Abstract
In this study we have examined the use of the ecdysone-inducible mammalian expression system (Invitrogen) for the regulation of expression of the predominant L-glutamate transporter EAAT2 (Excitatory Amino Acid Transporter) in HEK 293 cells.
HEK 293 cells which were stably transformed with the regulatory vector pVgRXR (EcR 293 cells) were used for transfection of the human EAAT2 cDNA using the inducible vector pIND and a clone designated HEK/EAAT2 was selected for further characterization.
Na+-dependent L-glutamate uptake activity (3.2 pmol min−1 mg−1) was observed in EcR 293 cells and this was increased approximately 2 fold in the uninduced HEK/EAAT2 cells, indicating a low level of basal EAAT2 activity in the absence of exogenous inducing agent. Exposure of HEK/EAAT2 cells to the ecdysone analogue Ponasterone A (10 μM for 24 h) resulted in a ⩾10 fold increase in the Na+-dependent activity.
L-glutamate uptake into induced HEK/EAAT2 cells followed first-order Michaelis-Menten kinetics and Eadie-Hofstee transformation of the saturable uptake data produced estimates of kinetic parameters as follows; Km 52.7±7.5 μM, Vmax 3.8±0.9 nmol min−1 mg−1 protein.
The pharmacological profile of the EAAT2 subtype was characterized using a series of L-glutamate transport inhibitors and the rank order of inhibitory potency was similar to that described previously for the rat homologue GLT-1 and in synaptosomal preparations from rat cortex.
Addition of the EAAT2 modulator arachidonic acid resulted in an enhancement (155±5% control in the presence of 30 μM) of the L-glutamate transport capacity in the induced HEK/EAAT2 cells.
This study demonstrates that the expression of EAAT2 can be regulated in a mammalian cell line using the ecdysone-inducible mammalian expression system.
Keywords: Excitatory amino acid transporter, EAAT2 pharmacology, L-glutamate, ecdysone, kainate, dihydrokainate, arachidonic acid
Introduction
L-glutamate is considered to be the predominant excitatory neurotransmitter in the mammalian central nervous system and its actions are mediated through activation of ligand-gated ion channels (the ionotropic receptors) and seven transmembrane spanning G-protein coupled receptors (the metabotropic receptors). Another important family of proteins which are critical for normal glutamatergic neurotransmission are the high-affinity Na+-dependent transporters present in both neuronal and astroglial plasma membranes (Gegelashvili & Schousboe, 1997). Historically the activity of these transporters was studied in brain slice and synaptosomal preparations and these approaches provided the basis for the existence of pharmacologically distinct transporter subtypes (Ferkany & Coyle, 1986; Robinson et al., 1991; 1993). Recent success in the isolation of complementary DNA sequences from both rodent and human species encoding multiple L-glutamate transporter subtypes has confirmed the presence of multiple transporter subtypes.
A family of five mammalian high-affinity Na+-dependent L-glutamate transporters has to date been identified by molecular cloning and the subtypes are designated EAAT1/GLAST, EAAT2/GLT-1, EAAT3/EAAC1, EAAT4 and EAAT5 (Arriza et al., 1994; 1997; Fairman et al., 1995; Kanai & Hediger, 1992; Pines et al., 1992; Storck et al., 1992). The designation EAAT (excitatory amino acid transporter) was applied following the isolation of the human subtypes 1–3 (Arriza et al., 1994) and reflects the fact that a number of excitatory amino acids are substrates for these transporters although L-glutamate is likely to be the predominant endogenous substrate. Localization studies have revealed that both EAAT1 and EAAT2 are expressed almost exclusively in astroglial cells (Rothstein et al., 1994) and are restricted to the central nervous system (CNS). In contrast, EAAT3 is predominantly neurone specific in the CNS and is also localized to a number of peripheral tissues (Conti et al., 1998; Rothstein et al., 1994). Expression of the EAAT4 subtype is high in cerebellum, in particular the Purkinje cells (Yamada et al., 1996), and EAAT5 is expressed primarily in the retina (Arriza et al., 1997). The family of EAAT proteins are responsible for the maintenance of low extracellular L-glutamate concentration and therefore prevent excitotoxicity. In addition, they participate in the termination of synaptic transmission with the reuptake of synaptically released L-glutamate.
Although five distinct EAAT subtypes have been identified it is evident that the predominant astroglial subtype EAAT2 accounts for the bulk of the L-glutamate transport activity which can be measured in adult cortex. This has been demonstrated by pharmacological characterization of the L-glutamate transport activity in synaptosome fractions prepared from cerebral cortex which can be completely blocked by the selective EAAT2 inhibitors dihydrokainic acid (DHK) and kainic acid (KA). The inhibitory potency estimated for DHK and KA in the native synaptosomal preparation is well correlated with their inhibitory potencies against the cloned rat and human EAAT2 (Dunlop et al., 1998; Robinson et al., 1993; Tan et al., 1999). In addition, the pharmacological profile of L-glutamate transport in synaptosomes is extremely well correlated with that described for the cloned GLT-1 and EAAT2 subtypes expressed in mammalian cell lines.
