Abstract
Pretraining administration of 8-hydroxy-2-di-n-propylamino-tetralin (8-OH-DPAT 0.1 mg kg−1), a 5-HT1A receptor agonist, or buspirone (1 mg kg−1), a 5-HT1A receptor partial agonist, markedly impaired passive avoidance retention in rats 24 h later. The effect of 8-OH-DPAT was prevented by the 5-HT1A receptor antagonists, NAN-190 and WAY-100635, at doses without any intrinsic effect.
N-ethoxycarbonyl-2-ethoxy-1,2-dihydroquinoline (EEDQ 10 mg kg−1), an alkylating agent that inactivates different G-protein coupled receptors, impaired retention performance when given 48 h pretraining. The disruptive effect of EEDQ was reversed by 8-OH-DPAT or buspirone, given 30 min before training.
Non-specific actions did not account for 8-OH-DPAT-induced reversal of the EEDQ effect since no significant difference in locomotor activity or in pain threshold was found between rats receiving EEDQ or EEDQ+8-OH-DPAT.
When NAN-190 (1 mg kg−1) or WAY-100635 (0.5 mg kg−1) were given before 8-OH-DPAT to EEDQ-pretreated animals, the reversal by 8-OH-DPAT of EEDQ-induced retention impairment was still more pronounced. However, no EEDQ reversal by 8-OH-DPAT was found when 5-HT1A receptors were protected by WAY-100635 (10 mg kg−1) 30 min before EEDQ.
In the hippocampus of EEDQ-treated rats, 5-HT7 receptors were less inactivated than 5-HT1A receptors and significant increases were found in 5-HT1A but not in 5-HT7 receptor mRNA levels. Ritanserin and methiothepin (10 mg kg−1 each), antagonists with higher affinity at 5-HT7 than at 5-HT1A receptors, prevented the retention impairment induced by EEDQ but did not significantly protect against 5-HT7 receptor inactivation.
The results indicate that the facilitatory effect of 8-OH-DPAT is not mediated through 5-HT1A receptors and suggest that other 8-OH-DPAT-sensitive receptors could be involved in the dual effect of 8-OH-DPAT on passive avoidance performance in rats.
Keywords: Passive avoidance, 5-HT1A receptors, 5-HT7 receptors, 8-OH-DPAT, EEDQ, hippocampus
Introduction
In animal models of cognition, there is considerable evidence that 5-hydroxytryptamine (5-HT) exerts complex effects on learning and memory by acting on different 5-HT receptor subtypes. Receptors for 5-HT may be grouped at present into seven families (5-HT1–5-HT7) based upon cDNA deduced primary sequences, signal transduction mechanisms and pharmacological profile (Boess & Martin, 1994). Systemic administration of 5-HT1A (see below) or 5-HT2 receptor agonists (Riekkinen, 1994) impairs passive avoidance performance. Stimulation of hippocampal 5-HT1B receptors impairs performance in a spatial learning task (Buhot et al., 1995). By contrast, activation of 5-HT4 receptors may enhance learning and memory (Fontana et al., 1997). It is also well known that 5-HT, by stimulating specific 5-HT receptor subtypes, facilitates short- and long-term memory in Aplysia (Bartsch et al., 1995). In regard to 5-HTergic antagonists, several studies have shown that 5-HT3 receptor antagonists are able to improve learning and memory and also antagonize the cognitive deficits related to aging or induced by anticholinergic drugs (e.g. review by Bentley & Barnes, 1995).
The hippocampus is one of the brain structures more crucially involved in cognitive functions (e.g. Jarrard, 1993). The hippocampus receives extensive innervation from 5-HT cell bodies in the raphe nuclei (Azmitia & Segal, 1978) and contains a high 5-HT1A receptor number (Pazos & Palacios, 1985). Several behavioural studies have demonstrated that administration of 5-HT1A receptor agonists, such as 8-hydroxy-2-(di-n-propylamino)tetralin (8-OH-DPAT), buspirone and tandospirone significantly impairs memory performance of rats and mice in various memory tasks, such as the Morris water maze and passive avoidance procedures (Rowan et al., 1990; Carli & Smanin, 1992; McNaughton & Morris, 1992; Riekkinen, 1994; Misane et al., 1998). Since the impairment of spatial learning in a water maze induced by systemically administered 8-OH-DPAT is attenuated by intrahippocampal infusion of the 5-HT1A receptor antagonists spiroxatrine or (+)WAY 100135 (Carli et al., 1995) and potentiated in rats with 5-HT depletion induced by intracerebroventricular injection of 5,7-dihydroxytryptamine (5,7-DHT) (Carli & Samanin, 1992), it is considered that postsynaptic 5-HT1A receptors in the hippocampus mediate the disruptive effect of 8-OH-DPAT on cognitive function. Furthermore, stimulation of postsynaptic 5-HT1A receptors by directly infusing 8-OH-DPAT into the dorsal hippocampus disrupts performance whereas infusion of 8-OH-DPAT into the median raphe nucleus improves performance accuracy in the delayed non-matching to position (DNMTP) task (Warburton et al., 1997).
EEDQ (N-ethoxycarbonyl-2-ethoxy-1,2-dihydroquinoline) is an alkylating agent that irreversibly inactivates G protein-coupled receptors and may serve as a useful tool in neuropharmacology to estimate receptor turnover rate and function (Battaglia et al., 1987; Keck & Lakoski, 1996). Irreversible receptor blockade by EEDQ involves activation of carboxyl groups, at or near the binding site of the receptor protein, that can react with any nucleophilic group in its vicinity to form the irreversible bond (Belleau et al., 1969). Although EEDQ has no chemical specificity, it does not affect all receptor types to the same extent. Among the different 5-HT receptor subtypes, 5-HT1A receptors are particularly sensitive to inactivation by EEDQ (Gozlan et al., 1994; Raghupathi et al., 1996).
Given the complex effects of 5-HT in learning, which are mediated through effects on multiple receptor subtypes (see above), it appeared of interest to examine the effect of 5-HTergic agents on passive avoidance learning in rats after varying degrees of 5-HT receptor inactivation by EEDQ. Rats treated with the alkylating agent showed a severe retention impairment in this test. Surprisingly, the effect of EEDQ was reversed by the selective 5-HT1A receptor agonist 8-OH-DPAT. We herein report this unexpected finding and we attempt to characterize the mechanism(s) involved in this paradoxical effect of 8-OH-DPAT. In this regard, an especial attention was paid to the possible role of 5-HT7 receptors.
Methods
Animals
Principles of laboratory animal care (NIH publication no. 85-23, revised 1985) were followed. Male Wistar rats weighing between 240–260 g were housed four to a cage with free access to food and water. Animals were maintained in a temperature-controlled environment (21–23°C) on a 12 h light/dark cycle with lights off at 20.00 h. Behavioural experiments were always performed during the light period (09.00–15.00 h) and animals were never used more than once.
