Abstract
The effect of the antidepressant drug, fluoxetine on neuronal delayed rectifier (KV) potassium (K) currents was investigated using perforated-patch whole-cell electrophysiological recording methods.
Fluoxetine was an effective inhibitor of KV currents in cerebellar granule neurons (CGNs) and also inhibited recombinant KV1.1 channels expressed in Chinese hamster ovary (CHO) cells.
Fluoxetine had an IC50 of 11 μM in CGNs but was slightly less potent on KV1.1 channels (IC50=55 μM). Interestingly, fluoxetine was a much more potent inhibitor of KV1.1 expressed in mammalian cells than has been found previously for the same homomeric channel expressed in Xenopus oocytes.
At concentrations that produced around 50% block, the shape of the KV currents in the presence of fluoxetine was simply scaled down when compared to control currents.
The effect of fluoxetine on KV currents in CGNs was neither voltage-dependent nor dependent on the channels being in their open state. Both of these observations suggest that fluoxetine does not act as a simple open channel blocking agent.
It is concluded that block of KV currents in mammalian neurons can occur at therapeutic levels of fluoxetine. This could lead to an increase in neuronal excitability and this effect may contribute to the therapeutic antidepressant action of fluoxetine.
Keywords: KV, potassium current, fluoxetine, cerebellar granule neurons, KV1.1
Introduction
Potassium (K) channels are among the most important signalling macromolecules in the nervous system. K channel activity determines the shape, frequency and duration of neuronal action potentials (Armstrong & Hille, 1998). In addition, K channels have a major role in the setting of the resting membrane potential of a neuron, which in turn may profoundly regulate neuronal excitability (Halliwell, 1990; Hille, 1992; Roeper & Pongs, 1996; Mathie et al., 1998). A diverse number of K channels have been identified in different neurons by functional electrophysiological studies (Cull-Candy et al., 1989; see also Rudy, 1988). These include the delayed rectifier currents (KV), A-type currents (KA), calcium-activated currents, inward rectifiers and M currents (Mathie et al., 1998). Mutations of specific K channels in both animals and man have been connected to certain CNS disorders, such as epilepsy (see review by Sanguinetti & Spector, 1997).
There are a number of compounds which act to inhibit neuronal K channels, thereby altering the electrical properties of the neurons. Block of K channels has been proposed as a potential therapeutic mechanism in the treatment of certain clinical disorders such as myaesthenia gravis, multiple sclerosis, Huntington's chorea and Alzheimer's disease. However such an action is more often regarded as underlying some of the more serious side effects (such as convulsions and dysrythymias) of a number of compounds currently in therapeutic use (Mathie et al., 1998). For example, previous work from our laboratory has shown that a number of clinically used antidepressant drugs such as amitriptyline and imipramine, are potent inhibitors of certain K+ currents in mammalian neurons, in particular the delayed-rectifier current, KV (Wooltorton & Mathie, 1993; 1995).
In this study, the effects on KV currents of a further clinically used antidepressant, fluoxetine, have been investigated. Fluoxetine, a selective serotonin reuptake inhibitor (SSRI), has been reported to have some blocking action on native KV currents in cultured human lens epithelium (Rae et al., 1995), on KV currents in PC12 cells; which are often regarded as a good neuronal model cell line; (Hahn et al., 1999), and on cloned KV channels expressed in Xenopus oocytes (Tytgat et al., 1997). In this study, we have examined the effects of fluoxetine on KV currents found in native neurons in the mammalian central nervous system and compared the potency of these effects with that seen in a more artificial system. Firstly, we have studied its effects on KV currents in rat cerebellar granule neurons (CGNs). These cells have been shown to have transient and sustained components to their whole-cell current which correspond to KA and KV conductances (e.g. Cull-Candy et al., 1989; Jalonen et al., 1990; Bardoni & Belluzzi, 1994; Watkins & Mathie, 1994). These can be studied in relative isolation with appropriate voltage protocols (see Methods and Watkins & Mathie, 1994). We have also examined the effects of fluoxetine in Chinese hamster ovary (CHO) cells permanently transfected with KV1.1 channels. We have chosen this particular recombinant channel for two reasons. Firstly, CGNs are known to express high levels of the KV channel subunit KV1.1 (Veh et al., 1995). Secondly, experiments using a behavioural model of anti-depressant action have suggested that inhibition of expression of KV1.1 channels using antisense oligonucleotides has the same effect as treatment with antidepressant drugs such as amitriptyline and imipramine (Galeotti et al., 1999).
Some of these results have been presented in abstract form (Yeung et al., 1998).
