Abstract
The mPer1 and mPer2 genes are putative mouse clock genes that regulate circadian oscillator present in the suprachiasmatic nucleus (SCN) neuron. While they are also expressed in the granular cell layer in the cerebellum, their function is unknown. In a first step to verify the physiological roles of mPer1 and mPer2 genes in the cerebellum, we examined the effects of benzodiazepines on the expression of the mPer1 and mPer2 genes.
mPer2 mRNA expression was higher at ZT16 than ZT4 in the mouse cerebellum.
High-dose administration of diazepam (10 mg kg−1) or triazolam (1 mg kg−1) reduced mPer1 mRNA level 1 h after treatment in the cerebellum.
Reduced expression of mPer1 by diazepam treatment was transient. No difference of mPer1 mRNA level between diazepam (10 mg kg−1)- and vehicle-treated group was observed 6 h after treatment.
Administration of high doses of tandospirone (30 mg kg−1), a non-benzodiazepine anxiolytic also reduced mPer1 mRNA expression 1 h after treatment.
Administration of high doses of clozapine (5 mg kg−1) or haloperidol (1 mg kg−1) impaired the rota-rod performance without affecting on mPer1 mRNA level.
Diazepam and tandospirone inhibited the expression of mPer1 mRNA in the primary cultured cerebellum granule cells.
Transient reductions of mPer1 mRNA levels by various benzodiazepines and tandospirone is associated with impairment of coordinated movement, such as rota-rod performance and equilibrium.
Keywords: mPer1, cerebellum, anxiolytics, diazepam, rota-rod, tandospirone
Introduction
Circadian rhythms are endogenously generated rhythms that persist under constant conditions with a period of about 24 h (Edmunds, 1988). The biological clock of mammals, which located in the suprachiasmatic nucleus (SCN) of hypothalamus, controls a variety of behavioural and physiological rhythmic phenomena, such as locomotor activity, sleep-wakefulness and secretion of various hormones (Hastings, 1997). Recent isolation of three mammalian homologues of the Drosophila clock gene, period (dPer), from mammals [Per1 (Tei et al., 1997; Sun et al., 1997), Per2 (Albrecht et al., 1997; Shearman et al., 1997; Takumi et al., 1998a) and Per3 (Zylka et al., 1998b; Takumi et al., 1998b)] and their circadian expression in the SCN suggests that molecular components and some mechanisms of mammalian circadian clock are evolutionarily conserved. This insight was further reinforced with recent isolation of other common clock components like timeless (Sangoram et al., 1998; Zylka et al., 1998a) or Clock (Allada et al, 1998) from Drosophila and mouse (Darlington et al., 1998). Interestingly, like dPer, all three mPer genes are expressed in various organs (Tei et al., 1997; Zylka et al., 1998a,1998b). In the mouse brain, mPer genes are most extensively expressed in the SCN but they are also expressed in the pyramidal cells of hippocampus and granule cells of cerebellum (Shearman et al., 1997). Circadian fluctuation of mPer1 mRNA in the hippocampus and cerebellum has also been reported (Sun et al., 1997). However, it is still unknown why these mPer genes are expressed in various regions of the brain and organs.
It is well known that normal function of cerebellum is required to perform co-ordinated motor movements (Thach et al., 1992 for review). The α1 and α6 subunits of GABAA receptor have been reported to express in granule cells of cerebellum (Khan et al., 1996). In addition, it is reported that benzodiazepine-induced motor impairment links to point mutation in cerebellar GABAA receptor containing α6 subunit (Korpi et al., 1993). Recently, it has been demonstrated that benzodiazepine reduces GABAA receptor α1 subunit protein expression in primary cultured cerebellar granule cells (Brown & Bristow, 1996). Thus, benzodiazepines may cause an impairment of co-ordinated motor activity through an interaction with GABAA receptor in the granule cells of the cerebellum. In a first step to clarify physiological roles of mPer genes in brain regions other than the SCN, we examined the mPer1 and mPer2 expression in the cerebellum when mice exhibited an impairment of rota-rod performance and equilibrium following high dose administration of anxiolytic drugs, such as benzodiazepines and tandospirone, and antipsychotic drugs, such as clozapine and haloperidol. We have shown here that mPer1 mRNA level was reduced in the cerebellum by diazepam, triazolam and tandospirone, but not by clozapine and haloperidol, even though high doses of these latter drugs also caused a deficit of co-ordinated motor movements as observed in the rota-rod and fixed bar tests. Interestingly, diazepam and tandospirone also reduced mPer1 mRNA level in the cultured cerebellar granule cells.
Methods
Animals
For all experiments, 4–6 week old male ddY mice (Takasugi, Japan) were group housed (8–10 per cage) and maintained at least 2 weeks under a 12 : 12 h light dark cycle with a light on at 8 : 30 h at 23°C. Animals were given food and water ad libitum. Animals were treated in accordance with the Law (No. 105) and Notification (No. 6) of the Japanese Government. Under the light dark cycle, zeitgeber time (ZT) 0 was designated as light on and ZT12 as light off.
Fixed-bar test
Mice were trained to stay on a fixed bar (8 or 20 mm in diameter, 50 cm in height) just before drug treatments. Only mice that stayed on the bar over 3 min were used for the subsequent tests. Groups of mice (n=5–8) were administered test compounds and put on the bar and tested for their ability to stay on the bar for 3 min. They were put on a bar again 1, 2, 6 and 12 h after the treatment and tested for staying for 3 min.
