Abstract
Acute SR 58611A (0.25 mg kg−1), was effective in reducing the blood glucose response to a glucose tolerance test (GTT) in normal lean (control) and spontaneously obese/diabetic CBA/Ca mice and to be equipotent to 1.25 mg kg−1 glibenclamide in lean mice.
Neither brown (BAT) nor white (WAT) adipose tissue lipogenesis was altered by acute SR 58611A (2–8 mg kg−1) in lean mice, but both increased significantly at the higher doses in the obese mice.
Acute SR 58611A produced a hypoglycaemia 40 min after dosing in lean and obese animals, the duration and potency of which was less than that of glibenclamide. Plasma insulin levels increased 20 min after acute SR 58611A and glibenclamide in lean and obese mice.
Chronic treatment (0.25 mg kg−1, 15 days) with SR 58611A increased its effectiveness in improving glucose tolerance, but did not affect the body weight (BW) or food intake of either lean or obese mice.
Acute and chronic SR 58611A prolonged the hypoglycaemic effect of exogenous insulin in lean but not obese mice.
In fed and fasted lean mice and in fasted obese mice chronic SR 58611A produced an acute hypoglycaemia 30 min post administration which was greater than after a single dose.
SR 58611A maintained its effectiveness in improving glucose tolerance in lean and obese mice over a dosing period of 15 days. The improvement in glucose tolerance was achieved at a dose less than that required to stimulate adipose tissue lipogenesis and which did not affect food intake or body weight.
Keywords: SR 58611A, β3-adrenoceptor agonist, glucose tolerance, lipogenesis, CBA/Ca mouse, non-insulin dependent diabetes mellitus (NIDDM), obesity
Introduction
Selective β3-adrenoceptor (β3-AR) agonists have been found to have a profound effect on energy balance in obese rats and mice; chronic administration results in a reduction in body fat content and increased thermogenesis, simultaneous to an unchanged food intake (Arch & Wilson, 1996). The increase in thermogenesis is thought to occur predominantly in brown adipose tissue, where it is accompanied by increases in β3-AR mRNA and mitochondrial uncoupling protein (Arbeeny et al., 1995). However it is possible that skeletal muscle might contribute to the response (Challis et al., 1988).
Rodent adipose tissue contains β1-, β2- and β3-AR, the latter being the predominant adrenoceptor in both brown (BAT) and white (WAT) adipose tissue (Hollenga & Zaagsma, 1989). The β3-AR has a much lower affinity for β agonist stimulation compared to either β1 and β2-AR. Functionally, this means that BAT and WAT are more responsive to sympathetic stimulation e.g. post-prandial or cold induced than to circulating adrenaline. Noradrenaline, acting through the β3-AR on BAT, activates lipolysis and thermogenesis, leading to a reduction in plasma free fatty acids and glucose (Foster & Frydman, 1978; Garcia-Sainz & Fain, 1982). Recently it has been suggested that an atypical β-AR (possibly a β3-AR) exists within skeletal muscle, its stimulation with BRL 37344, a selective β3-AR agonist, increases glucose uptake (Liu et al., 1996).
To date, β3-AR agonists have been disappointing in their effects on energy balance in humans, however there have been positive reports of their effects on some of the symptoms of obesity related NIDDM (non-insulin dependent diabetes mellitus) in man (Mitchell et al., 1989; Cawthorne et al., 1992; Connacher et al., 1992). The expression of β3-AR mRNA in human tissues has been confirmed (Krief et al., 1993) and the receptor has been cloned (Emorine et al., 1989). Other studies suggest that the receptor is functional in human adipocytes (Lonnqvist et al., 1993), although the rat adipocyte is more responsive to β3-AR agonist stimulation than the human adipocyte (Hollenga et al., 1990).
β3-AR agonists have been found to have anti-diabetic effects in animal models of type II diabetes; chronic dosing can improve glucose tolerance, increase insulin sensitivity and reduce fasting blood glucose levels (Cawthorne et al., 1992; 1984; Yoshida et al., 1994; Arbeeny et al., 1995). However, a review of the pharmaclogical effects of β3-AR agonists concluded that a compound which is highly selective for and efficacious at the human β3-AR has yet to be identified (Arch & Wilson, 1996).
We have already shown that acute SR 58611A increases adipose tissue hormone-sensitive lipase activity in normal lean CBA/Ca mice via an increase of cyclic AMP production (Shih & Taberner, 1995), but its effects on glucose and lipid metabolism in obese/diabetic mice are not known. The spontaneously obese diabetic mature male CBA/Ca mouse provides a good comparative model of human type II NIDDM by virtue of the late appearance and insidious onset of the syndrome, which is associated with hyperphagia, hyperinsulinaemia, hyperglycaemia, hypertriglyceridaemia, reduced glucose tolerance (Connelly & Taberner, 1989) and impaired adipose tissue lipogenesis (Mercer et al., 1992). It is well established that β3-AR agonists can activate adipose tissue lipolysis (Murphy et al., 1993) and an earlier preliminary study has indicated that the β3-AR partial agonist D 7114 can stimulate lipogenesis (Al Qatari & Taberner, 1992). Since a diminished lipogenic response to insulin is an established feature of insulin resistance in obesity (Mercer & Trayhurn, 1983), the present studies were undertaken to compare the acute dose response effect of a selective β3-AR agonist on lipogenesis and glucose tolerance in lean and obese-diabetic CBA/Ca mice, and to assess the chronic effects of the drug on glucose homoeostasis, food intake and body weight. The β3-AR agonist SR 58611A was chosen for study since it has been shown to be more selective than other agonists in terms of promoting lipolysis specifically through β3-AR activation (Galitzky et al., 1997).