Methods
Cell preparation
Cerebellar granule neurons (CGNs)
CGNs were isolated and cultured from 6–9 day old rats, of either sex, as previously described (Watkins & Mathie, 1996). Briefly, animals were killed by decapitation, the cerebellum removed and dissociated with a series of enzymatic solutions (trypsin, trypsin inhibitor and DNAse) and then centrifuged. The cells were subsequently resuspended in MEM (minimum essential medium) containing 10% FCS (foetal calf serum), 2.5% chick embryo extract, 25 mM KCl, 39 mM glucose, 2 mM L-glutamine and 50 IU ml−1 penicillin/50 μg ml−1 streptomycin antibiotic solution. The resulting CGN cell suspension was plated onto poly-L-lysine coated coverslips (80 μl per coverslip) to give a density of 106 cells ml−1. The coverslips were then incubated at 37°C in a humidified atmosphere of 95% O2 and 5% CO2. The cells were supplemented with 0.5 ml MEM and 10% FCS the next day, but were not used for at least 4 days post culture.
CHO cells expressing KV1.1
CHO cells expressing the recombinant KV1.1 channel, were kindly supplied by Dr David Owen (CeNeS Ltd.). Cells were grown in tissue culture in 25 ml flasks in a medium containing RPMI medium supplemented with 5% FCS, 4.1 mM L-glutamine, 50 mg ml−1 geneticin and 50 IU ml−1 penicillin/50 μg ml−1 streptomycin antibiotic solution. The flasks were maintained in an incubator at 37°C in a humidified atmosphere of 95% O2 and 5% CO2. The cells were diluted 1 in 10 every 3 days, plated onto poly-L-lysine coated coverslips and allowed to grow for at least 2 days prior to recording.
Solutions
The bathing (external) solution contained (mM): NaCl 120, KCl 2.5, MgCl2 2, CaCl2 0.5, glucose 5, HEPES 10, adjusted to pH 7.4 with NaOH. The pipette (internal) solution contained (mM): KCl 125, MgCl2 5, HEPES 5, BAPTA 0.1, adjusted to pH 7.4 with KOH. Both solutions were made up in distilled water.
Stock solutions of fluoxetine (HCl) were also made up in distilled water, and stored at −20°C until required. Perfusion solutions of fluoxetine were made up using the external solution, immediately before the experiment. Solutions were continuously perfused through the bath during recording at a rate of approximately 4–5 ml min−1.
Reagents were purchased from Sigma Chemical Company and Life Technologies (Gibco).
Current recordings and analysis
Currents were recorded in the whole cell perforated patch-clamp configuration by use of an Axopatch-1D amplifier (Axon Instruments, Foster City) and amphotericin B (240 μg ml−1) as the permeabilizing agent. All experiments were performed at room temperature. Patch pipettes were made from borosilicate glass, which had resistances between 5 and 10 MΩ.
Voltage protocols
The following two protocols were used to examine KV in isolation in CGNs and CHO cells expressing KV1.1.
Protocol 1: Cells were held at −70 mV, prepulsed to −50 mV for 30 ms before being stepped to a depolarizing test potential of +10 mV for 150 ms once every 6 s to evoke the sustained KV current, before being stepped back to −50 mV for 30 ms and then back to the holding potential.
Protocol 2: Cells were held at −70 mV, prepulsed to −50 mV for 30 ms before being stepped to the test potentials of −40 to +40 mV in 10 mV increments. Each test potential was applied once every 6 s, before being stepped back to −50 mV for 30 ms then back to the holding potential.
For both protocols, the amplitude of KV current was measured as a mean over 15 ms, 134 ms following the step to +10 mV (except during experiments to generate current-voltage relationships when the step potential varied). For some cells, tail current relaxations following the step back to −50 mV were measured to give complete isolation of the KV current component. Linear components of leak current were calculated from the prepulse step from −70 to −50 mV and were subtracted off-line where appropriate.
Currents were low-pass filtered at 5 kHz and recorded and analysed using an IBM-compatible PC, pClamp (version 6.0.3) with a Digidata 1200 interface (Axon Instruments), Excel 97 (Microsoft) and Origin version 3.5 (Microcal). Results were expressed as means±standard error of means (s.e.mean) and n as the number of cells. Statistical analyses were performed using Student's t-test. Results were considered significant at the P<0.05 level.
Results
Inhibition of KV in CGNs by fluoxetine
The delayed rectifier, KV, can be studied in relative isolation using a particular voltage protocol which is described in the Methods (protocol 1). The cells were held at a potential of −70 mV, prepulsed to −50 mV for 30 ms to obtain a measure of any background or leak currents (and during which KA channels are inactivated but KV channels remain closed), prior to stepping the current to a depolarizing potential of +10 mV for 150 ms to activate the KV current. The KV current was revealed by its distinctive profile, namely, a sustained current at a depolarized potential of +10 mV, which showed little or no inactivation over the duration of the step (see also Watkins & Mathie, 1994). This sustained component is blocked by tetraethylammonium ions and is sensitive to 4-aminopyridine (Watkins & Mathie, 1994).