Rota-rod test
Mice were trained to stay on a rod (25 mm in diameter, 25 cm in height) rotating at 15 r.p.m. just before drug treatments. Only mice that stayed on the rod over 3 min were used for the subsequent tests. Groups of mice (n=5–15) were administered test compounds and put on the rod and tested for staying on the rod for 3 min. They were again put on the rota-rod 1, 2, 6 and 12 h after the treatment and tested for staying on the rod for 3 min. The mice that dropped within 3 min were again tested immediately under the same conditions and the better score in the two tests was used for analysis.
Drugs
Diazepam (Wako, Japan), triazolam (Sigma), tandospirone citrate (Sumitomo Pharmaceuticals, Japan), clozapine (Sigma) and haloperidol (Wako, Japan) were administered intraperioneally in a volume of 10 ml kg−1 to mice. All compounds were suspended in 0.5% sodium carboxymethylcellulose (vehicle) (Tokyo Kasei, Japan). In the in vitro experiment, diazepam, tandospirone and nifedipine (Wako, Japan) were dissolved into DMSO with a final concentration of DMSO (1%) which had no effect on mPer1 mRNA level (data not shown).
RT–PCR analysis
The effects of anxiolytic drugs on mPer1 and mPer2 expression in the cerebellum were examined by RT–PCR. Mice entrained to the light dark cycle for 2 weeks were transferred to constant darkness for one extra daily cycle and mice were administered drugs or vehicle at ZT4 or ZT16 on the next day. An hour after injection, mice were deeply anaesthetized with ether and killed by decapitation. Some mice were killed 6 h after administration. The sagittal slices of 1 mm thickness were dissected from the middle vermis of cerebellum by razor blade. Total RNA from the cerebellum of individual animal (n=3–7) was extracted separately by Trizol Reagent (BRL). A One-Step RT–PCR System (BRL) was used for RT–PCR reaction of 100 ng of RNA. mPer1, mPer2 and glyceraldehyde-3-phosphate dehydrogenase (GAPDH) cDNAs were amplified simultaneously in a single tube using a GeneAmp 9900 (Perkin Elmer). Following the reverse transcription at 50°C for 30 min, samples were amplified by following parameters: 23 cycles of 94°C for 15 s, 55°C for 30 s, 72°C for 1 min and a 4°C hold. The primer pairs used for the amplification of each product are as follows: 5′-CCA GGC CCG GAG AAC CTT TTT-3′ and 5′-CGA AGT TTG AGC TCC CGA AGT G-3′ (mPer1); 5′-ACA CCA CCC CTT ACA AGC TTC-3′ and CGC TGG ATG ATG TCT GGC TC-3′ (mPer2); 5′-GAC CTC AAC TAC ATG GTC TAC A-3′ and TGG CCG TGA TGG CAT GGA CT-3′ (GAPDH). We found no amplification product without reverse transcription under this condition. The sizes of the PCR products of mPer1, mPer2 and GAPDH were 402, 779 and 436 bp, respectively. PCR products were run on 3% agarose gels and DNA in the corresponding bands was detected with an EDAS-120 system (Kodak).
Preparation of cultured granule neurons and treatment of cultures
A primary culture of mouse cerebellar granule neurons was prepared from 7–8 day old ICR mice (Takasugi, Japan), as previously reported (Fujita et al., 1999). In brief, cerebella were isolated and cut with small pieces and tripsinized at 37°C. The dissociated cells were seeded at about 3×105 onto the poly-L-lysine coated culture dish (35 mm diameter) (Iwaki, Japan). The cells were grown at 25 mM KCl in DMEM supplemented with 10% FCS at 37°C. After 2 days, the cells were given fresh medium with 10% FCS supplemented with 10 mM cytosine arabinoside and cultured for additional 2 days. Nifedipine, diazepam, tandospirone and vehicle were added directly to the medium. Total cellular RNAs were prepared 1.5 h after addition and analysed by RT–PCR.
Statistics
Statistical differences between drug treated groups were analysed by one way analysis of variance (ANOVA). After positive ANOVA results, individual differences between groups were tested by the Dunnett's two-tailed test. In cases where two groups are compared, their statistical differences were tested by Student's t-test. In the fixed bar tests and the rota-rod test, Fisher's test was used for statistical analysis.
Results
Expression of mPer1 and mPer2 in the cerebellum at day and night
Amounts of mPer1 and the mPer2 mRNA in the mouse cerebellum at ZT4 and ZT16 were measured by RT–PCR and normalized to GAPDH mRNA (Figure 1). GAPDH mRNA level was constant under the light-dark cycle in the cerebellum. In order to compare the present results with SCN data, we decided to use of a same reported RT–PCR method (Akiyama et al., 1999) to analyse the amounts of mPer mRNAs. While mPer1 mRNA was not so different between ZT4 and ZT16, mPer2 mRNA level was higher at ZT16 than at ZT4 (Figure 1b) (P<0.01, Student's t-test).
Figure 1.
