Abstract
Adenosine and the A1-adenosine receptor agonist R-PIA, exerted a negative inotropic effect in isolated, electrically driven left atria of wild-type mice.
In left atria of mice overexpressing the A1-adenosine receptor, adenosine and R-PIA exerted a positive inotropic effect.
The positive inotropic effect of adenosine and R-PIA in transgenic atria could be blocked by the A1-adenosine receptor antagonist DPCPX.
In the presence of isoprenaline, adenosine exerted a negative inotropic effect in wild-type atria but a positive inotropic effect in atria from A1-adenosine receptor overexpressing mice.
The rate of beating in right atria was lower in mice overexpressing A1-adenosine receptors compared with wild-type.
Adenosine exerted comparable negative chronotropic effects in right atria from both A1-adenosine receptor overexpressing and wild-type mice.
A1-adenosine receptor overexpression in the mouse heart can reverse the inotropic but not the chronotropic effects of adenosine, implying different receptor-effector coupling mechanisms.
Keywords: A1-adenosine receptors, chronotropy, inotropy, transgenic mice
Introduction
Adenosine is an endogenous compound that is generated in response to hypoxia and β-adrenergic stimulation in the heart (Bardenheuer et al., 1987; Berne, 1983). Adenosine exerts negative inotropic and chronotropic effects in mammalian hearts (Belardinelli et al., 1989; Ralevic & Burnstock, 1998; Schrader et al., 1977; Shryock & Belardinelli, 1997). The effects are mediated by A1-adenosine receptors. This conclusion is based on several lines of evidence. Though A1-, A2A-, A2B- and A3-adenosine receptors have been detected in the heart (Reppert et al., 1991; Salvatore et al., 1993; Xu et al., 1996), A1-adenosine receptor agonists like R-PIA exert negative inotropic and chronotropic effects. Moreover, the negative inotropic and chronotropic effects of adenosine are blocked by A1-adenosine receptor antagonists like DPCPX (von der Leyen et al., 1989). In atria of most species studied (guinea-pig, rat, ferret, rabbit, dog) including man, adenosine exerts a direct negative inotropic effect (Böhm et al., 1984; 1985; 1989; Dobson, 1983; Brückner et al., 1985; Drury & Szent-Györgyi, 1929). However, in ventricle of rat and ferret but not in man (and guinea-pig), adenosine exerts no direct negative inotropic effect (Song & Belardinelli, 1996). This can be explained by different receptor effector coupling. In atria, adenosine opens potassium channels and thereby shortens the duration of the action potential (Belardinelli & Isenberg, 1983). This reduces the time for influx of Ca2+ via the L-type calcium channel and can explain the negative inotropic effect of adenosine in the atrium. In ventricle of ferret and rat, adenosine acts via the same mechanism. However, in guinea-pig (and probably man) adenosine cannot open ventricular potassium channels (Song & Belardinelli, 1996). This could explain the lack of negative inotropy of adenosine alone in the ventricle from these species.
In contrast, in atria and ventricle of e.g. guinea-pig, rat, ferret and man, adenosine can antagonize the positive inotropic effects of β-adrenoceptor agonists like isoprenaline (Böhm et al., 1984; 1985; 1989; Brückner, 1985; Dobson et al., 1983). This has been termed the anti-adrenergic, or indirect effect of adenosine. Agonists at β-adrenoceptors stimulate the activity of adenylyl cyclase and thereby elevate intracellular cyclic AMP content. Cyclic AMP activates the cyclic AMP-dependent protein kinase which phosphorylates (besides other targets) phospholamban in the sarcoplasmic reticulum (SR) and the L-type calcium channels in the sarcolemma. Thereby, isoprenaline increases the current through L-type calcium channels in atrium and ventricle. Subsequent steps include a release of Ca2+ from the SR and finally the positive inotropic effect. This signal transduction pathway is attenuated by adenosine acting via A1-adenosine receptors which decreases cyclic AMP levels and can account for the anti-adrenergic effect of adenosine (Belardinelli & Isenberg, 1983; Fenton et al., 1991; Linden et al., 1985; George et al., 1991; Gupta et al., 1993). The direct and indirect effects of adenosine are abolished if atrium or ventricle is pretreated with pertussis toxin which covalently modifies and blocks inhibitory G-proteins (Böhm et al., 1986; 1988; Kurachi et al., 1986).
Recently, transgenic mice have been engineered which overexpress the A1-adenosine receptor in the heart (Matherne et al., 1997), using the alpha myosin heavy chain promoter and the rat A1-adenosine receptor. A1-adenosine receptor overexpression in these mice causes bradycardia in intact animals and in isolated perfused hearts (Gauthier et al., 1999). Moreover, the mice were significantly more resistant to both the functional and metabolic effects of ischaemia through their overexpressed adenosine receptors (Matherne et al., 1997; Headrick et al., 1998). However, whether the inotropic and chronotropic effects of adenosine are altered in atrium of these transgenic animals, has yet to be studied. Therefore, the purpose of this study was to investigate whether A1-adenosine receptor overexpression, (i) influences the inotropic effects of adenosine in atria, (ii) modulates the β-adrenergic inotropic response in atria and, (iii) alters the chronotropic effect of adenosine in atria.
Methods
The construct design and initial characterization of the transgenic lines has been previously reported (Matherne et al., 1997). In brief, the rat A1 cDNA was expressed under the control of the modified alpha myosin heavy chain (MHC) promoter. The construct was used to generate transgenic mice using standard techniques. Three transgenic lines were established (1, 5 and 8), which were then further analysed. In ligand binding experiments their Bmax for A1 adenosine receptors was about 2600, 3300 and 250 fmol mg−1 protein, respectively, whereas levels in wild type mice were around 8 fmol mg−1 protein.
