Abstract
5-HT and the prostanoid TP receptor agonists, U46619 and I-BOP, constricted the human umbilical artery with pEC50 values of 7.3±0.2, 6.7±0.1, and 7.3±0.2, respectively. The selective TP receptor antagonist, GR32191 (0.1 μM), shifted the concentration-effect curves to U46619 and I-BOP to the right, but had no effect on the response to 5-HT.
The natural prostaglandins, PGF2α and PGE2, caused concentration-dependent contraction with pEC50 values of 5.2±0.2 and 4.9±0.2, respectively. PGD2 was a partial agonist with a pEC50 of 5.24±0.03. GR32191 (0.1 μM) inhibited the responses to all of these compounds suggesting that they produce contraction by acting at TP receptors.
Sulprostone failed to elicit contraction in the human umbilical artery at concentrations up to 4.4 μM suggesting the absence of EP1 and EP3 receptors. Despite this, 17-phenyltrinor PGE2 and GR63799 both induced contraction at concentrations above 1 μM, but the effects were sensitive to GR32191 (0.1 μM).
Fluprostenol had no effect on the human umbilical artery at concentrations up to 17 μM suggesting the absence of FP receptors. Cloprostenol was ineffective in two tissues, but caused contraction in one tissue at the highest concentration tested (1.7 μM). However, this response was abolished in the presence of GR32191 (0.1 μM).
The effects of four TP receptor antagonists were assessed by global non-linear regression analysis. GR32191, SQ29548, SQ30741, and ICI192605 competitively inhibited responses to U46619 with pKb values of 8.0±0.1, 7.6±0.1, 7.0±0.2 and 8.1±0.1, respectively.
These results suggest that the human umbilical artery functionally expresses TP receptors, but not EP1, EP2 or FP receptors.
Keywords: Human umbilical artery, contraction, competitive antagonism, TP receptors, U46619
Introduction
The human isolated umbilical artery (HUA) contracts in response to prostaglandin (PG) E2 and PGF2α (Altura et al., 1972; Starling & Elliott, 1974; Tuvemo, 1978) and to the stable thromboxane A2 (TxA2)-mimetic, U46619 (Templeton et al., 1991; Toyofuku et al., 1995). While the effect of U46619 appears to be mediated via prostanoid TP receptors, since it is blocked by a selective TP receptor antagonist (Templeton et al., 1991; Crichton et al., 1993), the site of action of the natural prostanoids is unknown.
Five major types of prostanoid receptors are currently recognized and named DP, EP, FP, IP and TP according to which of the natural prostanoids (PGD2, PGE2, PGF2α, PGI2 or TxA2) is most potent (Coleman et al., 1994). EP receptors have been further subdivided into four subtypes named EP1, EP2, EP3 and EP4 (Coleman et al., 1994). Of these receptors, EP1, EP3, FP and TP generally mediate contraction in smooth muscle while DP, EP2, EP4 and IP predominantly mediate relaxation (Coleman et al., 1994). Thus, contraction of the HUA by PGE2 and PGF2α may indicate a role for EP1, EP3 and FP receptors in regulation of the umbilical circulation. However, the natural prostanoids are quite promiscuous in their activities, and no firm conclusions can be drawn about prostanoid receptor status without the use of appropriate, selective prostanoid receptor agonists and antagonists (Baxter et al., 1995).
In the present study we have characterized the excitatory prostanoid receptors in the HUA using both natural and synthetic prostanoid receptor agonists and a number of selective TP receptor antagonists. Some of these data have been communicated to the British Pharmacological Society (Boersma et al., 1997).
Methods
Tissue collection and preparation
Sections of umbilical cords within 20 cm of the placenta were collected from full-term vaginal or Caesarean births in cold buffered physiological salt solution (BPSS). Tissues were stored at 4°C and used within 24 h. Human umbilical arteries were dissected free of Wharton's Jelly and cut into transverse rings 3–5 mm in length. Endothelial cells were mechanically removed and removal of the cells was confirmed by histology.