Passive avoidance studies
A two-compartment passive avoidance apparatus was used (Artaiz et al., 1995). The apparatus consisted of an illuminated white compartment (42×44×46 cm) and a dark compartment (15×15×30 cm), both the white and black areas being equipped with a grid floor (Letica, model 1516). The two compartments were separated by a guillotine door. The rat was placed in the illuminated area and 3 s later, the door was raised. During 90 s, the animal explored the apparatus freely (habituation trial). Twelve minutes later, the rat was placed again in the illuminated chamber. When the rat entered the dark chamber, the guillotine door was closed and after 10 s the animal was returned to its home cage. Sixty minutes later, the animal was placed again in the white compartment (acquisition trial). When the rat entered the dark chamber the guillotine door was closed again and after 10 s an inescapable 2 mA scrambled electrical foot-shock was delivered for 3 s through the grid floor using a shock generator (Letica). A retention trial was given 24 h after the acquisition trial, by placing the rat in the illuminated compartment and measuring the response latency to re-enter the dark compartment using a cut-off time of 300 s. Results were expressed as mean retention latency for each group of rats. 8-OH-DPAT and buspirone were administered 30 min and EEDQ 48 h before the acquisition trial. The antagonists NAN-190 and WAY 100635 were administered 15 min before 8-OH-DPAT. In the receptor protection experiments, methiothepin or ritanserin were injected 30 min before EEDQ.
Spontaneous locomotor activity
Animals were placed in a black wooden open-topped box (65×65×45 cm high). Distance traveled in cm (locomotor activity) was measured during a 30 min period by using a digital VIDEOMEX-V system (Columbus Inst., U.S.A.) working with the appropriate computer program.
Pain threshold
An operant conditioning chamber with a grid floor connected to a scrambled shock generator (Coulbourn Inst., U.S.A.) was used to determine the pain threshold to electrical stimuli (Carli et al., 1992). The rats were allowed 15 min to habituate to the chamber before a series of inescapable shocks was delivered to the grid floor. Each series consisted of 10 stimulations at the following intensities in mA: 0.1, 0.2, 0.4, 0.6, 0.8, 1.0, 1.2, 1.4, 1.6 and 2. The shock duration was 2 s and the shocks were delivered at 30 s intervals. Thresholds for flinch (forepaws off the grid floor) and jump (removal of four paws from the grid floor) were measured.
[3H]-8-OH-DPAT binding to 5-HT1A receptors
[3H]-8-OH-DPAT binding studies were carried out according to the procedure previously described by Gozlan et al. (1983) with minor modifications. Briefly, the selected brain region was homogenized in ice-cold buffer Tris-HCl 50 mM (pH 7.7) and centrifuged at 49,000×g for 15 min at 4°C. The pellet was resuspended in the same buffer and incubated at 37°C for 15 min. After a second centrifugation under the same conditions, the resultant pellet was resuspended in 50 mM Tris-HCl buffer (pH 7.7) containing CaCl2 4 mM at a final tissue concentration of approximately 10 mg ml−1 (wet tissue weight). The incubation mixture contained 100 μl of tissue suspension, 50 μl of six increasing concentrations of the labelled ligand (0.4–2 nM) and 50 μl of incubation buffer with or without buspirone 10 μM. Tubes were incubated for 15 min at 37°C. After rapid filtration of the incubation mixture through GF/C Whatman filters, the filters were rinsed with 4×5 ml of ice-cold buffer using a Brandel harvester and placed in vials containing 4 ml of liquid scintillation cocktail (Biogreen3, Scharlau). All the determinations were carried out in duplicate. Data were subjected to Scatchard analysis to determine the number of binding sites (Bmax : fmol mg−1 of protein) and the dissociation constant (Kd : nM).
[3H]-5-CT binding to 5-HT7 receptors
[3H]-5-Carboxamidotryptamine ([3H]-5-CT) was used to label 5-HT7 receptors in rat hippocampus homogenates according to the method described by To et al. (1995) with minor modifications. Briefly, the hippocampus was homogenized in 50 mM Tris-HCl buffer, pH 7.4, and centrifuged once at 4°C for 10 min at 48,000×g. The tissue pellet was rehomogenized and incubated at 37°C for 20 min and was then centrifuged twice under the same conditions as above. After the last centrifugation, tissues were homogenized (10 mg ml−1) in 50 mM Tris-HCl, pH 7.4, containing 4 mM CaCl2, 1 mg ml−1 ascorbate, 0.01 mM pargyline. (−) Cyanopindolol, 1 μM, and 100 nM ergotamine were used to block 5-HT1 and 5-HT5 receptors respectively (cf. Boess & Martin, 1994). The incubation mixture contained 400 μl of tissue suspension, 50 μl of eight increasing concentrations of [3H]-5-CT (0.05–5 nM) and 50 μl of incubation buffer with or without 5-CT 10 μM. Tubes were incubated for 120 min at 23°C. The membrane fraction was separated by rapid filtration through GF/C Whatman filters. The filters were rinsed with 4×5 ml of ice-cold 50 mM Tris-HCl buffer (pH 7.4) using a Brandel harvester and placed in vials containing 4 ml of liquid scintillation cocktail (Biogreen3, Scharlau). All the determinations were carried out in duplicate. Data were subjected to Scatchard analysis as above.
5-HT1A and 5-HT7 receptor mRNA levels
5-HT1A and 5-HT7 receptor mRNA were quantified by using a reverse transcription-polymerase chain reaction (RT–PCR) technique. Briefly, mRNA was extracted from the hippocampus using a Quick-Prep MicroPurification kit (Pharmacia). Each sample of mRNA (0.4 μg) was transcribed and aliquots of the produced cDNA were amplified by PCR using specific oligonucleotides for the rat 5-HT1A receptor gene, corresponding to bases 724–743 and 839–856 (Albert et al., 1990; Aguirre et al., 1997) and for the rat 5-HT7 receptor gene, corresponding to bases 846–867 and 1157–1172 (Shen et al., 1993) in conditions which had been validated previously in our laboratory for optimal linearity and sensitivity. In separate experiments, β-actin was also amplified using specific primers. PCR was performed using a thermal cycler (Perkin Elmer 2400) in 30 cycles consisting of denaturation at 94°C for 1 min, 1 min of primer annealing, at 55 and 58°C for 5-HT1A and 5-HT7 amplification respectively, and extension at 72°C for 2 min followed by a final extension at 72°C for 15 min. Amplified products were resolved on 2% agarose gels and, after alkaline denaturation followed by neutralization, the gels were blotted overnight onto Hybond-N+ membranes (Amersham) and the DNA was fixed by UV cross linking (Stratagene). Membranes were prehybridized for 3 h and hybridized for 20 h at 42°C in hybridization solution using as probes oligonucleotides complementary to an internal sequence of the amplified fragments which were end-labeled with [γ32P]-dATP and T4 polynucleotide kinase (Promega). Membranes were washed in 2×SSC/0.1%SDS at room temperature and then twice at 55°C with 1×SSC/0.1%SDS for 15 min. Finally membranes received a last washing in 0.1×SSC/0.1%SDS at 65°C for 15 min. Blots were exposed to Kodak films with intensifying screens at −80°C. The intensity of hybridization bands were quantified by densitometric analysis using the ImageMaster 1-D program in an image analyzer (Pharmacia) and expressed as optical density (OD) ratio 5-HT receptor/β-actin.