Identification of transgenic mice
Genomic DNA was isolated from mice tail biopsies as follows: Approximately 0.5–1.0 cm of tail was agitated in 700 μl of a buffer containing 50 mM Tris (pH 8.0), 100 mM EDTA, 0.5% SDS and 10 mg ml−1 Proteinase K overnight at 55°C. Then 700 μl of phenol was added and the resulting two phases separated by centrifugation. To the aqueous phase, 700 μl of a mixture containing phenol/chloroform/isoamylalcohol 25 : 24 : 1 v v−1 was added and the two phases separated, again by centrifugation. The DNA present in the aqueous phase was precipitated with 3 M sodium acetate (pH 6.0) and 100% ethanol, and then washed with 70% v v−1 ethanol. The DNA pellet was dried under vacuum and then dissolved in PCR-water (Merck, Darmstadt, Germany). Each PCR reaction used 100 ng of genomic DNA. The forward primer 5′-CTCTGACAGAGAAGCAGGCACTTTACATGG-3′ was located within the alpha-MHC promoter region of the transgenic construct (Matherne et al., 1997). The reverse primer was located within the coding region of the transgene namely rat A1 receptor, 5′-CCAGTGACACGATGAAGCAGAAGGTGGCAT-3′. All PCR reactions were carried out in a total volume of 50 μl containing 20 mM Tris-HCl (pH 8.6 at 25°C), 16 mM (NH4)2 SO4, 200 μM each dNTP, 1.5 mM MgCl2 and 1.5 units Taq DNA polymerase (AGS, Heidelberg, Germany). Each reaction was subjected to 35 cycles of denaturation (1 min at 94°C), annealing (2 min at 59°C), and extension (2 min 72°C). PCR reactions were performed in a thermal cycler (Omnigene, model TR3 CM220, MWG-Biotech, Ebersberg, Germany) and PCR-products visualized on 2% agarose gels. Sizes of PCR-products were compared to DNA size markers (MBI Fermentas, Vilnius, Latvia). Only transgenic mice showed single PCR-products of the expected size (460 bp).
Measurement of contractile function and pacemaker activity
Cardiac preparations were studied from mice of both genders. The body weight ranged from 21.1 to 30.4 g. Body weight, total heart weight and atrial weight were not different between lineages (analysis of variances). Right and left atria were dissected from isolated mouse hearts and mounted in an organ bath. Left atrial preparations were continuously electrically stimulated (field stimulation) using a Grass stimulator SD 9, (Quincy, MA, U.S.A.) with each impulse consisting of 1 Hz, with a voltage of 10–15% above threshold and 5 ms duration. These conditions were chosen to ascertain comparability with our earlier work (mice: Neumann et al., 1998, guinea-pig: e.g. Boknik et al., 1997). Right atrial preparations were attached in the same set-up but were not electrically stimulated and allowed to contract spontaneously.
The bathing solution contained (in mM) NaCl 119.8, KCl 5.4, CaCl2 1.8, MgCl2 1.05, NaH2PO4 0.42, NaHCO3 22.6, Na2EDTA 0.05, ascorbic acid 0.28 and glucose 5.0, continuously gassed with 95% O2 and 5% CO2 and maintained at 35°C resulting in a pH of 7.4. Contractions were measured in an isometric set-up. Atria were attached with fine sutures to a hook in the organ bath and an isometric force transducer. Signals were amplified and continuously fed into a chart recorder (Föhr Medical Instruments, Egelsbach, Germany). Adenosine was cumulatively applied with 10 min for each concentration. Only one concentration of R-PIA (1 μM, 10 min) was used. R-PIA was applied after addition of adenosine deaminase (1 μg ml−1 for 30 min). DPCPX was applied for 30 min.
Chemicals
The following compounds were used: (−)-N6-phenylisopropyladenosine (R-PIA) and adenosine deaminase (both from Boehringer Mannheim, Mannheim, Germany), and 1,3-dipropyl-8-cyclopentylxanthine (DPCPX, from RBI, Natick, MA, U.S.A.). The other chemicals were analytical best grade. Twice distilled water was used throughout.
Statistics
Results are expressed as mean±s.e.mean. Significance was estimated by Student's t-test for paired or unpaired observations, as appropriate. In Figure 1 analysis of variance was used. A P-value smaller than 0.05 was considered significant.
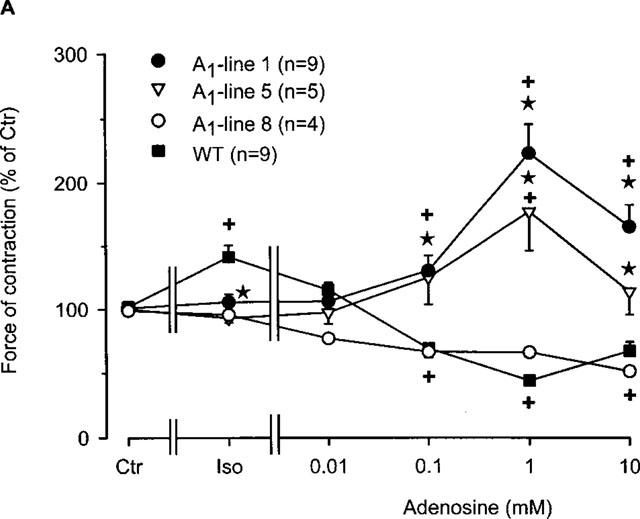
Figure 1.
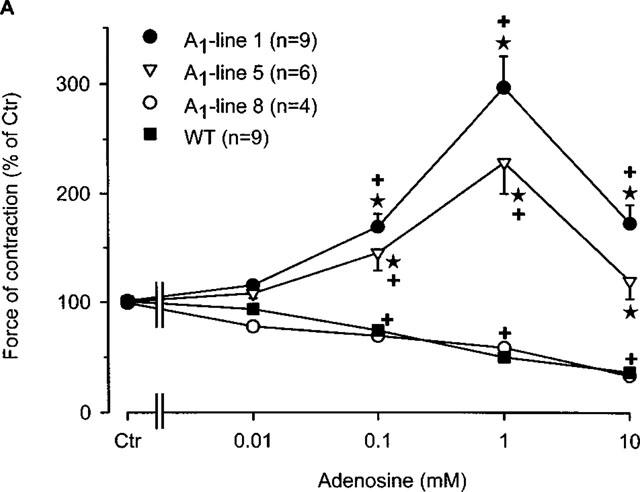
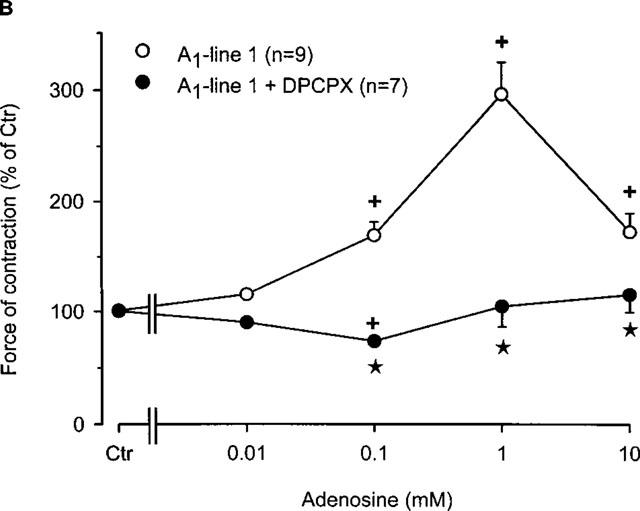
(A) Concentration response curve for the effect of adenosine on force of contraction in electrically driven left atria from wild-type mice and A1-adenosine receptor overexpressing mice. Adenosine was cumulatively applied to the organ bath. The transgenic lines exhibited increasing levels of receptor overexpression in the order of lines 8, 1 and 5. Numbers in brackets indicate the number of individual left atria. Asterisks indicate a significant difference between atrial groups, and crosses indicate a significant difference from Ctr. Differences between groups were significant using analysis of variances. (B) Concentration response curve for the effect of adenosine on force of contraction in electrically driven left atria from A1-adenosine receptor overexpressing mice (line 1) in the presence and absence of the A1 adenosine antagonist DPCPX (1 μM). Adenosine was cumulatively applied to the organ bath. Numbers in brackets indicate number of individual left atria. Asterisks indicate a significant difference between adenosine alone an in the presence of DPCPX, and crosses indicate a significant difference from Ctr. Differences between groups were significant using analysis of variances.