Methods
Animals
Adult male CBA/Ca mice were bred within the University of Bristol School of Medical Sciences and housed at 20–22°C with 35–45% humidity on a 12 h light-dark cycle (dark period 21.00–09.00 h). The mice were provided with tap water and a standard pelleted rodent diet ad libitum. All animals were aged 16 weeks or older, lean mice weighed 28–35 g. The obese/diabetic syndrome was classified as body weight >40 g and basal plasma glucose level >11 mM.
Drugs
SR 58611A (batch AW2 110) was supplied by Sanofi Winthrop and glibenclamide (lot No. 101–128) by Roussel Laboratories; both were stored in a light-free container at room temperature. Drugs were administered by intraperitoneal injection (i.p.), dissolved in physiological saline (0.9% NaCl w v−1) at room temperature to give a dose volume of 0.1 ml 10 g BW−1. Control mice received the equivalent volume of saline. Insulin (Human Actrapid, 100 iu ml−1, batch 3456456, NovoNordisk) was stored at 5°C and diluted with saline immediately before use.
Procedure
Acute experiments commenced between 09.00 and 10.00 h using mice which had been housed in groups of eight in the same cage for at least 2 weeks. Standard diet and water were available ad libitum, except during the glucose tolerance test (GTT). Blood samples, 20 μl, were taken by venesection of the tail vein following light ether anaesthesia. To establish a minimal effective dose of SR 58611A on GTT a dose range of 0.125–2.0 mg kg−1 was administered i.p. 20 min prior to GTT (see below). A dose range of glibenclamide (1.25–5.0 mg kg−1) was similarly assessed.
The dose response effect of SR 58611A on lipogenesis was assessed by giving 2.0–8.0 mg kg−1 60 min prior to measurement of lipogenesis in brown (BAT) and white (WAT) adipose tissue. Chronic drug treatment involved groups of eight mice receiving daily doses of SR 58611A (0.25 mg kg−1) or saline i.p. injection between 09.00 and 10.00 h for 15 days. Food and water was available ad libitum throughout. Body weight was recorded on a daily basis and food consumption per cage every other day. A GTT or comparative insulin sensitivity test (cIST) was carried out on day 15 after the final drug administration.
The acute and chronic effects of SR 58611A (0.25 mg kg−1) on GTT were assessed in lean and obese mice, and on IST in lean mice. The acute effects of SR 58611A and glibenclamide on basal blood glucose, plasma insulin and serum triglyceride levels were assessed in lean and obese mice.
Glucose tolerance test (GTT)
Following an overnight fast (food withdrawn at 17.00 h the previous day) study drugs or saline were administered 20 min (initial assessment) or 30 min prior to 1 g kg−1 BW glucose i.p. (zero time). Blood samples were taken immediately prior to administration of the drugs or saline, prior to the glucose and subsequently at 30 min intervals for a period of 180 min in the initial assessment and thereafter for 120 min. Measurement of blood glucose was carried out using a glucocheck strip (BM test 1-44, Boehringer Ltd.) and a Glucocheck II reflectometer.
Adipose tissue lipogenic rate
Fatty acid synthesis was measured in vivo by following the incorporation of 3H2O into adipose tissue fatty acids (Mercer & Trayhurn 1983). Fed mice were given SR 58611A or saline 60 min prior to 0.5 mCi 3H2O i.p. (Amersham Life Science). Animals were killed 60 min after receiving the tritiated water, blood samples were collected into heparinized tubes. Blood was centrifuged and plasma prepared. The interscapular BAT and a sample of epididymal WAT (<500 g) were cleaned, weighed and the lipid extracted into petroleum ether (Stansbie et al., 1976) then dried and weighed. Total extracted lipids and triplicate 10 μl plasma samples were solubilized in Emulsifier-safe LSC cocktail (Packard, Groningen) prior to scintillation counting. Lipogenic rate was calculated as μg H incorporated.h−1 mg tissue−1 (Mercer & Trayhurn, 1983).
Comparative insulin sensitivity test (cIST)
Comparative insulin sensitivity was assessed by measuring the blood glucose response to a single i.p. dose of insulin. Drugs or saline were administered 30 min prior to an acute i.p. dose of insulin, 2.5 iu kg−1 BW i.p. Blood samples for glucose analysis were taken prior to the administration of drugs or saline and to the insulin and subsequently at 60, 120, 180 and 240 min after the insulin.
Basal blood glucose levels (BGL)
Drugs or saline were given immediately after the initial blood sample, subsequent blood samples were taken at 20, 40, 60, 90 and 120 min after drug administration for blood glucose analysis. Blood sampling method and blood glucose analysis are as for GTT.
Plasma insulin levels
Blood samples were taken immediately prior to the drugs or saline and subsequently at 20 and 60 min. Due to the larger blood volume required each mouse was used to provide a control and one other sample. Plasma was stored at −20°C prior to assay. Plasma insulin was measured using Biotrak™ Rat insulin radio immuno-assay system (Amersham Life Science RPA 547) using centrifugation to separate the free and antibody bound insulin. The kit although marketed for rat insulin works equally well for mouse samples.
Serum triglyceride levels
Blood samples for triglyceride assay were taken before and 60 min after drugs or saline. A sample of 200 μl of blood was obtained from a tail vein under light ether anaesthesia and serum prepared by centrifugation using standard procedures. Duplicate aliquots of 20 μl serum were assayed using the lipase-glycerol-peroxidase method of Uwajima et al. (1980) on a Technicon RA 1000 autoanalyser.