KV was inhibited by fluoxetine, as illustrated in Figure 1. It can be seen that the inhibition of KV by fluoxetine was concentration-dependent. Figure 1A–C shows inhibition of KV by 3, 30 and 100 μM fluoxetine. As the concentration of fluoxetine added to the bath increased, the rate of inhibition increased. The time course of inhibition of KV in CGNs by 10 μM fluoxetine is illustrated in Figure 1D. With washout, there was some reversibility of the block at this concentration. The concentration-response relationship for block of the KV current by fluoxetine in CGNs is illustrated in Figure 1E. Concentrations of 3, 10, 30, 100 and 300 μM inhibited KV by 24±3% (mean±s.e.mean; n=6), 45±3% (n=6), 60±5% (n=7), 78±2% (n=5) and 83% (n=1) respectively. The fitted curve gave an IC50 for fluoxetine of 11 μM and a slope of 0.94. When the maximum response attainable was constrained to 83%, the fitted curve gave an IC50 of 12.5 μM and a slope of 1.24. At concentrations producing close to 50% inhibition of the KV current, the current profile was not altered, the current was simply scaled down in the presence of the drug.
Figure 1.
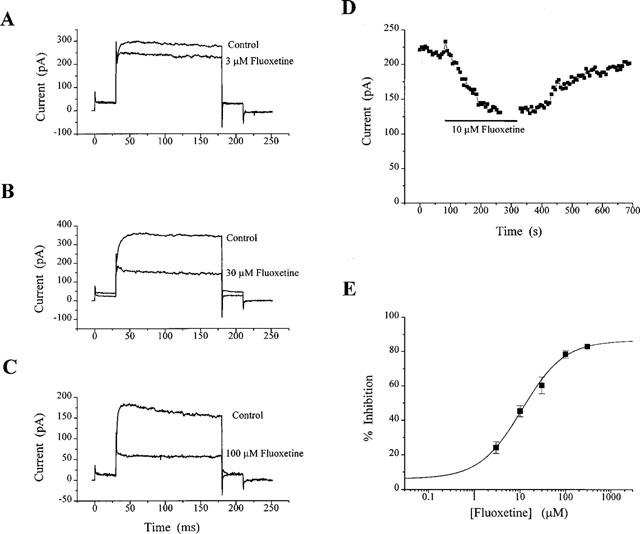
Concentration-dependent inhibition of KV currents in CGNs by fluoxetine. In the representative cells (A–C), 3, 30 and 100 μM fluoxetine produced 17, 59 and 63% inhibition respectively. Currents were activated by protocol 1 (see Methods). Each trace is the average current of four individual traces and all have been leak subtracted off-line. A time course plot in response to 10 μM fluoxetine is shown in (D). Each point is an average current taken over 15 ms, commencing 134 ms following the test step to +10 mV. The gap in the plot represents time during which an I-V relationship was obtained. The concentration-response curve for fluoxetine is shown in (E). Data points were fitted with a power function, giving a calculated IC50 value of 11 μM, with a slope of 0.94 and a maximal inhibition of 87%.
Inhibition of KV in CGNs is not voltage-dependent
Current-voltage relationships were obtained in CGNs using protocol 2 in the absence and presence of 10 μM fluoxetine (a concentration close to the IC50 for fluoxetine in these cells) and are shown in Figure 2A. The control plot demonstrates a non-linear increase in current at voltage steps to potentials more depolarized than −20 mV. It appears that this voltage is the threshold for activation of KV channels in these cells (see also Watkins & Mathie, 1994). The percentage inhibition of KV by 10 μM fluoxetine was measured at test potentials between 0 and +40 mV. Mean data obtained from 12 cells showed no significant difference across the voltages (Figure 2B); for example, at a test potential of 0 mV, current was inhibited by 37±6%, while at +40 mV, inhibition was 42±4% (n=12). These results suggest that block of KV in CGNs by fluoxetine shows little voltage-dependence.
Figure 2.
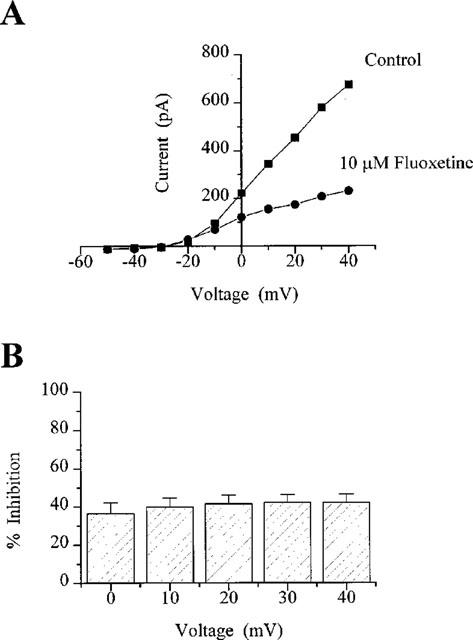
Inhibition of KV in CGNs showed little or no voltage-dependence. Currents were activated by protocol 2. The current-voltage relationship (A) shows current amplitudes in the absence and presence of 10 μM fluoxetine in a single CGN. (B) shows the percentage inhibition of KV by fluoxetine in 12 cells over the voltage range of 0 to +40 mV.
