEAU R. Antiarrhythmia effects of ceruloplasmin during reperfusion in the ischemic isolated rat heart. Can. J. Physiol. Pharmacol. 1995;73:1253–1261. doi: 10.1139/y95-177. [DOI] [PubMed] [Google Scholar]
- ATKINSON D.E. Functional roles of urea synthesis in vertebrates. Physiol. Zool. 1992;65:243–267. [Google Scholar]
- BECKMAN J.S., BECKMAN T.W., CHEN J., MARSHALL P.A., FREEMAN B.A. Apparent hydroxyl radical production by peroxynitrite: implications for endothelial injury from nitric oxide and superoxide. Proc. Natl. Acad. Sci. U.S.A. 1990;87:1620–1624. doi: 10.1073/pnas.87.4.1620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BERNIER M., HEARSE D.J., MANNING A.S. Reperfusion-induced arrhythmias and oxygen-derived free radicals. Studies with ‘anti-free radical' interventions and a free radical-generating system in the isolated perfused rat heart. Circ. Res. 1986;58:331–340. doi: 10.1161/01.res.58.3.331. [DOI] [PubMed] [Google Scholar]
- BURG M.B., KWON E.D., PETERS E.M. Glycerophophoscholine and betaine counteract the effect of urea on pyruvate kinase. Kidney Int. 1996;50 Suppl. 57:S100–S104. [PubMed] [Google Scholar]
- CATHERINE R.E.Free radicals and antioxidants in normal and pathological processes Oxidative Stress, Lipoproteins and Cardiovascular Dysfunction 1995London: Portland Press Ltd; 1–32.eds. Rice-Evans, C. & Bruckdorfer, K.R. pp [Google Scholar]
- CHAN P.H., YURKO M, FISHMAN R.A. Phospholipid degradation and cellular edema induced by free radicals in brain cortical slices. J. Neurochem. 1982;38:525–531. doi: 10.1111/j.1471-4159.1982.tb08659.x. [DOI] [PubMed] [Google Scholar]
- CHOW C.K.Interrelationship of cellular antioxidant defense systems Cellular Antioxidant Defense Mechanisms, Vol. II 1988CRC Press; 217–237.ed. Chow, C.K. pp [Google Scholar]
- COHEN D.M., GULLANS S.R., CHIN W.W. Urea signaling in cultured murine inner medullary collecting duct (mIMCD3) cells involves protein kinase C, inositol 1,4,5-trisphosphate (IP3), and a putative receptor tyrosine kinase. J. Clin. Invest. 1996;97:1884–1889. doi: 10.1172/JCI118619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DEMOPOULOS H.B., FLAMM E.S., PIETRONIGRO D.D., SELIGMAN M.L. The free radical pathology and the microcirculation in the central nervous system disorders. Acta Physiol. Scand. Suppl. 1980;492:91–118. [PubMed] [Google Scholar]
- DUMOULIN M.J., CHAHINE R., ATANASIU R., NADEAU R., MATEESCU M.A. Comparative antioxidant and cardioprotective effects of ceruloplasmin, superoxide dismutase and albumin. Arzneim.-Forsch./Drug Res. 1996;46:855–861. [PubMed] [Google Scholar]
- EDGE R., TRUSCOTT T.G. Prooxidant and antioxidant reaction mechanisms of carotene and radical interactions with vitamins E and C. Nutrition. 1997;13:992–994. doi: 10.1016/s0899-9007(97)00346-8. [DOI] [PubMed] [Google Scholar]
- GIOVANNETTI S., BARSOTTI G. Uremic intoxication. Nephron. 1975;14:123–133. doi: 10.1159/000180443. [DOI] [PubMed] [Google Scholar]
- GUYTON C.Textbook of medical physiology 1976Philadelphia: W.B. Saunders Company; 460–466.5th edition pp [Google Scholar]
- HALLIWELL B. Superoxide-dependent formation of hydroxyl radicals in the presence of iron chelates: is it a mechanism for hydroxyl radical production in biochemical systems. FEBS Lett. 1978;92:321–326. doi: 10.1016/0014-5793(78)80779-0. [DOI] [PubMed] [Google Scholar]
- HAMMOND B., KONTOS H.A., HESS M.L. Oxygen radicals in the adult respiratory distress syndrome, in myocardial ischemia and reperfusion injury and in cerebral vascular damage. Can. J. Physiol. Pharmacol. 1985;63:173–187. doi: 10.1139/y85-034. [DOI] [PubMed] [Google Scholar]
- HIMMELFARB J., HAKIM R.M. The use of biocompatible dialysis membranes in acute renal failure. Adv. Ren. Replace Ther. 1997;4 2 Suppl. 1:72–80. [PubMed] [Google Scholar]