Data analysis
All the data is presented as the mean with standard error of the number of independent observations. Groups of lean and obese mice were randomized as to treatment and the results combined from different experimental days. Statistical differences between individual groups were analysed, where appropriate, by Student's unpaired t-test; insulin data was analysed by a paired t-test. Data from the chronic experiments with repeated measures was analysed by ANOVA (Instat Graphpad for Macintosh) (Graphpad Software Inc. San Diego, U.S.A.).
Results
Glucose tolerance test–acute effective dose
The maximum rise in blood glucose occurred 30 min after the i.p. glucose challenge; SR 58611A (0.25 mg kg−1) significantly reduced the blood glucose response at 30, 60 and 90 min (P<0.05), the higher doses produced greater reductions in blood glucose response (see Figure 1a). Glibenclamide also significantly reduced the blood glucose response, the 1.25 mg kg−1 dose produced a comparable effect to 0.25 mg kg−1 SR 58611A (see Figure 1b). The two drugs had a similar time course of action. There was no significant fall in the blood glucose level between the administration of drug at any of these doses and the glucose challenge 20 min later. On the basis of these findings the standard i.p. doses of SR 58611A and glibenclamide used in all the subsequent experiments were 0.25 and 1.25 mg kg−1 respectively. The same doses were also found to be effective in obese mice.
Figure 1.
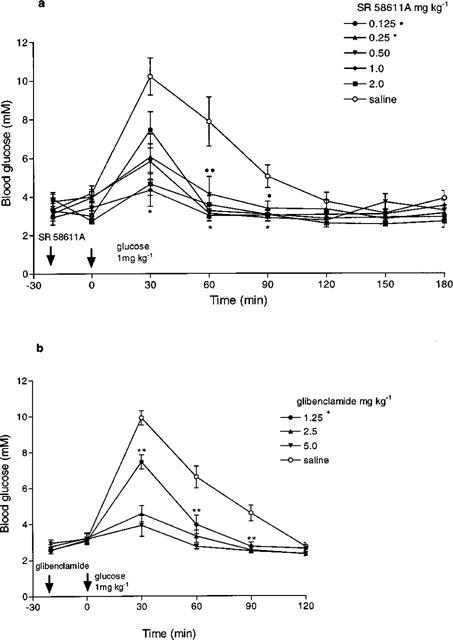
Dose-response effect of (a) SR 58611A and (b) glibenclamide on glucose tolerance in lean mice (n=6–7). Statistics are shown for 0.125 and 0.25 mg kg−1 SR 58611A and 1.25 mg kg−1 glibenclamide, *P<0.05, **P<0.01, ***P<0.001 compared to saline treated animals.
Lipogenesis–acute effective does
Unstimulated BAT lipogenesis was significantly lower in obese mice than in lean mice (P<0.01), but there was no difference in WAT lipogenesis. Acute SR 58611A did not produce a statistically significant change in BAT or WAT lipogenesis in lean mice over the dose range 2–10 mg kg−1 i.p. In contrast, there were significant increases (P<0.05) in BAT and WAT lipogenesis following SR 58611A in the obese mice (see Figure 2).
Figure 2.
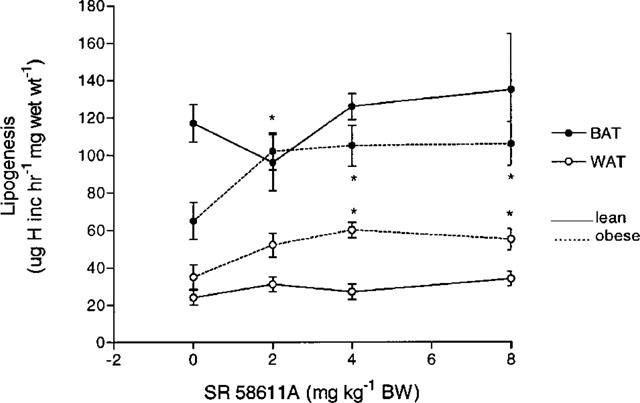
Effect of increasing doses of SR 58611A and of saline given 60 min prior to assessment of lipogenesis in brown and white adipose tissue in lean and obese diabetic mice. (n=6–8). *P<0.05, **P<0.01 compared to saline treated animals.
The wet weight of the interscapular BAT was significantly higher (P<0.01) in the obese mice at 261±19 mg (n=8) compared to that of the lean mice 162±12 mg (n=10), the obese mice also had greater amounts of subcutaneous, epididymal, perirenal and omental white adipose tissue, although this was not quantified.
Food intake and body weight–chronic drug effects
The day 1 mean body weights of the two groups of lean mice were 29.23±0.75 g and 30.19±0.96 g (n=8) and of the two obese groups 41.00±1.00 and 40.50±0.50 g (n=4). There were no significant changes in the body weights recorded over the treatment period for either the lean or obese animals (ANOVA). On day 15, the body weights of the SR 58611A-treated mice were: lean, 32.00±0.73 g; obese, 39.68±0.90 g. Similarly, there were no significant changes in the 48 h food intake recorded over the treatment periods for lean or obese mice.
Glucose tolerance–test acute and chronic drug effects
The effectiveness of the SR 58611A increased following chronic treatment in lean mice (see Figure 3). Blood glucose was significantly lower 30 min after the glucose load in the chronically treated mice compared to those receiving a single dose of SR 58611A (P<0.05). Blood glucose fell significantly in the chronically treated group 30 min after SR 58611A administration (P<0.01), this hypoglycaemic effect was not seen in the acutely treated group.