Drugs and chemicals
The source of the drugs used was as follows: [3H]-8-OH-DPAT (202 Ci mmol−1), [3H]-5-CT (84 Ci mmol−1) and [γ-32P]-dATP (3000 Ci mmol−1) were from Amersham (U.K.); 8-OH-DPAT-HBr, methiothepin mesylate, NAN-190-HBr and ritanserin (R.B.I. Natick, MA, U.S.A.); (−) cyanopindolol hemifumarate and ergotamine tartrate (Tocris, U.K.); pargyline and EEDQ (Sigma, U.K.); WAY-100635 (gift from Wyeth Labs. U.S.A.). All other chemicals were from Merck (Darmstadt, Germany). NAN-190 and ritanserin were suspended in saline with a drop of Tween 80 (Sigma). All other drugs were dissolved in saline except EEDQ which was dissolved in ethanol/water (1 : 1, v v−1).
Results
Passive avoidance
The 5-HT1A receptor agonist 8-OH-DPAT (0.1 mg kg−1 s.c.), administered 30 min before the acquisition trial significantly reduced retention latency 24 h later (Figure 1). The 5-HT1A receptor antagonists NAN-190 (1 mg kg−1 i.p.) and WAY-100635 (0.5 mg kg−1 i.p.), given 45 min before the acquisition trial, did not show any intrinsic effect on passive avoidance performance but fully prevented the retention impairment induced by 8-OH-DPAT (Figure 1).
Figure 1.
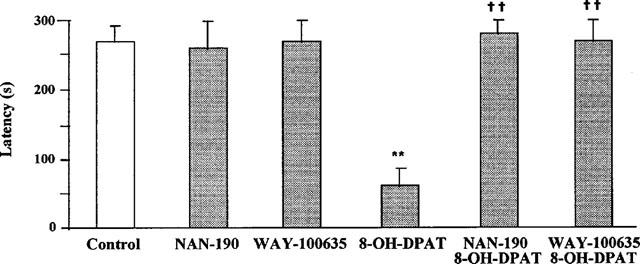
Impairment of passive avoidance retention in rats by 8-OH-DPAT and antagonism of the effect of 8-OH-DPAT by NAN-190 and WAY-100635. 8-OH-DPAT (0.1 mg kg−1 s.c.) given 30 min and NAN-190 (1 mg kg−1 i.p.) or WAY-100635 (0.5 mg kg−1 i.p.) 45 min before the acquisition session. Values are mean±s.e.mean of 10–30 animals. **P<0.01 vs control; ††P<0.01 vs 8-OH-DPAT (one-way ANOVA, F5,88=11.9, followed by Student-Newman-Keul's post hoc test).
The alkylating agent EEDQ (10 mg kg−1 s.c.) induced obvious signs of toxicity in rats and the animals lay immobile on the floor of the cages after injection. Forty-eight hours later, the animals were partially recovered. At this time, rats were exposed to the acquisition trial and a significant decrease in response latency was found 24 h later. The retention deficit induced by EEDQ was significantly antagonized by 8-OH-DPAT given 30 min before the acquisition trial (Figure 2). The reversal by 8-OH-DPAT of EEDQ-induced retention impairment was further enhanced when either of the 5-HT1A receptor antagonists, NAN-190 or WAY-100635 (same doses as above), which did not produce by themselves any change on the impairing effect of EEDQ (not shown), was also administered 15 min before 8-OH-DPAT (Figure 2). Buspirone (1 mg kg−1 i.p.), given 30 min before the acquisition trial, behaved like 8-OH-DPAT and reduced on its own retention latency 24 h later but counteracted the learning deficit induced by EEDQ (Figure 3). When 5-HT1A receptors were protected from inactivation by EEDQ (see below) by means of a high dose of WAY-100635 (10 mg kg−1 i.p.) given 30 min before EEDQ, no reversal by 8-OH-DPAT of the EEDQ-induced retention deficit was found (Figure 4).
Figure 2.
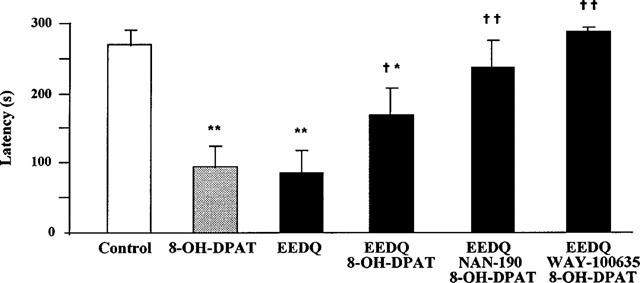
Effect of 8-OH-DPAT, NAN-190 and WAY-100635 on passive avoidance retention in rats pretreated with EEDQ (10 mg kg−1 s.c.) 48 h before. 8-OH-DPAT (0.1 mg kg−1 s.c.) given 30 min and NAN-190 (1 mg kg−1 i.p.) or WAY-100635 (0.5 mg kg−1 i.p.) 45 min before the acquisition session. Values are mean±s.e.mean of 10–25 animals. *P<0.05, **P<0.01 vs control; †P<0.05, ††P<0.01 vs 8-OH-DPAT (one-way ANOVA, F5,96=11.7, followed by Student-Newman-Keul's test).
Figure 3.
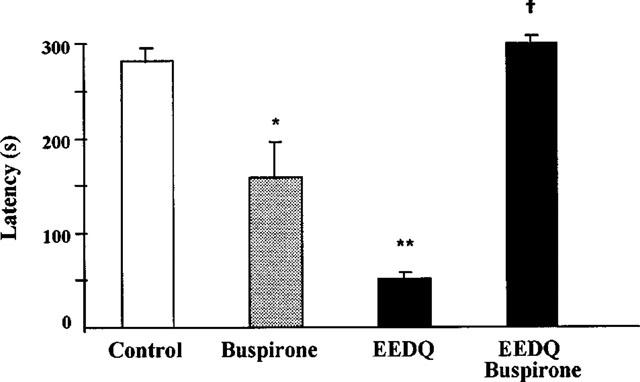
Effect of buspirone on passive avoidance retention in rats pretreated or not with EEDQ (10 mg kg−1 s.c.) 48 h before. Buspirone (1 mg kg−1 i.p.) given 30 min before the acquisition test. Values are mean±s.e.mean of 10–12 animals. *P<0.05, **P<0.01 vs control; †P<0.05 vs buspirone (one-way ANOVA, F3,40=11.8, followed by Student-Newman-Keul's test).
