Abstract
We investigated the effects of bepridil on the two components of the delayed rectifier K+ current, i.e., the rapidly activating (IKr) and the slowly activating (IKs) currents using tight-seal whole-cell patch-clamp techniques in guinea-pig ventricular myocytes, under blockade of L-type Ca2+ current with nitrendipine (5 μM) or D600 (1 μM).
Bepridil decreased IKs under blockade of IKr with E4031 (5 μM), in a concentration-dependent manner. The concentration-dependent inhibition of IKs by bepridil was fitted by a curve, assuming one-to-one interactions between the channel and the drug molecule. The concentration of half-maximal inhibition (IC50) was found to be 6.2 μM.
The effect of bepridil on IKr was assessed using an envelope-of-tails test. In the control condition, a ratio of the tail current to the time-dependent current measured during depolarization was large (>1) at shorter pulses (<200 ms), and it decreased to a steady state value of ∼0.4 with increases in the pulse duration. Bepridil at a concentration of 2 μM did not decrease this ratio at shorter pulses.
In a short-pulse (duration=50 ms) experiment that largely activates IKr, the drug was found to block IKr in a cooperative manner (Hill coefficient=3.03) and the IC50 was 13.2 μM.
These results suggest that bepridil at a clinical therapeutic concentration (∼2 μM) selectively blocks IKs but does not inhibit IKr. This may relate to the characteristic frequency-dependent effects of bepridil on the action potential duration (APD), e.g., the non-reverse use-dependent prolongation of APD.
Keywords: Bepridil, IKr, IKs, antiarrhythmic drug, ventricular myocytes
Introduction
Bepridil is a diarylaminopropylamine derivative with both anti-anginal and anti-arrhythmic effects: it dilates coronary vessels, limits consumption of oxygen, decreases the heart rate and prevents arrhythmias (Cosnier et al., 1977; Duchene-Marullaz et al., 1983; Pelleg et al., 1985; Marshall et al., 1983). Bepridil blocks the TTX-sensitive Na+ current (Yatani et al., 1986; Nawada et al., 1995; Sato et al., 1996), the T-type Ca2+ current (Cohen et al., 1992), and the L-type Ca2+ current (Yatani et al., 1986). It has also been shown that bepridil blocks the outward K+ currents including the inward rectifier (IK1) and delayed rectifier K+ current (IK) in sheep cardiac Purkinje fibres (Berger et al., 1989). The effects of bepridil on the action potential duration (APD) differ depending on species and preparations. Bepridil shortened the APD in rabbit ventricular myocardium (Anno et al., 1984; Gill et al., 1992), and in guinea-pig ventricular myocytes (Yatani et al., 1986; Nawada et al., 1995). In contrast, it prolonged the APD and the effective refractory period of the ventricular muscle in canine hearts (Kato & Singh, 1986). This difference may be related to the fact that the APD is determined by a critical balance between inward and outward ionic currents, both of which are affected by bepridil. Another possibility would be differential effects of this drug on outward K+ currents. The IK in mammalian ventricles consists of at least two different K+ channels, the rapidly activating IKr and the slowly activating IKs channels (Sanguinetti & Jurkiewicz, 1990). Each component has a distinct physiological role and expression levels differ from one species to another (Nair & Grant, 1997). If bepridil selectively suppresses either IKs or IKr, this may cause the differential effects on the APD, especially at different stimulation frequencies. Thus, the aim of the present study is to elucidate the effects of bepridil on the two components of IK, namely IKr and IKs, which are known to exist in guinea-pig ventricular myocytes. A part of this study has appeared elsewhere in abstract form (Wang et al., 1998).
Methods
Single ventricular myocytes were isolated from guinea-pig hearts using an enzymatic dissociation procedure described previously (Wang et al., 1996). The dissociated myocytes were allowed to settle in a chamber on an X-Y stage of an inverted microscope (TMD, Nikon, Tokyo, Japan). The cells were superfused with an external bathing solution containing (in mM): NaCl 137, KCl 5.4, CaCl2 1.8, NaH2PO4 0.16, NaHCO3 3, N-[2-hydroxyethyl] piperazine-N′-[2-ethanesulfonic acid] (HEPES) 5, glucose 5.5, (pH 7.4). All the experiments were performed at 35±0.5°C.
The whole-cell patch-clamp technique (Marty & Neher, 1983) was used to record the transmembrane ionic current by using a patch-clamp amplifier (CEZ-2100, Nihon Kohden, Japan). Patch pipettes were fabricated from borosilicate capillary-glass tubes (Narishige, Tokyo, Japan) using a puller (P-97, Sutter Instrument Co., Novato, CA, U.S.A.) and a heat-polisher (MF-83, Narishige, Tokyo, Japan). The electrodes were filled with an internal solution containing (in mM): KCl 140, EGTA 11, CaCl2 1, MgCl2 2, HEPES 10, ATP 5, CP 5, pH 7.2 by KOH. The tip resistances ranged from 1 to 3 MΩ. All experiments were performed in accordance with the Guidelines and Principles for Animal Experiments stipulated by the Animal Ethics Committee of the Oita Medical University and the Physiological Society of Japan.