Figure 3.
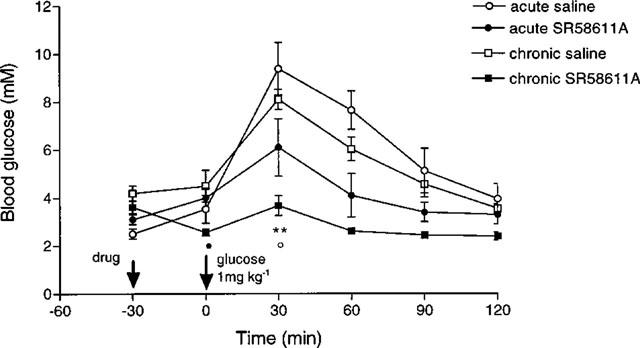
Effect of acute and chronic SR 58611A on glucose tolerance in lean mice (n=8). •P<0.01 versus −30 min; **P<0.001 versus saline control; ○P<0.05 versus acute SR 58611A.
Acute SR 58611A 0.25 mg kg−1 significantly attenuated the rise in blood glucose following a GTT at 30 (P<0.01) and 60 (P<0.05) min in obese mice (see Figure 4). After chronic dosing with SR 58611A blood glucose levels were also significantly lower 30 and 60 min post glucose administration in the SR 58611A obese group compared to their saline controls (P<0.001 and P<0.05 respectively).
Figure 4.
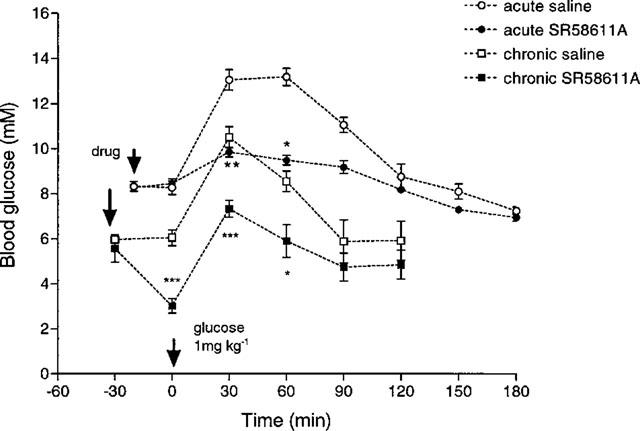
Effect of acute and chronic SR 58611A on glucose tolerance in obese diabetic mice (n=4). *P<0.05, **P<0.01, ***P<0.001 compared to saline treated animals.
In both the chronically treated SR 58611A and saline obese groups fasting blood glucose levels were significantly lower than in untreated controls (P<0.005) prior to drug treatment on day 15. Thirty minutes after SR 58611A the fasting blood glucose fell and was significantly lower than the saline treated group (P<0.001).
Comparative insulin sensitivity test (cIST)–acute and chronic drug effects
Administration of insulin produced a similar significant hypoglycaemia in all lean groups (P<0.01) at 60 min. In the lean mice the insulin induced hypoglycaemia was significantly increased by an acute dose of SR 58611A and glibenclamide (see Figure 5). In the obese mice the blood glucose levels were significantly lower (P<0.05) in the SR 58611A treated animals following insulin, but there were no significant differences thereafter.
Figure 5.
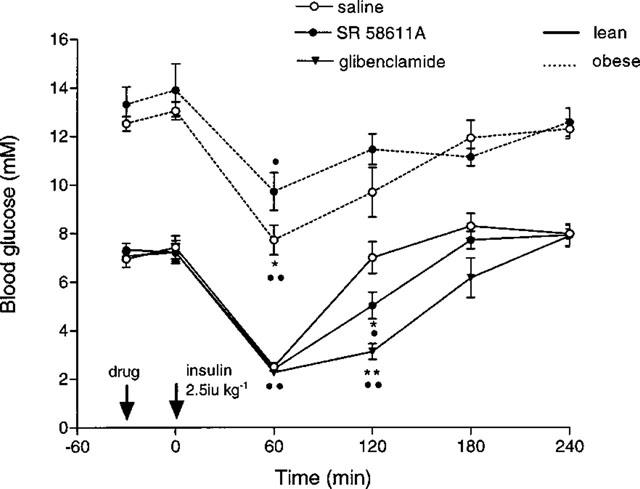
Effect of acute SR 58611A on the blood glucose response to exogenous insulin in lean and obese diabetic mice (n=6 lean, n=4–5 obese). *P<0.001 compared to saline treated animals; •P<0.01, ••P<0.001 versus 0 time.
The insulin-induced hypoglycaemia was greater in the chronically treated groups compared to acute treatment groups (see Figure 6) at 120, 180 and 240 min post insulin, although the differences in the drug treated groups were not statistically significant, the chronically treated saline group was significantly lower at 120 min (P<0.001). Following chronic treatment with SR 58611A the fed blood glucose level of the lean mice fell significantly (P<0.05) 30 min after SR 58611A treatment.
Figure 6.
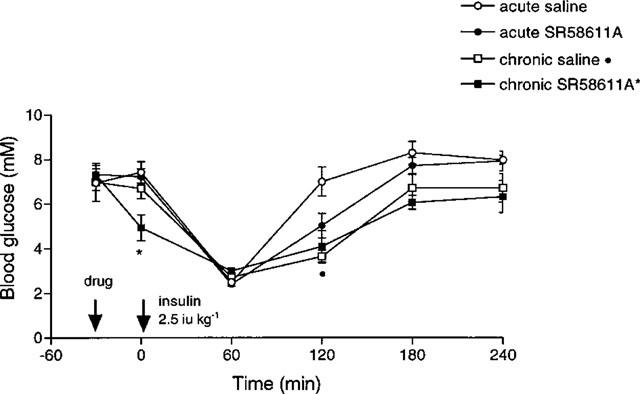
Effect of acute and chronic SR 58611A on the blood glucose response to exogenous insulin in lean mice (n=8). *P<0.05 versus −30 min, •P<0.001 versus acute saline.