Results
Inotropic effects
Figure 1A indicates that, as in other species, adenosine (alone) exerts a concentration-dependent negative inotropic effect on isolated electrically stimulated atria from wild-type mice (Figure 1A). The same negative inotropic effect is noted only in A1-adenosine receptor overexpressing atria with low level expression (line 8). Atria from mice which overexpress the A1-adenosine receptors to a higher degree (lines 1 and 5), demonstrated increased force of contraction (Figure 1A).
The positive inotropic effect of adenosine was further studied in line 1 since it has a high level (∼300 fold increase) of receptor expression and exhibited the most pronounced positive inotropic effect to adenosine. In the presence of the A1-adenosine receptor antagonist DPCPX, the positive inotropic effect of adenosine was abolished (Figure 1B). Interestingly, a small negative inotropic effect of adenosine was noted at 0.1 mM (Figure 1B). Thereafter, we tested the A1-adenosine receptor agonist R-PIA. We used 1 μM R-PIA because it was the maximally effective concentration. These experiments were performed in the presence of adenosine deaminase (given 30 min alone before drug addition) in order to exclude interference from endogenous adenosine. R-PIA decreased force of contraction in wild-type left atria but increased force of contraction in A1-adenosine receptor overexpressing atria (Figure 2A). The negative inotropic effect of R-PIA in wildtype atria was blocked by preincubation with DPCPX (Figure 2B). The positive inotropic effect of the A1-adenosine receptor agonist R-PIA on atria from line 1 transgenic mice was blocked by DPCPX and instead a small negative inotropic effect of R-PIA was observed (Figure 2B).
Figure 2.
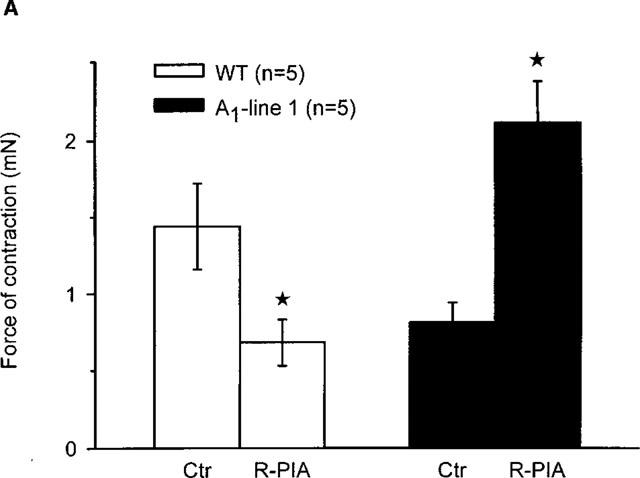
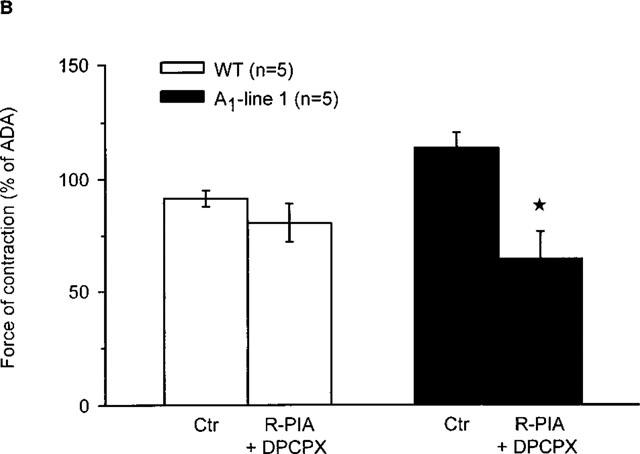
(A) Effect of the A1-adenosine receptor agonist R-PIA (1 μM) on force of contraction in electrically driven left atria from wild-type mice and A1-adenosine receptor overexpressing mice (line 1). Numbers in brackets indicate number of individual left atria. Asterisks indicate significant difference between Ctr (no addition of R-PIA) and treatment. First, the preparations were treated for 30 min with adenosine deaminase. Then R-PIA, as indicated, was added and incubation was for further 10 min. Thereafter, force of contraction was measured in mN (milli Newton, ordinate). (B) Effect of the A1-adenosine receptor agonist R-PIA (1 μM) in the presence of the A1-adenosine receptor antagonist DPCPX (1 μM) on force of contraction in electrically driven left atria from wild-type and A1-adenosine receptor overexpressing mice (line 1). First, the preparations were treated for 30 min with adenosine deaminase. Then, where indicated DPCPX was added. 30 min later, R-PIA (R-PIA+DPCPX) was added for 10 min. Ordinate: force of contraction in per cent of values after 30 min of treatment with adenosine deaminase (ADA). Numbers in brackets indicate number of individual left atria. Asterisks indicate a significant difference between Ctr (no addition of R-PIA or DPCPX) and treatment (DPCPX+R-PIA).