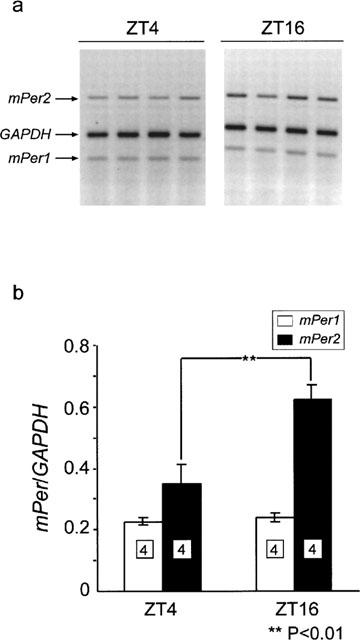
Expression of the mPer1 and mPer2 gene in the mouse cerebellum at ZT4 and ZT16. (a) Representative electrophoretic image of RT–PCR products of mPer1, mPer2 and GAPDH mRNA from ZT4 and ZT16. (b) Relative mRNA levels of mPer1 and mPer2 at ZT4 and ZT16 were shown by ratio to GAPDH mRNA levels. Numbers in boxes in the columns indicate the number of experiments. mPer2 mRNA is highly expressed at ZT16 than at ZT4 (**P>0.01, Student's t-test).
Effects of diazepam administration on mPer genes expression at day and night
mPer1 and mPer2 mRNA levels in the cerebellum 1 h after intraperitoneal administration of diazepam at ZT4 and ZT16 were examined. Diazepam treatment (10 mg kg−1) caused significant reduction of mPer1 mRNA in the cerebellum 1 h after treatment at both ZT (Figure 2b) (P<0.01 at ZT4 and P<0.05 at ZT16; Student's t-test). On the other hand, mPer2 mRNA levels 1 h after treatment were barely affected by same doses of diazepam administrations at both ZT.
Figure 2.
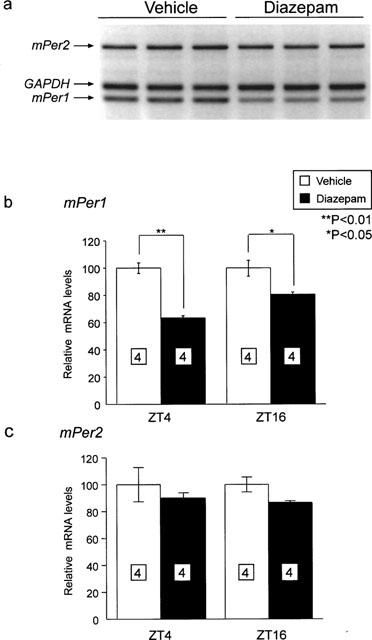
Effect of diazepam treatment on the expression of mPer1 and mPer2 at ZT4 and ZT16. (a) Representative example of electrophoretic image of RT–PCR products of mPer1, mPer2 and GAPDH mRNA from vehicle- or diazepam-treated mice at ZT16. mRNA levels of mPer1 (b) and mPer2 (c) of vehicle- or diazepam-treated group 1 h after injection at ZT4 and ZT16 were normalized to GAPDH mRNA. Vehicle-treated group was set to 100 in both ZT for avoiding the effects of daily change of mPer mRNA. Numbers in boxes in the columns indicate the number of experiments. mPer1 mRNA level was reduced by diazepam treatment at both ZT (**P<0.01 at ZT4, *P<0.05 at ZT16, Student's t-test).
Temporal effects of diazepam on mPer genes expression
To confirm whether reduction of mPer1 mRNA level after diazepam treatment is transient, mPer1 and mPer2 mRNA levels were examined 1 and 6 h after administration of diazepam at ZT16. Diazepam treatment (10 mg kg−1) caused a severe deficit of rota-rod performance and equilibrium 1 h after administration at ZT16 (Figure 3a). Thereafter, impairment was slowly restored to normal 6–12 h after administration. While both diazepam and vehicle-treated mice showed reduced mPer1 mRNA expression 1 h after injection (P<0.01, Student's t-test), no significant differences of mPer1 and mPer2mRNA levels were observed 6 h after administration (Figure 3b,c).
Figure 3.
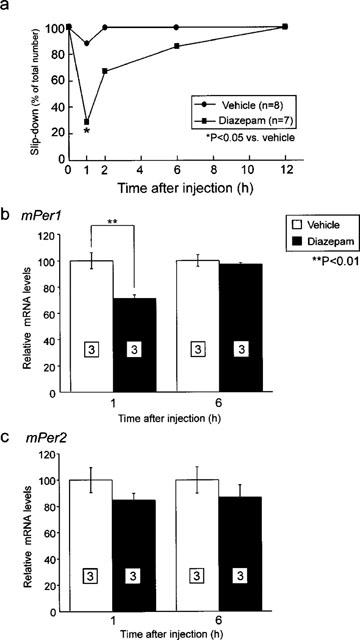
Temporal effect of diazepam administration on the expression of mPer1 and mPer2. (a) Temporal inhibition of cooperative motor activity by diazepam administration was examined by rota-rod test. Most severe inhibition of locomotor activity was observed at 1 h after injection. (*P<0.05 vs vehicle, Fisher's test) mRNA levels of mPer1 (b) and mPer2 (c) of vehicle- or diazepam-treated group 1 and 6 h after injection of vehicle or diazepam (10 mg kg−1) were normalized by GAPDH mRNA. Vehicle-treated group was set to 100 at both experiments. Numbers in boxes in the columns indicate the number of experiments. Reduction of mPer1 mRNA by diazepam (*P<0.01, Student's t-test) restored after 6 h after treatment.