Bepridil hydrochloride was a gift from Sankyo Pharmaceutical Co. (Tokyo, Japan); E4031 from Eisai Pharmaceutical Co. (Tokyo, Japan); and D600 and chromanol 293B from Hoechst Marion Roussel Deutschland GmbH (Frankfurt, Germany). Nitrendipine was purchased from Sigma Co. (St. Louis, MO, U.S.A.). Bepridil, nitrendipine and chromanol 293B were dissolved in dimethylsulphoxide (DMSO) to make the stock solution at concentrations of 10, 10 and 50 mM, respectively. E4031 was dissolved in distilled water at a concentration of 1 mM. D600 was dissolved in ethanol at a concentration of 1 mM. Each drug was stored at 4°C and diluted to desired final concentrations with Tyrode's solution just before use. The effect of each drug was assessed 4–5 min after application of the test solution.
The amplitude of IK tail current (IKtail) was defined as a difference current between the holding current recorded just before initiation of depolarizing pulse and the peak tail current evoked on return to a holding potential (−40 mV). The amplitude of the time dependent current during depolarization (IKdepo) was measured from an initial minimal current after depolarization to a terminal current at the end of the pulse.
The current signals were stored on video cassette tapes using a Pulse-Code-Modulation Processor (VR-10B, Instrutech Co., Elmont, NY, U.S.A.) with a band width of DC-15 kHz, and were digitized using a personal computer (NEC PC-9801RS, Tokyo, Japan) equipped with an analogue-digital converter (ADX98, Canopus Co, Kobe, Japan). All values were expressed as mean±s.e.mean. Student's t-test was used to determine the statistical significance between the means obtained before and after addition of the drug. A P-value of 0.05 or less was considered significant.
Results
We examined first the effects of bepridil on the delayed rectifier K+ current consisting of both IKr and IKs components. The L-type Ca2+ current was suppressed by nitrendipine (5 μM) or D600 (1 μM) and the Na+ current was inactivated by holding the membrane potential at −40 mV. Depolarizing voltage steps with a duration of 1 s were applied in 10 mV increments. Figure 1 shows representative current traces obtained before (Figure 1A) and after application of 10 μM bepridil (Figure 1B). It is apparent that bepridil decreased the amplitudes of both time-dependent outward currents that appeared during depolarizing pulses and the tail currents evoked on return to the holding potential. Figure 1C shows the current-voltage relationship of the bepridil-sensitive time-dependent current. The current was increased with increases in membrane potential, thereby suggesting that bepridil might have suppressed IKs, which is reported to possess a property of no inward-going rectification (Sanguinetti & Jurkiewicz, 1990), as it is observed in Figure 1C.
Figure 1.
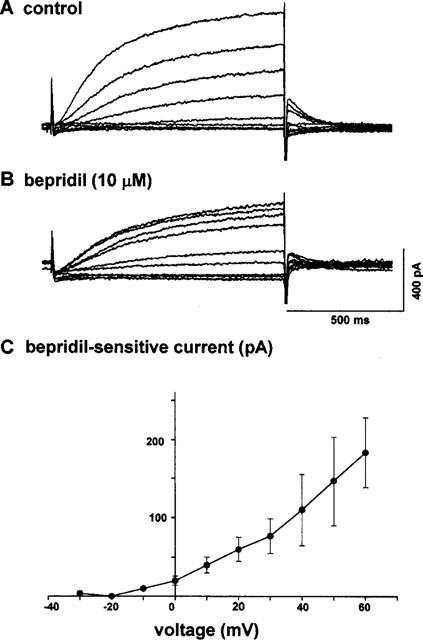
(A,B) Effects of bepridil on the outward current of a guinea-pig ventricular myocyte. Current families in the absence (A) and presence (B) of 10 μM bepridil. (C) Current-voltage relationship of bepridil-sensitive time-dependent current. The ordinate shows the difference in the time-dependent current at the end of the depolarizing pulses (abscissa), measured between corresponding traces in panels A and B. The lack of an apparent inward-going rectification in the I–V curve suggests that bepridil blocked the slowly activating component of the delayed-rectifier K+ current, i.e., IKs. The L-type Ca2+ current was blocked by 1 μM D600. Temperature, 35°C.