Further demonstration of the important role of EAAT2 comes from both gene knockdown and knockout of the EAAT2 subtype. Rothstein et al. (1996) used a chronic antisense oligonucleotide approach to inhibit the synthesis of each of the transporters EAAT 1-3. The most profound effects were demonstrated following the loss of EAAT2 which resulted in a 32 fold increase in striatal L-glutamate levels, as assessed by microdialysis, an observation strongly supportive of the notion that the activity of the EAAT2 subtype is essential for the maintenance of low extracellular L-glutamate levels. Consistent with the elevation of extracellular L-glutamate levels, a histological examination of EAAT2 deficient rats detected ultrastructural changes in both striatal and hippocampal neurones which were consistent with excitotoxicity. Generation of EAAT2 knockout mice by homologous recombination (Tanaka et al., 1997) results in a 94% loss of L-glutamate uptake activity in cortical synaptosomes prepared from homozygous mutants. In addition, homozygous mutant mice exhibited spontaneous and lethal seizures and were more susceptible than wild type animals to acute cortical injury following a cold-induced head injury. Again the phenotype of the EAAT2 knockout mouse is strongly supportive of a predominant role for EAAT2 in L-glutamate reuptake.
The critical role of EAAT2 in maintaining low extracellular L-glutamate concentrations suggests that this subtype may represent a novel target for neuroprotective agents capable of modulating its transport activity. It has been demonstrated that arachidonic acid produces a selective enhancement of EAAT2 transport activity and produces either a modest inhibitory activity or no effect on the EAAT1 and EAAT3 subtypes, respectively (Zerangue et al., 1995). The production of cell lines expressing the cloned EAATs is an important step in the development of molecules capable of inhibiting or enhancing transporter activity. In the process of generating a number of EAAT expressing cell lines we have investigated the use of the ecdysone-inducible expression system for the control of EAAT2 expression in mammalian cells. The insect steroid hormone ecdysone is responsible for the activation of metamorphosis in Drosophila melanogaster, an effect mediated by a heterodimer of the functional ecdysone receptor (EcR) and the product of the ultraspiracle gene (USP) which activates genes expression (Yao et al., 1993). It has been demonstrated (No et al., 1996) that responsiveness to the synthetic ecdysone analogues muristerone A or ponasterone A can be engineered in mammalian cells by co-expression of the EcR with the mammalian homologue of the USP gene, the retinoid X receptor (RXR). Using this system the expression of a chosen cDNA can be placed under the control of the EcR/RXR heterodimer which in theory activates genes expression only in the presence of exogenously supplied hormone. A number of advantages of the ecdysone system over tetracycline based inducible strategies have been claimed (see review by Saez et al., 1997) including its use as a naturally evolved inducible system, the lipophilic nature of the ecdysone analogues, short half life and favourable pharmacokinetics and lack of toxicity of teratogenicity or the synthetic analogues.
In this study, we have evaluated the use of the ecdysone-inducible mammalian expression system for the control of EAAT2 expression in HEK 293 cells which have a low endogenous capacity for Na+-dependent L-glutamate uptake. The results demonstrate that the expression of EAAT2 in HEK 293 cells can be regulated using the ecdysone system. The hallmark features of EAAT2, namely sensitivity to DHK and KA and enhancement in the presence of arachidonic acid, are faithfully reproduced following induction indicating that this cell line will be of use in the development of novel modulators of this transporter subtype.
Methods
Materials
L-[3H]-glutamate (20–60 Ci mmol−1) was obtained from Amersham (Arlington Heights, IL, U.S.A) and unlabelled L-glutamate from Sigma (St. Louis, MO, U.S.A.). Uptake inhibitors were purchased from Tocris Cookson Inc. (Ballwin, MO, U.S.A.) with the exception of threo-β-hydroxyaspartate, aspartate-β-hydroxamate and L-α-aminoadipic acid which were from Sigma. Reagents for the ecdysone inducible mammalian expression system were from Invitrogen (Carlsbad, CA, U.S.A.).
Expression of EAAT2 in EcR 293 cells
The cloning of the human EAAT2 subtype from a human cortex cDNA library has been described (Arriza et al., 1994) and the EAAT2 cDNA used in this study was obtained from Dr Susan Amara (Vollum Institute, Portland, OR, U.S.A.). EAAT2 cDNA was subcloned from pBluescript into the pIND mammalian expression vector and the resulting construct was transfected into EcR 293 cells (HEK 293 cells which were stably transformed with the regulatory vector pVgRXR) using lipofectamine. Resistance to G418 and zeocin was used to select for potential positive clones and a clonal cell line termed HEK/EAAT2 was established from the clone with the highest level of inducible EAAT2 expression. HEK/EAAT2 cells were maintained in Dulbecco's modified Eagle's medium supplemented with 10% foetal bovine serum, G418 (0.4 mg ml−1) and zeocin (0.4 mg ml−1) in a humidified atmosphere with 5% CO2 at 37°C. EcR 293 cells were maintained in the same medium minus G418.