Effects of anxiolytic drugs on mPer gene expression in vivo and rota-rod performance
Table 1 shows effects of various anxiolytic drugs (diazepam, triazolam and tandospirone) on performance of rota-rod test and fixed bar test. Administration of tandospirone (15 mg kg−1) showed no deficit of co-ordinated motor activity. In this case, mPer1 and mPer2 expression is barely inhibited (Figure 4). On the other hand, high dose administration of diazepam (10 mg kg−1), tandospirone (30 mg kg−1) and triazolam (1 mg kg−1) in that administration lead to significant depression of co-ordinated motor activity caused reduction of mPer1 mRNA but not mPer2 mRNA (Figure 4). Diazepam (1 mg kg−1) treatment that caused small inhibition of motor activity also reduced mPer1 mRNA level. These results showed that transient reduction of mPer1 mRNA levels by benzodiazepines correlated with an impairment of co-ordinated motor movements.
Table 1.
Effects of various drugs on rota-rod performance and equilibrium
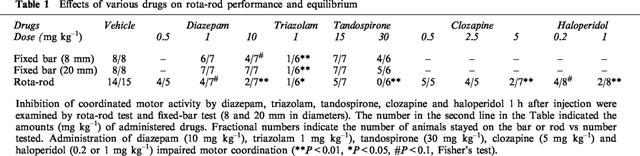
Figure 4.
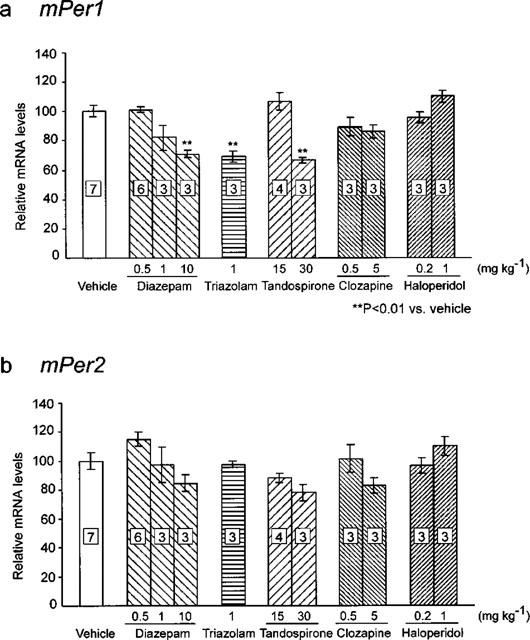
Effects of various drugs on mPer1 (a) and mPer2 (b) expression 1 h after administration. mRNA levels of mPer1 and mPer2 of vehicle-, diazepam-, triazolam-, tandospirone-, clozapine- and haloperidol-treated group 1 h after injection were normalized to GAPDH mRNA levels. The number in the Figure indicates the amounts (mg kg−1) of administered drugs. Numbers in boxes indicate the number of experiments. Vehicle-treated group was set to 100. Numbers in boxes in the columns indicate the number of experiments. High dose administration of diazepam (10 mg kg−1), triazolam (1 mg kg−1) and tandospirone (30 mg kg−1) reduced mPer1 expression (**P<0.01 vs vehicle, Student's t-test).
Effects of antipsychotic drugs on mPer gene expression in vivo and rota-rod performance
Table 1 shows effects of clozapine and haloperidol on performance of rota-rod test. Administration of clozapine (0.5 or 2.5 mg kg−1) and haloperidol (0.2 mg kg−1) showed no defict of co-ordinated motor activity. In all cases, mPer1 and mPer2 expression is barely inhibited (Figure 4). High dose clozapine (10 mg kg−1) administration or haloperidol administration (0.2 or 1 mg kg−1) led to significant depression of co-ordinated motor activity but did not reduce mPer1 and mPer2 mRNA level.
Effects of diazepam and tandospirone on mPer genes expression in vitro
As diazepam and tandospirone reduced mPer1 gene expression in vivo, we tried to see effects of these drugs on mPer1 and mPer2 mRNA levels using the primary granule cell culture of mouse cerebellum (Figure 5). We demonstrated that mPer1 expression in the cultured mouse cerebellar cells was reduced by nifedipine (10 μM) and transiently increased by addition of horse serum (50%) similar to rat-1 fibroblast cell culture as previously reported (Balsalobre et al., 1998). Addition of high concentrations of diazepam or tandospirone (each 100 μM) significantly reduced mPer1 expression (Figure 5).
Figure 5.
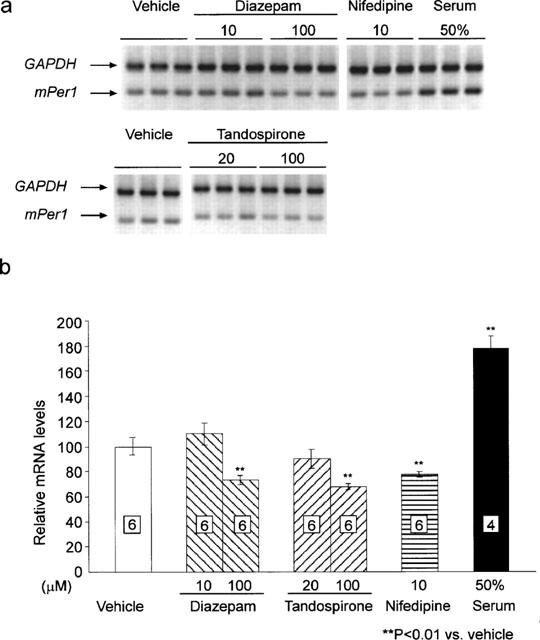
Effects of diazepam, tandospirone, nifedipine and 50% serum on mPer1 expression in cerebellar granule cell culture. (a) Representative example of electrophoretic image of RT–PCR products of mPer1, mPer2 and GAPDH mRNA from vehicle- diazepam- nifedipine- or high concentration of serum-treated granule cell culture. (b) mRNA levels of mPer1 1 h after treatment were normalized to GAPDH mRNA. Vehicle-treated group was set to 100. Numbers in boxes in the columns indicate the number of experiments. High dose treatment of diazepam (100 μM), tandospirone (100 μM) or nifedipine (10 μM) reduced mPer1 expression (**P<0.01 vs vehicle, Student's t-test).