Figure 4.
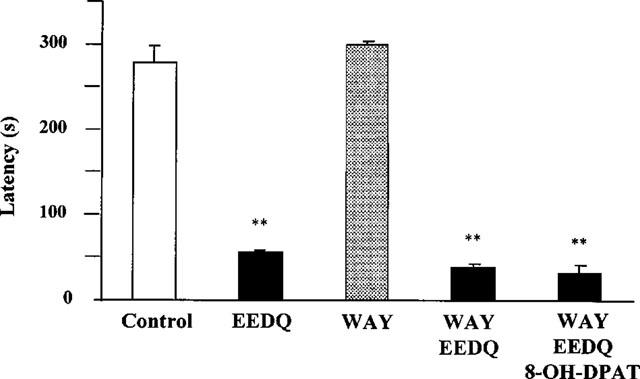
Effect of 8-OH-DPAT (0.1 mg kg−1 s.c.) on passive avoidance retention in rats pretreated with WAY-100635 (WAY, 10 mg kg−1 i.p.) and EEDQ (10 mg kg−1 s.c.) 48 h before. WAY was given 30 min before EEDQ. 8-OH-DPAT was given 30 min before the acquisition session. Values are mean±s.e.mean of 6–8 animals. **P<0.01 vs control (one-way ANOVA, F4,27=27.6, followed by Student-Newman-Keul's test).
In another experiment, a high dose of ritanserin (10 mg kg−1 i.p.), a 5-HT receptor antagonist which is virtually devoid of affinity at 5-HT1A receptors, was given 30 min before EEDQ and the rats were tested for retention performance 48 h later. It was then found that the retention deficit induced by EEDQ was almost fully prevented whereas 8-OH-DPAT still produced a moderate though significant increase in retention latency in rats previously treated with ritanserin+EEDQ as compared to rats pretreated only with EEDQ (Figure 5). When methiothepin (10 mg kg−1 i.p.), a 5-HT receptor antagonist with high affinity at 5-HT7 and other 5-HT1/5-HT2 receptor subtypes but lower affinity at 5-HT1A receptors, was used instead of ritanserin, the retention deficit induced by EEDQ was fully prevented (retention latencies for saline+EEDQ and methiothepin+EEDQ were 28±5 and 294±6 s respectively; mean±s.e.mean of six rats).
Figure 5.
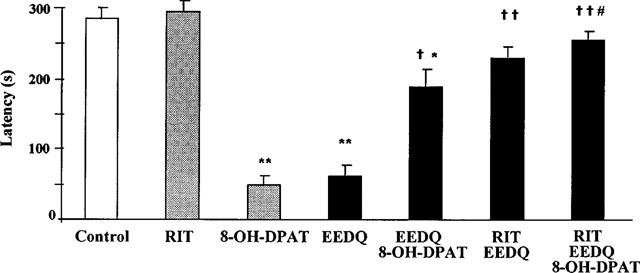
Prevention by ritanserin (RIT) 10 mg kg−1 i.p., given 30 min before EEDQ, of the impairing effect of EEDQ (10 mg kg−1 s.c.), given 48 h before the acquisition session, on passive avoidance retention in rats. Values are mean±s.e.mean of 10–12 animals. *P<0.05, **P<0.01 vs control; †P<0.05, ††P<0.01 vs EEDQ or 8-OH-DPAT; #P<0.05 vs EEDQ+8-OH-DPAT (one-way ANOVA, F6,68=9.4, followed by Student-Newman-Keul's test).
Locomotor activity
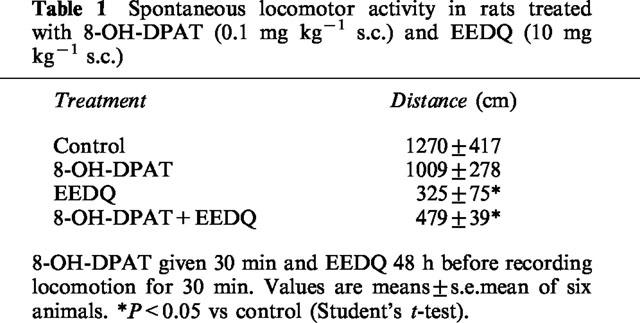
The administration of 8-OH-DPAT, at the same dose used in the passive avoidance studies (0.1 mg kg−1 s.c.), did not modify spontaneous locomotor activity as compared to saline-treated controls. Animals treated with EEDQ were still sedated 48 h later. Administration of 8-OH-DPAT did not modify the reduced locomotion induced by EEDQ. Locomotor activity data are depicted in Table 1.
Table 1.
Spontaneous locomotor activity in rats treated with 8-OH-DPAT (0.1 mg kg−1 s.c.) and EEDQ (10 mg kg−1 s.c.)
Pain threshold
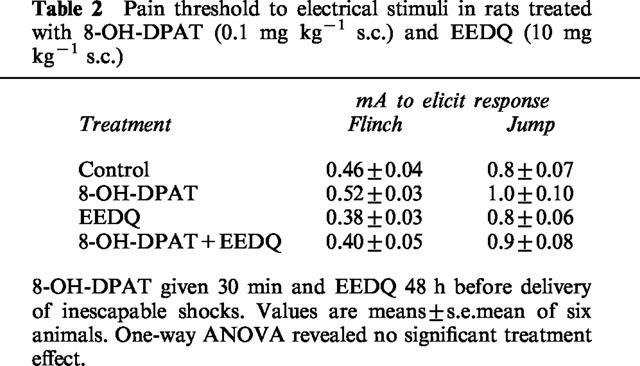
To assess whether the effect of drugs on passive avoidance performance could be due to non-specific actions on pain sensitivity, a nociception assay was carried out using electric current as the nocive stimulus. Neither 8-OH-DPAT nor EEDQ, at the same doses and time intervals used in the learning study, modified the thresholds for flinch and jump elicited by the electrical stimuli (Table 2).
Table 2.
Pain threshold to electrical stimuli in rats treated with 8-OH-DPAT (0.1 mg kg−1 s.c.) and EEDQ (10 mg kg−1 s.c.)
Radioligand binding assays
Preliminary studies were carried out in rat hippocampal homogenates to validate the method used for 5-HT7 receptor labelling. The affinity values (pKi) obtained for 5-CT, methiothepin and ritanserin were 9.16, 8.45 and 7.32 respectively (means of 3–4 experiments). These values were similar to those reported in the [3H]-5-HT or [125I]-LSD-labelled rat recombinant receptor (Shen et al., 1993). Displacement curves produced Hill coefficients lower than unity (0.50, 0.76 and 0.62 for 5-CT, methiothepin and ritanserin respectively) indicating the possibility of an additional low affinity binding site.