Basal blood glucose level (BGL)–acute drug effects
In the lean animals acute SR 58611A produced a fall in basal blood glucose levels which was significant at 40 (P<0.005) and 90 min (P<0.05), see Figure 7. Glibenclamide produced a greater fall in blood glucose levels, which were significantly depressed (P<0.005) throughout the test period 40–120 min. The response was similar in the obese animals, glibenclamide having a greater hypoglycaemic effect than SR 58611A. SR 58611A produced a significant fall in blood glucose at 40 min in obese animals (P<0.005); glibenclamide produced significant reductions (P<0.005) at all time points, 40–120 min.
Figure 7.
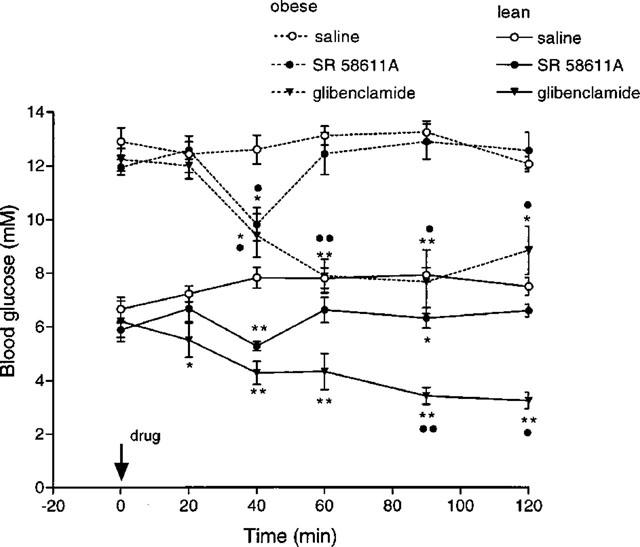
Effect of SR 58611A and glibenclamide on basal blood glucose levels in lean and obese diabetic mice (n=4). *P<0.05, **P<0.005 compared to saline treated animals; •P<0.05, ••P<0.005 versus zero time.
The effects of chronic dosing on the blood glucose response to SR 58611A are shown for lean fasted mice (Figure 3), obese fasted mice (Figure 4), and lean fed mice (Figure 6). In all three situations SR 58611A induced hypoglycaemia 30 min following its administration.
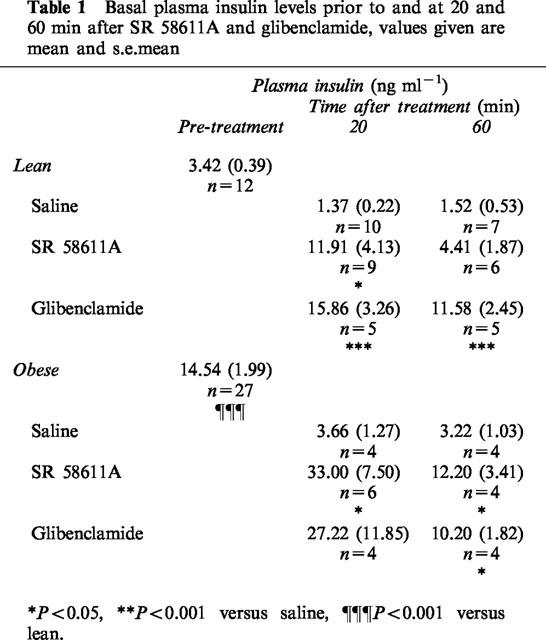
Acute drug effects on plasma insulin
Basal plasma insulin level was significantly increased 20 min after acute SR 58611A (P<0.05) but had returned to near control levels at 60 min (Table 1). Acute glibenclamide treatment resulted in a significant increase in plasma insulin at 20 and 60 min (P<0.001). Basal plasma insulin levels were significantly higher in the obese mice compared to lean animals (P<0.001) acute SR 58611A and glibenclamide produced similar increases in plasma insulin in obese animals.
Table 1.
Basal plasma insulin levels prior to and at 20 and 60 min after SR 58611A and glibenclamide, values given are mean and s.e.mean
Acute drug effects on serum triglycerides
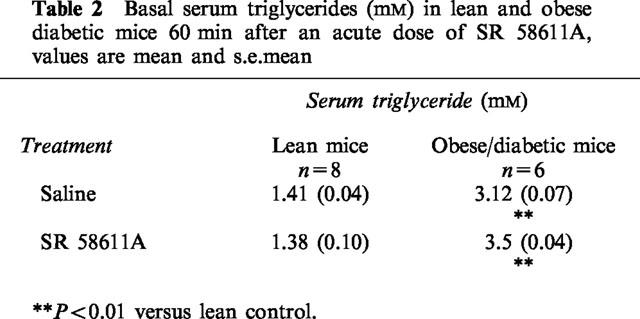
Serum triglyceride levels were significantly higher (P<0.01) in the obese mice compared to the lean mice (Table 2). There were no significant differences in serum triglyceride levels between the saline and SR 58611A treated groups.
Table 2.