Discussion
The present study demonstrated that the expression of mPer1 mRNA was rapidly reduced in the cerebellum by acute intraperitoneal injection of diazepam, triazolam and tandospirone, but not by clozapine and haloperidol. In addition, the present data showed that a transient reduction of mPer1 was mRNA levels by diazepam, triazolam and tandospirone associated with an impairment of co-ordinated motor movements. These results demonstrated the first evidence indicating a potential function for the mPer genes outside the SCN. mPer1 and mPer2 mRNA are rapidly induced after light exposure during the subjective night in the SCN (Shigeyoshi et al., 1997; Zylka et al., 1998b). A recent study showed that Per1 and Per2 mRNA are induced by transient application of a high concentration of serum in a few mammalian cell lines (Balsalobre et al., 1998). Their temporal induction of mPer1 and mPer2 mRNA is similar to those of light induction in the SCN. In our culture experiment, we observed a similar increase of mPer1 and mPer2 mRNA in the cerebellar granule cell culture after application of high concentration of serum. However, the mechanism of acute induction after light exposure or incubation in the presence of high concentration of serum is still unknown. It has been shown that the activation of N-methyl-D-aspartate receptor is required to produce the phase shift of SCN neuronal circadian rhythm (Shibata et al., 1994) as well as behavioural rhythms (Colwell et al., 1990). Thus, facilitation of glutamatergic receptors may be related to induction of mPer1 mRNA after light exposure and serum shock. In contrast to excitatory influence, activation of GABAA receptors by muscimol and benzodiazepines caused the inhibition of SCN neuronal firing (Tominaga et al., 1994; Liou et al., 1990). Thus, administration of benzodiazepines may inhibit granular cell activity in the cerebellum through the activation of GABAA receptor subunit 6. Although the level of mPer1 mRNA in the cerebellum was dose-dependently reduced by in vivo administration of diazepam, application of this drug in vitro required a high concentration (100 μM) to reduce the mPer1 mRNA. There are several reasons for the discrepancy, but at present we can not be precise. In the present culture conditions, there might be an insufficient number of benzodiazepine receptors to cause the inhibition of mPer1 mRNA. Actually, in the cultured granular cells, mPer1 mRNA was highly expressed, because extracellular KCl concentration was kept high to keep the neurons alive. It is well known that cerebellum receives several inputs from other brain areas such as cerebrum, hindbrain and spinal cord (Voogd & Glickstein, 1998). An alternative explanation for the disagreement may be that the normal morphological structure of cerebellum or secondary influence via inputs from other brain regions is necessary to cause the reduction of mPer1 mRNA by benzodiazepines.
What does the reduction of mPer1 mRNA expression mean? Amounts of mPer1 and mPer2 mRNA are oscillated in the SCN (Tei et al., 1997; Zylka et al., 1998b). A recent study showed that activation of mPer1 transcription in circadian oscillation is mediated by binding CLOCK-BMAL1 heteroduplexes to E-box elements in the promoter region of the mPer1 gene (Darlington et al., 1998). Increased expression of mPer1 is thought to induce an increase of mPER1 protein and in turn inhibit the CLOCK's activity and down-regulate mPer1 transcription (Darlington et al., 1998). Therefore, reduction of mPer1 mRNA by anxiolytics may cause a disinhibition upon the CLOCK's activity. Thus, anxiolytic drugs can produce an impairment of circadian clock mechanism in the cerebellum, suggesting that these drugs may also impair clock-controlled functions such as neuronal activity. Effects of anxiolytic drugs on mPer2 mRNA expression are much smaller than those on mPer1 mRNA expression. Light induction of mPer2 mRNA in the SCN is about 30 min slower than mPer1 (Takumi et al., 1998a). These results taken together, suggest that various regulatory mechanisms exist in the expression of the mPer1 and mPer2 gene in different cell groups.
It has been proposed that the role of the cerebellar cortex is to combine simpler elements of movement into complex coordinated action (Thach et al., 1992). In the rota-rod test, the mGluR1, GluRδ2 and NMDA2A/NMDA2C receptor mutant mice have been shown to fall off the rota-rod with a deficit of long-term depression in the cerebellum (Aiba et al., 1994; Kashiwabuchi et al., 1995; Kadotani et al., 1996). Thus, normal function of glutamatergic neurotransmission in mossy fibre-granule cell synapses may be important for performance of motor co-ordination. Further genetic dissection of mPer1 gene, possibly with knockout mice is useful to identify the role of these genes in the cerebellum.