An anti-adrenergic effect of adenosine also termed ‘indirect' effect has been known for years. This effect was also noted in wild-type atria in the present study. Addition of the β-adrenoceptor agonist, isoprenaline, significantly increased force of contraction in wild-type atria (Figure 3A). Additionally, adenosine completely abolished this positive inotropic effect of isoprenaline (Figure 3A). Adenosine concentrations of ⩾0.1 mM, reduced force of contraction to values like those prior to addition of isoprenaline. Interestingly, the positive inotropic effect of 10 nM isoprenaline was attenuated or even lacking in left atria exhibiting A1-adenosine receptor overexpression. In the high level overexpressing lines (i.e. lines 1 and 5), adenosine exerted a positive inotropic effect which peaked at 1 mM (Figure 3A). In low level overexpressing atria (line 8), adenosine exerted a negative inotropic effect similar to the wild-type. We tested also whether the effect of adenosine in the presence of isoprenaline was sensitive to A1-receptor blockade by DPCPX. In the presence of DPCPX the positive inotropic effect of isoprenaline was again readily apparent (Figure 3B). Moreover, DPCPX in the presence of adenosine was able to decrease isoprenaline-stimulated force of contraction in transgenic atria (Figure 3B). Similarly to previous results, the negative inotropic effect was largest at 1 mM adenosine.
Figure 3.
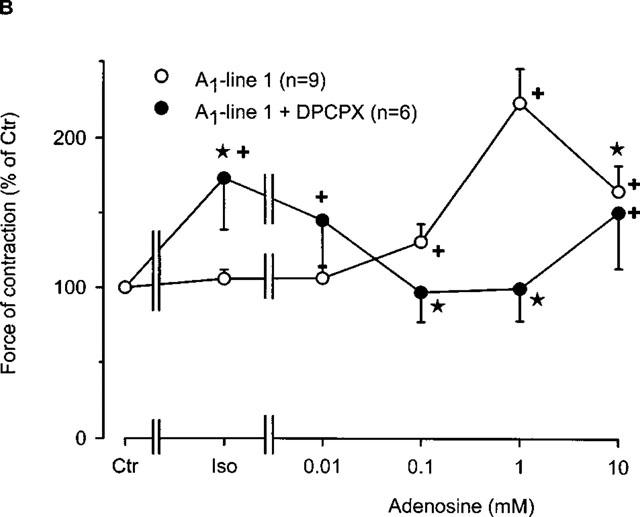
(A) Concentration-response curve for the effect of adenosine in the presence of 10 nM isoprenaline on force of contraction in electrically driven left atria from wild-type and A1-adenosine receptor overexpressing mice. Initially, isoprenaline was applied to the organ bath. Once, the effect of isoprenaline was stable, adenosine was then cumulatively added. The transgenic lines of mice exhibited increasing levels of receptor overexpression in the order of lines 8, 1 and 5. Numbers in brackets indicate number of individual left atria. Asterisks indicate a significant difference between wild-type and transgenic lines and crosses indicate a significant difference from Ctr. (B) Concentration-response curve for the effect of adenosine in the presence or absence of the A1-adenosine receptor antagonist DPCPX on force of contraction in electrically driven left atria from A1-adenosine receptor overexpressing mice (line 1). DPCPX was added to all atria prior to application of treatments. Isoprenaline (10 nM) was initially applied and thereafter, adenosine was cumulatively added to the organ bath. Numbers in brackets indicate number of individual left atria. Asterisks indicate a significant difference between treatments and crosses indicate a significant difference from Ctr.
Since, the effect of 10 nM isoprenaline on force of contraction was attenuated in transgenic atria overexpressing A1-adenosine receptors compared to wild-type atria (Figure 3A), we established complete concentration response curves for the effect of isoprenaline on force of contraction in left atria (Figure 4). It may also be noted that the developed force of contraction is lower in lines 1 and 5 than in the wild-type and line 8 preparations, and that hence, the maximum positive inotropic effect to isoprenaline is smaller. The negative logarithm of EC50 values (in M) for the positive inotropic effect of isoprenaline in wild-type, lines 1, 5 and 8 were 7.66±0.09, 6.73±0.17, 6.72±0.13 and 7.35±0.15. Hence, there was a difference in the EC50 values between wild-type mice versus lines 1 as well as line 5 (P<0.05).
Figure 4.
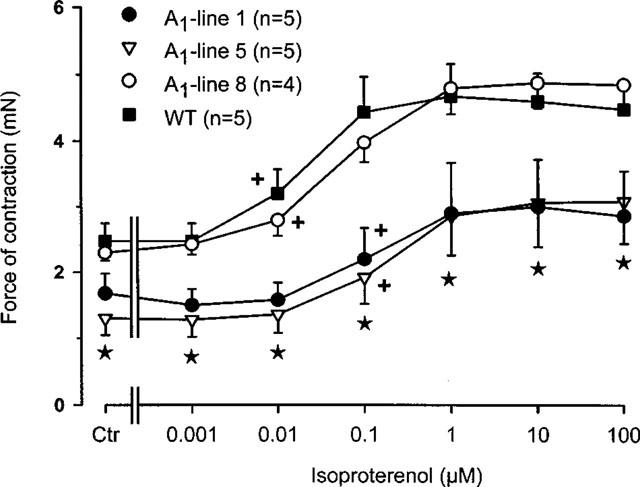
Concentration-response curve for the effect of isoprenaline on force of contraction in electrically driven left atria from wildtype and A1-adenosine receptor overexpressing mice. Isoprenaline (10 nM) was cumulatively applied to the organ bath. The transgenic lines of mice exhibited increasing levels of receptor overexpression in the order of lines 8, 1 and 5. Numbers in brackets indicate number of individual right atria. Asterisks indicate a significant difference between wild-type and transgenic lines and crosses indicate a significant difference from Ctr.
Chronotropic effects
Similar to the inotropic effect, adenosine is known to exert a negative chronotropic effect in mammalian hearts. Hence, this section investigates the beating rate of right atrial preparations and the effect of A1-adenosine receptor overexpression. Figure 5A clearly shows that adenosine exerted a concentration-dependent negative chronotropic effect in right atria of both wild-type and A1-adenosine receptor overexpression mice. Of note, A1-adenosine receptor overexpressing mice from line 1 showed a significant bradycardia compared to preparations from wild-type atria (Figure 5A). Although the atrial preparations started from different basal rates of beating, adenosine still exerted a concentration-dependent negative chronotropic effect in transgenic preparations. Interestingly, all transgenic atria stopped beating at an adenosine concentration of 10 mM.