Inhibition of KV in CGNs does not require the channels to be open
For this set of experiments, a slightly modified version of protocol 1 was used to distinguish whether the block by fluoxetine depended on KV channels being in their open state. This was determined by ceasing the depolarizing pulses (and thus the opening of KV channels) during application of the drug. This is illustrated for a single experiment in Figure 3A,B.
Figure 3.
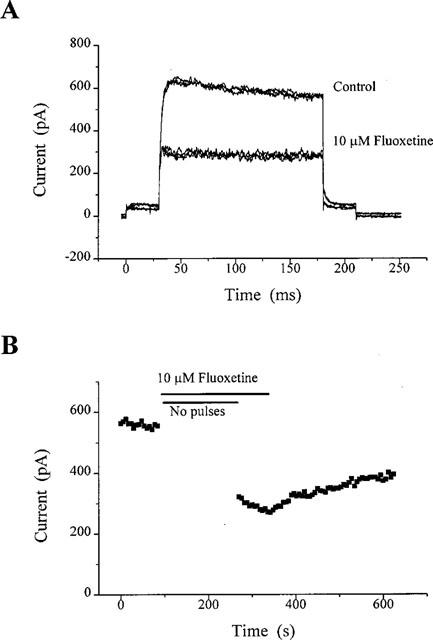
Inhibition of KV in CGNs by 10 μM fluoxetine does not require the channels to be open. Currents were activated by adapting protocol 1 (see Results). Current recordings in (A) were taken under control and drug conditions. The latter represents maximal inhibition. Only three traces have been shown for clarity, which have not been leak subtracted. The time-course relationship (B) illustrates the protocol more clearly, and inhibition of KV by fluoxetine during the 3 min period devoid of depolarizing pulses to +10 mV. This particular cell produced a 51% inhibition. Some recovery was possible upon wash-out.
The time course of the experiment is shown in Figure 3B. Depolarizing test pulses were stopped for 3 min and fluoxetine was simultaneously added to the bath. In control experiments (see Figure 1D), 3 min is sufficient to obtain maximal block by 10 μM fluoxetine. Immediately on resumption of pulses, block of KV was found to be fully established and was 51% in this cell (Figure 3B). In six cells, using an identical protocol, block of KV was 41±5%. Thus block of KV in CGNs by fluoxetine occurs in the absence of depolarizing pulses, i.e. block occurs despite the channels being in the closed state.
Inhibition of KV1.1 in CHO cells
The effects of fluoxetine on recombinant KV1.1 channels were also investigated, as illustrated in Figures 4 and 5. Recordings from CHO cells expressing KV1.1 shows the classic delayed rectifier type profile (see also Robertson & Owen, 1993). Also illustrated is the time course of fluoxetine inhibition of KV1.1 channels in these cells. It is clear that fluoxetine (10–300 μM) is not as potent at inhibiting recombinant KV1.1 channels as it is on KV in CGNs (Figure 4A–D; compare with Figure 1). Block of KV1.1 with 30 μM was rapid, and was fully reversible (Figure 4C). However at the highest concentration used in CHO cells (300 μM) the inhibition was extremely rapid but irreversible over the time course of the recording (not shown).
Figure 4.
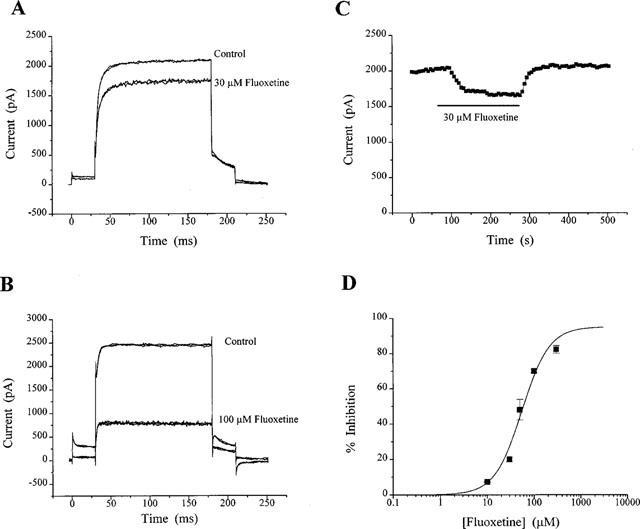
Inhibition of recombinant KV1.1 in CHO cells by fluoxetine. Currents were activated by protocol 1. (A) shows recordings of control currents and the maximal inhibition by 30 μM fluoxetine. The corresponding time-course plot for this cell (C) shows rapid onset of action with full recovery of the block. Another current recording is shown in (B), but in response to 100 μM fluoxetine. (D) illustrates the concentration-response curve to fluoxetine. Each data point is the average inhibition of at least three cells. The IC50 was calculated to be 55 μM, with a slope of 1.49 and a maximal inhibition of 95%.