The high dose of tandospirone, a 5HT1A receptor agonist, caused a reduction of mPer1 mRNA expression in the cerebellum in vivo as well as in vitro again with an impairment of rota-rod performance, however its low doses affected neither mPer1 mRNA nor co-ordinated motor activity and equilibrium. The administration of high doses of haloperidol and clozapine caused an impairment of rota-rod performance and the dose range used in the present experimental study is well in accordance with a previous paper by Millan et al. (1999). However, haloperidol and clozapine did not affect the expression of mPer1 mRNA in the cerebellum. The present result suggested that the reduction mPer1 mRNA in the cerebellum may not be related to the anxiolytic activity, because small dose of tandospirone (15 mg kg−1) is sufficient to produce the anxiolytic activity (Shimizu et al., 1992) and clozapine is known to possess anxiolytic properties in certain experimental models (Wiley et al., 1993). Why did high dose of tandospirone reduce both mPer1 mRNA and rota-rod performance? Tandospirone exhibits the high affinity to 5-HT1A receptors (Shimizu et al., 1988), but clozapine shows mild agonistic properties at these receptors (Newman-Tancredi et al., 1996). Although there is a report that shows a low expression of 5-HT1Areceptors in the cerebellum (Kia et al., 1996), tandospirone reduced mPer1 mRNA expression in vivo and in vitro. Therefore, the present results suggest that a high dose of tandospirone affects the cerebellar function. Alternatively, high dose of tandospirone may affect 5-HT1A receptors in the hindbrain and/or spinal cord to cause an impairment of motor coordination. In the present experiment, haloperidol did not affect the expression of mPer1 mRNA in the cerebellum, although this chemical impaired the rota-rod performance. Therefore, it is suggested that impairment of rota-rod performance itself did not affect the expression of mPer1 mRNA. Although Yokoyama et al. (1994) reported the high expression of D2 receptors in the cerebellum and extrapyramidal, brain areas such as striatum may be responsible for haloperidol-induced impairment of rota-rod performance. Actually, the haloperidol induced decrement in co-ordinated locomotor activity was observed only after a high dose was injected into the cerebellum (Barik & de Beaurepaire, 1996). Thus, we conclude that there is no contribution of D2 receptors on the mPer1 mRNA expression in the cerebellum.
In summary, present study showed a reduction of mPer1 mRNA level in the cerebellum by benzodiazepines and a 5-HT1A receptor agonist, but not by antipsychotics closely associated with impairment of co-ordinated motor activity.
Acknowledgments
This study was partially supported by grants to S. Shibata from Research Project for Future Program (RFTF96L00310) and Japanese Ministry of Education, Science, Sports and Culture (09470018 11233207, 1170248).
Abbreviations
- SCN
suprachiasmatic nucleus
- ZT
zeitgeber time
References
- AIBA A., KANO M., CHEN C., STANTON M.E., FOX G.D., HERRUP K., ZWINGMAN T.A., TONEGAWA S. Deficient cerebellar long-term depression and impaired motor learning in mGluR1 mutant mice. Cell. 1994;79:377–388. [PubMed] [Google Scholar]
- AKIYAMA M., KOUZU Y., TAKAHASHI S., WAKAMATSU H., MORIYA T., MAETANI M., WATANABE S., TEI H., SAKAKI Y., SHIBATA S. Inhibition of light- or glutamate-induced mPer1 expression represses the phase shifts into the mouse circadian locomotor and suprachiasmatic firing rhythms. J. Neurosci. 1999;19:1115–1121. doi: 10.1523/JNEUROSCI.19-03-01115.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ALBRECHT U., SUN Z.S., EICHELE G., LEE C.C. A differential response of two putative mammalian circadian regulators, mPer1 and mPer2, to light. Cell. 1997;91:1055–1064. doi: 10.1016/s0092-8674(00)80495-x. [DOI] [PubMed] [Google Scholar]
- ALLADA R., WHITE N.E., SO W.V., HALL J.C., ROSBASH M. A mutant Drosophila homologue of mammalian Clock disrupts circadian rhythms and transcription of period and timeless. Cell. 1998;93:791–804. doi: 10.1016/s0092-8674(00)81440-3. [DOI] [PubMed] [Google Scholar]
- BALSALOBRE A., DAMIOLA F., SCHIBLER U. A serum shock induces circadian gene expression in mammalian tissue culture cells. Cell. 1998;93:929–937. doi: 10.1016/s0092-8674(00)81199-x. [DOI] [PubMed] [Google Scholar]