Isometric contractions
Rings were suspended on stainless-steel hooks and mounted in individual 5, 10 or 15 ml jacketed muscle baths containing oxygenated (95% O2 and 5% CO2) physiological salt solution (PSS) at 37°C. One hook was anchored in the bath while the other was attached with silk thread to a FT-03 force displacement transducer writing to a 7D 8-channel polygraph (Grass Instruments). A resting tension of 30 mN was applied to each tissue ring. Individual rings were washed and allowed to equilibrate for 3 h under these conditions during which time spontaneous tone developed. Tissues were then challenged with 60 mM KCl. Once the maximum response to the KCl challenge was achieved, tissues were washed three times and allowed to equilibrate for 20 min to allow baseline to be reached again. The KCl challenge was performed a total of three times.
Agonist potency
Eighty minutes after the last KCl challenge had been washed out, concentration-effect experiments were performed by cumulative addition of agonists to produce approximately half log unit increases in the bath concentration per addition. When the response to the last agonist addition had reached a plateau, the PSS was washed from the bath and replaced with deionized water in order to induce a hypotonic shock. The contraction produced by the hypotonic shock was used to normalize all drug-induced responses (Boersma et al., 1997).
Concentration-effect curves were constructed from the data obtained by fitting a form of the logistic equation:
where E is the effect of the agonist, Emin is the effect in the absence of agonist, Emax is the maximum agonist-induced effect, C is the molar concentration of the agonist, nH is the Hill coefficient and pEC50 is the negative log of the molar concentration of the agonist that produces a half-maximal response. In experiments where antagonists were used to verify the selectivity of the response, they were added to the bath 60 min before the start of the concentration-effect experiment.
TP receptor antagonist activity
Because of the difficulty in completely washing out responses to high concentrations of U46619, only one concentration-effect experiment could be performed reliably on each tissue ring. Therefore, global non-linear regression analysis (Lew & Angus, 1995) which does not require that concentration-effect curves using different antagonist concentrations be obtained from the same tissue rings, was employed to analyse antagonists' effects. Separate rings from the same artery were incubated in the absence or in the presence of antagonist for 1 h prior to and throughout the duration of an agonist concentration-effect experiment. Concentration-effect parameters were calculated as described above.
The pEC50 values for U46619 in the absence and in the presence of various concentrations of antagonist were plotted against the molar concentration of antagonist (linear scale) and fit by non-linear regression to the equation:
where [B] is the molar concentration of the antagonist and −logc is a constant equal to the difference between the antagonist pKb and the agonist pEC50 in the absence of antagonist. Deviations from simple competitive antagonism were assessed using the ‘power departure' equation:
and the ‘quadratic departure' equation:
as described by Lew & Angus (1995).
Effects of drugs on stable contractions
Stable contractions were obtained to either U46619 (1 or 3 μM) or KCl (60 mM). Responses were allowed 30 min to equilibrate. Thereafter, putative inhibitory compounds were added cumulatively as described for agonist potency experiments.
Drugs and chemicals