Figure 5.
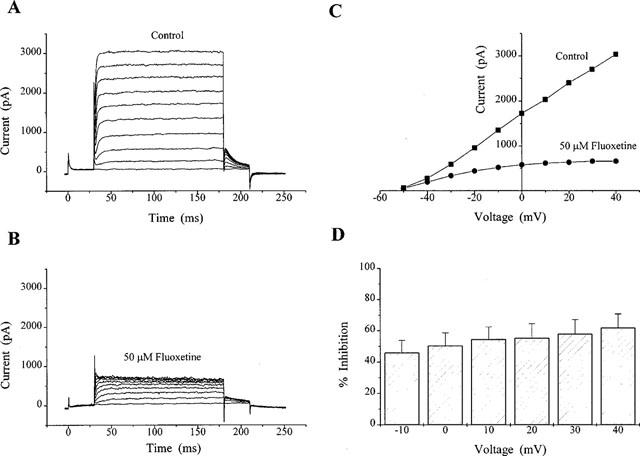
Fluoxetine inhibition of KV1.1 at different voltages. Currents were evoked using protocol 2. (A and B) illustrate current recordings in the absence and presence of 50 μM fluoxetine in a single CHO cell. The corresponding current-voltage relationship is shown in (C). The degree of inhibition over the voltage range −10 to +40 mV for three cells is shown in (D).
A concentration-response curve for fluoxetine inhibition of KV1.1 was obtained for concentrations of fluoxetine between 10 and 300 μM (Figure 4D). At concentrations of 10, 30, 50, 100 and 300 μM fluoxetine, inhibitions of the KV1.1 current were calculated to be 7±1% (n=3), 20±1%, (n=3), 48±6% (n=11), 70±1% (n=3) and 82±2% (n=3) respectively. The inhibition of KV1.1 at 10 and 30 μM was significantly less (P<0.001) than that seen for KV in CGNs. The fitted data here gave an IC50 of 55 μM (or 53 μM when the maximum response was constrained to 82%), which is significantly higher than that calculated for KV in CGNs.
Application of 50 μM fluoxetine (as with the CGN experiments, a concentration close to the IC50 for fluoxetine in these cells) produced an obvious decrease in current amplitude across a range of test voltages (Figure 5A–C) whether measuring absolute currents at the test potential or tail-current relaxations on stepping back to −50 mV (see Methods). The mean inhibition for three cells at test potentials ranging from −10 to +40 mV is shown in Figure 5D. Currents were inhibited by 46±9% at −10 mV but 62±9% at +40 mV.
Discussion
In this study, it has been shown that fluoxetine is an effective inhibitor of the KV current in CGNs and that it also inhibited recombinant KV1.1 channels expressed in CHO cells. The block of KV currents in CGNs by fluoxetine is the first demonstration of the action of this compound on KV currents in native mammalian neurons, however, the effects of fluoxetine have been investigated on K channels and other ion channels present in non-neuronal cells and in cell lines. It has been reported previously that fluoxetine blocks native KV currents in human lens epithelium (Rae et al., 1995) and K currents in both canine and human jejunal circular smooth muscle cells (Farrugia, 1996). Much less potently, fluoxetine (IC50 of 600–700 μM) inhibits KV1.1 channels transfected into Xenopus oocytes (Tytgat et al., 1997). In addition fluoxetine inhibits K+, Na+ and Ca2+ currents in PC12 cells (Hahn et al., 1999), while Pancrazio et al. (1998) reported that fluoxetine was as effective as tricyclic antidepressants (TCAs; e.g. amitriptyline) at inhibiting the Na+ currents in bovine adrenal chromaffin cells. It has been shown by Lavoie et al. (1997) that fluoxetine is capable of inhibiting calcium uptake via calcium channels in rat hippocampal synaptosomes and more recently, it was reported by Maertens et al. (1999) that fluoxetine produces a reversible block of both the volume-sensitive chloride current and the Ca2+-activated Cl− current in cultured bovine pulmonary artery endothelial cells. Thus fluoxetine has previously been shown to have blocking actions on a number of different ion channel types in non-neuronal cells and in cell lines.