- BARIK S., DE BEAUREPAIRE R. Evidence for a functional role of the dopamine D3 receptors in the cerebellum. Brain Res. 1996;737:347–350. doi: 10.1016/0006-8993(96)00964-x. [DOI] [PubMed] [Google Scholar]
- BROWN M.J., BRISTOW D.R. Molecular mechanisms of benzodiazepine-induced down-regulation of GABAA receptor α1 subunit protein in rat cerebellar granule cells. Br. J. Pharmacol. 1996;118:1103–1110. doi: 10.1111/j.1476-5381.1996.tb15512.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- COLWELL C.S., RALPH M.R., MENAKER M. Do NMDA receptors mediate the effects of light on circadian behavior. Brain Res. 1990;523:117–120. doi: 10.1016/0006-8993(90)91643-u. [DOI] [PubMed] [Google Scholar]
- DARLINGTON T.K., WAGER-SMITH K., CERIANI M.F., STAKNIS D., GEKAKIS N., STEEVES T.D.L., WEITZ C.J., TAKAHASHI J.S., KAY S.A. Closing the circadian loop: CLOCK-induced transcription of its own inhibitors per and tim. Science. 1998;280:1599–1603. doi: 10.1126/science.280.5369.1599. [DOI] [PubMed] [Google Scholar]
- EDMUNDS L.N. Cellular and Molecular Basis of Biological Clocks. Springer: New York; 1988. [Google Scholar]
- FUJITA Y., KATAGI J., TABUCHI A., TSUCHIYA T., TSUDA M. Coactivation of serectogranin-II and BDNF genes mediated by calcium signals in mouse cerebellar granule cells. Mol. Brain. Res. 1999;63:316–324. doi: 10.1016/s0169-328x(98)00299-x. [DOI] [PubMed] [Google Scholar]
- HASTINGS M.H. Central clocking. Trends Neurosci. 1997;20:459–464. doi: 10.1016/s0166-2236(97)01087-4. [DOI] [PubMed] [Google Scholar]
- KADOTANI H., HIRANO T., MASUGI M., NAKAMURA K., NAKAO K., KATSUKI M., NAKANISHI S. Motor discoordination results from combined gene disruption of the NMDA receptor NR2A and NR2C subunits, but not from single disruption of the NR2A or NR2C subunit. J. Neurosci. 1996;16:7859–7867. doi: 10.1523/JNEUROSCI.16-24-07859.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KASHIWABUCHI N., IKEDA K., ARAKI K., HIRANO T., SHIBUKI K., TAKAYAMA C., INOUE Y., KUTSUWADA T., YAGI T., KANG Y. Impairment of motor coordination, Purkinje cell synapse formation, and cerebellar long-term depression in GluRδ2 mutant mice. Cell. 1995;81:245–252. doi: 10.1016/0092-8674(95)90334-8. [DOI] [PubMed] [Google Scholar]
- KHAN Z.U., GUTIERREZ A., DE BLAS A.L. The α1 and α6 subunits can coexist in the same cerebellar GABAA receptor maintaining their individual benzodiazepine-binding specificities. J. Neurochem. 1996;66:685–691. doi: 10.1046/j.1471-4159.1996.66020685.x. [DOI] [PubMed] [Google Scholar]
- KIA H.K., MIQUEL M.C., BRISORGUEIL M.J., DAVAL G., RIAD M., EL MESTIKAWY S., HAMON M., VERGE D. Immunocytochemical localization of serotonin1A receptors in the rat central nervous system. J. Comp. Neurol. 1996;365:289–305. doi: 10.1002/(SICI)1096-9861(19960205)365:2<289::AID-CNE7>3.0.CO;2-1. [DOI] [PubMed] [Google Scholar]
- KORPI E.R., KLEINGOOR C., KETTENMANN H., SEEBURG P.H. Benzodiazepine-induced motor impairment linked to point mutation in cerebellar GABAA receptor. Nature. 1993;361:356–359. doi: 10.1038/361356a0. [DOI] [PubMed] [Google Scholar]
- LIOU S.Y., SHIBATA S., ALBERS H.E., UEKI S. Effects of GABA and anxiolytics on the single unit discharge of suprachiasmatic neurons in rat hypothalamic slices. Brain Res. Bull. 1990;25:103–107. doi: 10.1016/0361-9230(90)90259-3. [DOI] [PubMed] [Google Scholar]
- MILLAN M.J., BROCCO M., GOBERT A., SCHREIBER R., DEKEYNE A. S-16924 [(R)-2-[1-[2-(2,3-dihydro-benzo[1,4]dioxin-5-yloxy)-ethyl]-pyrrolidine -3yl] -1-(4-fluorophenyl)-ethanone], a novel, potential antipsychotic with marked serotonin1A agonist properties: III. Anxiolytic actions in comparison with clozapine and haloperidol. J. Pharmacol. Exp. Ther. 1999;288:1002–1014. [PubMed] [Google Scholar]
- NEWMAN-TANCREDI A., CHAPUT C., VERRIELE L., MILLAN M.J. Clozapine is a partial agonist at cloned human serotonin 5-HT1A receptors. Neuropharmacology. 1996;35:119–121. doi: 10.1016/0028-3908(95)00170-0. [DOI] [PubMed] [Google Scholar]
- SANGORAM A.M., SAEZ L., ANTOCH M.P., GEKAKIS N., STAKNIS D., WHITELEY A., FRUECHTE E.M., VITATERNA M.H., SHIMOMURA K., KING D.P., YOUNG M.W., WEITZ C.J., TAKAHASHI J.S. Mammalian circadian autoregulatory loop: a timeless ortholog and mPer1 interact and negatively regulate CLOCK-BMAL1-induced transcription. Neuron. 1998;21:1101–1113. doi: 10.1016/s0896-6273(00)80627-3. [DOI] [PubMed] [Google Scholar]