L-glutamate uptake
Uptake studies were performed at room temperature in 24 well plates precoated with poly-D-lysine. Two days before the uptake assay cells were harvested by addition of Dulbecco's phosphate buffered saline (D-PBS) containing 5 mM EDTA, resuspended in fresh growth medium and seeded at a density of 1×105 cells well−1 (0.5 ml) in 24 well plates precoated with poly-D-lysine. On the day before the assay 0.5 ml ponasterone A was added to each well using a 2× solution (20 μM) prepared by first adding 25 μl ethanol to 250 μg ponasterone A to produce a 20 mM stock solution which was then diluted further (1 : 1000) with growth medium. The uninduced wells also received a final equivalent (0.05%) of ethanol. All uptake assays were in D-PBS except for the experiments where Na+-dependency was evaluated in which we used a HEPES buffered saline (HBS) solution of the following composition: (mM), HEPES 10, Tris Base 5, NaCl 140, KCl 2.5, CaCl2 1.2, MgCl2 1.2, K2HPO4 1.2, glucose 10, pH 7.4, with equimolar replacement of Na+ with choline for the Na+-free conditions. Cells were washed three times with D-PBS or HBS before incubation with substrate for 10 min at room temperature. In preliminary experiments it was established that the uptake of L-glutamate into HEK/EAAT2 cells was linear at least up to 30 min (not shown). For the saturation experiments the substrate solution contained L-glutamate in the concentration range 1–300 μM and 2 μCi assay−1 L-[3H]-glutamate as radiotracer. Experiments with transport inhibitors and arachidonic acid were undertaken in the presence of 10 μM L-glutamate containing 2 μCi assay−1 L-[3H]-glutamate. Uptake assays were terminated after 10 min by rapid aspiration followed by two washes with ice-cold D-PBS and accumulated radioactivity was measured by liquid scintillation counting after the addition of 0.5 ml 0.5 N NaOH to each well.
Data analysis
Data from the saturation analysis were linearized using the Eadie-Hofstee transformation for the estimation of kinetic parameters. Results from the inhibitor studies were expressed as per cent control L-glutamate uptake observed in the absence of added compound after correction for non-specific uptake defined as the accumulation of radiolabel observed in the presence of 1 mM unlabelled L-glutamate. Inhibitor IC50 values were estimated by non-linear regression analysis and were converted to Ki values according to the equation Ki=IC50/1+[S]/Km (Cheng & Prusoff, 1973) where [S] equals the concentration of L-glutamate substrate and Km equals the estimated Km for L-glutamate transport obtained from the Eadie-Hofstee transformation.
Results
Inducible expression of EAAT2 in HEK cells
EcR 293 cells, derived from HEK 293 cells by incorporation of the regulatory vector pVgRXR, were chosen for the purposes of this study by virtue of their commercial availability. This required transfection with a single plasmid containing the gene of interest which, in this case, was the human EAAT2 subtype incorporated into the pIND vector. The pIND and pVgRXR vectors are components of the ecdysone-inducible mammalian expression system from Invitrogen. The pIND vector incorporates a multiple cloning site for insertion of a gene of interest under the control of a minimal heat shock promoter and five upstream ecdysone response elements. The pVgRXR vector encodes for the ecdysone and retinoid X receptors under the control of CMV and RSV promoters, respectively. Selection of stable transfectants is permitted by the neomycin resistance gene in pIND, and the zeocin resistance gene in pVgRXR. 293 cells can serve as a suitable host for the transfection of L-glutamate transporters due to their low endogenous capacity for transport. Following transfection an EAAT2 expressing cell line was selected based on a preliminary evaluation of Na+-dependent L-glutamate transport capacity in 30 different clones, and the line which exhibited the best inducibility was chosen for further characterization and was designated HEK/EAAT2.
In order to examine the inducibility of the EAAT2 subtype in HEK/EAAT2 cells we compared the effect of treatment with the ecdysone analogue ponasterone A between EcR 293 cells and HEK/EAAT2 cells generated using the pIND/EAAT2 construct. EcR 293 cells exhibited a small Na+-dependent L-glutamate uptake capacity which was unaltered by treatment with 10 μM ponasterone A for 24 h (Figure 1). Treatment of HEK/EAAT2 cells with 10 μM ponasterone A for 24 h produced an 11.7 fold increase in the L-glutamate transport capacity (Figure 1) demonstrating the inducible nature of EAAT2 expression in these cells. Figure 1 also serves to illustrate that the transport of L-glutamate by HEK/EAAT2 cells was predominantly (96%) extracellular Na+-dependent, as determined by equimolar replacement of Na+ with choline in the incubation buffer. Finally, there was also a 2.4 fold increase in the transport capacity measured in the uninduced HEK/EAAT2 cells relative to EcR-293 cells (Figure 1).
Figure 1.