U46619 (9,11-dideoxy-9α,11α-methanoepoxy prostaglandin F2α), I-BOP ([1S[1α,2α(Z),3β(1E,3S*),4α)]]-7-[3-[3-hydroxy-4-(4-iodophenoxy) -1-butenyl] - 7-oxabicyclo - [2.2.1]hept - 2 -yl]5-heptanoic acid), PGD2, PGE2, 17-phenyltrinor PGE2, PGF2α, and fluprostenol were obtained from Cayman Chemical (Ann Arbor, MI, U.S.A.). Cloprostenol was purchased from Coopers Agropharm (Ajax, ON, Canada). Indomethacin and 5-hydroxytryptamine (5-HT) were obtained from Sigma (Oakville, ON, Canada). The following compounds were received as gifts: sulprostone and cicaprost from Schering (Berlin, Germany); GR32191 ([1R-[1α(Z),2β,3β,5α]]-(+)-7-[5-[[(1,1′-biphenyl) - 4 - yl]methoxy] - 3 - hydroxy-2-(1-piperidinyl)cyclopentyl] - 4 - heptenoic acid) and GR63799X [1R-[1α(Z),2β(R*),3α]]-4-(benzoylamino)phenyl 7-[3-hydroxy-2-(2-hydroxy-3-phenoxypropoxy)-5-oxocyclopentyl] - 4- heptenoate from Glaxo-Wellcome (Stevenage, U.K.); BW245C (5-(6-carboxyhexyl) - 1 - (3-cyclohexyl - 3 - hydroxypropyl)hydantoin) from Wellcome (Beckenham, U.K.); ICI192605 (4(Z)-6-[(2,4,5 cis)2-(2-chlorophenyl)-4-(2-hydroxy phenyl)1,3-dioxan-5-yl]hexenoic acid) from Zeneca (Alderley Park, U.K.); SQ29548 ([1S-(1α,2β(5Z),3β,4α]]-7-[3-[[2-[(phenylamino)carbonyl]hydrazino]methyl]-7-oxabicyclo[2.2.1]hept-2-yl]-5-heptenoic acid) and SQ30741 ([1S-(1α,2α (Z),3α,4α]-7-[3-[[[[(1-Oxoheptyl)amino]acetyl]amino]methyl]-7-oxabicyclo[2.2.1]hept-2-yl]-5-heptenoic acid) from the Squibb Institute for Medical Research (Princeton, NJ, U.S.A.). All other chemicals were from BDH (Toronto, ON, Canada). Cloprostenol came as a solution in isotonic citrate buffer while sulprostone was in ethyl acetate. Indomethacin was prepared as described by Curry et al. (1982). A stock solution of 5-HT was prepared in double distilled water. All other drugs were made as solutions in ethanol. Immediately before experiments, appropriate serial dilutions of drugs were made in double distilled water from concentrated stock solutions.
Solutions
The buffered saline had the following composition (mM): N-2-hydroxyethylpiperazine-N′-2-ethanesulphonic acid 5.0, NaCl 150, KCl 4.6, MgSO4 1.2, CaCl2 2.5, glucose 11.1, and indomethacin 0.01, pH 7.4. The PSS was composed as follows (mM): KCl 4.6, MgSO4 1.16, NaH2PO4 1.16, CaCl2 2.5, NaCl 115.5, NaHCO3 21.9, glucose 11.1 and indomethacin 0.03.
Statistics
All data are expressed as means±s.e.mean. Slopes and maxima of the agonist concentration-effect curves in the presence and absence of antagonist were compared using a one-way ANOVA to check for parallelism. The goodness-of-fit among the simple (equation 2), ‘power departure' (equation 3) and ‘quadratic departure' (equation 4) forms of the global non-linear regression equation were compared using the F-test as described by Lew & Angus (1995). In all cases, values of P<0.05 were considered significant.
Results
U46619 caused concentration-dependent contraction of HUA. Contractions were slow to develop and tonic in nature at low agonist concentrations, with relatively slow phasic contractions often superimposed on the tonic background at higher concentrations. 5-HT and the TP receptor selective agonist, I-BOP, were also potent constrictors of HUA. Responses to U46619 and I-BOP were blocked by the selective TP receptor antagonist GR32191 (0.1 μM), but the 5-HT response was not GR32191-sensitive. Sample traces appear in Figure 1 showing the effect of U46619 alone (Figure 1a) and in the presence of 0.1 μM GR32191 (Figure 1b). Mean concentration-effect curves are given in Figure 2 and concentration-effect parameters are shown in Table 1.
Figure 1.
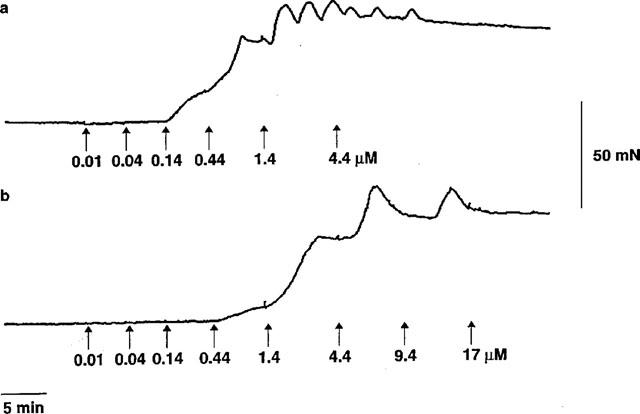
Sample traces showing the effect of cumulative addition of U46619 on tension development by rings of human umbilical artery in the absence (a) and presence (b) of 0.1 μM GR32191.
Figure 2.