- SHEARMAN L.P., ZYLKA M.J., WEAVER D.R., KOLAKOWSKI LFJ.R., REPPERT S.M. Two period homologs: circadian expression and photic regulation in the suprachiasmatic nuclei. Neuron. 1997;19:1261–1269. doi: 10.1016/s0896-6273(00)80417-1. [DOI] [PubMed] [Google Scholar]
- SHIBATA S., WATANABE A., HAMADA T., ONO M., WATANABE S. N-methyl-D-aspartate induces phase shifts in circadian rhythm of neuronal activity of rat SCN in vitro. Am. J. Physiol. 1994;267:R360–R364. doi: 10.1152/ajpregu.1994.267.2.R360. [DOI] [PubMed] [Google Scholar]
- SHIGEYOSHI Y., TAGUCHI K., YAMAMOTO S., TAKEKIDA S., YAN L., TEI H., MORIYA T., SHIBATA S., LOROS J.J., DUNLAP J.C., OKAMURA H. Light-induced resetting of a mammalian circadian clock is associated with rapid induction of the mPer1 transcript. Cell. 1997;91:1043–1053. doi: 10.1016/s0092-8674(00)80494-8. [DOI] [PubMed] [Google Scholar]
- SHIMIZU H., KUMASAKA Y., TANAKA H., HIROSE A., NAKAMURA M. Anticonflict action of tandospirone in a modified Geller-Seifter conflict test in rats. Jpn. J. Pharmacol. 1992;58:283–289. doi: 10.1254/jjp.58.283. [DOI] [PubMed] [Google Scholar]
- SHIMIZU H., TATSUNO T., HIROSE A., TANAKA H., KUMASAKA Y., NAKAMURA M. Characterization of the putative anxiolytic SM-3997 recognition sites in rat brain. Life Sci. 1988;42:2419–2427. doi: 10.1016/0024-3205(88)90340-2. [DOI] [PubMed] [Google Scholar]
- SUN Z.S., ALBRECHT U., ZHUCHENKO O., BAILEY J., EICHELE G., LEE C.C. RIGUI, a putative mammalian ortholog of the Drosophila period gene. Cell. 1997;91:1003–1011. doi: 10.1016/s0092-8674(00)80366-9. [DOI] [PubMed] [Google Scholar]
- TAKUMI T., MATSUBARA C., SHIGEYOSHI Y., TAGUCHI K., YAGITA K., MAEBAYASHI Y., SAKAKIDA Y., OKUMURA K., TAKASHIMA N., OKAMURA H. A new mammalian period gene predominantly expressed in the suprachiasmatic nucleus. Genes Cells. 1998a;3:167–176. doi: 10.1046/j.1365-2443.1998.00178.x. [DOI] [PubMed] [Google Scholar]
- TAKUMI T., TAGUCHI K., MIYAKE S., SAKAKIDA Y., TAKASHIMA N., MATSUBARA C., MAEBAYASHI Y., OKUMURA K., TAKEKIDA S., YAMAMOTO S., YAGITA K., YAN L., YOUNG M.W., OKAMURA H. A light-independent oscillatory gene mPer3 in mouse SCN and OVLT. EMBO J. 1998b;17:4753–4759. doi: 10.1093/emboj/17.16.4753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TEI H., OKAMURA H., SHIGEYOSHI Y., FUKUHARA C., OZAWA R., HIROSE M., SAKAKI Y. Circadian oscillation of a mammalian homologue of the Drosophila period gene. Nature. 1997;389:512–516. doi: 10.1038/39086. [DOI] [PubMed] [Google Scholar]
- THACH W.T., GOODKIN H.P., KEATING J.G. The cerebellum and the adaptive coordination of movement. Annu. Rev. Neurosci. 1992;15:403–442. doi: 10.1146/annurev.ne.15.030192.002155. [DOI] [PubMed] [Google Scholar]
- TOMINAGA K., SHIBATA S., HAMADA T., WATANABE S. GABAA receptor agonist muscimol can reset the phase of neural activity rhythm in the rat suprachiasmatic nucleus in vitro. Neurosci. Lett. 1994;166:81–84. doi: 10.1016/0304-3940(94)90845-1. [DOI] [PubMed] [Google Scholar]
- VOOGD J., GLICKSTEIN M. The anatomy of the cerebellum. Trends. Neurosci. 1998;21:370–375. doi: 10.1016/s0166-2236(98)01318-6. [DOI] [PubMed] [Google Scholar]
- WILEY J.L., COMPTON A.D., PORTER J.H. Effects of four antipsychotics on punished responding in rats. Pharmacol. Biochem. Behav. 1993;45:263–267. doi: 10.1016/0091-3057(93)90237-n. [DOI] [PubMed] [Google Scholar]
- YOKOYAMA C., OKAMURA H., NAKAJIMA T., TAGUCHI J., IBATA Y. Autoradiographic distribution of [3H]YM-09151-2, a high-affinity and selective antagonist ligand for the dopamine D2 receptor group, in the rat brain and spinal cord. J. Comp. Neurol. 1994;344:121–136. doi: 10.1002/cne.903440109. [DOI] [PubMed] [Google Scholar]
- ZYLKA M.J., SHEARMAN L.P., LEVINE J.D., JIN X., WEAVER D.R., REPPERT S.M. Molecular analysis of mammalian timeless. Neuron. 1998a;21:1115–1122. doi: 10.1016/s0896-6273(00)80628-5. [DOI] [PubMed] [Google Scholar]
- ZYLKA M.J., SHEARMAN L.P., WEAVER D.R., REPPERT S.M. Three period homologs in mammals: differential light responses in the suprachiasmatic circadian clock and oscillating transcripts outside of brain. Neuron. 1998b;20:1103–1110. doi: 10.1016/s0896-6273(00)80492-4. [DOI] [PubMed] [Google Scholar]