Basal serum triglycerides (mM) in lean and obese diabetic mice 60 min after an acute dose of SR 58611A, values are mean and s.e.mean
Discusison
Previous studies have found β3-AR agonists to be effective at improving glycaemic control and insulin sensitivity in obese/diabetic animals at doses less than were required to reduce body weight (Rochet et al., 1988; Cawthorne et al., 1992). The dose of SR 58611A found here to improve glucose tolerance in lean and obese mice (0.25 mg kg−1) was less than that required to stimulate lipogenesis in either the brown or white adipose tissue of obese animals, which might explain its lack of effect on food intake and body weight over a 15 day period. The lack of effect of SR 58611A on lipogenesis in the lean mice was not unexpected, it is not unusual for β3-AR agonists to exert little or no effect on energy balance in lean animals (Arch & Wilson, 1996). The increased WAT and BAT lipogenesis in the obese mice following acute SR 58611A could be due to a direct effect of the SR 58611A on the β3-AR of the adipose tissue, or a consequence of an increase in circulating insulin levels and/or an increase in insulin sensitivity (see below). These results suggest that SR 58611A would have greater potency in the treatment of NIDDM than obesity, and would be of use in the treatment of both NIDDM with and without obesity.
Comparison of acute SR 58611A and glibenclamide, a sulphonylurea which improves glucose tolerance by direct stimulation of insulin release from pancreatic β cells, showed SR 58611A to have a greater relative potency than glibenclamide with respect to improving glucose tolerance (lean mice only); 0.25 mg kg−1 SR 58611A produced a comparable effect to 1.25 mg kg−1 glibenclamide. These doses, given acutely, were both hypoglycaemic and hyperinsulinaemic in lean and obese mice and increased sensitivity to exogenous insulin in lean mice. However the glibenclamide dose produced a greater and more prolonged hypoglycaemia and hyperinsulinaemia than SR 58611A. The similarities in action of the two drugs suggest that SR 58611A might act acutely to stimulate insulin release like glibenclamide. In vivo, acute BRL26830A, a β3-AR agonist, can stimulate insulin release in lean animals (Sennit et al., 1985); an acute effect which is blocked by pre-administration of propranolol (Yoshida, 1992). It is also possible that acute SR 58611A can directly increase glucose uptake; the early β3 agonist BRL 35135A was shown to stimulate glucose uptake into skeletal muscle independently of insulin action (Abe et al., 1993). Our finding that SR 58611A also increased sensitivity to exogenous insulin implies that it may have more than one site of action.
Chronic dosing of obese animals with selective β3-AR agonists has been reported to improve glucose tolerance over 14 days (Wilbraham et al., 1994; Young et al., 1985) and to normalize plasma glucose levels over 14–28 days (Arbeeny et al., 1995; Wilbraham et al., 1994; Ghorbani & Himms-Hagen, 1997). Chronic dosing with SR 58611A confirmed these findings. We have also shown increased effectiveness of SR 58611A by chronic administration in terms of improving glucose tolerance in lean and obese mice and increasing the hypoglycemic effect of a single dose in fed and fasted lean mice and fasted obese mice. It is likely that the chronic dosing regime increased the insulin sensitivity of the animals; the increased response to exogenous insulin following chronic dosing in lean animals supports this hypothesis.
Fourteen day administration of selective β3-AR agonists has previously been reported to increase white adipocyte insulin binding and receptor number in lean and obese diabetic mice (Yoshida et al., 1994; Young et al., 1985), both actions which would tend to improve insulin sensitivity. Such chronically induced changes are unlikely to explain the positive effect of acute SR 58611A on the sensitivity to insulin. It is possible that this acute effect is mediated by a different mechanism to the chronic effect. Chronic treatment of the obese mice with SR 58611A or saline reduced their fasting blood glucose levels, an effect not seen in the lean animal. Repeated dosing may familiarize the animals with handling and thus minimize the effects of handling on blood glucose levels, although our findings suggest that obese mice are more susceptible to this effect than their lean littermates.
Acute BRL 35135 increased glucose uptake in skeletal muscle and adipose tissue, but the effect was lost after 14 days in all tissues except BAT and soleus muscle (Liu & Stock, 1995). We have shown acute SR 58611A to be less effective in improving insulin sensitivity in obese animals compared to the lean, but given the improved glucose tolerance observed after chronic treatment of both groups it would be useful in future to assess the effects of chronic dosing on insulin sensitivity of specific tissues in the obese mice.
In conclusion, the increased effectiveness of SR 58611A following chronic dosing enhances its potential value for long term use and supports the hypothesis that β3-ARs are resistant to desensitization, since the β3-AR lacks the phosphorylation site associated with agonist-induced desensitization and has a low affinity for circulating catecholamines (Giacobino, 1995). SR 58611A clearly maintains its effectiveness in improving glucose homeostasis in both lean and obese mice over 14 days. This improvement is achieved at a dose less than is required to stimulate adipose tissue lipogenesis and at a dose which does not affect food intake or body weight. It is possible that SR 58611A has more potential in the treatment of the glucose intolerance in diabetes than in obesity.
Acknowledgments
We are grateful to Sanofi Winthrop, Milan for supplying SR 58611A and for supporting this work. C.A. Williams is a Daphne Jackson Fellow supported by the University of Bristol and Lloyds Bank Plc.