Figure 5.
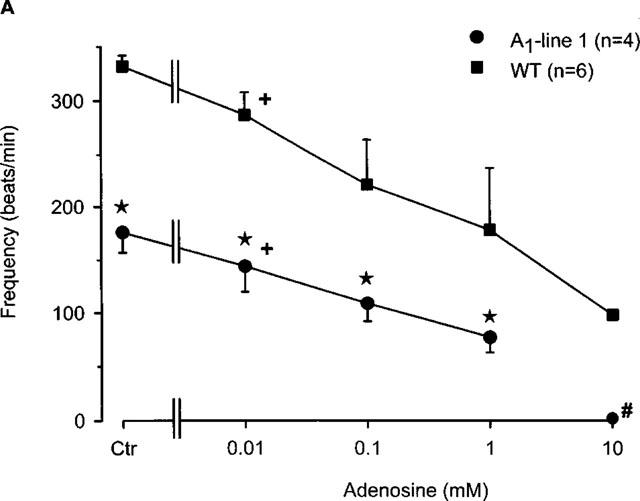
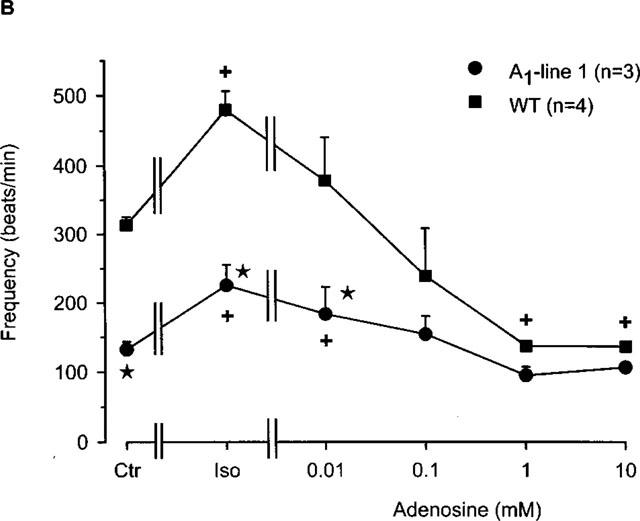
(A) Concentration response curve for the effect of adenosine on the frequency of beating in right atria from wild-type and A1-adenosine receptor overexpressing mice (line 1). Adenosine was cumulatively applied to the organ bath. Numbers in brackets indicate number of individual right atria. Asterisks indicate a significant difference between wild-type and transgenic mice and crosses indicate a significant difference from Ctr. # Indicates asystole. (B) Concentration-response curve for the effect of adenosine in the presence of 10 nM isoprenaline on frequency of beating in right atria from wild-type and A1-adenosine receptor overexpressing mice (line 1). Isoprenaline was initially applied and thereafter, adenosine was cumulatively applied to the organ bath. Numbers in brackets indicate the number of individual right atria. Asterisks indicate a significant difference between wild-type and transgenic mice and crosses indicate a significant difference from Ctr.
It is well known that adenosine antagonizes the positive chronotropic effect of isoprenaline in many species (Ralevic & Burnstock 1998; Shryock & Belardinelli, 1997). This effect was also noted in mouse right atria in the current study. Infusion of 10 nM isoprenaline significantly increased rate of beating in both the wild-type and transgenic atria (Figure 5B). Upon addition of adenosine, the positive chronotropic effects of isoprenaline were completely abolished in a concentration-dependent fashion in both wild-type and transgenic atria. The beating frequency of wild-type atria decreased significantly to below basal rates, whereas the transgenic atria showed a negative chronotropic response to adenosine but the frequency did not fall below that of baseline. Wild-type atrial beating at ⩾1 mM adenosine concentrations approached that of the transgenic atrial (Figure 5B) beating frequency. Interestingly, these results indicate that the positive inotropic effect of isoprenaline is attenuated after A1-adenosine receptor overexpression whereas the positive chronotropic action is unaffected.
Finally, we investigated whether the negative chronotropic effects of adenosine were DPCPX-sensitive. In wild-type atria, the antagonist DPCPX (Figure 6A), attenuated the negative chronotropic effect of adenosine (Figure 6A) up to an adenosine concentration of 0.1 mM. The antagonist was then unable to block the adenosine effect at concentrations of 1 mM and above probably due to competitive binding. Moreover, it is conceivable that in wild-type mice the chronotropic effect mediated by the A1-adenosine receptors is not predominant. There was little, if any effect of DPCPX on the negative chronotropic effect of adenosine in transgenic right atria (Figure 6A). Further studies with R-PIA produced similar results (data not shown).
Figure 6.