Thus, we examined the effect of bepridil on IKs, more selectively, after blockade of IKr with 5 μM E4031 (Sanguinetti & Jurkiewicz, 1990). In order to activate IKs, we applied various levels of depolarizing pulses with a duration of 3 s at an interpulse interval of 10 s (Figure 2A). Bepridil (10 μM) decreased both the time-dependent outward current during depolarization (IKdepo) and the tail current (IKtail) evoked on clamping back to the holding potential (−40 mV). In Figure 2B, the bepridil-induced suppression of IKtail (evoked after depolarization to +60 mV) was plotted against various drug concentrations used. The data were well fitted by an equation assuming one-to-one interactions between the receptors (channels) and the drug molecules. The concentration of half-maximal inhibition (IC50) was calculated to be 6.2 μM.
Figure 2.
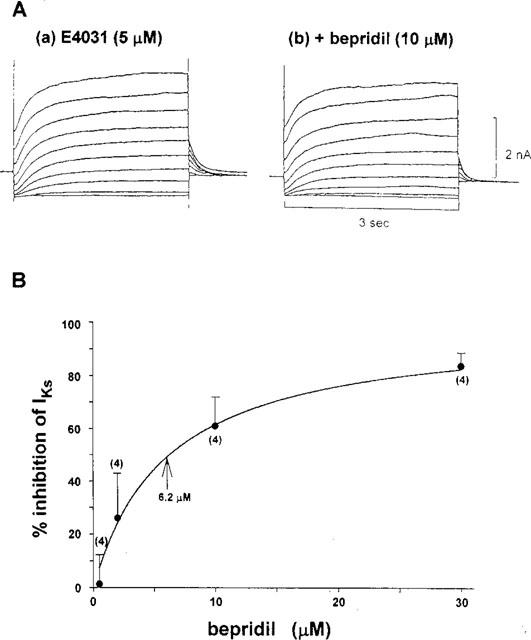
(A) Effects of 10 μM bepridil on the slowly activating delayed rectifier K+ current, IKs, which was recorded after blockade of the rapidly activating component of the current, IKr, with 5 μM E4031. (B) Concentration-dependent effect of bepridil on IKs. The per cent inhibition of the tail current evoked after depolarization to +60 mV (duration of 1 s) was plotted against the drug concentrations tested. The data were fitted by the following equation, by assuming one-to-one interaction between the channel and the drug molecule: Fractional inhibition (per cent inhibition of IKs)=Imax *C/ (C+Kd), where Imax is the maximal inhibition and Kd is a dissociation constant. Imax and Kd were calculated to be 100% and 6.2 μM.
To examine whether or not bepridil blocks IKs in a voltage-dependent manner, the per cent current inhibition caused by 10 μM bepridil was plotted against voltage for either the tail current (open circles) or the time-dependent current measured at the end of depolarization (filled circles) in Figure 3. The data indicate that the bepridil-induced block of IKs is not voltage-dependent.
Figure 3.
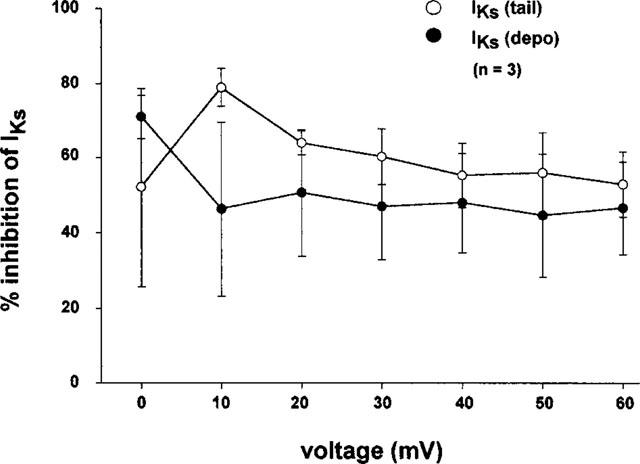
Voltage-independent effects of bepridil on IKs. The experiments were conducted in the presence of 5 μM E4031. Each data point and vertical bar indicate the mean and s.e.mean from three experiments.
We then examined the effects of bepridil on IKr using the envelope-of-tails test (Noble & Tsien, 1969; Sanguinetti & Jurkiewicz, 1991). Depolarizing voltage steps from −40 mV to a test potential of +50 mV were applied with stepwise increases in the pulse duration from 50 ms to 3 s. Figure 4A shows representative current traces obtained before (Figure 4A-a) and after application of 2 μM bepridil (Figure 4A-b). The ratio of the peak of IKtail to the amplitude of the time-dependent current (IKdepo) was plotted against the duration of the depolarizing pulses (Figure 4B). Under the control condition (open circles), the ratio (IKtail/IKdepo) for the short pulses (<200 msec) was much larger than that for longer pulses, indicating the presence of two types of IK (Sanguinetti & Jurkiewicz, 1991). Application of 2 μM bepridil shifted the curve upward over all pulse durations tested, without decreasing the IKtail/IKdepo ratio at shorter pulses, indicating that the drug at this concentration does not inhibit IKr (cf. Discussion). To obtain further support for this notion, we conducted the envelope-of-tails test on the bepridil-sensitive current, as shown in Figure 4C. The bepridil-sensitive current was obtained by subtraction of the current recorded in the presence of bepridil from that in the absence of the drug. It is apparent that the IKtail/IKdepo ratio was almost constant regardless of the pulse duration. In other words, the 2 μM bepridil-sensitive current represents only a single component of the delayed rectifier K+ current, that is IKs.