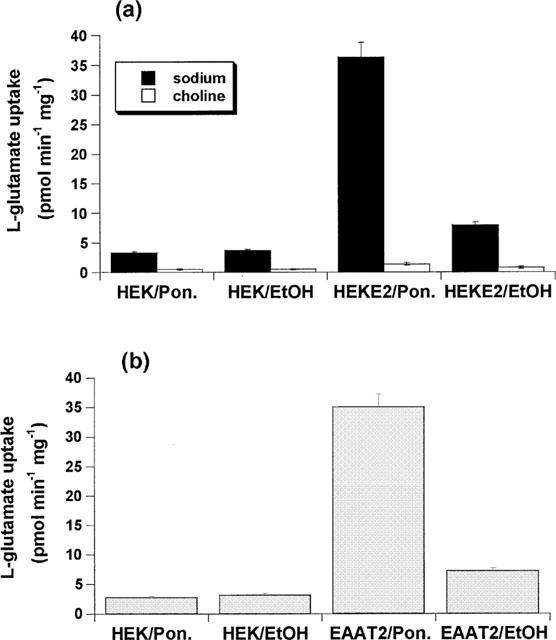
Effect of 10 μM ponasterone A induction on L-glutamate transport activity in non-EAAT2 transfected EcR 293 cells (HEK) and in cells expressing EAAT2 (HEK E2). (a) L-glutamate uptake was evaluated in Na+-containing and Na+-free HBS and the Na+-dependent L-glutamate uptake (b) was determined after correction for the activity observed in the absence of extracellular Na+.
Kinetic and pharmacological properties of expressed EAAT2
By measuring the uptake of L-glutamate into induced HEK/EAAT2 cells over a range of substrate concentrations (1–300 μM) the transport activity was shown to be both saturable and high-affinity (μM range) in nature (Figure 2), as would be expected for the EAAT2 protein. Eadie-Hofstee transformation of the saturable uptake data was used to generate estimates of the kinetic parameters Km and Vmax which were 52.7±7.5 μM and 3.82±0.91 nmol min−1 mg−1 protein, respectively (n=3). The selective EAAT2 inhibitors DHK and KA produced a concentration-dependent inhibition of L-glutamate uptake into induced HEK/EAAT2 cells with Ki estimates of 34.3±5.8 and 37.3±15.7 μM, respectively (Figure 3). The pharmacological profile of the EAAT2 subtype generated using 12 known L-glutamate transport inhibitors is presented in more detail in Table 1 with the estimated Ki values reported in their rank order of inhibitory potency. The conformationally restricted analogue of L-glutamate L-CCG-III ((2S,3S,4R)-2-(carboxycyclopropyl)glycine) which exhibits 1–3 μM potency for the inhibition of L-glutamate uptake into synaptosomes and glial vesicles (Nakamura et al., 1993) was the most potent inhibitor of the compounds examined producing a Ki estimate of 4.0±1.6 μM. The (2S,3R,4S) isomer L-CCG-IV was the weakest of the compounds tested which showed some measurable activity producing 46% inhibition at 1 mM, an observation consistent with the weak activity observed for the same compound in synaptosomes (Nakamura et al., 1993). L-α-aminoadipic acid was ineffective as an inhibitor of EAAT2 when tested at concentrations up to 1 mM. Assessment of the effect of the EAAT2 modulator arachidonic acid on L-glutamate uptake by induced HEK/EAAT2 cells was performed by measuring uptake in the absence and presence of increasing concentrations of arachidonate. As depicted in Figure 4 arachidonate produced an enhancement of the L-glutamate uptake into HEK/EAAT2 cells with a maximum effect of 155±5% control observed at 30 μM AA. There was no enhancement of the endogenous uptake activity in EcR 293 cells in the presence of arachidonate (data not shown), indicating that the expressed EAAT2 subtype is selectively enhanced by this agent. This observation is consistent with the predominant expression of the EAAT3 subtype in HEK 293 cells (Toki et al., 1998), and the reported lack of effect of arachidonate on the cloned EAAT3 subtype (Zerangue et al., 1995).
Figure 2.
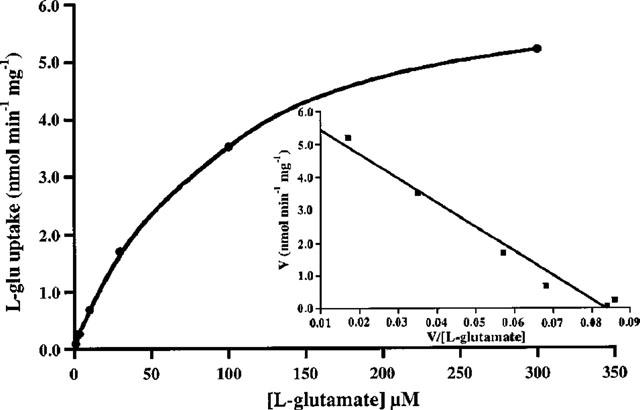
Saturation analysis of L-glutamate uptake into induced HEK/EAAT2 cells. Cells were incubated in the presence of increasing concentrations of substrate and the Na+-dependent L-glutamate uptake was measured and expressed as nmol min−1 mg−1 protein with correction for the activity observed in the absence of extracellular Na+. Inset: Eadie-Hofstee transformation of the saturable uptake data was used to estimate the kinetic parameters Km and Vmax which were 73.8 μM and 6.2 nmol min−1 mg−1 protein, respectively, for the experiment shown. Data from three independent experiments generated values of 52.7±7.5 μM for Km and 3.8±0.9 nmol min−1 mg−1 protein for Vmax.
Figure 3.