- JACKSON C.V., MICKELSON J.K., STUINGER K., RAO P.S., LUCCHESI B.R. Electrolysis induced myocardial dysfunction. A novel method for the study of free radical mediated tissue injury. J. Pharmacol. Methods. 1986;15:305–320. doi: 10.1016/0160-5402(86)90010-0. [DOI] [PubMed] [Google Scholar]
- LUKASH A.I., KARTASHEV I.P., ANTIPINA T.V. Participation of iron ions in antioxidant action of urea. Ukrainskii Biokhimicheshkii Zhurnal. 1980;52:462–465. [PubMed] [Google Scholar]
- MANNING A.S. Reperfusion-induced arrhythmias: Do free radicals play a critical role. Free Radical Biol. Med. 1988;4:305–316. doi: 10.1016/0891-5849(88)90051-2. [DOI] [PubMed] [Google Scholar]
- MANNING A.S., HEARSE D.J. Reperfusion-induced arrhythmias: mechanisms and prevention. J. Mol. Cell. Cardiol. 1984;16:497–518. doi: 10.1016/s0022-2828(84)80638-0. [DOI] [PubMed] [Google Scholar]
- MARSH D.J., KNEPPER M.A.Renal Handling of Urea Handbook of Physiology, section 8, Renal Physiology 1992New York: Oxford University Press; 1317–1348.ed. Windhager, E.E. pp [Google Scholar]
- MATEESCU M.A., CHAHINE R., ROGER S., ATANASIU R., YAMAGUCHI N., LALUMIERE G., NADEAU R. Protection of myocardial tissue against deleterious effects of oxygen free radicals by ceruloplasmin. Arzneim. Forsch./Drug Res. 1995;45:476–480. [PubMed] [Google Scholar]
- MATEESCU M.A., DUMOULIN M.J., WANG X.T., NADEAU R., MONDOVI B. A new physiological role of copper amine oxidases: Cardioprotection against reactive oxygen intermediates. J. Physiol. Pharmacol. 1997;48 Suppl. 2:110–121. [Google Scholar]
- MATHEIS G., SHERMAN M.P., BUCKBERG G.D., HAYBRON D.M., YOUNG H.H., IGNARRO L.J. Role of L-arginine-nitric oxide pathway in myocardial reoxygenation injury. Am. J. Physiol. 1992;262:H616–H620. doi: 10.1152/ajpheart.1992.262.2.H616. [DOI] [PubMed] [Google Scholar]
- MCCORD J.M., ROY R.S., SCHAFFER S.W. Free radicals and myocardial ischemia. The role of xanthine oxidase. Adv. Myocardiol. 1985;5:183–189. [PubMed] [Google Scholar]
- MEERSON F.Z., BELKINA L.M., SAZONTOVA T.G., SALTYKOVA V.A., ARKHIPENKO YU V. The role of lipid peroxidation in pathogenesis of arrhythmias and prevention of cardiac fibrillation with antioxidants. Basic Res. Cardiol. 1987;82:123–137. doi: 10.1007/BF01907060. [DOI] [PubMed] [Google Scholar]
- MEIJER A.J., LAMERS W.H., CAMULEAU R.A.F.M. Nitrogen metabolism and ornithine cycle function. Physiol. Rev. 1990;70:701–748. doi: 10.1152/physrev.1990.70.3.701. [DOI] [PubMed] [Google Scholar]
- MONDOVI B., WANG X.T., PIETRANGELI P., WANG R., NADEAU R., MATEESCU M.A. New aspects on the physiological role of copper amine oxidases. Curr. Topics Medicinal Chem. 1997;2:31–43. [Google Scholar]
- MURPHY R.A. What is special about smooth muscle? The significance of covalent crossbridge regulation. FASEB J. 1994;8:311–318. doi: 10.1096/fasebj.8.3.8143937. [DOI] [PubMed] [Google Scholar]
- OPIE L.H. Reperfusion injury and its pharmacologic modification. Circulation. 1989;80:1049–1062. doi: 10.1161/01.cir.80.4.1049. [DOI] [PubMed] [Google Scholar]
- PRABHAKAR S.S., ZEBALLOS G.A., MONTOYA-ZAVALA M., LEONARD C. Urea inhibits inducible nitric oxide synthase in macrophage cell line. Am. J. Physiol. 1997;273:C1882–C1888. doi: 10.1152/ajpcell.1997.273.6.C1882. [DOI] [PubMed] [Google Scholar]
- REDDY C.C., SCHOLZ R.W., THOMAS C.E., MASSARO E.J. Vitamin E dependent reduced glutathine inhibition of rat liver microsomal lipid peroxidation. Life Sci. 1982;31:371–480. doi: 10.1016/0024-3205(82)90486-6. [DOI] [PubMed] [Google Scholar]
- SEEGMILLER J.E.Disorders of Purine and Pyrimidine Metabolism Contemporary Metabolism, Vol. 1 1979New York: Plenum Press; ed. Freinkel. E [Google Scholar]