Figure 4.
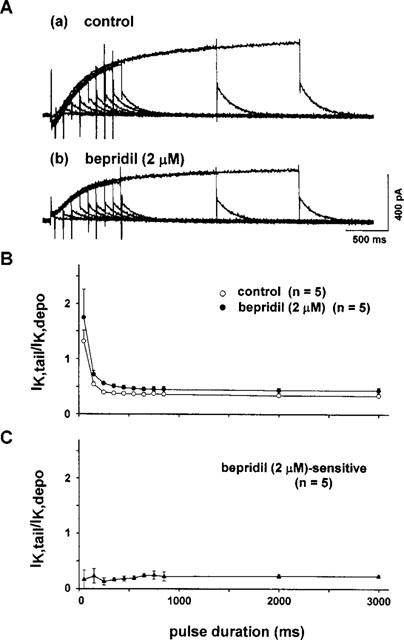
(A) Envelope-of-tails test in the absence (a) and presence of 2 μM bepridil (b). Depolarizing pulses to +50 mV from −40 mV were applied by increasing the pulse duration. (B) The ratio of the tail current (IKtail) to the time-dependent current during the pulse (IKdepo) was plotted against the pulse duration. Bepridil shifted the curve upward, preserving a large value in the IKtail/IKdepo ratio at the short pulse duration of ⩽200 ms. (C) The IKtail/IKdepo ratio of the bepridil-sensitive current, which was obtained by subtracting the current in the presence of bepridil from the current in the absence of bepridil. The absence of a large IKtail/IKdepo ratio at shorter pulse durations suggests that bepridil at 2 μM did not block IKr, but did block IKs (cf. Figure 7C).
Using the same protocol, the effect of bepridil on IKr was then examined using a higher drug concentration of 10 μM (Figure 5A). Bepridil again shifted the curve upward, indicating the suppression of IKs by the drug. However, the envelope-of-tails test of the current blocked by 10 μM bepridil (the drug-sensitive current) showed a slight increase in the IKtail/IKdepo ratio for short pulses (<0.1 s) as compared to the longer ones. These results suggest that 10 μM bepridil inhibits some fraction of IKr in addition to the blockade of IKs.
Figure 5.
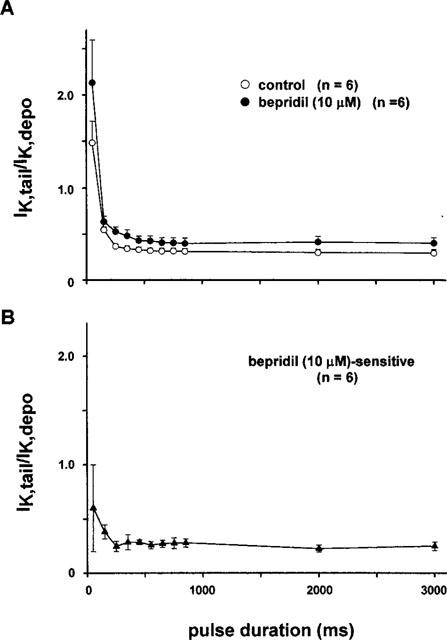
(A) Envelope-of-tails test in the absence and presence of 10 μM bepridil. (B) Envelope-of-tails test of the current blocked by 10 μM bepridil. A slight increase in the IKtail/IKdepo ratio at shorter pulses suggests that a higher concentration of bepridil (∼10 μM) blocks both IKr and IKs.
To study the effect of bepridil on IKr more quantitatively, we employed a short test-pulse protocol (duration=50 ms) during which a negligible amount of IKs was activated, but there was a sufficient activation of IKr. The tail current evoked on returning to −40 mV was not influenced by 50 μM chromanol 293 B, a specific blocker of IKs (34.7±5.6 pA for the control vs 35.1±6.5 pA in the presence of 50 μM 293 B, n=4). In contrast, the amplitude of the tail current was markedly decreased from 86.7±14.5 pA to 18.3±6 pA, or by 80% (n=3) after application of E4031 (5 μM). This finding lends support to the notion that under the experimental condition, the tail current was composed of only one component of IK, namely IKr alone. Figure 6A shows the effects of 2 and 10 μM bepridil on the tail currents (IKr) thus obtained; bepridil produced little (2 μM) or considerable (10 μM) depression on these tail currents. The concentration-dependent inhibition of the drug on the tail current, i.e., IKr, is summarized and plotted in Figure 6B. The inhibition was nil or small at low concentrations (2–10 μM) but was augmented rather steeply at higher concentrations (>20 μM). As the overall contour of the inhibition-concentration relationship seemed somewhat different from that for IKs (Figure 2B), we fitted the data with the following Hill equation: Per cent inhibition of IKr=K·Ch/(1+K·Ch)·100, where C is the drug concentration, K is the apparent dissociation constant and h is the Hill coefficient. The best fit was obtained when K=0.0004 and h=3.03, indicating that the drug interacts with the channel in a highly cooperative manner. The concentration of half-maximal inhibition of IKr was found to be 13.2 μM.