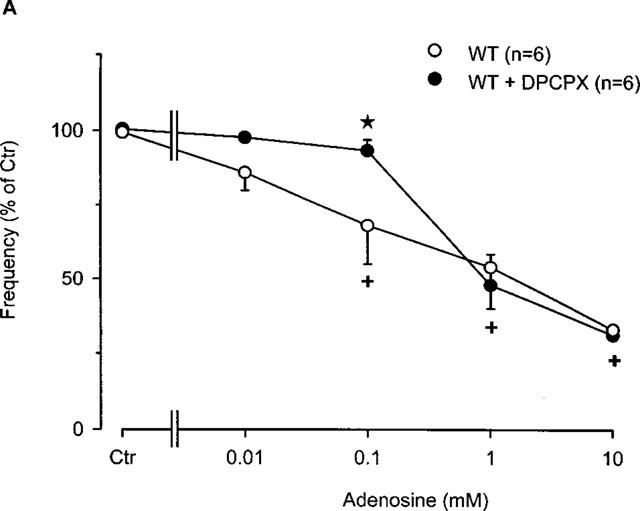
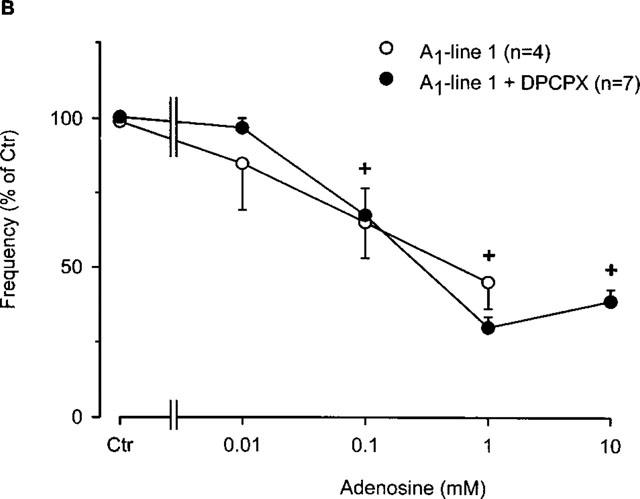
(A) Concentration-response curve for the effect of adenosine in the presence or absence of the A1-adenosine receptor antagonist DPCPX (1 μM) on frequency of beating in right atria from wild-type mice. Adenosine was cumulatively applied to the organ bath. Numbers in brackets indicate number of individual right atria. Asterisks indicate a significant difference between treatments and crosses indicate a significant difference from Ctr. (B) Concentration-response curve for the effect of adenosine in the presence or absence of the A1-adenosine receptor antagonist DPCPX (1 μM) on frequency of beating in right atria from A1-adenosine receptor overexpressing mice (line 1). Adenosine was cumulatively applied to the organ bath. Numbers in brackets indicate number of individual right atria. Asterisks indicate a significant difference between treatments and crosses indicate a significant difference from Ctr.
Discussion
It is well known that the endogenous compound adenosine exerts negative inotropic and chronotropic effects in the mammalian heart (Ralevic & Burnstock, 1998; Shryock & Belardinelli, 1997). These functional effects are now known to be mediated by the A1-adenosine receptor. Some endogenous cardioprotective mechanisms have also been shown to be secondary to A1-adenosine receptor activation. A1-adenosine receptor overexpression in a murine model (Matherne et al., 1997) has been shown to be protective both functionally and metabolically in ischaemia-reperfusion injury (Matherne et al., 1997; Gauthier et al., 1999; Headrick et al., 1998). While some cardiac function studies have been performed (Gauthier et al., 1998; 1999) the complete effects of A1-adenosine receptor overexpression on inotropy and chronotropy have yet to be investigated.
The role of A1-adenosine receptors in inotropic function has been well documented. In this study, adenosine exerted a negative inotropic effect in wild-type atria, similar to previous results from this and other laboratories (Dobson 1983; Linden et al., 1985; Brückner et al., 1985; Böhm et al., 1984; 1989). Atria overexpressing the A1-adenosine receptors to a high degree (∼300 fold increase) exhibited a positive inotropic response to adenosine in two lines (lines 1 and 5) of mice (Figure 1A). Another line of transgenic mice (line 8) with atria exhibiting a low degree of overexpression (∼30 fold) showed a negative inotropic response, which was similar to wild-type. Moreover, the change from a negative to a positive inotropic response to adenosine in these atria may be correlated with the degree of A1-adenosine receptor overexpression, supporting a potential link between both parameters. Recent results from Gauthier et al., (1998) alluded to this possibility, when they investigated the effect of A1-adenosine receptor overexpression on the inotropic response to isoprenaline in the isolated working heart.
One might argue that the positive inotropic effects of adenosine in these transgenic atria are not mediated by the A1-adenosine receptors but by compensatory altered expression of other genes encoding for A2- or A3-adenosine receptors. Indeed, compensatory alterations in transgenic mice have been shown (Rockman et al., 1996). For instance, a SR protein (SERCA) has been shown to be down regulated when another SR protein (phospholamban) was overexpressed in the mouse heart (Neumann et al., 1998). Interestingly, β-receptor density has also been found to be altered in hearts which had high level A1-adenosine receptor overexpression (Gauthier et al., 1999). Furthermore, a thorough characterization of important regulatory genes in A1-overexpressing mice is still currently unavailable. Assuming that the levels of G-proteins are not altered but that the G-proteins are able to freely associate to autonomic receptors, the overexpressed A1-adenosine receptors may couple to stimulatory G-proteins resulting in a positive inotropic action in the atrium. Keeping these points in mind, adenosine as well as the A1-adenosine receptor agonist R-PIA elicited a positive inotropic effect in transgenic atria (Figure 2A). Moreover, these positive inotropic effects were both blocked by DPCPX, an A1-adenosine receptor antagonist (Figures 1B and 2B). Hence, it would seem unlikely that the positive inotropic effects of adenosine in transgenic atria are mediated by receptors other than A1-adenosine receptors. Interestingly, adenosine induced a positive inotropic effect even in the presence of isoprenaline in transgenic atria (Figure 3A). This indicates that the mechanism of the positive inotropic effect does not interfere with the β-adrenergic system in the atrium. Transgenic lines 1 and 5 exhibited a positive inotropic response at adenosine concentrations of ⩾0.1 mM while line 8 produced similar results to wild-type. This correlation coupled with the DPCPX sensitivity (Figure 3B) both support the conclusion that adenosine in the presence of isoprenaline exerted its positive inotropic effect via A1-adenosine receptors.
The results from the present study raise a variety of questions, including (i) what could be the mechanism(s) of the positive inotropic effect of adenosine? and (ii) what might be the second messenger for the positive inotropic effect of adenosine? One can speculate that A1-overexpression has the same biochemical effect as uncoupling the Gi pathway from the A1-adenosine receptor. Stimulation of this receptor can lead to an increase in PLC activity in the heart which can increase production of IP3 and thus potentially lead to positive inotropic effects.
The effect of A1-adenosine overexpression on the β-adrenergic system has recently been investigated. Hearts overexpressing the A1-adenosine receptor exhibited a blunted inotropic response to catecholamines (Gauthier et al., 1998). The positive inotropic effect of isoprenaline in this study appears to change EC50 in transgenic atria overexpressing A1-adenosine receptors at a higher degree (Figure 4, lines 1 and 5). This could be due to a changed affinity of β-adrenoceptors in the high level overexpressing transgenic lines, similar to the results recently obtained by Gauthier et al. (1998). Another, more simplified explanation, is a ‘tonic' inhibition of the β-adrenergic system by endogenously produced adenosine which inhibits via the overexpressed A1-adenosine receptor. This hypothesis is consistent with the fact that DPCPX pre-treatment enhanced the positive inotropic effect of isoprenaline in transgenic atria (Figure 3B).