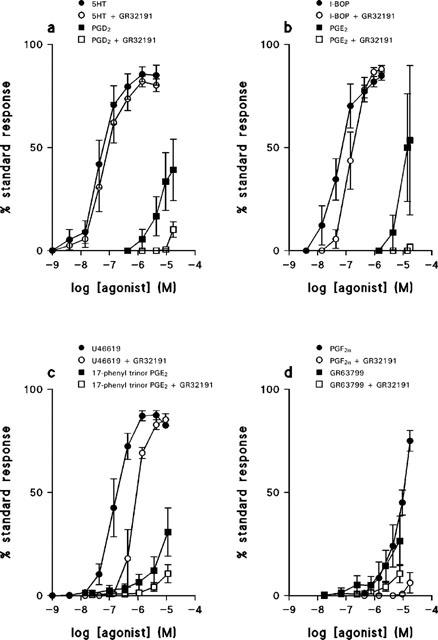
Mean concentration-effect curves for contractile agonists on tension development by rings of human umbilical artery in the absence and presence of GR32191, 0.1 μM. (a) 5-HT, n=6; PGD2, n=3; (b) I-BOP, n=3; PGE2, n=3; (c) U46619, n=5; 17-phenyltrinor PGE2, n=3; (d) PGF2α, n=4; GR63799, n=3. Values are means±s.e.mean.
Table 1.
Comparison of concentration-effect parameters for various agonists in the presence and absence of GR32191
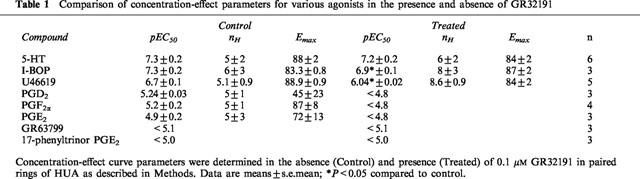
The natural prostaglandins, PGE2 and PGF2α, were low-potency contractile agonists, PGD2 was also of low potency and did not achieve the same maximum response as the TP receptor selective compounds. The FP receptor selective agonist, fluprostenol, had no effect on the HUA at concentrations up to 17 μM (n=3) whereas another FP receptor selective agonist, cloprostenol, was ineffective at concentrations up to 1.7 μM in two preparations, but caused a contraction at 1.7 μM in a third preparation. The EP1/EP3 receptor selective agonist, sulprostone, was ineffective at concentrations up to 4.4 μM (n=3). Both the EP1 receptor selective 17-phenyltrinor PGE2 and the EP3 receptor selective GR63799 produced contractions with a very low potency, maximum response was not attained over the concentration range used. Responses to the natural prostaglandins, cloprostenol, 17-phenyltrinor PGE2, and GR63799 were all GR32191-sensitive. (Figure 2, Table 1).
Stable contractions of HUA to U46619 or KCl were not affected in any way by cumulative addition of PGE2 up to 42 μM, cicaprost up to 0.3 μM, or BW245C up to 42 μM.
Four TP receptor selective antagonists, GR32191, SQ29548, SQ30741 and ICI192605 produced progressive rightward shifts in the concentration-effect curves to U46619 (Figure 3). In no case did any antagonist significantly affect either the slope or maximum of the concentration-effect curve. The effect of the concentration of each antagonist on the pEC50 for U46619 is shown in Figure 4. For all four antagonists the data shown in Figure 4 were best fit by the simple form of the global non-linear regression equation (equation 1), from which the following pKb values were determined: GR32191, 8.0±0.1; SQ29548, 7.6±0.1; SQ30741, 7.0±0.2; ICI192605, 8.1±0.1.
Figure 3.
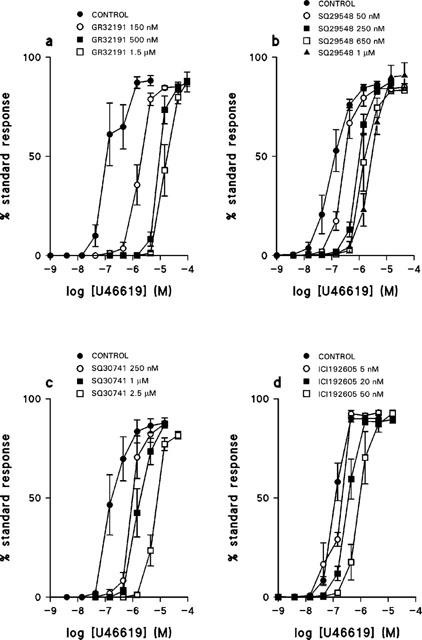
The effect of TP receptor antagonists on the response of human umbilical artery to U46619. Mean concentration-effect curves for U46619 in the absence and presence of (a) GR32191 150 nM, 500 nM, 1.5 μM, n=4–5; (b) SQ29548 50 nM, 250 nM, 650 nM, 1 μM, n=6–9; (c) SQ30741 250 nM, 1 μM, 2.5 μM, n=4–6; (d) ICI192605 5 nM, 20 nM, 50 nM, n=4–6. Values are means±s.e.mean.