Figure 6.
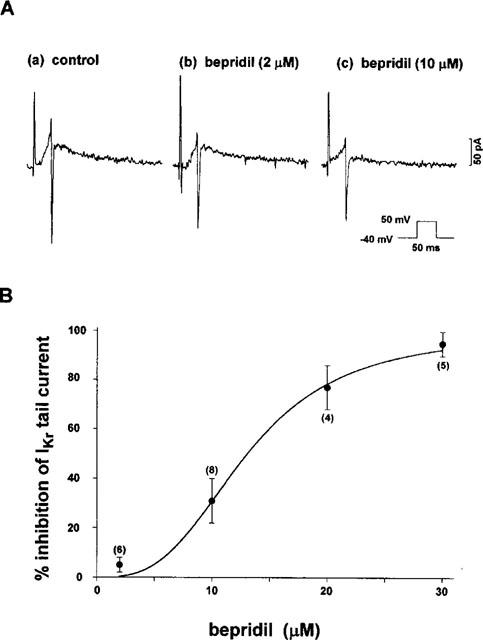
Effects of bepridil on IKr tail current. (A) Typical examples of the effect of bepridil at 2 and 10 μM on IKr evoked by short depolarizing pulses (duration=50 ms; see inset). (B) The concentration-dependent inhibition of bepridil on IKr, examined using a short-pulse protocol. The data were fitted by a Hill equation: Fractional inhibition (per cent inhibition of IKr)={K·Ch/(1+K·Ch)}·100, where C, K, and h are the drug concentration, dissociation constant, and Hill coefficient, respectively. Best fit was obtained when K=0.0004 and h=3.03. The figures in parentheses indicate the number of cells tested.
Discussion
The major findings in the present study are as follows: (1) Bepridil decreased the IKs measured after blockade of IKr with the use of E4031 (5 μM). The concentration of half-maximal inhibition, IC50 was 6.2 μM. (2) In an envelope-of-tails test, 2 μM bepridil did not decrease, but rather increased the IK tail/IKdepo ratio at short pulses (<0.2 s), suggesting that the drug does not inhibit IKr at this concentration. However, a higher concentration (10 μM) of the drug slightly depressed IKr. (3) The concentration-dependent effect of bepridil on IKr was studied using the tail current activated by short depolarizing pulses (duration=50 ms) which largely represented IKr. High concentrations (⩾10 μM) of bepridil depressed IKr, with an IC50 of 13.2 μM and the blockade occurred in a cooperative manner (Hill coefficient=3.03).
Effects of bepridil on the delayed rectifier K+current
We found that bepridil at concentrations relevant to the clinical therapeutic concentrations suppressed IKs. This was confirmed by two different sets of experiments. In the envelope-of-tails test (Noble & Tsien, 1969), bepridil at a concentration of 2 μM did not change the shape of the curve, but shifted the curve upward (Figure 4B). This parallel shift can be interpreted from the diagram illustrated in Figure 7. When the pulse duration is short (<200 ms), the amplitude of IKr seen during the depolarizing pulse is small due to its strong inward-going rectification. On return to −40 mV, a large tail current of IKr appeared as the channel quickly recovered from rectification (Sanguinetti & Jurkiewicz, 1990) (left, Figure 7A). In contrast, as IKs is activated very slowly, its contribution to either IKdepo or IKtail is very small when the duration of the depolarizing pulse is short (<200 ms) (left, Figure 7B). Consequently, the amplitude of the IKtail evoked by a short pulse is much larger than that of IKdepo (left, Figure 7C). On the other hand, the longer depolarization (∼1000 ms) activates IKs considerably (Figure 7B) (in addition to the activation of IKr) and increases the contribution of IKs to the overall current of either IKdepo or IKtail (right, Figure 7C) and causes the IKtail/IKdepo ratio to converge to a small certain constant value (∼0.4, ‘IKr+IKs' curve of Figure 7D). In the plots of IKtail/IKdepo vs pulse duration (Figure 7D), the idealistic relationship of IKr alone (i.e. under nearly complete blockade of IKs) could be a straight line, as shown at the top of Figure 7D, the ratio of which is large and determined mostly by the rectification property of IKr. However, in the presence of IKr alone (i.e. under nearly complete blockade of IKs) the IKtail/IKdepo ratio would be fairly small and constant as shown at the bottom of Figure 7D, which could be determined mostly by the difference in the driving force for potassium ions at the different potentials (−40 and +50 mV). In the presence of both IKr and IKs, the ratio was large for short pulses and became smaller as the pulse duration was increased (IKr+IKs, Figure 7D). In the present study, 2 μM bepridil shifted the relationship upward (Figure 4B), suggesting that the relative contribution of IKr to total IK was slightly increased as the drug partially suppressed IKs.