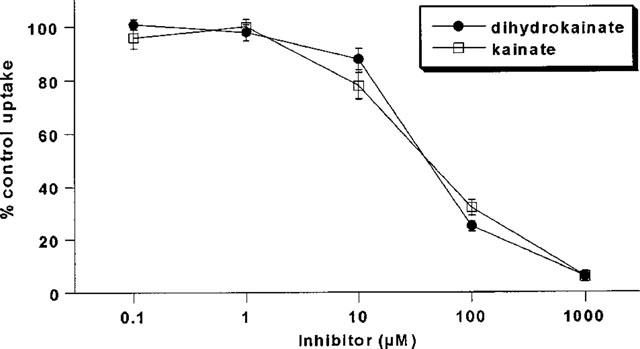
Log-concentration effect curves for dihydrokainate and kainate inhibition of L-glutamate uptake into induced HEK/EAAT2 cells.
Table 1.
Ki values (mean±s.e.mean) for L-glutamate uptake inhibitors on cloned EAAT2 activity expressed in HEK cells
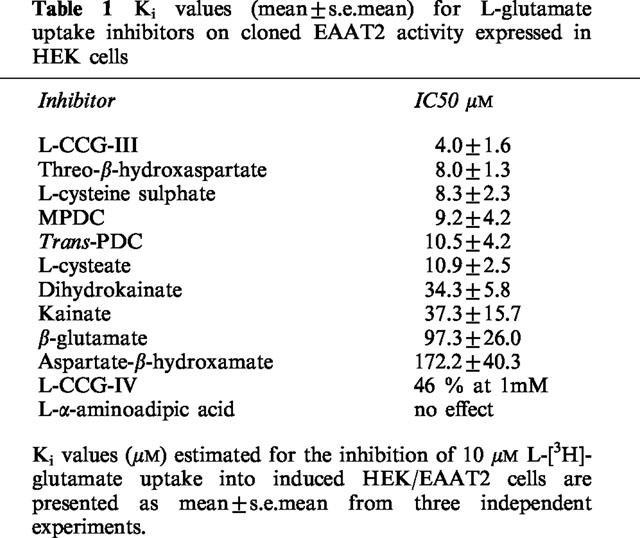
Figure 4.
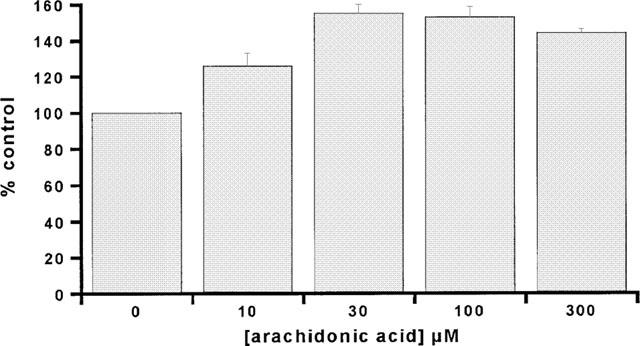
Effect of arachidonic acid on the uptake of L-glutamate into HEK/EAAT2 cells. L-glutamate transport was examined in the absence and presence of increasing concentrations of arachidonic acid. Data are expressed as per cent control uptake measured in the absence of arachidonic acid. The maximum enhancement of uptake activity observed was 155±5% control in the presence of 30 μM arachidonate.
Discussion
In this study we have evaluated the use of the ecdysone-inducible mammalian expression system (No et al., 1996) for the control of EAAT2 expression in HEK 293 cells. The use of the ecdysone system for the control of gene expression in vitro has been demonstrated previously in both transiently transfected CV-1 cells and in a stable cell line derived from HEK 293 using β-gal as the reporter gene (No et al., 1996). Additionally, the same study described the generation of transgenic mice in which muristerone (ecdysone) dependent gene expression was demonstrated in the absence of any toxic side effects associated with administration of insect steroid hormone. The results presented here provide the first example of the use of the ecdysone system for the control of expression of a mammalian Na+-coupled amino acid transporter in a stable cell line.
The family of EAAT proteins (five members cloned to date) are critical in the maintenance of L-glutamate homeostasis in the brain and they prevent excitotoxicity by limiting the extracellular L-glutamate concentration below a threshold required for excessive activation of excitatory amino acid receptors (see reviews by Kanner, 1993; Kanai et al., 1993; Gegelashvili & Schousboe, 19997; Robinson & Dowd, 1997). Furthermore, they participate in the termination of L-glutamate transmission with reuptake of synaptically released transmitter. The transporter under investigation, EAAT2, represents the predominant L-glutamate transporter expressed in adult brain and this subtype accounts for the bulk of the transport activity which can be measured in synaptosomal preparations. Therefore the function of the EAAT2 subtype is critical to the normal functional of the brain and this subtype represents a potential therapeutic target for the development of agents capable of modulating EAAT2 activity.