Figure 4.
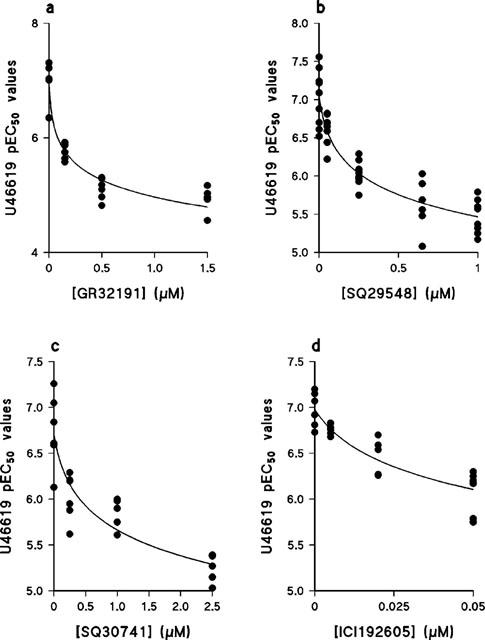
The effect of TP receptor selective antagonists on the pEC50 value of U46619 in human umbilical artery. Values were obtained from the data shown in Figure 3. The lines show the best fit of the data to equation 2, from which the pKb values given in the text were determined. (a) GR32191, n=4–5; (b) SQ29548, n=6–9; (c) SQ30741, n=4–6; (d) ICI192605, n=4–6.
Discussion
Conditions of high oxygen tension were used in the present study so that the results obtained could be directly compared to similar studies in other human vascular smooth muscles such as uterine artery (Baxter et al., 1995) and pulmonary artery (Qian et al., 1994). Under these conditions HUA spontaneously generates TxA2 (Templeton et al., 1991). This spontaneously generated TxA2 is responsible for the oxygen-induced contraction of HUA, and also potentiates the constrictor response to some exogenous agonists, including 5-HT (Templeton et al., 1991). In order to determine accurately the potency of contractile agonists, we had to ensure that there was no potentiation by endogenous TxA2 in the present experiments. Indomethacin (30 μM) was included in our incubation medium to prevent both the oxygen-induced generation of TxA2 and the release of prostanoids by other agonists. The lack of a significant effect of the TP receptor-selective antagonist GR32191 (Lumley et al., 1989) on the responses to 5-HT, while it did antagonize similar responses to U46619 (Figure 2, Table 1), demonstrates that interference by endogenous TxA2 was effectively eliminated in our experiments.
Wylam et al. (1993) used oxygen tension and indomethacin concentrations very similar to ours, and reported a pEC50 value for 5-HT of 6.6 in HUA, this value compares favourably to ours (Table 1). Templeton et al. (1991) used conditions of low oxygen tension without indomethacin, and reported a pEC50 value for U46619 of 8.5 in HUA, this value is considerably lower than ours (Table 1). We cannot explain the increased potency of U46619 at low oxygen tension, but it is unlikely to result from potentiation of the response by agonist-induced TxA2 release since responses to U46619 at low oxygen tension were unaffected by a thromboxane synthase inhibitor (Templeton et al., 1991). The experiments of the present study also differed from those of Templeton et al. (1991) by the absence of endothelium in the former case and its presence in the latter.