Figure 7.
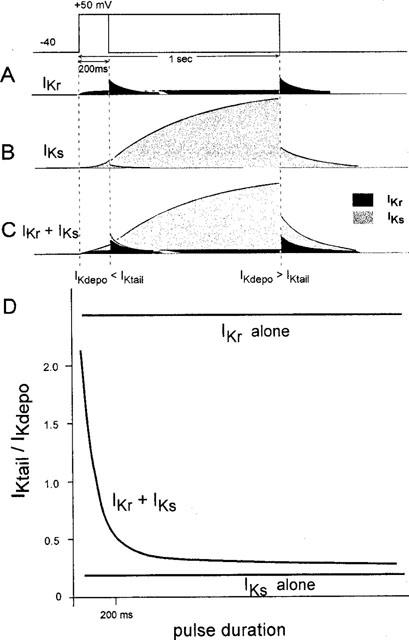
A scheme explaining the bepridil-induced changes in the profile of an envelope-of-tails test. The ordinate unit in panel D is arbitrary. See text for details.
Selective block of IKs by 2 μM bepridil was further supported in the plot of the IKtail/IKdepo ratio of the bepridil-sensitive current (Figure 4C). The ratio was almost constant regardless of the pulse duration, and the bepridil (2 μM)-sensitive component of the IKtail/IKdepo ratio shown in Figure 4C shared the characteristic common to the ‘IKs alone' curve schematically drawn at the bottom of Figure 7D. These results strongly suggest that 2 μM bepridil selectively inhibits IKs. In contrast, a much higher concentration of bepridil (∼10 μM) may inhibit both IKr and IKs, since the relationship of the 10 μM bepridil-sensitive current showed a biphasic pattern (Figure 5B). These results suggest that at concentrations relevant to the plasma therapeutic concentrations in clinical use (2–3 μM, Hollingshead et al., 1992), bepridil selectively blocks IKs with no practical effect on IKr.
In an alternative experiment, we studied the concentration-inhibition relationships of the IKr block by bepridil using a short-pulse protocol. The duration of depolarization for 50 ms was too short to activate IKs, because the tail current elicited on return to −40 mV was insensitive to chromanol 293 B (50 μM), a putative IKs blocker (Busch et al., 1996). The tail current was almost completely abolished, however, by E4031 (5 μM). This apparently IKr-selective tail current was suppressed by bepridil in a highly cooperative manner (Figure 6). Such findings imply that the IKr channel does not have a single binding site, but rather multiple (≈percnt;3) sites for bepridil. Due to this cooperation observed in the bepridil action on IKr, the blocking effect of this drug on the IKr at low concentrations might have become much smaller than expected from the case when there is no cooperation. Indeed 2 μM bepridil inhibited IKr only less than 5% (Figure 6B).
Bepridil on action potential duration
Most class III drugs selectively block IKr. The resulting reverse-use-dependent effect, i.e. marked prolongation of the APD at low heart rates and lack of prolongation at higher heart rates, hampered the effectiveness of these drugs as antiarrhythmic agents (Nair & Grant, 1997). Thus agents that selectively block the IKs may have a potential usefulness in effectively suppressing tachyarrhythmias due to re-entry (Nair & Grant, 1997). The present study demonstrated that bepridil at therapeutic concentrations (2–3 μM) preferentially blocked IKs. This effect may underlie at least in part the previous finding that bepridil prolonged the APD in a use-dependent manner in guinea-pig ventricular papillary muscles (Nobe et al., 1993). In the latter report, bepridil significantly lengthened the APD only at the highest stimulation frequency of 5 Hz.