While A1-adenosine receptor overexpression has improved cardioprotection, these hearts have been shown to exhibit a resting bradycardia (Matherne et al., 1997; Gauthier et al., 1998; Headrick et al., 1998). Here, A1-adenosine overexpression significantly reduced spontaneous rate of beating in right atrial preparations of the high level transgenic mice (Figure 5A, line 1). This indicates a negative chronotropic effect of endogenous adenosine. This also confirms and extends the findings in intact transgenic mice (Matherne et al., 1997).
Conflicting inotropic and chronotropic effects
The apparent conflicting effects of A1-adenosine receptor overexpression on chronotropy and inotropy deserve some consideration. We speculate that with overexpression of A1-adenosine receptors the ‘extra' receptors may promiscuously couple with effectors that mediate inotropy which could overwhelm the weak negative inotropic effects of adenosine that are present in wild-type hearts. Since overexpression results in so many ‘extra' receptors it is not surprising that the usually strong negative chronotropic effects are accentuated in this model.
The beneficial cardioprotective functions mediated by adenosine and the A1-adenosine receptor are well suited to clinical application. It will be important, however, to understand the complete physiological effects of A1-adenosine receptor overexpression on cardiac function before clinical application is possible. In summary, the data acquired in this study indicates that overexpression of A1-adenosine receptors enhances the negative chronotropic effects of adenosine but reverses the negative inotropic effects of adenosine in atrial preparations of transgenic mice.
Acknowledgments
This work was supported by the Deutsche Forschungsgemeinschaft, the Innovative Medizinische Forschung, Münster, the I7KF Münster and NIH RO1 HL59419. Dr Matherne is a Recipient of an AHA Established Investigator Grant.
Abbreviations
- DPCPX
1,3-dipropyl-8-cyclopentylxanthine
- R-PIA
(−)-N6-R-phenylisopropyladenosine
References
- BARDENHEUER H., WHELTON B., SPARKS H.V., JR Adenosine release by the isolated guinea pig heart in response to isoproterenol, acetylcholine, and acidosis: The minimal role of vascular endothelium. Circ. Res. 1987;61:594–600. doi: 10.1161/01.res.61.4.594. [DOI] [PubMed] [Google Scholar]
- BELARDINELLI L., ISENBERG G. Isolated atrial myocytes: adenosine and acetylcholine increase in potassium conductance. Am. J. Physiol. 1983;244:H291–H294. doi: 10.1152/ajpheart.1983.244.5.H734. [DOI] [PubMed] [Google Scholar]
- BELARDINELLI L., LINDEN J., BERNE R.M. The cardiac effects of adenosine. Prog. Cardiovasc. Dis. 1989;32:73–97. doi: 10.1016/0033-0620(89)90015-7. [DOI] [PubMed] [Google Scholar]
- BERNE R.M. Cardiac nucleotides in hypoxia: Possible role in regulation of coronary blood flow. Am. J. Physiol. 1983;204:317–322. doi: 10.1152/ajplegacy.1963.204.2.317. [DOI] [PubMed] [Google Scholar]
- BÖHM M., BRÜCKNER R., HACKBARTH I., HAUBITZ B., LINHART R., MEYER W., SCHMIDT B., SCHMITZ W., SCHOLZ H. Adenosine inhibition of catecholamine-induced increase in force of contraction in guinea-pig atrial and ventricular heart preparations. Evidence against a cyclic AMP- and cyclic GMP-dependent effect. J. Pharmacol. Exp. Ther. 1984;230:483–492. [PubMed] [Google Scholar]
- BÖHM M., BRÜCKNER R., NEUMANN J., SCHMITZ W., SCHOLZ H., STARBATTY J. Role of guanine nucleotide-binding protein in the regulation by adenosine of cardiac potassium conductance and force of contraction. Evaluation with pertussis toxin. Naunyn-Schmiedeberg's Arch. Pharmacol. 1986;332:403–405. doi: 10.1007/BF00500095. [DOI] [PubMed] [Google Scholar]
- BÖHM M., BRÜCKNER R., SCHÄFER H., SCHMITZ W., SCHOLZ H. Inhibition of the effects of adenosine on force of contraction and the slow calcium inward current by pertussis toxin is associated with myocardial lesions. Cardiovasc. Res. 1988;22:87–94. doi: 10.1093/cvr/22.2.87. [DOI] [PubMed] [Google Scholar]
- BÖHM M., MEYER W., MÜGGE A., SCHMITZ W., SCHOLZ H. Functional evidence for the existence of adenosine receptors in the human heart. Eur. J. Pharmacol. 1985;116:323–326. doi: 10.1016/0014-2999(85)90170-0. [DOI] [PubMed] [Google Scholar]
- BÖHM M., PIESKE M., UNGERER M., ERDMANN E. Characterization of A1-adenosine receptors in human atrial and ventricular myocardium from diseased human hearts. Circ. Res. 1989;65:1201–1211. doi: 10.1161/01.res.65.5.1201. [DOI] [PubMed] [Google Scholar]
- BOKNIK P., NEUMANN J., SCHMITZ W., SCHOLZ H., WENZLAFF H. Characterization of biochemical effects of CGS 21680C, an A2-adenosine receptor agonist, in the mammalian ventricle. J. Cardiovasc. Pharmacol. 1997;30:750–758. doi: 10.1097/00005344-199712000-00009. [DOI] [PubMed] [Google Scholar]
- BRÜCKNER R., FENNER A., MEYER W., NOBIS T.M., SCHMITZ W., SCHOLZ H. Cardiac effects of adenosine and adenosine analogs in guinea-pig atrial and ventricular preparations. J. Pharmacol. Exp. Ther. 1985;234:766–774. [PubMed] [Google Scholar]
- DOBSON J.G., JR Adenosine reduces catecholamine contractile responses in oxygenated and hypoxic atria. Am. J. Physiol. 1983;245:H468–H474. doi: 10.1152/ajpheart.1983.245.3.H468. [DOI] [PubMed] [Google Scholar]