The relatively high potency of U46619, and its sensitivity to GR32191 (Figure 2, Table 1) support the presence of excitatory TP receptors in HUA (Templeton et al., 1991), and this argument is given further weight by the GR32191-sensitive response to another TP receptor-selective agonist, I-BOP (Coleman et al., 1994) (Figure 2, Table 1). The potency ratio of the TP receptor agonists (EC50 U46619/EC50 I-BOP) in HUA (4) is similar to the value of 6.3 found in human myometrium (Senchyna & Crankshaw, 1996), but less than reported from human saphenous vein and human platelets (Dorn, 1991) where the values were 19 and 17, respectively. Evidence for differences in potency ratios for TP receptor agonists between different human tissue preparations has been used to support an argument for the existence of TP receptor subtypes (Krauss et al., 1996), and may do so in this case. However, definitive experiments comparing pKb values for a range of antagonists in a range of tissues have not been reported.
Constrictor responses of HUA to PGE2 and PGF2α (Figure 2, Table 1) confirm earlier reports (Altura et al., 1972; Starling & Elliott, 1974; Tuvemo, 1978), but the low potencies of these compounds and their sensitivities to GR32191 suggest that their action might be mediated via TP receptors. A similar conclusion also appears appropriate for PGD2, which was a partial agonist in the present study. The lack of significant effects of the FP receptor agonists fluprostenol and cloprostenol (Coleman et al., 1994) over the range of concentrations that we tested confirms the absence of a significant population of functional FP receptors in HUA and strengthens the claim that PGF2α's effects are TP receptor-mediated. Similarly, the lack of effect of sulprostone suggests that neither EP3 nor EP1 receptors are functionally present. Both the EP1 receptor-selective agonist 17-phenyltrinor PGE2 (Coleman et al., 1994) and the EP3 receptor selective agonist GR63799 (Coleman et al., 1994) had constrictor effects, but these were of very low potency, and were sensitive to GR32191 (Figure 2, Table 1). In the human isolated pulmonary artery, which does appear to express functional EP3 receptors, sulprostone and GR63799 are full agonists with pEC50 values of 8.3 and 7.1, respectively (Qian et al., 1994). The response of the human isolated pulmonary artery to sulprostone is insensitive to TP receptor antagonists (Qian et al., 1994). We therefore conclude that our data do indeed support the absence of functional EP1 and EP3 receptors from HUA. The constrictor effects of PGE2, 17-phenyltrinor PGE2 and GR63799 must therefore also be ascribed to TP receptors.
In the human isolated uterine artery PGE2, PGF2α, and PGD2 are also of low potency and GR32191-sensitive (Baxter et al., 1995), but in that preparation PGD2 is a full agonist. The partial agonism of PGD2 in HUA might be a consequence of a lower density of TP receptors in HUA compared to human uterine artery. Such a notion is supported by the 14 fold higher potency of U46619 in human uterine artery than in HUA.
Failure of BW245C, PGE2, and cicaprost to reverse U46619 or KCl-induced contractions argues against the operational expression of DP, EP2, EP4 or IP receptors under the conditions of our experiment. It is, therefore, unlikely that activation of inhibitory prostanoid receptors contributed to any of the effects seen in the present study. In other work, PGI2 was able to partially relax endothelium-denuded HUA with a very low potency (Chaudhuri et al., 1993). This suggests that operational expression of IP receptors in HUA is present, but at a very low level. The work of Chaudhuri et al. (1993) used rings of HUA taken from the middle section of the cord whereas the present study used rings from the placental end. Others have reported differences in operational expression of prostanoid receptors along the length of the umbilical artery (Duckworth et al., 1998).
The effects of all four antagonists on concentration-effect curves to U46619 are consistent with surmountable, competitive antagonism at a single site in HUA. These compounds are all recognized as competitive TP receptor antagonists (Ogletree et al., 1985; 1986; Jessup et al., 1988; Lumley et al., 1989). The absence of deviation from simple competitive behaviour by any of the antagonists tested argues against simultaneous operational expression of more than one subtype of TP receptor in HUA. The order of antagonist potency, ICI192605>GR32191>SQ29548 found in the present study agrees with that found in human myometrium (Senchyna & Crankshaw, 1996), using a different technique, although the pKb values are slightly lower in the present study. A pKb value for SQ30741 in human tissue has not been reported previously, and although cross-species comparisons may be misleading, its lower potency than SQ29548 in the present study is consistent with results from rat and guinea-pig smooth muscles (Ogletree & Allen, 1991). Taken together, the antagonist data reported here add further support to the contention that U46619 produces constriction of HUA by action at TP receptors. There has been a suggestion of the existence of a distinct receptor for isoprostanes that is substantially ‘TP-like' in its operational properties (Fukunaga et al., 1993), in a subsequent paper we investigate the interactions of isoprostanes with the HUA (Oliveira et al., 1999).