Multiple effects of bepridil
Bepridil has multiple inhibitory effects on sarcolemmal ionic currents including the L-type (Yatani et al., 1986) and T-type Ca2+ currents (Cohen et al., 1992), the delayed-rectifier K+ current, the transient outward current (Berger et al., 1989) as well as the K+ current activated by intracellular Na+ (Mori et al., 1998). The drug also modulates contractile proteins and increases the Ca2+ sensitivity (Solaro et al., 1986; Ozaki et al., 1999). The main target of bepridil is believed to be the L-type Ca2+ current, because the concentration of half-maximal inhibition was as low as 0.5 μM (Yatani et al., 1986). Bepridil has multiple intracellular and sarcolemmal targets and these effects in concert may form a characteristic profile of the drug action. For example, the drug enhances the Ca2+ sensitivity of the contractile proteins, which may counteract the depression of contractile tension due to a decrease in ICa (Solaro et al., 1986; Ozaki et al., 1999).
We demonstrated that bepridil has differential effects on the two components of the delayed-rectifier K+ current, IKr and IKs: the IC50 for inhibition of IKr and IKs was 13.2 and 6.2 μM, respectively. In addition, since the inhibition of IKr occurred in a cooperative manner (Hill coefficient=3.03), the inhibition of IKr by low concentrations of bepridil is much attenuated. Therefore, we conclude that bepridil at therapeutic concentrations (2–3 μM, Hollingshead et al., 1992) blocks IKs, with no practical effect on IKr. The differential inhibition of two delayed-rectifier K+ currents by bepridil may be important in understanding the antiarrhythmic effects of this drug.
Acknowledgments
We thank Dr Hans-J Lang for the gift of chromanol 293 B and Ms K Moriyama for her secretarial services.
Abbreviations
- APD
action potential duration
- ICa
the L-type Ca2+ current
- IK
the delayed rectifier K+ current
- IKdepo
time-dependent current during depolarizing pulse
- IKr
the rapidly activating delayed-rectifier K+ current
- IKs
the slowly activating delayed-rectifier K+ current
- IKtail
tail current of the delayed rectifier K+ current
References
- ANNO T., FURUTA T., ITOH M., KODAMA I., TOYAMA J., YAMADA K. Electromechanical effects of bepridil on rabbit isolated hearts. Br. J. Pharmacol. 1984;81:41–47. doi: 10.1111/j.1476-5381.1984.tb10741.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BERGER F., BORCHARD U., HAFNER D. Effects of the Ca entry blocker bepridil on repolarizing and pacemaker currents in sheep cardiac Purkinje fibres. Naunyn-Schmiedeberg's Arch. Pharmacol. 1989;339:638–646. doi: 10.1007/BF00168656. [DOI] [PubMed] [Google Scholar]
- BUSCH A.E., SUESSBRICH H., WALDEGGER S., SAILER E., GREGER R., LANG H.J., LANG F., GIBSON K.J., MAYLIE J.G. Inhibition of IKs in guinea pig cardiac myocytes and guinea pig IsK channels by the chromanol 293B. Pflügers. Arch. Eur. J. Physiol. 1996;432:1094–1096. doi: 10.1007/s004240050240. [DOI] [PubMed] [Google Scholar]
- COHEN C.J., SPIRES S., VAN SKIVER D. Block of T-type Ca channels in guinea pig atrial cells by antiarrhythmic agents and Ca channel antagonists. J. Gen. Physiol. 1992;100:703–728. doi: 10.1085/jgp.100.4.703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- COSNIER D., DUCHENNE-MARULLAZ P., RISPAT G., STREICHENBERGER G. Cardiovascular pharmacology of bepridil, (1[3 isobutoxy 2 (benzylphenyl) amino] propyl pyrrolidine hydrochloride) a new potential anti-anginal compound. Arch. Int. Pharmacodyn. Ther. 1977;225:133–151. [PubMed] [Google Scholar]
- DUCHENE-MARULLAZ P., KANTELIP J.-P., TROLESE J.-F. Effects of bepridil, a new antianginal agent, on ambulatory electrocardiography in human volunteers. J. Cardiovasc. Pharmacol. 1983;5:506–510. doi: 10.1097/00005344-198305000-00024. [DOI] [PubMed] [Google Scholar]
- GILL A., FLAIM S.F., DAMIANO B.P., SIT S.P., BRANNAN M.D. Pharmacology of bepridil. Am. J. Cardiol. 1992;69:11D–16D. doi: 10.1016/0002-9149(92)90953-v. [DOI] [PubMed] [Google Scholar]
- HOLLINGSHEAD L.M., FAULDS D., FITTON A. Bepridil. A review of its pharmacological properties and therapeutic use in stable angina pectoris. Drugs. 1992;44:835–837. doi: 10.2165/00003495-199244050-00009. [DOI] [PubMed] [Google Scholar]