- DRURY A.N., SZENT-GYÖRGYI A. The physiological activity of adenine compounds with especial reference to their action upon the mammalian heart. J. Physiol. (Lond.). 1929;68:213–237. doi: 10.1113/jphysiol.1929.sp002608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FENTON R.A., MOORE E.D., FAY F.S., DOBSON J.G., JR Adenosine reduces the Ca2+ transients of isoproterenol-stimulated rat ventricular myocytes. Am. J. Physiol. 1991;261:C1107–1014. doi: 10.1152/ajpcell.1991.261.6.C1107. [DOI] [PubMed] [Google Scholar]
- GAUTHIER N.S., HEADRICK J.P., MATHERNE G.P. Myocardial function in the working mouse heart overexpressing cardiac A1 adenosine receptors. J. Mol. Cell. Cardiol. 1998;30:193–198. doi: 10.1006/jmcc.1997.0585. [DOI] [PubMed] [Google Scholar]
- GAUTHIER N.S., MORRISON R.R., BYFORD A.M., JONES R., HEADRICK J.P., MATHERNE G.P.Functional genomics of transgenic overexpression of A1 adenosine receptors in the heart Drug Devel. Res. 1999. in press
- GEORGE E.E., ROMANO F.D., DOBSON J.G., JR Adenosine and acetylcholine reduce isoproterenol-induced protein phosphorylation of rat myocytes. J. Mol. Cell. Cardiol. 1991;23:749–764. doi: 10.1016/0022-2828(91)90984-t. [DOI] [PubMed] [Google Scholar]
- GUPTA R.C., NEUMANN J., DURANT P., WATANABE A.M. A1-adenosine-receptor mediated inhibition of isoproterenol-stimulated protein phosphorylation in ventricular myocytes. Evidence against a cyclic AMP-dependent effect. Circ. Res. 1993;72:65–74. doi: 10.1161/01.res.72.1.65. [DOI] [PubMed] [Google Scholar]
- HEADRICK J.P., GAUTHIER N.S., BERR S.S., MATHERNE G.P. Transgenic A1 adenosine receptor overexpression improves myocardial energy state during ischemia reperfusion. J. Mol. Cell. Cardiol. 1998;30:1059–1064. doi: 10.1006/jmcc.1998.0672. [DOI] [PubMed] [Google Scholar]
- KURACHI Y., NAKAJIMA T., SUGIMOTO T. On the mechanism of activation of muscarinic potassium channels by adenosine in isolated atrial cells. Pflügers Arch. 1986;407:264–274. doi: 10.1007/BF00585301. [DOI] [PubMed] [Google Scholar]
- LINDEN J., HOLLEN C.E., PATEL A. The mechanism by which adenosine and cholinergic agents reduce contractility in rat myocardium. Correlation with cyclic adenosine monophosphate and receptor densities. Circ. Res. 1985;56:728–735. doi: 10.1161/01.res.56.5.728. [DOI] [PubMed] [Google Scholar]
- MATHERNE G.P., LINDEN J., BYFORD A.M., GAUTHIER N.S., HEADRICK J.P. Transgenic A1 adenosine receptor overexpression increases myocardial resistance to ischemia. Proc. Natl. Acad. Sci., USA. 1997;94:6541–6546. doi: 10.1073/pnas.94.12.6541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NEUMANN J., BOKNIK P., DEPAOLI-ROACH A.A., FIELD L.J., ROCKMAN H.A., KOBAYASHI Y.M., KELLEY J.S., JONES L.R. Targeted overexpression of phospholamban to mouse atrium depresses Ca2+ transport and contractility. J. Mol. Cell. Cardiol. 1998;30:1991–2002. doi: 10.1006/jmcc.1998.0760. [DOI] [PubMed] [Google Scholar]
- RALEVIC V., BURNSTOCK G. Receptors for purines and pyrimidines. Pharmacol. Rev. 1998;50:415–492. [PubMed] [Google Scholar]
- REPPERT S.M., WEAVER D.R., STEHLE J.H., RIVKEES S.A. Molecular cloning and characterization of a rat A1-adenosine receptor that is widely expressed in brain and spinal cord. Mol. Endocrinol. 1991;5:1037–1048. doi: 10.1210/mend-5-8-1037. [DOI] [PubMed] [Google Scholar]
- ROCKMAN H.A., HAMILTON R., JONES L.R., MILANO C.A., MAO L., LEFKOWITZ R.J. Enhanced myocardial relaxation in vivo in transgenic mice overexpressing the β2-adrenergic receptor is associated with reduced phospholamban protein. J. Clin. Invest. 1996;97:1618–1623. doi: 10.1172/JCI118587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SALVATORE C.A., JACOBSON M.A., TAYLOR H.E., LINDEN J., JOHNSON R.G. Molecular cloning and characterization of the human A3 adenosine receptor. Proc. Natl. Acad. Sci. USA. 1993;90:10365–10369. doi: 10.1073/pnas.90.21.10365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SCHRADER J., BAUMANN G., GERLACH E. Adenosine as inhibitor of myocardial effects of catecholamines. Pflügers Arch. 1977;372:29–35. doi: 10.1007/BF00582203. [DOI] [PubMed] [Google Scholar]
- SHRYOCK J.C., BELARDINELLI L. Adenosine and adenosine receptors in the cardiovascular system: biochemistry, physiology, and pharmacology. Am. J. Cardiol. 1997;79:2–10. doi: 10.1016/s0002-9149(97)00256-7. [DOI] [PubMed] [Google Scholar]
- SONG Y., BELARDINELLI L. Electrophysiological and functional effects of adenosine on ventricular myocytes of various mammalian species. Am. J. Physiol. 1996;271:C1233–C1243. doi: 10.1152/ajpcell.1996.271.4.C1233. [DOI] [PubMed] [Google Scholar]
- VON DER LEYEN H., SCHMITZ W., SCHOLZ H., SCHOLZ J., LOHSE M.J., SCHWABE U. Effects of 1,3-dipropyl-8-cyclopentylxanthine (DPCPX), a highly selective adenosine receptor antagonist, on force of contraction in guinea-pig atrial and ventricular preparations. Naunyn-Schmiedeberg's Arch. Pharmacol. 1989;340:204–209. doi: 10.1007/BF00168970. [DOI] [PubMed] [Google Scholar]
- XU H., STEIN B., LIANG B.T. Characterization of a stimulatory adenosine A2a receptor in adult rat ventricular myocyte. Am. J. Physiol. 1996;39:H1655–H1661. doi: 10.1152/ajpheart.1996.270.5.H1655. [DOI] [PubMed] [Google Scholar]