The maximal apparent reduction in 5-HT1A and 5-HT7 receptor density was observed 4 h after administration of EEDQ (10 mg kg−1 s.c.). At this time point, 5-HT1A receptor number was decreased by 95% in the hippocampus and by 83% in the brainstem region including the raphe nuclei (Figure 6A,B) whereas 5-HT7 receptor number was decreased in the hippocampus by 69%. Forty-eight hours after EEDQ, the density of 5-HT1A receptors in the hippocampus and brainstem remained significantly lower than in controls (−52 and −38% respectively). The reduction in 5-HT7 receptor density in the hippocampus 48 h after EEDQ did not reach statistical significance (Figure 6C). In all experiments, no significant differences were found in Kd values for [3H]-OH-DPAT or [3H]-5-CT binding (data not shown).
Figure 6.
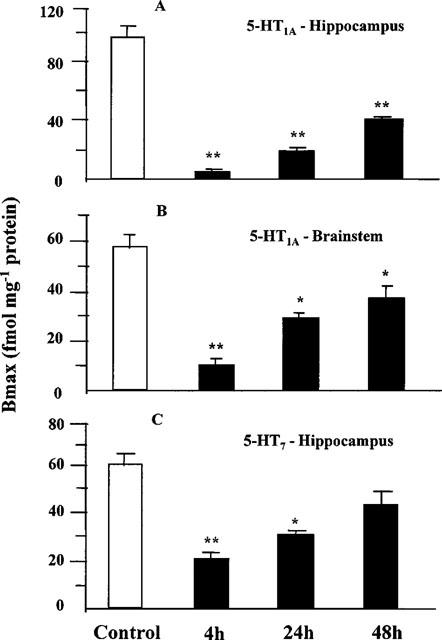
Effect of EEDQ (10 mg kg−1 s.c.) on 5-HT1A and 5-HT7 receptor density in rat brain regions. Values are mean±s.e.mean of 6–8 rats. *P<0.05; **P<0.01 (one-way ANOVA (A: F3,20=23.5; F3,25=19.2; C: F3,28=22.8) followed by Student-Newman-Keul's post hoc test).
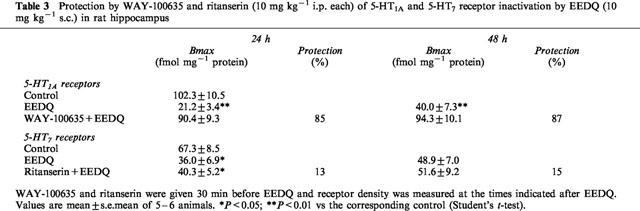
In protection experiments, high doses of WAY-100635 or ritanserin (10 mg kg−1 each) were given 30 min before EEDQ. Whereas 5-HT1A receptors were almost entirely protected by WAY-100635, protection of 5-HT7 receptors by ritanserin did not reach statistical significance (Table 3).
Table 3.
Protection by WAY-100635 and ritanserin (10 mg kg−1 i.p. each) of 5-HT1A and 5-HT7 receptor inactivation by EEDQ (10 mg kg−1 s.c.) in rat hippocampus
5-HT receptor mRNA levels
In the hippocampus, changes in 5-HT1A receptor mRNA levels were found after EEDQ. Four and 24 h after treatment, a moderate though significant increase in the optical density of the corresponding cDNA signal was found. Conversely, no change was found in the signal for the 5-HT7 receptor (Figure 7).
Figure 7.
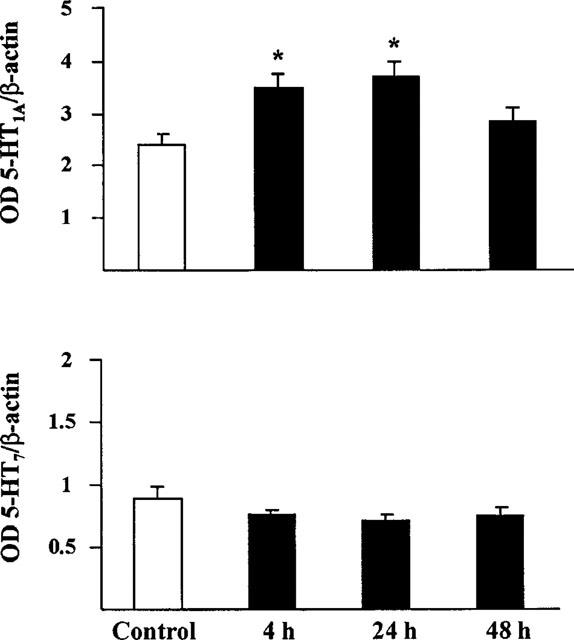
Effect of EEDQ (10 mg kg−1 s.c.) on 5-HT1A and 5-HT7 receptor mRNA expression in the hippocampus of rats killed at different times after treatment. Values are mean±s.e.mean (n=5) expressed as optical density ratio of the hybridization signal of the 5-HT receptor compared with β-actin on the same blot. *P<0.05 vs control (one-way ANOVA, F3,16=11.02, followed by Student-Newman-Keul's test).
Discussion
Pretraining administration of the 5-HT1A receptor agonist 8-OH-DPAT or the partial agonist buspirone significantly impaired passive avoidance retention in rats. The impairment caused by 8-OH-DPAT was blocked by the 5-HT1A receptor antagonists NAN-190 and WAY-100635, at doses without any intrinsic effect on the learning task. The alkylating agent EEDQ also impaired passive avoidance performance. The disruptive effect of EEDO was counteracted by 8-OH-DPAT or buspirone and prevented by ritanserin or methiothepin. When 5-HT1A receptors were protected from EEDQ-induced inactivation by a high-dose of WAY-100635, 8-OH-DPAT did not reverse the disruptive effect of EEDQ.
The impairment in passive avoidance performance by systemic 8-OH-DPAT is consistent with previous data demonstrating deficits not only in passive avoidance retention (Carli et al., 1992; Misane et al., 1998) but also in other cognitive tasks such as spatial learning in a water maze (Kang et al., 1998) and DNMTP (Warburton et al., 1997). A disruption in passive avoidance performance by buspirone has been also reported (Rowan et al., 1990). Activation of 5-HT1A receptors causes hyperpolarization of pyramidal neurons in CA1 and CA3 fields of the rat hippocampus due to an increase in a potassium conductance (Beck et al., 1992). Concerning the phenomenon of long-term potentiation (LTP), which represents a possible neural basis for learning and memory, it has been reported that the induction of hippocampal LTP is potentiated by NAN-190 through blockade of 5-HT1A receptors (Sakai & Tanaka, 1993).