- SHVETA V.N., DAVYDOV V.V. The characteristics of antioxidant action in the heart of old rats under stress. Eksp. Klin. Farmakol. 1994;57:838–845. [PubMed] [Google Scholar]
- SILVER S.M., DESIMONE J.A., SMITH D.A., STERNS R.H. Dialysis disequilibrium syndromes (DDS) in the rat: Role of the ‘reverse urea effect'. Kidney Int. 1992;42:161–166. doi: 10.1038/ki.1992.273. [DOI] [PubMed] [Google Scholar]
- SIMPSON P.J., LUCCHESI B.R. Free radicals and myocardial ischemia and reperfusion. J. Lab. Clin. Med. 1987;110:13–30. [PubMed] [Google Scholar]
- SMITH H.W. The retention and physiological role of urea in Elasmobranchii. Biol. Rev. 1936;11:49–82. [Google Scholar]
- STOCKS J., GUTTERIDGE J.M.C., SHARP R.J., DORMANDY T.L. The inhibition of lipid autoxidation by human serum and its relationship to serum proteins and alpha-tocopherol. Clin. Sci. 1974;47:223–231. doi: 10.1042/cs0470223. [DOI] [PubMed] [Google Scholar]
- TAKANO H., MANCHIKALAPUDI S., TANG X.L., QIU Y., RIZVI A., JADOON A.K., ZHANG Q., BOLLI R. Nitric oxide synthase is the mediator of late preconditioning against myocardial infarction in conscious rabbits. Circulation. 1998;98:441–449. doi: 10.1161/01.cir.98.5.441. [DOI] [PubMed] [Google Scholar]
- THOMPSON-GORMAN S.L., ZWEIER J.L. Evaluation of the role of xanthine oxidase in myocardial reperfusion injury. J. Biol. Chem. 1990;265:6656–6663. [PubMed] [Google Scholar]
- VAJRAGUPTA O., PATHOMSAKUL A., MATAYATSUK C., RUANGREANGYINGYOD L., WONGKRAJANG Y., FOYE W.O. Synthesis and antihypertensive activity of N-(alkyl/alkenyl/aryl)-N-heterocyclic ureas and thioureas. J. Pharm. Sci. 1996;85:258–261. doi: 10.1021/js930295x. [DOI] [PubMed] [Google Scholar]
- VISWESWARAN P., MASSIN E.K., DUBOSE T.D., Jr Mannitol-induced acute renal failure. J. Am. Soc. Nephrol. 1997;8:1028–1033. doi: 10.1681/ASN.V861028. [DOI] [PubMed] [Google Scholar]
- WALKER M.J.A., CURTIS M.J., HEARSE D.J., CAMPBELL R.W.F., JANSE M.J., YELLON D.M., COBB S.M., COKER S.J., HARNESS J.B., HARRON D.W.G., HIGGINS A.J., JULIAN D.J., LAB M.J., MANNING M.S., NORTHOVER B.J., PARRATT J.R., RIEMERSMA R.A., RIVA E., RUSSEL D.C., SHERIDAN D.J., WINSLOW E., WOODWARD B. The Lambeth Conventions: guidelines for the study of arrhythmias in ischemia, infarction and reperfusion. Cardiovasc. Res. 1988;22:447–455. doi: 10.1093/cvr/22.7.447. [DOI] [PubMed] [Google Scholar]
- WANG P., ZWEIER J.L. Measurement of nitric oxide and peroxynitrite generation in the postischemic heart. Evidence for peroxynitrite-mediated reperfusion injury. J. Biol. Chem. 1996;271:29223–29230. doi: 10.1074/jbc.271.46.29223. [DOI] [PubMed] [Google Scholar]
- WAYNER D.D.M., BURTON G.W., INGOLD K.U., LOCKE S. Quantitative measurement of the total, peroxyl radical-trapping antioxidant capability of human blood plasma by controlled peroxidation. The important contribution made by plasma proteins. FEBS Lett. 1985;187:33–37. doi: 10.1016/0014-5793(85)81208-4. [DOI] [PubMed] [Google Scholar]
- WOODWARD B., ZAKARIA M.N. Effect of some free radical scavengers on reperfusion induced arrhythmias in the isolated rat heart. J. Mol. Cell. Cardiol. 1995;17:485–493. doi: 10.1016/s0022-2828(85)80053-5. [DOI] [PubMed] [Google Scholar]
- WRIGHT P.A. Nitrogen excretion: three end products, many physiological roles. J. Exp. Biol. 1995;198:273–281. doi: 10.1242/jeb.198.2.273. [DOI] [PubMed] [Google Scholar]
- XIA Y., KHATCHIKIAN G., ZWEIER J.L. Adenosine deaminase inhibition prevents free radical-mediated injury in the postischemic heart. J. Biol. Chem. 1996;271:10096–10102. doi: 10.1074/jbc.271.17.10096. [DOI] [PubMed] [Google Scholar]
- ZWEIER J.L., WANG P., KUPPUSAMY P. Direct measurement of nitric oxide generation in the ischemic heart using electron paramagnetic resonance spectroscopy. J. Biol. Chem. 1995;270:304–307. doi: 10.1074/jbc.270.1.304. [DOI] [PubMed] [Google Scholar]