- KATO R., SINGH B.N. Effects of bepridil on the electrophysiological properties of isolated canine and rabbit myocardial fibers. Am. Heart. J. 1986;111:271–279. doi: 10.1016/0002-8703(86)90139-0. [DOI] [PubMed] [Google Scholar]
- MARSHALL R.J., MUIR A.W., WINSLOW E. Effects of antiarrhythmic drugs on ventricular fibrillation thresholds of normal and ischemic myocardium in the anesthetized rat. Br. J. Pharmacol. 1983;78:165–171. doi: 10.1111/j.1476-5381.1983.tb09377.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MARTY A., NEHER E.Tight-seal whole-cell recording Single-Channel Recording 1983New York: Plenum; 107–122.ed. Sackmann B. Neher E. pp [Google Scholar]
- MORI K., KOBAYASHI S., SAITO T., MASUDA Y., NAKAYA H. Inhibitory effects of class I and IV antiarrhythmic drugs on the Na+-activated K+ channel current in guinea pig ventricular cells. Naunyn-Schmiedeberg's Arch. Pharmacol. 1998;358:641–648. doi: 10.1007/pl00005306. [DOI] [PubMed] [Google Scholar]
- NAIR L.A., GRANT A.O. Emerging class 3 antiarrhythmic agents: mechanism of action and proarrhythmic potential. Cardiovasc. Drug. Ther. 1997;11:149–167. doi: 10.1023/a:1007784814823. [DOI] [PubMed] [Google Scholar]
- NAWADA T., TANAKA Y., HISATOME I., SASAKI N., OHTAHARA A., KOTAKE H., MASHIBA H., SATO R. Mechanism of inhibition of the sodium current by bepridil in guinea-pig isolated ventricular cells. Br. J. Pharmacol. 1995;116:1775–1780. doi: 10.1111/j.1476-5381.1995.tb16662.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NOBE S., AOMINE M., ARITA M. Bepridil prolongs the action potential duration of guinea pig ventricular muscle only at rapid rates of stimulation. Gen. Pharmac. 1993;24:1187–1196. doi: 10.1016/0306-3623(93)90367-7. [DOI] [PubMed] [Google Scholar]
- NOBLE D., TSIEN R.W. Outward membrane currents activated in the plateau range of potential in cardiac Purkinje fibres. J. Physiol (Lond.) 1969;200:205–231. doi: 10.1113/jphysiol.1969.sp008689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- OZAKI M., ZAIZEN H., KIYOSUE T., NASU M., ARITA M. Effect of bepridil on intracellular calcium concentration and contraction in cultured rat ventricular myocytes. J. Cardiovasc. Pharmacol. 1999;33:492–499. doi: 10.1097/00005344-199903000-00021. [DOI] [PubMed] [Google Scholar]
- PELLEG A., PARDO Y., BELHASSEN B., SHARGORDSKY B., CHAGNAC A., LANIADO S. Effect of verapamil and bepridil on occlusion and reperfusion arrhythmias in the canine heart. Cardiology. 1985;72:193–201. doi: 10.1159/000173873. [DOI] [PubMed] [Google Scholar]
- SANGUINETTI M.C., JURKIEWICZ N.K. Two components of cardiac delayed rectifier K+ current: differential sensitivity to block by class III antiarrhythmic agents. J. Gen. Physiol. 1990;96:195–215. doi: 10.1085/jgp.96.1.195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SANGUINETTI M.C., JURKIEWICZ N.K. Delayed rectifier outward K+ current is composed of two currents in guinea pig atrial cells. Am. J. Physiol. 1991;260:H393–H399. doi: 10.1152/ajpheart.1991.260.2.H393. [DOI] [PubMed] [Google Scholar]
- SATO N., NISHIMURA M., KAWAMURA Y., WARD A.C., KIKUCHI K. Block of Na+ channel by bepridil in isolated guinea-pig ventricular myocytes. Eur. J. Pharmacol. 1996;314:373–379. doi: 10.1016/s0014-2999(96)00567-5. [DOI] [PubMed] [Google Scholar]
- SOLARO R.J., BOUSQUET P., JOHNSON J.D. Stimulation of cardiac myofilament force, ATPase activity and troponin C Ca2+ binding by bepridil. J. Pharmacol. Exp. Ther. 1986;238:502–507. [PubMed] [Google Scholar]
- WANG J.C., KIYOSUE T., ARITA M. Bepridil selectively blocks the delayed rectifier K+ current (IKs) in guinea pig hearts. J. Mol. Cell. Cardiol. 1998;30:A305. [Google Scholar]
- WANG D.-W., KIYOSUE T., SATO T., ARITA M. Effects of class I antiarrhythmic drugs, cibenzoline, mexiletine and flecainide on the delayed rectifier K+ current in guinea-pig ventricular myocytes. J. Mol. Cell. Cardiol. 1996;28:893–903. doi: 10.1006/jmcc.1996.0084. [DOI] [PubMed] [Google Scholar]
- YATANI A., BROWN A.M., SCHWARTZ A. Bepridil block of cardiac calcium and sodium channels. J. Pharmacol. Exp. Ther. 1986;237:9–17. [PubMed] [Google Scholar]