An almost obligatory step in the pursuit of modulators of any defined molecular target is the generation of stable cell lines expressing the protein which can be utilized for the purposes of screening. A frequently encountered problem with the approach of stable constitutive expression is the loss of expression over time as the cells are maintained. An inducible expression strategy has the potential to overcome this problem with the ability to switch on expression of the gene of interest before assay. In addition, inducible expression approaches are becoming more important in the generation of transgenic animals to overcome developmental changes which may arise as a consequence of constitutive transgene expression. Successful demonstration of an inducible expression strategy in vitro would be advantageous before embarking on such an approach in vivo. With this in mind we set out to develop EAAT2 expressing cell lines using strategies for both constitutive and inducible expression. In addition to the results presented here we have described a cell line with constitutive expression of EAAT2, using Madin-Darby Canine Kidney cells as a host (Dunlop et al., 1998), which maintains a similar level of expression of the EAAT2 protein at least up to 50 passages (unpublished observations). These cell lines have allowed us to profile the pharmacology of the EAAT2 subtype and to develop screens for novel modulators of this protein.
In the original report on the cloning of the human EAAT2 subtype (Arriza et al., 1994) the pharmacological profile for inhibition of L-glutamate uptake was characterized using transient expression in cos-7 cells. The Ki values determined for DHK and KA for the inhibition of cos-7/EAAT2 uptake were 23 and 59 μM, respectively, values which are in good agreement with those described here (34.3 and 37.3 μM, respectively) for EAAT2 expressed in HEK. Moreover, the Ki values reported for a number of other L-glutamate uptake inhibitors using cos-7/EAAT2 expression are in excellent agreement with those determined here using the HEK/EAAT2 cells. Recently, the pharmacology of the rat homologue, GLT-1, has been described in some detail using two cell lines exhibiting equivalent levels of transport capacity (Tan et al., 1999). As would be predicted from the high degree of sequence homology between the rat and human subtypes the pharmacological profiles of GLT-1 and EAAT2 are very similar. With respect to the effects of the selective inhibitors DHK and KA on L-glutamate uptake mediated by GLT-1, IC50 values in the range 32–50 and 53–70 μM, respectively, were reported. Finally, the pharmacological profiles of GLT-1 and EAAT2 are well correlated with the pharmacology of L-glutamate transport into rat cortical synaptosomes (Robinson et al., 1991; 1993; Dunlop et al., 1998; Tan et al., 1999) suggesting that the expression of GLT-1 may be sufficient to account for synaptosomal L-glutamate uptake activity.
Besides the sensitivity to the inhibitors DHK and KA the selective enhancement of EAAT2 activity observed in the presence of arachidonic acid further distinguishes this subtypes from both EAAT1 and EAAT3 (Zerangue et al., 1995). Application of arachidonic acid in the presence of L-glutamate substrate has been shown to enhance transporter mediated currents for both the EAAT2 and EAAT4 subtypes when expressed in Xenopus oocytes (Zerangue et al., 1995; Tzingounis et al., 1998). In the case of the EAAT2 subtype this effect has been shown to be attributed to an increase in L-glutamate uptake capacity, whereas arachidonic acid activates a proton current associated with EAAT4 in the absence of any detectable effect on L-glutamate transport (Tzingounis et al., 1998). We observed a 1.55 fold increase in the uptake activity of EAAT2 when arachidonic acid was co-applied with L-glutamate substrate, demonstrating that this property of the cloned EAAT2 can be reproduced in this cell based system. Arachidonic acid which can be released following activation of ionotropic L-glutamate receptors or G-protein coupled receptors linked to activation of phospholipase A2 may act as an endogenous modulator of certain EAAT subtypes.
During the course of development and characterization of the HEK/EAAT2 cell line the feasibility for the inducible expression of the rat homologue GLT-1 was established in a mutant CHO cell line with low endogenous Na+-dependent L-glutamate transport (Levy et al., 1998). It is worth pointing out that the same authors were unable to maintain stable expression of GLT-1 in their CHO cell line, thereby providing a good example for the use of an inducible expression strategy in vitro. The system used for the inducible expression of GLT-1 was the Tet-On system which allows for expression to be switched on by addition of the tetracycline analogue doxycycline to the culture medium. Upon treatment of cells with doxycycline for 24–48 h the uptake of 0.2 μM D-aspartate was increased 117–280 fold above the level of activity observed in the absence of doxycycline. It is impossible to draw direct comparisons between the levels of activity attained in the CHO/GLT-1 cells to those observed here for the HEK/EAAT2 cells without appropriate kinetic comparison of the two lines. Nevertheless, both studies have demonstrated the feasibility for the control of expression of GLT-1/EAAT2 using two different inducible strategies. Importantly, the maximum capacity for uptake estimated in our HEK/EAAT2 cell line is comparable to the levels of activity which can be measured in synaptosome fractions (typically 1–2 nmol min−1 mg−1 protein) indicating that the expression level in this cell line is physiologically relevant and that the activity is not over-expressed.
In summary, the expression of the predominant human high-affinity Na+-dependent L-glutamate transporter EAAT2 can be regulated in vitro using the ecdysone inducible mammalian expression system. The pharmacological profile of the expressed EAAT2 is similar to that described for both the rat homologue GLT-1 and in synaptosomal fractions prepared from rat cortex.