A number of compounds, such as tetrapentylammonium ion and clofilium (French & Shoukimas, 1981; Reeve & Peers, 1992) block KV channels by binding to a site inside the pore of the channel and acting as open-channel blocking agents. For fluoxetine, however, the relatively consistent amount of block of KV currents in CGNs observed across the range of positive potentials tested suggests that it does not have a preference for open channels. In addition in the absence of depolarizing pulses, inhibition of the KV current by fluoxetine was still achieved. Again, this implies that fluoxetine can act to block channels whether they are open or closed.
Rae et al. (1995) suggested that the block of KV currents in human lens epithelium was due to an action of fluoxetine on segments of the channel located intracellularly, however, Maertens et al. (1999) altered the proportion of uncharged to charged fluoxetine by changing extracellular pH and found that it was the uncharged form of the molecule which mediates block of the voltage-regulated anion channel in endothelial cells and suggested that the most likely mechanism of action of fluoxetine was that it had hydrophobic interactions with the channel within the membrane bilayer. The results in this study with fluoxetine are similar to those obtained in previous work on inhibition of neuronal KV currents by certain TCAs and related compounds (Wooltorton & Mathie, 1993; 1995). Wooltorton & Mathie (1995) demonstrated that amitriptyline and its permanently charged analogue, N-methylamitriptyline, when applied internally, had no effect on the KV current. Furthermore, Kuo (1998) showed that imipramine and a number of structurally related compounds inhibit the transient KA current of rat hippocampal neurons and hypothesized that there is a receptor for imipramine which consists of an aliphatic and aromatic site on the external side of the channel pore. By analogy with these experiments on TCAs, it seems reasonable to suggest that fluoxetine may act either by binding to an extracellular site on the channel or a hydrophobic site within the lipid bilayer. It seems less likely that fluoxetine acts intracellularly by gaining access to the channel region via the membrane. Finally, although unlikely, we cannot completely rule out the possibility that direct block of the KV channel by fluoxetine and related compounds may not be the only method of inhibition (see Farrugia, 1996). Intracellular biochemical processes such as tyrosine phosphorylation may also be affected which may, in turn, reduce the activity of KV channels (e.g. Lev et al., 1995). However, in experiments using conventional whole-cell recording we found the same degree of inhibition as that seen in perforated patch recordings which suggests that a diffusional biochemical messenger is unlikely to be involved.
The exact subunits constituting the KV channels in CGNs have not been identified, and the genes encoding many different KV channels have been found to be expressed to varying degrees throughout the developing rat brain (Kues & Wunder, 1992). However, since KV1.1 was found to be particularly abundant in the granule layer of the cerebellum (Veh et al., 1995), it was of some interest to compare the block by fluoxetine of native KV currents with that of recombinant KV1.1 channels. When challenged with fluoxetine, block of KV1.1 channels was observed but the drug was less potent than in CGNs (IC50=55 μM compared to 11 μM). Variations between the sensitivity of recombinant KV1.1 channels and KV channels in CGNs, may indicate that either a different subunit, or more likely, a more complex subunit composition exists in the native cells.
The effects of fluoxetine on KV1.1 currents have previously been studied in Xenopus oocytes (Tytgat et al., 1997). These authors showed that the inhibition of KV1.1 by fluoxetine was much less potent (IC50 between 600 and 700 μM at 0 mV) than observed in this study for KV1.1 in a mammalian expression system. Moreover, while inhibition of KV1.1 by fluoxetine was reversible in the mammalian expression system this was not the case in Xenopus oocytes. Thus there is a difference in potency of fluoxetine on KV1.1 channels depending on the recombinant system used. It is possible that fluoxetine has easier access to the channel components in mammalian cells.
In man, following therapeutic doses of fluoxetine, the drug concentrations present in both plasma and brain have been measured. It was found that the latter contains approximately 20 times as much as in the blood (reaching levels ranging from 5 to 70 μM, see Karson et al., 1993; Altamura et al., 1994). This suggests that substantial block of KV currents in mammalian neurons can occur at therapeutic levels of fluoxetine and could lead to an increase in neuronal excitability. In clinical use, one noted side effect of fluoxetine in the CNS is that it can induce convulsions and the use of fluoxetine is cautioned in epilepsy and during concurrent electroconvulsive therapy. Furthermore, it is of some interest that Galeotti et al. (1999), using an established animal model to indicate antidepressant activity, have shown that inhibiting the expression of KV1.1 channels through the use of antisense oligonucleotides has the same effect as treating animals with antidepressant drugs such as imipramine and amitriptyline. This suggests that block of KV channels such as KV1.1 by antidepressant drugs, an effect which has been observed for all such drugs so far examined (see Mathie et al., 1998), may be an important contributory factor to their antidepressant action.
Acknowledgments
This work was supported by the MRC. Thanks to David Owen for supplying MK-1 cells, Maurice Gittos for many useful discussions and David Boyd for reading an earlier version of the manuscript.