Pretreatment with EEDQ, an alkylating agent that inactivates different G-protein coupled receptors (Belleau et al., 1969), produced a marked sedation and also impaired passive avoidance learning. Unexpectedly, the acquisition deficit induced by EEDQ was reversed by the 5-HT1A receptor full agonist 8-OH-DPAT and also by the partial agonist buspirone. EEDQ-reversal by 8-OH-DPAT was not achieved at the cost of any change in spontaneous locomotor activity since, at the dose used, 8-OH-DPAT neither modified locomotion nor antagonized the sedating effect of EEDQ. No significant difference in the pain threshold to electrical stimuli applied to the grid floor was either found between rats receiving EEDQ or the combined treatment EEDQ+8-OH-DPAT. Hence the results obtained in the passive avoidance studies do not seem to be the consequence of the signs of short-term neurotoxicity elicted by EEDQ. It appeared of interest to examine the dynamics of 5-HT1A receptor inactivation by EEDQ on postsynaptic receptors of the hippocampus and also on somatodendritic receptors of the raphe nuclei. It was found that receptor inactivation was more marked in the hippocampus than in the brainstem, both 4 and 48 h after EEDQ administration, as could be expected from the larger presynaptic 5-HT1A receptor reserve (Yocca et al., 1992). It is known that infusion of a high dose of 8-OH-DPAT into the median raphe nucleus improves performance accuracy in the DNMTP task while infusion of 8-OH-DPAT into the dorsal hippocampus impairs performance (Warburton et al., 1997). These findings strongly suggest that the amnesic effects of the 5-HT1A agonist are mediated by postsynaptic 5-HT1A receptors. Even though postsynaptic 5-HT1A receptors in the hippocampus were inactivated to a larger extent than the presynaptic 5-HT1A receptors of the brainstem 48 h after EEDQ, we can exclude the possibility that the agonistic activity of 8-OH-DPAT at presynaptic 5-HT1A receptors was involved in the reversal of EEDQ-induced retention found in the present study, as the reversing effect of 8-OH-DPAT was further enhanced in rats receiving the 5-HT1A antagonists NAN-190 or WAY-100635, suggesting a non-5-HT1A receptor-mediated effect. Indeed, the retention latency of EEDQ-pretreated rats receiving an additional combined treatment of 8-OH-DPAT and a 5-HT1A receptor antagonist was virtually identical to that of controls. Furthermore, no reversal by 8-OH-DPAT of the EEDQ effect was found when 5-HT1A receptor inactivation was prevented by pretreatment with a high WAY-100635 dose (cf. Gozlan et al., 1994). Consequently, it appears that the facilitatory effect of 8-OH-DPAT in EEDQ-pretreated rats is not mediated at all through 5-HT1A receptor stimulation.
8-OH-DPAT shows a low affinity at non-5-HTergic receptors or other 5-HT receptor subtypes, one exception being the 5-HT7 receptor (Shen et al., 1993). 8-OH-DPAT not only binds to the latter receptor type but also stimulates cyclic AMP formation in rat frontocortical astrocytes through 5-HT7 receptors (Shimizu et al., 1998). We found that EEDQ injection produced a less pronounced inactivation of 5-HT7 receptors than of 5-HT1A receptors in the hippocampus. The enhanced 5-HT1A receptor mRNA expression 4 and 24 h after EEDQ treatment suggests increased receptor synthesis to compensate for the marked receptor inactivation (Rahupathi et al., 1996). In an analogous fashion, the lack of changes in 5-HT7 receptor mRNA levels after EEDQ treatment may be interpreted in terms of lower receptor inactivation, although the missmatch between 5-HT7 mRNA and [3H]-5-CT-labelled 5-HT7 receptors in some hippocampal subfields (Gustafson et al., 1996) may perhaps yield conflicting results. To test the hypothesis that the paradoxical effect of 8-OH-DPAT, and also of buspirone, which has been reported to possess some affinity at 5-HT7 receptors (Boess & Martin, 1994), was mediated through 5-HT7 receptor activation, we planned a protection experiment with ritanserin, a 5-HT receptor antagonist with moderate-high affinity at 5-HT7 receptors and virtually devoid of affinity at 5-HT1A receptors (Boess & Martin, 1994). In keeping with previous protection experiments against EEDQ receptor inactivation (Gozlan et al., 1994), a very high dose of ritanserin (10 mg kg−1) was used. Ritanserin, given before EEDQ, prevented the retention deficit and also enhanced the ability of 8-OH-DPAT to reverse the effect of EEDQ. The retention impairment by EEDQ was also prevented, by methiothepin, an antagonist with higher affinity at 5-HT7 than at 5-HT1A receptors (Boess & Martin, 1994). Binding assays showed, however, that ritanserin only exerted a limited protection (c. 15%) on 5-HT7 receptor inactivation. Indeed, the moderate affinity of ritanserin at 5-HT7 receptors, as compared with the much higher affinity of WAY-1006365 at 5-HT1A receptors (Kd=0.37 nM; Khawaja et al., 1995) makes it unlikely a full selective protection of 5-HT7 receptors with any of the readily available pharmacological tools. It is hard to believe that the modest 5-HT7 receptor protection by ritanserin may account for the impressive prevention of the disruptive effect of EEDQ on passive avoidance performance. Other 5-HT1/5-HT2 receptor subtypes with high affinity for both methiothepin and ritanserin (Boess & Martin, 1994) may perhaps be involved. Moreover, according to binding affinities, 8-OH-DPAT at the low dose of 0.1 mg kg−1 may not be acting at 5-HT7 receptors, and it is still less likely that buspirone (1 mg kg−1) may have some effect at this 5-HT receptor type.
The issue of a dual effect for 8-OH-DPAT is not new, and both excitatory and inhibitory effects have been reported for this agonist in other studies non related to learning and memory (Millan, 1995; Clarke et al., 1997). Thus, the facilitatory action of 8-OH-DPAT in decerebrated and spinalized rabbits cannot be blocked by selective 5-HT1A antagonists, so it was proposed that non-5-HT1A receptors, such as 5-HT7 or 5-HT1D receptors, should be involved in this effect, as these receptors were ritanserin-sensitive (Clarke et al., 1997). The latter possibility seems unlikely in the present study. Even though a high potency in terms of cyclic AMP response has been shown for 8-OH-DPAT in C6 glial cells transfected with the human 5-HT1D receptor (Pauwels & Colpaert, 1996), most binding studies have demonstrated a moderate to low affinity of 8-OH-DPAT at 5-HT1D receptors from different species in different expression systems (Boess & Martin, 1994). Moreover, it seems unlikely that 5-HT1A and 5-HT1D receptors, both of them coupled to adenylate cyclase inhibition, play an opposing role in passive avoidance learning. Obviously, the multiple possibilities of interaction of 5-HT1A receptors with cholinergic and glutamatergic systems (e.g. reviews by Cassel & Jeltsch, 1994; Meneses & Hong, 1997) should also be considered at the time of interpreting the present results.