Abbreviations
- DHK
dihydrokainic acid
- D-PBS
Dulbecco's phosphate buffered saline
- EAAT
excitatory amino acid transporter
- EDTA
ethylenediaminetetraacetic acid
- HEK
human embryonic kidney
- HEPES
N-2-hydroxyethylpiperazine-N′-2-ethane sulphonic acid
- KA
kainic acid
- L-CCG-III
(2S,3S,4R)-2-(carboxycyclopropyl)-glycine
- L-CCG-IV
(2S,3R,4S)-2-(carboxycyclopropyl)glycine
- MPDC
L-anti-endo-3,4-methanopyrrolidine-dicarboxylic acid
- trans-PDC
L-trans-pyrrolidine-2,4-dicarboxylic acid.
References
- ARRIZA J.L., ELIASOF S., KAVANAUGH M.P., AMARA S.G. Excitatory amino acid transporter 5, a retinal glutamate transporter coupled to a chloride conductance. Proc. Natl. Acad. Sci. U.S.A. 1997;94:4155–4160. doi: 10.1073/pnas.94.8.4155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ARRIZA J.L., FAIRMAN W.A., WADICHE J.I., MURDOCH G.H., KAVANAUGH M.P., AMARA S.G. Functional comparisons of three glutamate transporter subtypes cloned from human cortex. J. Neurosci. 1994;14:5559–5569. doi: 10.1523/JNEUROSCI.14-09-05559.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CHENG Y-C., PRUSOFF W.H. Relationship between the inhibition constant (Ki) and the concentration of inhibitor which causes 50% inhibition (I50) of an enzymatic reaction. Biochem. Pharmacol. 1973;22:3099–3108. doi: 10.1016/0006-2952(73)90196-2. [DOI] [PubMed] [Google Scholar]
- CONTI F., DEBIASI S., MINELLI A., ROTHSTEIN J.D., MELONE M. EAAC1, a high-affinity glutamate transporter localised to astrocytes and GABAergic neurones besides pyramidal cells in the rat cerebral cortex. Cerebral Cortex. 1998;8:108–116. doi: 10.1093/cercor/8.2.108. [DOI] [PubMed] [Google Scholar]
- DUNLOP J., MCILVAIN H.B., LOU Z., FRANCO R. The pharmacological profile of L-glutamate transport in human NT2 neurones is consistent with excitatory amino acid transporter 2. Eur. J. Pharmacol. 1998;360:249–256. doi: 10.1016/s0014-2999(98)00675-x. [DOI] [PubMed] [Google Scholar]
- FAIRMAN W.A., VANDENBERG R.J., ARRIZA J.L., KAVANAUGH M.P., AMARA S.G. An excitatory amino-acid transporter with properties of a ligand-gated chloride channel. Nature. 1995;375:599–603. doi: 10.1038/375599a0. [DOI] [PubMed] [Google Scholar]
- FERKANY J., COYLE J. Heterogeneity of sodium-dependent excitatory amino acid uptake mechanisms in rat brain. J. Neurosci. Res. 1986;16:491–503. doi: 10.1002/jnr.490160305. [DOI] [PubMed] [Google Scholar]
- GEGELASHVILI G., SCHOUSBOE A. High affinity glutamate transporters: regulation of expression and activity. Mol. Pharmacol. 1997;52:6–15. doi: 10.1124/mol.52.1.6. [DOI] [PubMed] [Google Scholar]
- KANAI Y., HEDIGER M.A. Primary structure and functional characterisation of a high-affinity glutamate transporter. Nature. 1992;360:467–471. doi: 10.1038/360467a0. [DOI] [PubMed] [Google Scholar]
- KANAI Y., SMITH C.P., HEDIGER M.A. The elusive transporters with a high affinity for glutamate. Trends Neurosci. 1992;16:365–370. doi: 10.1016/0166-2236(93)90094-3. [DOI] [PubMed] [Google Scholar]
- KANNER B.I. Glutamate transporters from brain. A novel neurotransmitters transporter family. FEBS Letts. 1993;325:95–99. doi: 10.1016/0014-5793(93)81421-u. [DOI] [PubMed] [Google Scholar]
- LEVY L.M., ATTWELL D., HOOVER F., ASH J.E., BJORAS M., DANBOLT N.C. Inducible expression of the GLT-1 glutamate transporter in a CHO cell line selected for low endogenous glutamate uptake. FEBS Lett. 1998;422:339–342. doi: 10.1016/s0014-5793(98)00036-2. [DOI] [PubMed] [Google Scholar]
- NAKAMURA Y., KATAOKA K., ISHIDA M., SHINOZAKI H. (2S,3S,R4)-2-(carboxycyclopropyl)glycine, a potent and competitive inhibitor of both glial and neuronal uptake of glutamate. Neuropharmacology. 1993;32:833–837. doi: 10.1016/0028-3908(93)90137-r. [DOI] [PubMed] [Google Scholar]