Abbreviations
- CGN
cerebellar granule neurons
- CHO
Chinese hamster ovary
- FCS
foetal calf serum
- KV
delayed rectifier currents
- KA
A-type currents
- MEM
minimum essential medium
- SSRI
selective serotonin reuptake inhibitor
- TCA
tricyclic antidepressant
References
- ALTAMURA A.C., MORO A.R., PERCUDANI M. Clinical pharmacokinetics of fluoxetine. Clin. Pharmacokinet. 1994;26:201–214. doi: 10.2165/00003088-199426030-00004. [DOI] [PubMed] [Google Scholar]
- ARMSTRONG C.M., HILLE B. Voltage-gated ion channels and electrical excitability. Neuron. 1998;20:371–380. doi: 10.1016/s0896-6273(00)80981-2. [DOI] [PubMed] [Google Scholar]
- BARDONI R., BELLUZZI O. Modifications of A-current kinetics in mammalian central neurones induced by extracellular zinc. J. Physiol. 1994;479:389–400. doi: 10.1113/jphysiol.1994.sp020304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CULL-CANDY S.G., MARSHALL C.G., OGDEN D. Voltage-activated membrane currents in rat cerebellar granule neurons. J. Physiol. 1989;414:179–199. doi: 10.1113/jphysiol.1989.sp017683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FARRUGIA G. Modulation of ionic currents in isolated canine and human jejunal circular smooth muscle cells by fluoxetine. Gastroenterology. 1996;110:1438–1445. doi: 10.1053/gast.1996.v110.pm8613049. [DOI] [PubMed] [Google Scholar]
- FRENCH R.J., SHOUKIMAS J.J. Blockage of squid axon potassium conductance by internal tetra-N-alkylammonium ions of various sizes. Biophys. J. 1981;34:271–291. doi: 10.1016/S0006-3495(81)84849-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GALEOTTI N., GHELARDINI C., CALDARI B., BARTOLINI A. Effect of potassium channel modulators in mouse forced swimming test. Br. J. Pharmacol. 1999;126:1653–1659. doi: 10.1038/sj.bjp.0702467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HAHN S.J., CHOI J.-S., RHIE D.-J., OH C.-S., JO Y.-H., KIM M.-S. Inhibition by fluoxetine of voltage-activated ion channels in rat PC12 cells. Eur. J. Pharmacol. 1999;367:113–118. doi: 10.1016/s0014-2999(98)00955-8. [DOI] [PubMed] [Google Scholar]
- HALLIWELL J.V.K+ channels in the central nervous system Potassium Channels: Structure, Classification, Function and Therapeutic Potential 1990Ellis Horwood Ltd; 348–381.N.S. Cook (ed), pp [Google Scholar]
- HILLE B. Ionic Channels of Excitable Membranes 1992Sinauer Press: Sunderland, MA; 2nd edn [Google Scholar]
- JALONEN T., JOHANSSON S., HOLOPAINEN I., OJA S.S., ÅRHEM P. Single-channel and whole-cell currents in rat cerebellar granule cells. Brain Res. 1990;535:33–38. doi: 10.1016/0006-8993(90)91820-7. [DOI] [PubMed] [Google Scholar]
- KARSON C.N., NEWTON J.E., LIVINGSTON R., JOLLY J.B., COOPER T.B., SPRIGG J., KOMOROWSKI R.A. Human brain fluoxetine concentrations. J. Neuropsychiatry Clin. Neurosci. 1993;5:322–329. doi: 10.1176/jnp.5.3.322. [DOI] [PubMed] [Google Scholar]
- KUES W.A., WUNDER F. Heterogeneous expression patterns of mammalian potassium channel genes in developing and adult rat brain. Eur. J. Neurosci. 1992;4:1296–1308. doi: 10.1111/j.1460-9568.1992.tb00155.x. [DOI] [PubMed] [Google Scholar]
- KUO C.-C. Imipramine inhibition of transient K+ current: An external open channel blocker preventing fast inactivation. Biophys. J. 1998;75:2845–2857. doi: 10.1016/S0006-3495(98)77727-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LAVOIE P.-A., BEAUCHAMP G., ELLIE R. Atypical antidepressants inhibit depolarization-induced calcium uptake in rat hippocampus synaptosomes. Can. J. Physiol. Pharmacol. 1997;75:983–987. doi: 10.1139/cjpp-75-8-983. [DOI] [PubMed] [Google Scholar]
- LEV S., MORENO H., MARTINEZ R., CANOLL P., PELESE E., MUSACCHIO J.M., PLOWMAN G.D., RUDY B., SCHLESSINGER J. Protein tyrosine kinase PYK2 involved in Ca2+-induced regulation of ion channel and MAP kinase functions. Nature. 1995;376:737–745. doi: 10.1038/376737a0. [DOI] [PubMed] [Google Scholar]