Abbreviations
- β3-AR
β3-adrenoceptor
- BAT
brown adipose tissue
- BGL
basal blood glucose level
- BW
body weight
- cIST
comparative insulin sensitivity test
- GTT
glucose tolerance test
- i.p.
intraperitoneal injection
- NIDDM
non-insulin dependent diabetes mellitus
- WAT
white adipose tissue
References
- ABE H., MINOKOSHI Y., SHIMAZU T. Effect of a β3-adrenergic agonist, BRL35135A, on glucose uptake in rat skeletal muscle in vivo and in vitro. J. Endocrinol. 1993;139:479–486. doi: 10.1677/joe.0.1390479. [DOI] [PubMed] [Google Scholar]
- AL QATARI M., TABERNER P.V. Effect of ICI D7114, a selective β3-adrenoceptor agonist, on BAT lipogenesis in CBA/Ca obese mice. J. Physiol. 1992;452:176P. [Google Scholar]
- ARBEENY C.M., MEYERS D.S., HILLYER D.E., BERGQUIST K.E. Metabolic alterations associated with the antidiabetic affect of β3-adrenergic receptor agonists in obese mice. Am. J. Physiol. 1995;268:E678–E684. doi: 10.1152/ajpendo.1995.268.4.E678. [DOI] [PubMed] [Google Scholar]
- ARCH J.R.S., WILSON S. Prospects for β3-adrenoceptor agonists in the treatment of obesity and diabetes. Int. J. Obesity. 1996;20:191–199. [PubMed] [Google Scholar]
- CAWTHORNE M.A., CARROLL M.J., LEVY A.L., LISTER C.L., SENNITT M.V., SMITH S.A., YOUNG P. Effects of novel β-adrenoceptor agonists on carbohydrate metabolism: relevance for the treatment of non-insulin dependent diabetes. Int. J. Obesity. 1984;8:93–102. [PubMed] [Google Scholar]
- CAWTHORNE M.A., SENNITT M.V., ARCH J.R.S., SMITH S.A. BRL 35135, a potent and selective atypical β-adrenoceptor agonist. Am. J. Clin. Nutr. 1992;55:252S–257S. doi: 10.1093/ajcn/55.1.252s. [DOI] [PubMed] [Google Scholar]
- CHALLIS R.A.J., LEIGHTON M.B., WILSON S., THURLBY P.L., ARCH J.R.S. An investigation of the β-adrenoceptor that mediates metabolic responses to the novel agonist BRL 28410 in rat soleus muscle. Biochem. Pharmacol. 1988;37:947–950. doi: 10.1016/0006-2952(88)90186-4. [DOI] [PubMed] [Google Scholar]
- CONNACHER A.A., BENNET W.M., JUNG R.T., RENNIE M.J. Metabolic effects of three weeks administration of the β-adrenoceptor agonist BRL 26830A. Int. J. Obesity. 1992;16:685–694. [PubMed] [Google Scholar]
- CONNELLY D.M., TABERNER P.V. Characterisation of the spontaneous diabetes obesity syndrome in mature male CBA/Ca mice. Pharmacol. Biochem. Behaviour. 1989;34:255–259. doi: 10.1016/0091-3057(89)90308-0. [DOI] [PubMed] [Google Scholar]
- EMORINE L.J., MARULLO S., BRIEND-SUTREN M.-M., PATEY G., TATE K., DELAVIER-KLUTCHKO C., STROSBERG D.A. Molecular characterization of the human β3-adrenergic receptor. Science. 1989;245:1118–1121. doi: 10.1126/science.2570461. [DOI] [PubMed] [Google Scholar]
- FOSTER D.O., FRYDMAN M.L. Nonshivering thermogenesis in the rat. II. Measurement of blood flow with microspheres point to brown adipose tissue as the dominant site of the calorigenesis induced by noradrenaline. Can. J. Physiol. Pharmacol. 1978;56:110–122. doi: 10.1139/y78-015. [DOI] [PubMed] [Google Scholar]
- GALITZKY J., LANGIN D., VERWAERDE P., MONTASTRUC J.-L., LAFONTAN M., BERLAN M. Lipolytic effects of conventional β3-adrenoceptor agonists and of CGP 12, 177 in rat and human fat cells: preliminary pharmacological evidence for a putative β4-adrenoceptor. Br. J. Pharmacol. 1997;122:1244–1250. doi: 10.1038/sj.bjp.0701523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GARCIA-SAINZ J.A., FAIN J.N. Regulation of adipose tissue metabolism by catecholamines: roles of alpha1, alpha2 and beta-adrenoceptors. Trends Pharmacol. Sci. 1982;3:201–203. [Google Scholar]
- GHORBANI M., HIMMS-HAGEN J. Appearance of brown adipocytes in white adipose tissue during CL316,243-induced reversal of obesity and diabetes in Zucker fa/fa rats. Int. J. Obesity. 1997;21:465–475. doi: 10.1038/sj.ijo.0800432. [DOI] [PubMed] [Google Scholar]
- GIACOBINO J.-P. β3-Adrenoceptor: an update. Eur. J. Endocrinol. 1995;132:377–385. doi: 10.1530/eje.0.1320377. [DOI] [PubMed] [Google Scholar]
- HOLLENGA C., HAAS M., DEINUM J.T., ZAAGSMA J. Discrepancies in lipolytic activities induced by β-adrenoceptor agonists in human and rat adipocytes. Horm. Metab. Res. 1990;22:17–21. doi: 10.1055/s-2007-1004839. [DOI] [PubMed] [Google Scholar]