In summary, under conditions of high oxygen tension, the HUA functionally expresses only one type of excitatory prostanoid receptor, namely the TP receptor. Although several natural and synthetic prostanoids are capable of constricting the HUA, they all produce their effects via TP receptors.
Acknowledgments
We thank the Labour & Delivery staff at Chedoke McMaster Hospital for helping us to collect umbilical cords. We are grateful to all those who provided drugs used in this study. The Medical Research Council of Canada supported this work.
Abbreviations
- BPSS
buffered physiological salt solution
- HUA
human umbilical artery
- PG
prostaglandin
- PSS
physiological salt solution
- TxA2
thromboxane A2
References
- ALTURA B.M., MALAVIYA D., REICH C.F., ORKIN L.R. Effects of vasoactive agents on isolated human umbilical arteries and veins. Am. J. Physiol. 1972;222:345–355. doi: 10.1152/ajplegacy.1972.222.2.345. [DOI] [PubMed] [Google Scholar]
- BAXTER G.S., CLAYTON J.K., COLEMAN R.A., MARSHALL K., SANGHA R., SENIOR J. Characterization of the prostanoid receptors mediating constriction and relaxation of human isolated uterine artery. Br. J. Pharmacol. 1995;116:1692–1696. doi: 10.1111/j.1476-5381.1995.tb16393.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BOERSMA J.I., JANZEN K.M., CRANKSHAW D.J. Characterization of excitatory prostanoid receptors in the human umbilical artery in vitro. Br. J. Pharmacol. 1997;122:135P. doi: 10.1038/sj.bjp.0702965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CHAUDHURI G., CUEVAS J., BUGA G.M., IGNARRO L.J. NO is more important than PGI2 in maintaining low vascular tone in feto- placental vessels. Am. J. Physiol. 1993;265:H2036–H2043. doi: 10.1152/ajpheart.1993.265.6.H2036. [DOI] [PubMed] [Google Scholar]
- COLEMAN R.A., SMITH W.L., NARUMIYA S. International Union of Pharmacology classification of prostanoid receptors: properties, distribution, and structure of the receptors and their subtypes. Pharmacol. Rev. 1994;46:205–229. [PubMed] [Google Scholar]
- CRICHTON C.A., TEMPLETON A.G.B., MCGRATH J.C., SMITH G.L. Thromboxane A2 analogue, U46619, potentiates calcium-activated force in human umbilical artery. Am. J. Physiol. 1993;264:H1878–H1883. doi: 10.1152/ajpheart.1993.264.6.H1878. [DOI] [PubMed] [Google Scholar]
- CURRY S.H., BROWN E.A., KUCK H., CASSIN S. Preparation and stability of indomethacin solutions. Can. J. Physiol. Pharmacol. 1982;60:988–992. doi: 10.1139/y82-139. [DOI] [PubMed] [Google Scholar]
- DORN G.W., II Tissue- and species-specific differences in ligand binding to thromboxane A2 receptors. Am. J. Physiol. 1991;261:R145–R153. doi: 10.1152/ajpregu.1991.261.1.R145. [DOI] [PubMed] [Google Scholar]
- DUCKWORTH N., MARSHALL K., SENIOR J., CLAYTON J.K. A preliminary study of the TP-receptor population along the length of the human isolated umbilical artery. Br. J. Pharmacol. 1998;125:98P. [Google Scholar]
- FUKUNAGA M., MAKITA N., ROBERTS L.J., II, MORROW J.D., TAKAHASHI K., BADR K.F. Evidence for the existence of F2-isoprostane receptors on rat vascular smooth muscle cells. Am. J. Physiol. 1993;264:C1619–C1624. doi: 10.1152/ajpcell.1993.264.6.C1619. [DOI] [PubMed] [Google Scholar]
- JESSUP C.L., JESSUP R., WAYNE M. The effects of ICI 192,605, a selective thromboxane A2 receptor antagonist, on platelets. Br. J. Pharmacol. 1988;95:676P. [Google Scholar]