- NO D., YAO T-P., EVANS R.M. Ecdysone-inducible expression in mammalian cells and transgenic mice. Proc. Natl. Acad. Sci. U.S.A. 1996;93:3346–3351. doi: 10.1073/pnas.93.8.3346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PINES G., DANBOLT N.C., BJORAS M., ZHANG Y., BENDAHAN A., EIDE L., KOEPSELL H., STORM-MATHISEN J., SEEBERG E., KANNER B.I. Cloning and expression of a rat brain L-glutamate transporter. Nature. 1992;360:464–467. doi: 10.1038/360464a0. [DOI] [PubMed] [Google Scholar]
- ROBINSON M.B., DOWD L.A. Heterogeneity and functional properties of subtypes of sodium-dependent glutamate transporters in the mammalian central nervous system. Adv. Pharmacol. 1997;37:69–115. doi: 10.1016/s1054-3589(08)60948-5. [DOI] [PubMed] [Google Scholar]
- ROBINSON M.B., HUNTER-ENSOR M., SINOR J. Pharmacologically distinct sodium-dependent L-[3H]glutamate transport processes in rat brain. Brain Res. 1991;544:196–202. doi: 10.1016/0006-8993(91)90054-y. [DOI] [PubMed] [Google Scholar]
- ROBINSON M.B., SINOR J., DOWD L.A., KERWIN J.F., Jr Subtypes of sodium-dependent high-affinity L-[3H]glutamate transport activity: pharmacologic specificity and regulation by sodium and potassium. J. Neurochem. 1993;60:167–179. doi: 10.1111/j.1471-4159.1993.tb05835.x. [DOI] [PubMed] [Google Scholar]
- ROTHSTEIN J.D., DYKES-HOBERG M., PARDO C.A., BRISTOL L.A., JIN L., KUNCL R.W., KANAI Y., HEDIGER M.A., WANG Y., SCHIELKE J.P., WELTY D.F. Knockout of glutamate transporters reveals a major role for astroglial transport in excitotoxicity and clearance of glutamate. Neuron. 1996;16:675–686. doi: 10.1016/s0896-6273(00)80086-0. [DOI] [PubMed] [Google Scholar]
- ROTHSTEIN J.D., MARTIN L., LEVEY A.I., DYKES-HOBERG M., JIN L., WU D., NASH N., KUNCL R.W. Localisation of neuronal and glial glutamate transporters. Neuron. 1994;13:713–725. doi: 10.1016/0896-6273(94)90038-8. [DOI] [PubMed] [Google Scholar]
- SAEZ E., NO D., WEST A., EVANS R.M. Inducible gene expression in mammalian cells and transgenic mice. Curr. Opin. Biotech. 1997;8:608–616. doi: 10.1016/s0958-1669(97)80037-7. [DOI] [PubMed] [Google Scholar]
- STORCK T., SCHULTE S., HOFMANN K., STOFFEL W. Structure, expression and functional analysis of a Na+-dependent glutamate/aspartate transporter from rat brain. Proc. Natl. Acad. Sci. U.S.A. 1992;89:10955–10959. doi: 10.1073/pnas.89.22.10955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TAN J., ZELENAIA O., CORREALE D., ROTHSTEIN J.D., ROBINSON M.B. Expression of the GLT-1 subtype of Na+-dependent glutamate transporter: Pharmacological characterisation and lack of regulation by protein kinase C. J. Pharm. Exp. Ther. 1999;289:1600–1610. [PubMed] [Google Scholar]
- TANAKA J., ICHIKAWA J., WATANABE M., TANAKA K., INOUE Y. Extra-junctional localisation of glutamate transporter EAAT4 at excitatory Purkinje cell synapses. Neuroreport. 1997;8:2461–2464. doi: 10.1097/00001756-199707280-00010. [DOI] [PubMed] [Google Scholar]
- TOKI H., NAMIKAWA K., SU Q., KIRYU-SEO S., SATO K., KIYAMA H. Enhancement of extracellular glutamate scavenge system in injured motoneurons. J. Neurochem. 1998;71:913–919. doi: 10.1046/j.1471-4159.1998.71030913.x. [DOI] [PubMed] [Google Scholar]
- TZINGOUNIS A.V., LIN C.L., ROTHSTEIN J.D., KAVANAUGH M.P. Arachidonic acid activates a proton conductance in the rat glutamate transporter EAAT4. J. Biol. Chem. 1998;273:17315–17317. doi: 10.1074/jbc.273.28.17315. [DOI] [PubMed] [Google Scholar]
- YAMADA K., WATANABE M., SHIBATA T., TANAKA K., WADA K., INOUE Y. EAAT4 is a post-synaptic glutamate transporter at Purkinje cell synapses. Neuroreport. 1996;7:2013–2017. doi: 10.1097/00001756-199608120-00032. [DOI] [PubMed] [Google Scholar]
- YAO T-P., FORMAN B.M., JIANG Z., CHERBAS L., CHEN J-D., MCKEOWN M., CHERBAS P., EVANS R.M. Functional ecdysone receptor is a product of EcR and Ultraspiracle genes. Nature. 1993;366:476–479. doi: 10.1038/366476a0. [DOI] [PubMed] [Google Scholar]
- ZERANGUE N., ARRIZA J.L., AMARA S.G., KAVANAUGH M.P. Differential modulation of human glutamate transporter subtypes by arachidonic acid. J. Biol. Chem. 1995;270:6433–6435. doi: 10.1074/jbc.270.12.6433. [DOI] [PubMed] [Google Scholar]