- HOLLENGA C., ZAAGSMA J. Direct evidence for the atypical nature of functional β-adrenoceptors in rat adipocytes. Br. J. Pharmacol. 1989;98:1420–1424. doi: 10.1111/j.1476-5381.1989.tb12692.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KRIEFF S., LONNQVIST F., RAIMBAULT S., BAUDE B., VAN SPROUSEN A., ARNER P., STROSBERG D.A., RICQUIER D., EMORINE L.J. Tissue distribution of β3-adrenergic receptor mRNA in man. J. Clin. Invest. 1993;91:344–349. doi: 10.1172/JCI116191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LIU Y.-L., CAWTHORNE M.A., STOCK M.J. Biphasic effects of the β-adrenoceptor agonist BRL 37344, on glucose utilization in rat isolated skeletal muscle. Br. J. Pharmacol. 1996;117:1355–1361. doi: 10.1111/j.1476-5381.1996.tb16736.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LIU Y.-L., STOCK M.J. Acute effects of the β3-adrenoceptor agonist, BRL 35135, on tissue glucose utilisation. Br. J. Pharmacol. 1995;114:888–894. doi: 10.1111/j.1476-5381.1995.tb13287.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LONNQVIST F., KRIEF S., STROSBERG D.A., NYBERG B., EMORINE L.J., ARNER P. Evidence for a functional β3-adrenoceptor in man. Br. J. Pharmacol. 1993;110:929–936. doi: 10.1111/j.1476-5381.1993.tb13902.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MERCER S.W., DENTON R.M., TABERNER P.V. The development of resistance to the lipogenic effects of insulin in brown tissue and white adipose tissue of spontaneously type II diabetic male CBA/Ca mice. Int. J. Biochem. 1992;24:941–944. doi: 10.1016/0020-711x(92)90101-6. [DOI] [PubMed] [Google Scholar]
- MERCER S.W., TRAYHURN P. Development changes in fatty acid synthesis in interscapular brown adipose tissue of lean and genetically obese (ob/ob) mice. Biochem. J. 1983;212:393–398. doi: 10.1042/bj2120393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MITCHELL T.H., ELLIS R.D.M., SMITH S.A., ROBB G., CAWTHORNE M.A. Effects of BRL 35135, a β-adrenoceptor agonist with novel selectivity, on glucose tolerance and insulin sensitivity in obese subjects. Int. J. Obesity. 1989;13:757–766. [PubMed] [Google Scholar]
- MURPHY G.J., KIRKHAM D.M., CAWTHORNE M.A., YOUNG P. Correlation of β3-adrenoceptor-induced activation of cAMP-dependent protein kinase with activation of lipolysis in rat white adipocytes. Biochem. Pharmacol. 1993;46:575–581. doi: 10.1016/0006-2952(93)90540-d. [DOI] [PubMed] [Google Scholar]
- ROCHET N., TANTI J.F., VAN OBBERGHEN E., LE MARCHAND-BRUSTEL Y. Effect of a thermogenic agent, BRL26830A, on insulin receptors in obese mice. Am. J. Physiol. 1988;255:E101–E109. doi: 10.1152/ajpendo.1988.255.2.E101. [DOI] [PubMed] [Google Scholar]
- SENNITT M.V., ARCH J.R., SIMSON D.L., SMITH S.A., CAWTHORNE M.A.Anti-hyperglycaemic action of BRL 26830, a novel beta-adrenoceptor agonist, in mice and rats Biochem. Pharmacol. 1985151279–1285.f [DOI] [PubMed] [Google Scholar]
- SHIH M.-F., TABERNER P.V. Selective activation of brown adipose tissue hormone-sensitive lipase and cAMP production in mouse by β3-adrenoceptor agonists. Biochem. Pharmacol. 1995;50:601–608. doi: 10.1016/0006-2952(95)00185-3. [DOI] [PubMed] [Google Scholar]
- STANSBIE D., BROWNSEY R.W., CRETTAZ M., DENTON R.M. Acute effects in vivo of anti-insulin serum on rates of fatty acid synthesis and activities of acetyl-coenzyme A carboxylase and pyruvate dehydrogenase in liver and epididymal adipose tissue of fed rats. Biochem. J. 1976;160:413–416. doi: 10.1042/bj1600413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- UWAJIMA T., AKITA H., ITO K., AISAKA K., TERADA O. Formation and purification of a new enzyme glycerol oxidase and stoichiometry of the enzyme reaction. Agric. Biol. Chem. 1980;44:399–406. [Google Scholar]
- WILBRAHAM J.M., WOOTTON C.L., MARTIN D.A., HOLLOWAY B.R. β3-adrenoceptor agonist, Zeneca ZD2079, improves glucose homeostasis in insulin resistant rodents. Diabetologia. 1994;37:A212. [Google Scholar]
- YOSHIDA T. The antidiabetic β3-adrenoceptor agonist BRL26830A works by release of endogenous insulin. Am. J. Clin. Nutr. 1992;55:237S–241S. doi: 10.1093/ajcn/55.1.237s. [DOI] [PubMed] [Google Scholar]
- YOSHIDA T., SAKANE N., WAKABAYASHI Y., UMEKAWA T., KONDO M. Anti-obesity and anti-diabetic effects of CL 316,243, a highly specific β3-adrenoceptor agonist in yellow KK mice. Life Sci. 1994;54:491–498. doi: 10.1016/0024-3205(94)00408-0. [DOI] [PubMed] [Google Scholar]
- YOUNG P., KING L., CAWTHORNE M.A. Increased insulin binding and glucose transport in white adipocytes isolated from C57B2/6 ob/ob mice treated with the thermogenic β-adrenoceptor agonist BRL 26830. Biocem. Biophys. Res. Commun. 1985;133:457–461. doi: 10.1016/0006-291x(85)90928-3. [DOI] [PubMed] [Google Scholar]