- KRAUSS A.H.P., WOODWARD D.F., GIBSON L.L., PROTZMAN C.E., WILLIAMS L.S., BURK R.M., GAC T.S., ROOF M.B., ABBAS F., MARSHALL K., SENIOR J. Evidence for human thromboxane receptor heterogeneity using a novel series of 9,11-cyclic carbonate derivatives of prostaglandin F2α. Br. J. Pharmacol. 1996;117:1171–1180. doi: 10.1111/j.1476-5381.1996.tb16712.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LEW M.J., ANGUS J.A. Analysis of competitive agonist-antagonist interactions by nonlinear regression. Trends Pharmacol. Sci. 1995;16:328–337. doi: 10.1016/s0165-6147(00)89066-5. [DOI] [PubMed] [Google Scholar]
- LUMLEY P., WHITE B.P., HUMPHREY P.P.A. GR32191, a highly potent and specific thromboxane A2 receptor blocking drug on platelets and vascular and airways smooth muscle in vitro. Br. J. Pharmacol. 1989;97:783–794. doi: 10.1111/j.1476-5381.1989.tb12017.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- OGLETREE M.L., ALLEN G.T. Interspecies differences in thromboxane receptors: Studies with thromboxane receptor antagonists in rat and guinea-pig smooth muscles. J. Pharmacol. Exp. Ther. 1991;260:789–794. [PubMed] [Google Scholar]
- OGLETREE M.L., HARRIS D.N., GREENBERG R., HASLANGER M.F., NAKANE M. Pharmacological actions of SQ 29,548, a novel selective thromboxane antagonist. J. Pharmacol. Exp. Ther. 1985;234:435–441. [PubMed] [Google Scholar]
- OGLETREE M.L., HARRIS D.N., HEDBERG A., NAKANE M., REID S.J. SQ 30,471, a selective TXA2-receptor antagonist in vitro. Pharmacologist. 1986;28:186. [Google Scholar]
- OLIVEIRA L., STALLWOOD N., CRANKSHAW D.J.Effects of some isoprostanes on the human umbilical artery in vitro Br. J. Pharmacol. 1999(in press) [DOI] [PMC free article] [PubMed]
- QIAN Y.M., JONES R.L., CHAN K.M., STOCK A.I., HO J.K. Potent contractile actions of prostanoid EP3-receptor agonists on human isolated pulmonary artery. Br. J. Pharmacol. 1994;113:369–374. doi: 10.1111/j.1476-5381.1994.tb16997.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SENCHYNA M., CRANKSHAW D.J. Characterization of the prostanoid TP receptor population in human nonpregnant myometrium. J. Pharmacol. Exp. Ther. 1996;279:262–270. [PubMed] [Google Scholar]
- STARLING M.B., ELLIOTT R.B. The effects of prostaglandins, prostaglandin inhibitors, and oxygen on the closure of the ductus arteriosus, pulmonary arteries and umbilical vessels in vitro. Prostaglandins. 1974;8:187–203. doi: 10.1016/0090-6980(74)90042-2. [DOI] [PubMed] [Google Scholar]
- TEMPLETON A.G.B., MCGRATH J.C., WHITTLE M.J. The role of endogenous thromboxane in contractions to U46619, oxygen, 5-HT and 5-CT in the human isolated umbilical artery. Br. J. Pharmacol. 1991;103:1079–1084. doi: 10.1111/j.1476-5381.1991.tb12303.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TOYOFUKU K., NISHIMURA J., KOBAYASHI S., NAKANO H., KANAIDE H. Effects of U46619 on intracellular Ca++ concentration and tension in human umbilical artery. Am. J. Obstet. Gynecol. 1995;172:1414–1421. doi: 10.1016/0002-9378(95)90471-9. [DOI] [PubMed] [Google Scholar]
- TUVEMO T. Action of prostaglandins and blockers of prostaglandin synthesis on the isolated human umbilical artery. Adv. Prostaglandin Thromboxane Res. 1978;4:271–274. [PubMed] [Google Scholar]
- WYLAM M.E., SAMSEL R.W., SCHUMACKER P.T., UMANS J.G. Extracellular calcium and intrinsic tone in the human umbilical artery. J. Pharmacol. Exp. Ther. 1993;266:1475–1481. [PubMed] [Google Scholar]