- MAERTENS C., WEI L., VOETS T., DROOGMANS G., NILIUS B. Block by fluoxetine of volume-regulated anion channels. Br. J. Pharmacol. 1999;126:508–514. doi: 10.1038/sj.bjp.0702314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MATHIE A., WOOLTORTON J.R.A., WATKINS C.S. Voltage-activated potassium channels in mammalian neurons and their block by novel pharmacological agents. Gen. Pharmacol. 1998;30:13–24. doi: 10.1016/s0306-3623(97)00034-7. [DOI] [PubMed] [Google Scholar]
- PANCRAZIO J.J., KAMATCHI G.L., ROSCOE A.K., LYNCH C., III Inhibition of neuronal Na+ channels by antidepressant drugs. J. Pharmacol. Exp. Ther. 1998;284:208–214. [PubMed] [Google Scholar]
- RAE J.L., RICH A., ZAMUDIO A.C., CANDIA O.A. Effect of Prozac on whole cell ionic currents in lens and corneal epithelia. Am. J. Physiol. 1995;269:C250–C256. doi: 10.1152/ajpcell.1995.269.1.C250. [DOI] [PubMed] [Google Scholar]
- REEVE H.L., PEERS C. Blockade of delayed-rectifier K+ currents in neuroblastoma X glioma hybrid (NG 108-15) cells by clofilium, a class III antidysrhythmic agent. Br. J. Pharmacol. 1992;105:458–462. doi: 10.1111/j.1476-5381.1992.tb14275.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ROBERTSON B., OWEN D.G. Pharmacology of a cloned potassium channel from mouse brain (MK-1) expressed in CHO cells: effects of blockers and an ‘inactivation peptide'. Br. J. Pharmacol. 1993;109:725–735. doi: 10.1111/j.1476-5381.1993.tb13634.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ROEPER J., PONGS O. Presynaptic potassium channels. Curr. Opin. Neurobiol. 1996;6:338–341. doi: 10.1016/s0959-4388(96)80117-6. [DOI] [PubMed] [Google Scholar]
- RUDY B. Diversity and ubiquity of K channels. Neuroscience. 1988;25:729–749. doi: 10.1016/0306-4522(88)90033-4. [DOI] [PubMed] [Google Scholar]
- SANGUINETTI M.C., SPECTOR P.S. Potassium channelopathies. Neuropharmacol. 1997;36:755–762. doi: 10.1016/s0028-3908(97)00029-4. [DOI] [PubMed] [Google Scholar]
- TYTGAT J., MAERTENS C., DAENENS P. Effect of fluoxetine on a neuronal, voltage-dependent potassium channel (KV1.1) Br. J. Pharmacol. 1997;122:1417–1424. doi: 10.1038/sj.bjp.0701545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VEH R.W., LICHTINGHAGEN R., SEWING S., WUNDER F., GRUMBACH I.M., PONGS O. Immunohistochemical localization of five members of the KV1 channel subunits: contrasting subcellular locations and neuron-specific co-localizations in rat brain. Eur. J. Neurosci. 1995;7:2189–2205. doi: 10.1111/j.1460-9568.1995.tb00641.x. [DOI] [PubMed] [Google Scholar]
- WATKINS C.S., MATHIE A. Modulation of the gating of the transient outward potassium current of rat isolated cerebellar granule neurons by lanthanum. Pflügers Archiv. 1994;428:209–216. doi: 10.1007/BF00724499. [DOI] [PubMed] [Google Scholar]
- WATKINS C.S., MATHIE A. A non-inactivating K+ current sensitive to muscarinic receptor activation in rat cultured cerebellar granule neurons. J. Physiol. 1996;491:401–412. doi: 10.1113/jphysiol.1996.sp021224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WOOLTORTON J.R.A., MATHIE A. Potent block of potassium currents in rat isolated sympathetic neurones by tricyclic antidepressants and structurally related compounds. Br. J. Pharmacol. 1993;110:1126–1132. doi: 10.1111/j.1476-5381.1993.tb13931.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WOOLTORTON J.R.A., MATHIE A. Potent block of potassium currents in rat isolated sympathetic neurones by the uncharged form of amitriptyline and related tricyclic compounds. Br. J. Pharmacol. 1995;116:2191–2200. doi: 10.1111/j.1476-5381.1995.tb15053.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- YEUNG S.Y., MILLAR J.A., BOYD D.F., JONES G., GITTOS M.W., MATHIE A. Block of neuronal KV potassium currents by fluoxetine and iprindole. Br. J. Pharmacol. 1998;125:41P. doi: 10.1038/sj.bjp.0702955. [DOI] [PMC free article] [PubMed] [Google Scholar]