In summary, it can be concluded that 8-OH-DPAT exerts a dual effect on passive avoidance retention in the rat. Deficits in passive avoidance performance are probably due to stimulation of postsynaptic 5-HT1A receptors in the hippocampus. However, 8-OH-DPAT is also able to facilitate passive avoidance learning after inactivation by EEDQ of hippocampal 5-HT1A receptors and this effect is further enhanced by 5-HT1A receptor antagonists. Moreover, such a facilitation is not observed when 5-HT1A receptors are protected from EEDQ inactivation. Accordingly, other non-5-HT1A but 8-OH-DPAT-sensitive receptors should be involved in this facilitatory effect.
Acknowledgments
Supported by EC-BIO4C-960752 and CEPA/CICYT-970315 (Spain). We are grateful to Fundación Ramón Areces (A. Otano) and to Ministry of Education, Spain (A.Garcia-Osta) for fellowships. We thank Ms María Espelosín for her excellent technical assistance.
Abbreviations
- 5-CT
5-carboxamidotryptamine
- 5,7-DHT
5,7-dihydroxytryptamine
- 8-OH-DPAT
8-hydroxy-2-(di-n-propylamino)tetralin
- DNMTP
delayed non-matching to position
- EEDQ
N-ethoxycarbonyl-2-ethoxy-1,2-dihydroquinoline
- LTP
long-term potentiation
References
- AGUIRRE N., FRECHILLA D., GARCÍA-OSTA A., LASHERAS B., DEL RÍO J. Differential regulation by methylenedioxymethamphetamine of 5-hydroxytryptamine1A receptor density and mRNA expression in rat hippocampus, frontal cortex and brainstem: the role of corticosteroids. J. Neurochem. 1997;68:1099–1105. doi: 10.1046/j.1471-4159.1997.68031099.x. [DOI] [PubMed] [Google Scholar]
- ALBERT P.R., ZHOU Q.-Y., VAN TOL H.H.M., BUNZOW J.R., CIVELLI O. Cloning, functional expression and mRNA tissue distribution of the rat 5-hydroxytryptamine1A receptor gene. J. Biol. Chem. 1990;265:5825–5832. [PubMed] [Google Scholar]
- ARTAIZ I., ROMERO G., ZAZPE A., MONGE A., CALDERÓ M.M., ROCA J., LASHERAS B., DEL RÍO J. The pharmacology of VA21B7: an atypical 5-HT3 receptor antagonist with anxiolytic-like properties in animal models. Psychopharmacology. 1995;117:137–148. doi: 10.1007/BF02245179. [DOI] [PubMed] [Google Scholar]
- AZMITIA E.C., SEGAL M. An autoradiographic analysis of the differential ascending projections of the dorsal and median raphe nuclei in the rat. J. Comp. Neurol. 1978;179:641–668. doi: 10.1002/cne.901790311. [DOI] [PubMed] [Google Scholar]
- BARTSCH D., GHIRARDI M., SKEHEL P.A., KARL K.A., HERDER S.P., CHEN M., BAILEY C.H., KANDEL E.R. Aplysia CREB2 represses long-term facilitation: relief of repression converts transient facilitation into long-term functional and structural change. Cell. 1995;83:979–992. doi: 10.1016/0092-8674(95)90213-9. [DOI] [PubMed] [Google Scholar]
- BATTAGLIA G., NORMAN A.B., CREESE Y. Differential serotonin2 receptor recovery in mature and senescent rat brain after irreversible receptor modification: effect of chronic reserpine treatment. J. Pharmacol. Exp. Ther. 1987;243:69–75. [PubMed] [Google Scholar]
- BECK S.G., CHOI K.C., LIST T.J. Comparison of 5-hydroxytryptamine1A-mediated hyperpolarization in CA1 and CA3 hippocampal pyramidal cells. J. Pharmacol. Exp. Ther. 1992;263:350–359. [PubMed] [Google Scholar]
- BELLEAU B., DITULLIO V., GODIN D. The mechanism of irreversible adrenergic blockade by N-carbethoxydihydroquinolines. Model studies with typical serine hydrolases. Biochem. Pharmacol. 1969;18:1039–1044. doi: 10.1016/0006-2952(69)90107-5. [DOI] [PubMed] [Google Scholar]
- BENTLEY K.R., BARNES J.M. Therapeutic potential of serotonin 5-HT3 antagonists in neuropsychiatric disorders. CNS Drugs. 1995;3:363–392. [Google Scholar]
- BOESS F.G., MARTIN I.L. Molecular biology of 5-HT receptors. Neuropharmacology. 1994;33:275–317. doi: 10.1016/0028-3908(94)90059-0. [DOI] [PubMed] [Google Scholar]
- BUHOT M.-C., PATRA S.K., NAÏLI S. Spatial memory deficits following stimulation of hippocampal 5-HT1B receptors in the rat. Eur. J. Pharmacol. 1995;285:221–228. doi: 10.1016/0014-2999(95)00407-c. [DOI] [PubMed] [Google Scholar]
- CARLI M., LUSCHI R., GAROFALO P., SAMANIN R. 8-OH-DPAT impairs spatial but not visual learning in a water maze by stimulating 5-HT1A receptors in the hippocampus. Behav. Brain Res. 1995;67:67–74. doi: 10.1016/0166-4328(94)00105-o. [DOI] [PubMed] [Google Scholar]
- CARLI M., SAMANIN R. 8-Hydroxy-2-(di-n-propylamino)tetralin impairs spatial learning in a water maze: Role of postsynaptic 5-HT1A receptors. Br. J. Pharmacol. 1992;105:720–726. doi: 10.1111/j.1476-5381.1992.tb09045.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CARLI M., TRANCHINA S., SAMANIN R. 8-Hydroxy-2-(di-n-propylamino)tetralin, a 5-HT1A receptor agonist, impairs performance in a passive avoidance task. Eur. J. Pharmacol. 1992;211:227–234. doi: 10.1016/0014-2999(92)90533-a. [DOI] [PubMed] [Google Scholar]
- CASSEL J.C., JELTSCH H. Serotonergic modulation of cholinergic function in the central nervous system: Cognitive implications. Neuroscience. 1995;69:1–41. doi: 10.1016/0306-4522(95)00241-a. [DOI] [PubMed] [Google Scholar]
- CLARKE R.W., OGILVIE J., HOUGHTON A.K. Enhancement and depression of spinal reflexes by 8-hydroxy-2-(di-n-propylamino)tetralin in the decerebrated and spinalized rabbit: involvement of 5-HT1A- and non-5-HT1A-receptors. Br. J. Pharmacol. 1997;122:631–638. doi: 10.1038/sj.bjp.0701430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FONTANA D.J., DANIELS S.E., WONG E.H., CLARK R.D., EGLEN R.M. The effects of novel, selective 5-hydroxytryptamine4 receptor ligands in rat spatial navigation. Neuropharmacology. 1997;36:689–696. doi: 10.1016/s0028-3908(97)00055-5. [DOI] [PubMed] [Google Scholar]
- GOZLAN H., EL MESTIKAWY S., PICHAT L., GLOWINSKI J., HAMON M. Identification of presynaptic serotonin autoreceptors using a new ligand: 3H-PAT. Nature. 1983;305:140–142. doi: 10.1038/305140a0. [DOI] [PubMed] [Google Scholar]
- GOZLAN H., LAPORTE A.M., THIBAULT S., SCHECHTER L.E., BOLAÑOS F., HAMON M. Differential effects of N-ethoxycarbonyl-2-ethoxy-1,2-dihydroquinoline (EEDQ) on various 5-HT receptor binding sites in the rat brain. Neuropharmacology. 1994;33:423–431. doi: 10.1016/0028-3908(94)90072-8. [DOI] [PubMed] [Google Scholar]
- GUSTAFSON E.L., DURKIN M.M., BARD J.A., ZGOMBICK J., BRANCHEK T.A. A receptor autoradiographic and in situ hybridization analysis of the distribution of the 5-HT7 receptor in the rat brain. Br. J. Pharmacol. 1996;117:657–666. doi: 10.1111/j.1476-5381.1996.tb15241.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- JARRARD L.E. On the role of the hippocampus in learning and memory in the rat. Behav. Neural. Biol. 1993;60:9–26. doi: 10.1016/0163-1047(93)90664-4. [DOI] [PubMed] [Google Scholar]
- KANT G.J., WYLIE R.M., CHU K., GHOSH S. Effects of the serotonin agonists 8-OH-DPAT, buspirone, and DOI on water maze performance. Pharmacol. Biochem. Behav. 1998;59:729–735. doi: 10.1016/s0091-3057(97)00553-4. [DOI] [PubMed] [Google Scholar]
- KECK B.J., LAKOSKI J.M. Age-related assessment of central 5-HT1A receptors following irreversible inactivation of N-ethoxycarbonyl-2-ethoxy-1,2-dihydroquinoline (EEDQ) Brain Res. 1996;728:130–134. doi: 10.1016/s0006-8993(96)00481-7. [DOI] [PubMed] [Google Scholar]
- KHAWAJA X., EVANS N., REILLY Y., ENNIS C., MINCHIN M.C.W. Characterisation of the binding of [3H]WAY-100635, a novel 5-HT1A receptor antagonist, to rat brain. J. Neurochem. 1995;64:2716–2726. doi: 10.1046/j.1471-4159.1995.64062716.x. [DOI] [PubMed] [Google Scholar]
- MCNAUGHTON N., MORRIS R.G.M. Buspirone produces a dose-related impairment in spatial navigation. Pharmacol. Biochem. Behav. 1992;43:167–171. doi: 10.1016/0091-3057(92)90653-w. [DOI] [PubMed] [Google Scholar]
- MENESES A., HONG E. Role of 5-HT1A receptors in acquisition, consolidation and retrieval of learning. CNS Drug Rev. 1997;3:68–82. [Google Scholar]
- MILLAN M.J. Serotonin (5-HT) and pain: A reappraisal of its role in the light of receptor multiplicity. Seminars Neurosci. 1995;7:409–419. [Google Scholar]
- MISANE Y., JOHANSSON C., ÖGREN S.O. Analysis of the 5-HT1A receptor involvement in passive avoidance in the rat. Br. J. Pharmacol. 1998;125:499–509. doi: 10.1038/sj.bjp.0702098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PAUWELS P.J., COLPAERT F.C. Stereoselectivity of 8-OH-DPAT enantiomers at cloned human 5-HT1D receptor sites. Eur. J. Pharmacol. 1996;300:137–139. doi: 10.1016/0014-2999(95)00876-4. [DOI] [PubMed] [Google Scholar]
- PAZOS A., PALACIOS J.M. Quantitative autoradiographic mapping of serotonin receptors in the rat brain. I. Serotonin-1 receptors. Brain Res. 1985;346:205–230. doi: 10.1016/0006-8993(85)90856-x. [DOI] [PubMed] [Google Scholar]
- RAGHUPATHI R.K., BROUSSEAU D.A., M MCGONIGLE P. Time-course of recovery of 5-HT1A receptors and changes in 5-HT1A receptor mRNA after irreversible inactivation with EEDQ. Mol. Brain Res. 1996;38:233–242. doi: 10.1016/0169-328x(95)00311-f. [DOI] [PubMed] [Google Scholar]
- RIEKKINEN P., JR 5-HT1A and muscarinic acetylcholine receptors jointly regulate passive avoidance behavior. Eur. J. Pharmacol. 1994;262:77–90. doi: 10.1016/0014-2999(94)90030-2. [DOI] [PubMed] [Google Scholar]
- ROWAN M.J., CULLEN W.K., MOULTON B. Buspirone impairment of performance of passive avoidance and spatial learning tasks in the rat. Psychopharmacology. 1990;122:137–146. doi: 10.1007/BF02244613. [DOI] [PubMed] [Google Scholar]
- SAKAI Y., TANAKA C. Inhibitory modulation of long-term potentiation via the 5-HT1A receptor in slices of the rat hippocampal dentate gyrus. Brain Res. 1993;613:326–330. doi: 10.1016/0006-8993(93)90921-9. [DOI] [PubMed] [Google Scholar]
- SHEN Y., MONSMA F.J., JR, METCALF M.A., JOSE P.A., HAMBLIN M.W., SIBLEY D.R. Molecular cloning and expression of a 5-hydroxytryptamine7 serotonin receptor subtype. J. Biol. Chem. 1993;268:18200–18204. [PubMed] [Google Scholar]
- SHIMIZU M., NISHIDA A., ZENSHO H., MIYATA M., YAMAWAKI S. Agonist-induced desensitization of adenylyl cyclase activity mediated by 5-hydroxytryptamine7 receptors in rat frontocortical astrocytes. Brain Res. 1998;784:57–62. doi: 10.1016/s0006-8993(97)01185-2. [DOI] [PubMed] [Google Scholar]
- TO Z.P., BONHAUS D.W., EGLEN R.M., JAKEMEN L.B. Characterization and distribution of putative 5-HT7 receptors in guinea-pig brain. Br. J. Pharmacol. 1995;115:107–116. doi: 10.1111/j.1476-5381.1995.tb16327.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WARBURTON E.C., HARRISON A.A., ROBBINS T.W., EVERITT B.J. Contrasting effects of systemic and intracerebral infusions of the 5-HT1A receptor agonist 8-OH-DPAT on spatial short-term working memory in rats. Behav. Brain Res. 1997;84:247–258. doi: 10.1016/s0166-4328(96)00154-4. [DOI] [PubMed] [Google Scholar]
- YOCCA F.D., IBEN L., MELLER E. Lack of apparent receptor reserve at postsynaptic 5-hydroxytryptamine1A receptors negatively coupled to adenylyl cyclase activity in rat hippocampal membranes. Mol. Pharmacol. 1992;41:1006–1072. [PubMed] [Google Scholar]


